Abstract
Mutations in SETD2 are among the most prevalent drivers of renal cell carcinoma (RCC). We identified a novel single nucleotide polymorphism (SNP) in SETD2, E902Q, within a subset of RCC patients, which manifests as both an inherited or tumor-associated somatic mutation. To determine if the SNP is biologically functional, we used CRISPR-based genome editing to generate the orthologous mutation within the Drosophila melanogaster Set2 gene. In Drosophila, the homologous amino acid substitution, E741Q, reduces H3K36me3 levels comparable to Set2 knockdown, and this loss is rescued by reintroduction of a wild-type Set2 transgene. We similarly uncovered significant defects in spindle morphogenesis, consistent with the established role of SETD2 in methylating α-Tubulin during mitosis to regulate microtubule dynamics and maintain genome stability. These data indicate the Set2 E741Q SNP affects both histone methylation and spindle integrity. Moreover, this work further suggests the SETD2 E902Q SNP may hold clinical relevance.
Keywords: renal cell carcinoma, SETD2, Drosophila, SNP, models of human disease, H3K36me3
A novel mutation in the tumor suppressor SETD2 identified from renal cell carcinoma patients compromises epigenetic modification and mitotic fidelity when introduced in Drosophila, hinting at clinical relevance.
Introduction
Renal cell carcinoma (RCC) is expected to occur in over 81,800 individuals in the United States in 2023 (Siegel et al. 2023) and is the 14th most common cancer worldwide, affecting over 430,000 people each year (Ferlay et al. 2021). Clear cell RCC (ccRCC) is the most common histologic subtype, accounting for ∼80% of all RCCs.
The most common somatic mutations in ccRCC occur in VHL (52%), PBRM1 (31%), SETD2 (15%), BAP1 (13%), MTOR (8%), KDM5C (8%), TP53 (7%), and LRP1B (5%) (Tate et al. 2019). Among these, SETD2 is the H3K36 methyltransferase (H3K36me3) associated with gene activation (Bannister et al. 2005; Edmunds et al. 2008). In addition to its H3K36me3 activity, SETD2 also methylates α-Tubulin at lysine 40 (αTubK40me3) (Park et al. 2016). Consequently, SETD2 loss impairs microtubule polymerization and stability, resulting in spindle and cytokinesis errors and genomic instability (Park et al. 2016; Kearns et al. 2021; Xie et al. 2021).
Inactivation of SETD2 is observed in ccRCC and is coincident with H3K36me3 depletion (Duns et al. 2010; Li et al. 2016). While mutations in SETD2 are commonly associated with RCC, it is also somatically mutated in several other cancers, including meningeal, skin, liver, endometrial, colon, cervix, prostate, and lung cancers (Tate et al. 2019). Given that SETD2 is a critical tumor suppressor, identifying disease-relevant mutations may improve clinical outcomes.
While examining circulating tumor DNA (ctDNA) from RCC patients, we identified a novel single nucleotide polymorphism (SNP; rs58906143, chr3:47163422 C→G (hg19); E902Q (The SNP is a C to G. However, since the RNA is transcribed from the reverse strand, the RNA mutation is G2704C. This results in an amino acid alteration of GAG (glutamic acid) to CAG (glutamine) or the E902Q mutation.)) within SETD2. SETD2 is evolutionarily conserved across eukaryotes and related to the set2 gene originally identified in Saccharomyces cerevisiae (Strahl et al. 2002). As the SETD2 E902Q SNP resides outside the previously characterized functional domains, we sought to investigate its biological relevance by introducing the homologous missense mutation in Drosophila Set2 (E741Q) by CRISPR/CAS-9 genome editing (Larschan et al. 2007; Stabell et al. 2007). Modeling the putative human disease mutation in Drosophila Set2 led to reduced H3K36me3 activity. Set2 E741Q also impaired spindle integrity in rapidly dividing syncytial embryos, suggesting that α-Tub modification may be compromised. Expression of a genomic rescuing Set2 construct restored H3K36me levels and mitotic fidelity. Taken together, these data argue that the SETD2 SNP identified in RCC patients is biologically functional and may be relevant to human disease.
Materials and methods
Sample acquisition
Patients at Emory University Hospital with a solid renal mass concerning for RCC were identified prospectively. Controls were identified during an annual physical exam (Healthy controls, without imaging) or after screening patients for current (within 1 year) CT scan proving no solid renal mass (Radiologic control group, Atlanta Veterans Affairs Medical Center). Blood was collected, and plasma and buffy coat were banked. All provided informed consent.
Blood was drawn in EDTA tubes (BD Vacutainer, VWR, Radnor, PA, USA) placed on ice and processed within 2 h by centrifugation at 1300 g, 4°C, for 20 min. Buffy coat was collected and stored at −80°C for future DNA purification. Plasma was collected and transferred to a 50 mL conical tube, and a second spin was executed at 3200 g, 4°C, for 10 min. Plasma was transferred to a clean 50 mL conical tube and stored at −80°C for future ctDNA purification. Buffy coat DNA was purified using the Flexigene DNA kit (Qiagen, Germantown, MD, USA). ctDNA was purified using either QiAmp-Circulating Nucleic Acid Kit (Qiagen, Mansfield, MA, USA) or GenElute UltraMag Cell-Free DNA Kit (MilliPore Sigma, Burlington, MA, USA) according to manufacturer's protocols. ctDNA was concentrated after purification using Vivaspin 500 centrifugal filter unit (VWR). The Qubit dsDNA HS Assay Kit (ThermoFisher, Waltham, MA, USA) was used to measure DNA concentration. DNA was stored at −20°C.
Initial experimental determination of SNP
Fluidigm Access Array (South San Francisco, CA, USA) amplicons were designed to interrogate the coding sequence and splice sites of 14 genes primarily based on their known association with RCC and urothelial carcinoma. The array included VHL, PBRM1, SETD2, BAP1, and KDM5C.
Sequencing and SNP genotyping
To identify variants, DNA libraries were prepared from germline genomic DNA (gDNA) with a customized Fluidigm Access Array library prep and sequenced on an Illumina MiSeq instrument (San Diego, CA, USA). The Fluidigm 48.48 Access Array Integrated Fluidic Circuit library preparation method was described previously (Obeng et al. 2020). The libraries were sequenced (2 × 150, 300 cycles) in the same Illumina MiSeq sequencing run using the version 2 reagent kit. Demultiplexed FASTQ data files were processed through a robust, reproducible analysis workflow to identify variants in the coding regions of the 14 genes (Obeng et al. 2020). All variants were manually inspected and confirmed using the Integrative Genomics Viewer version 2.3.42.
Determination of somatic mutation in ctDNA
Libraries were prepared from 20 ng of ctDNA using Agilent's SureSelcetXT HS library prep kit (Agilent Technologies, Santa Clara, CA, USA), adding unique sample and molecular barcodes. Sequencing libraries were then enriched using a custom Agilent SureSelect hybrid capture panel incorporating our RCC-targeted 16 gene panel (Supplementary Table 1). Enriched libraries are sequenced from 200 to 250nt target regions in 96 sample batches on an Illumina MiSeq (San Diego, CA, USA) using paired-end 100 bp chemistry. Barcoding, library preparation, targeted hybrid capture, and sequencing were supported through the Emory Integrated Genomics core. Data analytics was performed utilizing Agilent SureCall 4.1.2.11.
Genotyping of samples
The majority of patients samples were genotyped for rs58906143 using TaqMan SNP Genotyping Assays C_90007756_10 (SNP ID: rs58906143) (ThermoFisher). Assays were done by the Emory Integrated Genomics Core according to manufacturer's protocol.
Genome-wide SNP assay
Ancestry analysis was conducted in cases and controls using Affymetrix's Precision Medicine Research Array, which is optimized for multiethnic coverage and functional variants. The genomic DNA was standardized to 50 ng/µL and processed following standard protocols, including hybridization, incubation, and scanning (Affymetrix protocol). Genome-wide SNP assays were performed by AKESOgen (Peachtree Corners, GA, USA). Genome-wide SNP data were processed and quality controlled using standard measures (e.g. call rate, sex-mismatch, outlier of population structure and Hardy–Weinberg Equilibrium) and merged with phenotypic data for genome-wide analysis (Raghavan et al. 2022). Some samples from the original genotype database were filtered out due to high relatedness. Total number of samples from combined genome-wide SNP assay and genotyping data was 867. A PCA plot of the data is shown in Supplementary Fig. 1. There were 52 mismatched by ancestry between the genome-wide SNP data and the self-reported data. Because racial disparities in RCC survival are well documented (Siegel et al. 2023), we compared individuals from African Ancestry (AA) and European Ancestry (EA).
Fly stocks
The following Drosophila strains and transgenic lines were used: y1w1118 [Bloomington Drosophila Stock Center (BDSC) #1495] was used as the wild-type (WT) control unless otherwise noted. The maternal driver maternal α-Tubulin67C-GAL4 (mat-GAL4; BDSC #7063) was used to drive P(UAS-Set2.IR) (BDSC #24108) to express dsRNA against Set2 (Larschan et al. 2007; Mukai et al. 2015). The functional Set2 rescue construct, P(Set2+10.3)2 (BDSC #77917), spans the Set2 genomic region (Larschan et al. 2007) and was used to test for rescue of Set2 CRISPR mutants. mat-GAL4, UASp-GFP-αTub (Lucas and Raff 2007) was used to visualize microtubule dynamics. In all experiments, mutant or transgenic embryos represent progeny derived from mutant or transgenic mothers to examine maternal effects. CRISPR mutant stocks are maintained over FM7 balancers, although homozygous females and hemizygous males are readily recoverable. Flies were raised on Bloomington formula Drosophila medium (Lab-Express; Ann Arbor, MI, USA) and crosses were maintained at 25°C in a light- and temperature-controlled incubator chamber.
Construction of set2 CRISPR lines
Human SETD2 was aligned to Drosophila Set2-RA in MAFFT software (https://mafft.cbrc.jp/alignment/server/) (Katoh and Standley 2013) and EMBOSS (https://www.ebi.ac.uk/Tools/psa/emboss_needle) (Needleman and Wunsch 1970) to identify E741 as the Drosophila amino acid orthologous to hSETD2 E902. Set2E741Q point mutants were genetically engineered via CRISPR-CAS9 technology by Rainbow Transgenic Flies, Inc. (Camarillo, CA, USA) by mutating GAG (glutamic acid) to CAG (glutamine) using the guide RNA (aacaccgttgccagagatgaagg; PAM underlined) and a single-stranded oligo DNA nucleotide (ssODN) 5′-aacaatcaaaaggggcaaaagcaaacaccgttgccagagatgaagCagcccgagaaacccgtcgccgaaactgtgtccaaaaaggagaaggcaatg −3′, where the underlined base indicates the introduced point mutation (G->C). Four mutant and 3 control (nonmutated) lines were identified by DNA sequencing using the forward Set2 primer: 5′- aagactgaggcagcgaaaga −3′. We used 3 mutant and one control line in this study: Set2[E741Q]2, Set2[E741Q]4, Set2[E741Q]10, and Set2CTRL#31. Line Set2[E741Q]4 was genetically combined with the Set2 transgene, P(Set2 + 10.3)2 (Larschan et al. 2007) for rescue experiments.
Immunofluorescence
Ovaries were prepared for immunofluorescence as described (Benner et al. 2019). Briefly, ovaries from 0 to 1-day females fed on yeast paste for 24 h were dissected in phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde diluted into PBTX (PBS, 0.3% Triton x-100) for 15 min, washed several times with PBTX, blocked in 1% BBT [PBS supplemented with 0.1% Tween-20, 2% normal goat serum (NGS) and 1% globulin-free bovine serum albumin (BSA)] for at least 30 min, and incubated overnight at 4°C with nutation in primary antibodies diluted into 1% BBT. The following primary antibodies were used: rat anti-Vasa [1:10, Developmental Studies Hybridoma Bank (DSHB)] and rabbit anti-Histone H3K36me3 (1:250, ab194677, Abcam; Cambridge, UK). In a pilot experiment, mouse anti-H3K36me3 (1:10, gift from Hiroshi Kimura) was the primary antibody (Kimura et al. 2008). The next day, samples were rinsed in PBST (PBS supplemented with 0.1% Tween-20) and further blocked in 1% BBT supplemented with 2% NGS prior to incubation with secondary antibodies for 2 h at room temperature with nutation. Samples were washed well in PBST, teased apart under a dissecting scope using insect pins, then mounted in Aqua-Poly/Mount mounting medium (Polysciences, Inc.; 87001-902, VWR).
For visualization of MTs, embryos were prepared as described (Theurkauf 1994; Lerit and Gavis 2011). Briefly, embryos were fixed in a 1:1 mixture of 37% paraformaldehyde: heptane for 3 min with intermittent mixing, washed 3 times in PBS, and manually devitellinized using 30G PrecisionGlide needles (BD). Embryos were blocked in 0.1% BBT (PBS supplemented with 0.1% BSA and 0.1% Tween-20), further blocked with Image-iT FX signal enhancer (Fisher Scientific) prior to incubation with mouse anti-α-Tubulin DM1α (1:500, Sigma-Aldrich T6199) and rabbit antiphosphoH3 Ser10 (pH3; 1:1000, EMD Millipore) antibodies.
The following secondary antibodies were used: Alexa Fluor goat antirabbit 488, goat antirabbit 568, goat antirat 568, or goat antimouse 488 (1:500, Molecular Probes). DAPI was used at 10 ng/ml (D1306; Thermo Fisher Scientific).
Microscopy
Images were acquired using a Nikon 40× 1.3-NA Plan Fluor oil immersion objective on a Nikon Ti-E inverted microscope fitted with a Yokogawa CSU-X1 spinning disk head (Yokogawa Corp. of America), Orca Flash 4.0 v2 CMOS camera (Hamamatsu Corp.), and a Nikon LU-N4 solid-state laser launch (15 mW; 405, 488, and 561 nm) at ambient temperature (∼25°C). The microscope was powered through Nikon Elements AR software on a 64-bit HP Z440 workstation (Hewlett-Packard).
Individual germaria were imaged with 1 μm optical sections through the depth of the tissue, while spindles from fixed embryos were imaged with 0.5 μm step sizes across approximately 10 μm of tissue.
Images were prepared for live imaging as previously described (Lerit et al. 2015). Briefly, 1–2 h old dechorionated embryos were aligned on an agar slab, transferred to a #1.5 glass coverslip coated in sticky glue, coated in series 700 halocarbon oil (Sigma), and inverted onto an optically clear, gas permeable membrane (Sarstedt) fitted with 2 #1 glass coverslip spacers. Embryos were imaged using a Nikon 40× 1.3-NA Plan Fluor oil immersion objective using 0.5 μm optical sections over 3 μm total depth at 60 s time intervals. Multiple nuclear divisions were captured per embryo, and at least 5 embryos were imaged per genotype.
Image analysis
Images were analyzed using Fiji software (NIH) (Schindelin et al. 2012), and the experimenter was blinded from genotype using a custom macro. For germaria, maximum intensity projections spanning up to 3 μm were rendered, and the background subtracted integrated density of the H3K36me3 channel was measured from N = 25 GSCs per genotype per replicate using the circular ROI tool. The analysis was repeated over 3 or more independent biological replicates. For live imaging, inactivated, bent, or multipolar spindles were scored from randomized timelapse videos. Individual optical sections were maximum intensity projected and displayed in greyscale using Fiji. For fixed embryos, the same spindle parameters were assessed from maximum-projected images.
Immunoblotting
Ovarian extracts were prepared from ovaries dissected from 5 well-fed 1-day old females. Ovaries were homogenized in 150 μL 0.1% PBST using a disposable plastic pestle and cordless motor. Forty microliters of 5 × SDS-loading buffer was added to each lysate, and the samples were boiled at 95°C for 10 min. Extracts were stored at −20°C or immediately resolved on a 12% SDS-PAGE and transferred onto a 0.2 μm nitrocellulose membrane (GE Healthcare, 10600001) by wet-transfer in a buffer containing 25 mM Tris-HCl, pH 7.6, 192 mM glycine, 10% methanol and 0.02% SDS or by semi-dry transfer using the Trans-Blot Turbo Transfer System (Bio-Rad, 1704150). Membranes were blocked for 1 h at room temperature in 5% dry milk diluted in TBST (Tris-based saline with 0.01% Tween-20), washed well with TBST, cut, and incubated overnight at 4°C with primary antibodies. The following primary antibodies were used: rabbit anti-H3K36me3 (1:1000, Abcam ab194677) and mouse anti-β-Tubulin E7 (βTub; 1:20,000, DSHB; M. Klymkowsky, University of Colorado, USA). While revising this manuscript, Abcam discontinued ab94677. Subsequently, for several immunoblots, we employed the rabbit anti-H3K36me3 antibody ab9050 (1:750) using 3% BSA in TBST for dilution and blocking. After washing with TBST, membranes were incubated for 1.5 h in secondary antibodies goat antimouse HRP and goat antirabbit HRP diluted 1:2500 in TBST. Bands were visualized with Clarity ECL substrate (Bio-Rad, 1705061) on a Bio-Rad ChemiDoc imaging system. Densiometry of bands was measured from independent biological replicates in Fiji using the Analyze Gels function. Values of H3K36me3 were normalized to the βTub load control and expressed as a fold-change from the WT control. Similar results were obtained with ab194677 vs ab9050.
Embryonic hatch rate analysis
Embryonic viability was scored from 24-h collections of eggs collected on yeasted grape juice agar plates (#47-102, Genesee Scientific; San Diego, CA, USA), transferred to fresh grape plates marked with a grid, then aged for 24 h at 25°C. The remaining unhatched embryos were counted and scored. For Set2 experiments, homozygous Set2 virgin females were mated to Set2/Y males.
Fecundity analysis
40–50 female and 20–25 male 0- to 2-day old Drosophila were prefed then allowed to lay on yeasted grape juice agar plates for 2 h. For Set2 experiments, homozygous Set2 virgin females were mated to Set2/Y males. Embryos were collected on 30 μm nitex nylon mesh (#57-105, Genesee Scientific; San Diego, CA, USA) by rinsing with water to dislodge any embryos from the yeast paste then counted under a dissection microscope. Embryo lays were scored daily over 3 consecutive days and are presented as an average number of embryos laid per female per day (Piper and Partridge 2016).
Statistical analysis
Enrichment of rs58906143 was determined using the SciPy and statsmodels libraries in Python. Data were analyzed using Fisher's exact test and logistic regression followed by a Bonferroni correction for multiple comparisons.
Drosophila data were plotted and statistical analysis performed using Microsoft Excel and GraphPad Prism software. Normality distributions were determined using a D’Agnostino and Pearson normality test. Data were then analyzed by Student's 2-tailed t-test, chi-square test, ANOVA, or a nonparametric Kruskal–Wallis test followed by Dunn's multiple comparison relative to the indicated control. Data are plotted as mean ± SD and are representative results from 3 independent experiments.
Results
Identification of the rs58906143 SNP from an initial sample set
An initial observation that the rs58906143 [chr3:47163422 C→G (hg19), E902Q] SNP may be overrepresented in RCC cases compared to controls was made from a cohort of 187 individuals with 117 RCC cases and 70 controls whose buffy coat had been banked and SETD2 had been sequenced for a prior study of circulating tumor DNA (ctDNA; unpublished). In this initial dataset, 151 SNPs were considered. For rs58906143, there was a G allele in 16/117 cases (13.7%) and 0/70 (0%) controls (uncorrected Fisher's exact test P-value = 6.13 × 10−4, Bonferroni-corrected P-value = 0.0923, undefined odds ratio). The prevalence of the SNP varies with different ancestries. For African ancestry (AA) patients, there was a G allele in 13/28 (46.4%) cases and 0/14 (0%) controls (uncorrected Fisher's exact test P-value = 1.60 × 10−3, Bonferroni-corrected P-value = 0.242, undefined odds ratio). For European ancestry (EA) patients, there was a G allele in 2/82 cases (2.4%) and 0/53 (0%) controls (uncorrected Fisher's exact test P-value = 0.520, Bonferroni-corrected P-value = 1, undefined odds ratio). Despite our early observation that the rs58906143 SNP may be overrepresented, these results failed to be statistically significant.
Prevalence of rs58906143 in the general population
The population frequency of the SNP varies by ancestry with EA patients having the C allele 99.5% of the time, and AA patients having the C allele approximately 78% of the time vs the G allele at 22% frequency (Sherry et al. 2001). Different population studies have the AA frequency of the G allele at slightly different rates (e.g. 10.1% of 3,364 AAs in the ALFA allele frequency database (Phan et al. 2020) vs 24.6% of 1006 AAs in the 1000 Genomes database [1000 Genomes Project Consortium et al. 2015)], while larger studies all show the G allele at approximately 22% (ExAC N = 10,222 (Lek et al. 2016); gnomAD-Exomes N = 16,162 (Karczewski et al. 2020); and the PAGE study N = 35,512 (Wojcik et al. 2019)). The PAGE study is the largest single cohort of AAs genotyped and found the G allele at a frequency of 21.1%. Our data are consistent with these studies.
Frequency of the rs58906143 SNP in an expanded cohort
Given these initial observations, we expanded our pilot study to include a total of 849 individuals, including 493 RCC cases and 356 controls. We genotyped each individual at the chr3:47163422 position using a TaqMan assay to evaluate the overrepresentation of the rs58906143 SNP and omitted the analysis of other SNPs except for the 151 original loci. In parallel, we performed a 900,000 SNP GWAS analysis to determine the ancestry of each individual, but these results were independent of our evaluation of the overrepresentation of the rs58906143 SNP. The details of this GWAS are in the Materials and Methods section. In the expanded dataset, there was a G allele in 72/493 cases (14.6%) and 24/356 (6.7%) controls (uncorrected Fisher's exact test P-value = 3.95 × 10−4, Bonferroni-corrected = 0.0596, odds ratio = 2.37). For AA patients, there was a G allele in 50/119 (42.0%) cases and 21/71 (29.6%) controls (uncorrected Fisher's exact test P-value = 0.0912, Bonferroni-corrected P-value = 1, odds ratio = 1.73). For EA patients, there was a G allele in 19/335 cases (5.7%) and 3/269 (1.1%) controls (uncorrected Fisher's exact test P = 3.50 × 10−3, Bonferroni-corrected P-value = 0.529, odds ratio = 5.33). Since RCC has a higher prevalence rate in older and male populations, we also investigated the prevalence of rs58906143 in RCC cases and controls while controlling for age, sex, and ancestry (unregularized logistic regression; SNP coefficient P = 3.40 × 10−3, Bonferroni-corrected P-value = 0.514, SNP logistic regression coefficient = 0.805). The frequency of the rs58906143 SNP in RCC cases vs controls again did not reach statistical significance, largely because the G allele was absent in controls in the pilot dataset but not in the expanded dataset. The demographic variation of these groups is shown in Tables 1 and 2.
Table 1.
Patient demographics.
| Control | RCC | |||||
|---|---|---|---|---|---|---|
| Total | EA | AA | Total | EA | AA | |
| Total | 57.5 ± 11.4 | 56.5 ± 11.5 | 59.5 ± 11.0 | 60.9 ± 11.1 | 61.3 ± 11.1 | 61.3 ± 10.2 |
| C/C | 57.1 ± 11.1 | 56.6 ± 11.4 | 59.7 ± 9.19 | 61.0 ± 11.3 | 61.4 ± 11.2 | 59.1 ± 11.5 |
| C/G + G/G | 63.7 ± 12.8 | 53.7 ± 18.5 | 65.1 ± 11.8 | 59.9 ± 10.3 | 59.0 ± 10.2 | 60.2 ± 10.4 |
The age (mean ± standard deviation) of all patients whose genotype was determined (European Ancestry, African Ancestry) broken down by genotype and ancestry.
Table 2.
Number of patients in EA and AA RCC and control cohorts based on genotype.
| RCC | Control | RCC | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Both | Female | Male | Both | Both | Both | Population control | ||
| EA | Total | 109 | 226 | 335 | 56 | 213 | 269 | |||
| CC | 107 | 209 | 316 | 54 | 212 | 266 | 92.9% | 98.9% | 99.93% | |
| CG & GG | 2 | 17 | 19 | 2 | 1 | 3 | 5.7% | 1.1% | 0.07% | |
| AA | Total | 49 | 70 | 119 | 14 | 57 | 71 | |||
| CC | 28 | 41 | 69 | 7 | 43 | 50 | 58.0% | 70.4% | 78.9% | |
| CG & GG | 21 | 29 | 50 | 7 | 14 | 21 | 42.0% | 29.6% | 21.1% | |
rs58906143 as a somatic mutation in RCC
To determine whether the rs58906143 SNP occurs as a somatic mutation, we sequenced both tumor tissue and ctDNA from 50 ccRCC patients. Of the 50 individuals, 10 (20%) had the E902Q mutation (Table 3). A somatic mutation in SETD2 was not previously reported for ccRCC. In contrast, somatic mutations in VHL are more common in ccRCC cases. Nevertheless, other inherited tumor syndromes, such as breast cancers, may have both inherited and somatic mutations in BRCA (Tate et al. 2019). Of note, the rs58906143 SNP is not reported as a somatic mutation in RCC in either cBioPortal (Cerami et al. 2012) (search included all available RCC datasets) or COSMIC V98 (Tate et al. 2019) databases as of July 2023.
Table 3.
The E858Q/E902Q mutation was detected as a somatic mutation in ctDNA from 10 of 50 clear cell renal cell carcinoma patients.
| SETD2 E902Q | ||
|---|---|---|
| Patient ID | % somatic mutation (duplicates) | |
| RCC114 | 3.0 | 7.1 |
| RCC141 | 0.9 | 0.2 |
| RCC151 | 1.6 | 0.3 |
| RCC212 | 1.1 | 0.1 |
| RCC315 | 34.0 | 38.1 |
| RCC348 | 4.9 | 5.6 |
| RCC528 | 1.1 | 0.4 |
| RCC596 | 2.4 | 3.5 |
| RCC608 | 2.3 | 4.2 |
| RCC740 | 1.3 | 0 |
Duplicates for each patient were analyzed. % represents the percentage of the G allele in the sequence. In samples RCC212 and RCC740, only one of the duplicates showed a somatic mutation.
Modeling the SNP in Drosophila
SETD2 encodes a protein of 2564 amino acids with several conserved functional domains. Residing at the carboxy terminus is an AWS (associated with SET) domain of unknown function adjacent to a SET (Su(var)3-9, Enhancer of zeste, Trithorax) domain responsible for methyltransferase activity, as originally identified in Drosophila Su(var)3-9 (Rea et al. 2000). Downstream of the SET and post-SET domains are a WW (tryptophan–tryptophan) domain, important for protein–protein interactions, and the SRI (Set2 Rbp1 interacting) domain, which mediates association with RNA polymerase II (Kizer et al. 2005). The E902Q mutation resides upstream of any known SETD2 functional domains within a structurally undefined region (Fig. 1a; Apweiler et al. 2000; Jumper et al. 2021).
Fig. 1.
Functional domains and location of E902Q in the human SETD2 protein and the homologous E741Q in Drosophila melanogaster Set2-RA protein. a) The missense SNP described in this report (rs58906143, E902Q) location (arrow) is in the amino half of the protein, while the 5 known functional domains (AWS, SET, post-SET, WW, and SRI domains) are in the carboxy half. Note the domain architecture is similar in comparison to Drosophila melanogaster Set2. The orthologous E741Q mutation is shown (arrow). (Not drawn to scale.) b) A partial alignment of hSETD2 and Drosophila Set2-RA (dSet2) proteins with the conserved glutamic acid (E) highlighted.
Given its incidence in ccRCC, we sought to model the rs58906143 SNP in a genetically tractable system to examine its biological relevance. Drosophila Set2 is genetically and functionally orthologous to human SETD2 (Larschan et al. 2007; Stabell et al. 2007). Alignment of human SETD2 and Drosophila melanogaster Set2 isoform Set2-RA reveals 21.5% identity and 34.7% similarity, and SETD2 E902 is conserved with Drosophila Set2 E741 (Fig. 1a, b). Using CRISPR/CAS-9, we introduced the E741Q mutation, orthologous to the missense E902Q mutation present in our ccRCC cohort, into Drosophila. Mutant progeny were scored for germline transmission by DNA sequencing, yielding 4 independent Set2E741Q lines and 3 nonmutated sibling control lines (Set2CTRL) stably maintained over balancer chromosomes to prevent recombination.
Set2 E741Q is a hypomorphic allele
Set2 is an essential gene, and null mutants are late larval lethal (Larschan et al. 2007). Within the female germline, Set2 is required for oogenesis, as null mothers display impaired germline differentiation (Larschan et al. 2007; Mukai et al. 2015). Given we were able to recover homozygous Set2 E741Q adults, we assayed embryonic hatch rate and female fecundity. However, we noted no significant difference between WT or Set2CTRL relative to Set2RNAi or Set2 E741Q animals (Fig. 2a, b). These data suggest Set2E741Q likely represents an incomplete loss-of-function allele dispensable for germline differentiation and early embryonic development.
Fig. 2.
Drosophila Set2 E741Q does not impair fitness. a) Quantification of the fecundity rate, measured as the number of eggs laid per female over 2-h, from the indicated age-matched genotypes. Each dot represents a measurement over 3 consecutive days. Mean ± SD for WT = 5.5 ± 0.8, Set2RNAi = 5.7 ± 0.4, Set2CTRL31= 5.5 ± 0.4, and Set2[E741Q]4= 4.9 ± 0.2 eggs laid per female. b) Quantification of hatch rate. Each dot represents the percent of unhatched embryos from N∼200 embryos from 3 independent replicate experiments from the indicated genotypes. Mean ± SD for WT = 13 ± 3.3, Set2RNAi = 17 ± 5, Set2CTRL31= 8.2 ± 1.3, and Set2[E741]4= 17.7 ± 5.3% unhatched embryos. n.s., not significant by a Kruskal–Wallis test followed by a Dunn's multiple comparison post-test.
The E741Q mutation reduces the SET2 methyltransferase activity
To begin to ascertain whether Set2 E741Q compromises methyltransferase activity, we first monitored H3K36me3 levels from total ovarian extracts. Previous work showed depletion of Set2 by RNAi was sufficient to reduce H3K36me3 by western blotting of cultured Drosophila cells and by immunofluorescence in the female germline (Larschan et al. 2007; Mukai et al. 2015). Using the maternal mat-GAL4 driver to express Set2RNAi in germline cells did not significantly reduce H3K36me3 as detected by immunoblot, likely due to the high remaining levels of H3K36me3 within somatically derived lineages. We similarly examined several independent Set2E741Q homozygotes or transheterozygotes and noted variable reductions in H3K36me3 levels, which could be restored upon expression of a rescuing genomic Set2 construct (Fig. 3) (Larschan et al. 2007). Given this biological variability and lack of statistical significance, these data suggest Set2 E741 may not necessarily regulate protein stability or overall H3K36me3 levels.
Fig. 3.
H3K36me3 levels in Drosophila Set2 E741Q mutants. Immunoblots of relative H3K36me3 levels within 0–1 day total ovarian extracts relative to the βTub loading control, as quantified and normalized to the WT control (below). n.s., not significant or *P < 0.05 by one-way ANOVA followed by Dunnett's multiple comparison post-test.
Germline stem cells (GSCs) of the Drosophila ovary are sensitive to epigenetic regulation, with dysregulation leading to GSC loss or germline tumor phenotypes (Buszczak et al. 2009; Benner et al. 2019). Supporting this idea, Set2 regulates H3K36me3 levels in the female germarium required for germline differentiation (Mukai et al. 2015). As a more sensitive assay to examine Set2 methyltransferase activity in defined cellular lineages, we therefore examined H3K36me3 levels in female GSCs by immunofluorescence.
Consistent with prior work, we confirmed depletion of Set2 by RNAi using the mat-GAL4 driver was sufficient to reduce H3K36me3 levels (Mukai et al. 2015). H3K36me3 signals were significantly reduced in GSCs from Set2RNAi animals relative to wild-type (WT) controls (Fig. 4a, b; mean ± SD = 3.49 ± 1.29 from N = 28 WT vs 1.00 ± 0.61 from N = 60 Set2RNAi GSCs; P < 0.0001 by Kruskal–Wallis test). We similarly assessed H3K36me3 levels from multiple independent E741Q CRISPR isolates and control lines through triplicated experiments. Among these, the mutant lines Set2[E741Q]4 and Set2[E741Q]10 showed significant reductions in H3K36me3 relative to WT and comparable to Set2RNAi (Fig. 4a, b; mean ± SD = 1.30 ± 0.70 from N = 44 Set2[E741Q]4 GSCs and 1.31 ± 0.83 from N = 17 Set2[E741Q]10 GSCs; both P < 0.0001 by Kruskal–Wallis test relative to WT). Unexpectedly, one E741Q CRISPR line, Set2[E741Q]2, resembled WT and CRISPR controls (Fig. 4a, b; mean ± SD = 3.48 ± 1.49 from N = 40 Set2[E741Q]2 GSCs). Given each of our CRISPR mutants were confirmed by DNA sequencing, we reasoned a second mutation within the Set2[E741Q]2 background might account for this apparent genetic suppression. To test this idea, we placed Set2[E741Q]2 in trans to the Set2[E741Q]4 allele and examined H3K36me3 levels in GSCs from Set2[E741Q]2/Set2[E741Q]4 transheterozygous females. Set2[E741Q]2/Set2[E741Q]4 GSCs showed a significant reduction in H3K36me3, demonstrating the E741Q mutation results in reduced H3K36me3 activity in multiple independent lines (Fig. 4a, b; mean + SD = 1.51 + 1.01 from N = 19 Set2[E741Q]2/Set2[E741Q]4 GSCs).
Fig. 4.
Drosophila Set2 E741Q reduces H3K36me3 in the female germline. a) Images show maximum intensity projections of germaria from the indicated genotypes stained with DAPI (blue), Vasa (red), and H3K36me3 (green) antibodies. Boxed regions contain GSCs and are enlarged in the insets, where ovals mark representative GSCs used for H3K36me3 intensity measurements. b) Quantification of the H3K36me3 intensity in GSCs of the indicated genotypes. Each dot represents a measurement from a single GSC. Data were normalized to the mean intensity of Set2RNAi; n.s., not significant and ****P < 0.0001 by a Kruskal–Wallis test followed by a Dunn's multiple comparison post-test relative to WT. c) Quantification of the H3K36me3 intensity in GSCs of the indicated genotypes. Each dot represents a measurement from a single GSC. Data were normalized to the mean intensity of Set2[E741Q]4; **P < 0.01 by an unpaired, 2-tailed t-test. Scale bars: 10 μm and (insets) 5 μm.
To rule out the possibility the observed reduction in H3K36me3 levels was due to off-target effects associated with CRISPR/CAS-9 genome editing, we similarly examined the nonedited CRISPR control line, Set2CTRL31, which appeared phenotypically normal (Fig. 4a, b; mean + SD = 2.59 ± 1.41 from N = 36 Set2CTRL31 GSCs; P = 0.1982 by Kruskal–Wallis test relative to WT). Moreover, introduction of a rescuing Set2 transgene was sufficient to restore H3K36me3 activity in the CRISPR mutant background [Fig. 4a, c; 1.6-fold more H3K36me3 in Set2[E741Q]4; P[Set2]WT than Set2[E741Q]4; P < 0.01 by unpaired t-test (Larschan et al. 2007)]. Taken together, these data reveal a requirement for Set2 E741 for normal methyltransferase activity, as the E741Q SNP reduced the enrichment of H3K36me3 within GSC nuclei. Furthermore, these findings hint that the homologous SETD2 E902Q mutation may similarly compromise H3K36me3 levels in humans, although this remains to be tested.
Set2 contributes to spindle integrity
In addition to modifying H3K36, SETD2-mediated trimethylation of αTubK40 on spindle microtubules is required for mitotic fidelity and microtubule stability (Park et al. 2016; Xie et al. 2021). In contrast, contributions of Drosophila Set2 to microtubule dynamics remain relatively unexplored. However, the recovery of smaller homozygous mutant eye clones relative to controls suggests Set2 may promote mitotic fidelity (Larschan et al. 2007).
To examine whether Drosophila Set2 influences microtubule dynamics or mitotic progression, we visualized spindle integrity in syncytial embryos. Drosophila embryos proceed through 14 rounds of abridged, synchronous nuclear cycles (NCs) prior to cellularization (Edgar et al. 1994). From NC 10–14, the embryo develops as a syncytial blastoderm, wherein thousands of nuclei migrate to and divide just beneath the cortical surface (Foe and Alberts 1983). To examine maternal effects, embryos were harvested from Set2 homozygous mutant mothers or from mothers in which Set2 was depleted by RNAi (see Materials and Methods section).
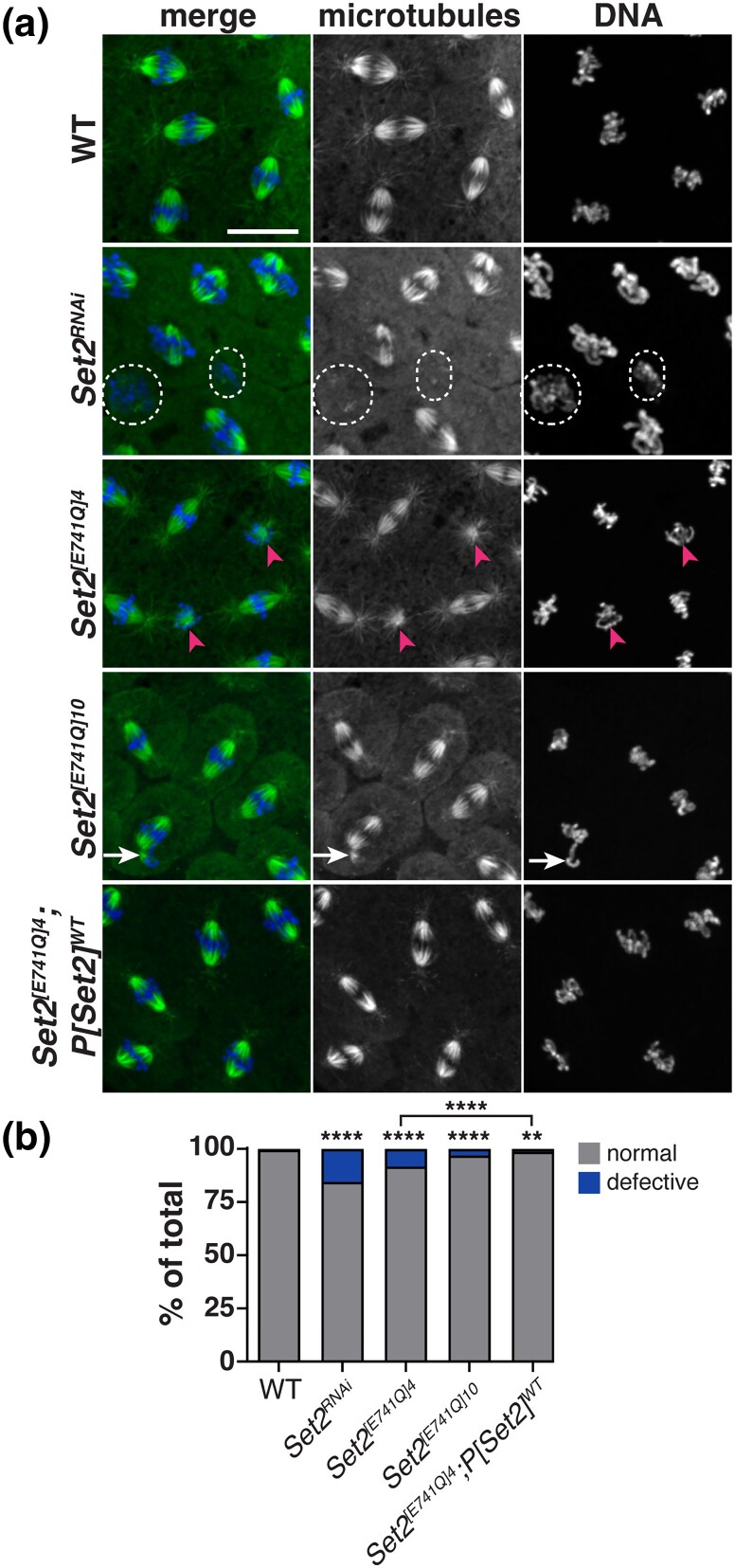
We quantified the frequency of mitotic spindle defects, defined as bent, multipolar, or inactivated spindles, from fixed control, Set2RNAi, and Set2E701Q embryos. Elevated rates of aberrant spindles were detected in Set2RNAi embryos relative to WT (dashed circles, Fig. 5a, b; N = 136/880 defective spindles from N = 8 Set2RNAi embryos vs N = 8/1977 defective spindles from N = 10 WT embryos; P < 0.0001 by chi-square test), consistent with a role for Set2 in promoting spindle integrity. Examination of multiple Set2 E701Q isolates revealed a similar increase in the frequency of spindle errors (Fig. 5a, b; N = 98/1183 defective spindles from N = 9 Set2[E741Q]4 embryos and N = 31/998 defective spindles from N = 10 Set2[E741Q]10 embryos; both P < 0.0001 relative to WT by chi-square test), suggesting Set2 E701 normally supports mitotic fidelity. Consistent with this model, expression of the rescuing Set2 transgene was able to significantly restore spindle organization in Set2[E741Q]4 mutants (N = 20/1643 defective spindles from n = 20 Set2[E741Q]4; P[Set2]WT embryos; P < 0.0001 relative to Set2[E741Q]4 by chi-square test), albeit not to fully WT levels.
Fig. 5.
Drosophila Set2 supports spindle morphogenesis. a) Maximum intensity projected images of fixed syncytial blastoderm embryos stained to visualize mitotic spindles using αTub (microtubules; green) antibodies and DAPI to label DNA (blue). Examples of spindle inactivation (dashed circles), monopolar spindles (arrowheads), and loss of bipolarity (arrow) are shown. b) Quantification of spindle defects (i.e. bent, inactivated, or multipolar) tabulated from N = 8/1977 spindles from n = 10 WT embryos; 136/880 spindles from n = 8 Set2RNAi embryos; N = 98/1183 spindles from n = 9 Set2[E741Q]4 embryos N = 31/998 spindles from n = 10 Set2[E741Q]10 embryos; and N = 20/1643 spindles from n = 20 Set2[E741Q]4; P[Set2]WT NC 11–13 embryos. Significance determined by chi-square test relative to WT. Scale bar: 10 μm.
To study the role of Set2 in spindle morphogenesis in greater detail, we visualized mitosis in rapidly cycling, live control and Set2RNAi embryos expressing GFP-αTub from NC 10–13 (Fig. 6a). While control embryos showed normal, synchronized cell cycle progression marked by the formation of evenly spaced bipolar mitotic spindles during mitosis (control; Fig. 6a), most Set2RNAi embryos (∼80%, N = 5/6 embryos) showed spindle defects (Set2RNAi; Fig. 6a, dashed lines). Aberrant spindle formation was preceded by microtubule-organizing center inactivation during interphase and nuclear envelope breakdown (NEB; Set2RNAi, Fig. 6a). Subsequently, a cluster of anucleated centrosomes remained at the embryonic cortex in the following interphase (interphase—NC 12; Set2RNAi, Fig. 6a). Such anucleated centrosomes are a hallmark of nuclear fall-out, the developmental response whereby damaged nuclei are displaced from the cortex to prevent their propagation (Sullivan et al. 1993; Takada et al. 2003). Significantly more abnormal mitotic spindles were detected in Set2RNAi embryos relative to controls (N = 36/324 defective spindles from N = 6 Set2RNAi embryos vs N = 6/527 defective spindles from N = 5 control embryos; P < 0.0001 by chi-square test). These data further implicate Set2 in spindle morphogenesis within syncytial embryos.
Fig. 6.
Drosophila Set2 E741Q affects mitotic fidelity in cycling embryos. a) Maximum projected still images from videos of cycling Drosophila syncytial embryos of the indicated genotypes expressing UASp-GFP-αTubulin (GFP-αTub) to visualize microtubules. Time is displayed as minutes:s and is relative to NEB onset. Dashed lines highlight errors in microtubule organization throughout the timelapse. For Set2[E741Q]4, multiple clusters of mitotic errors are labeled. b) Defective spindles (i.e. bent, inactivated, or multipolar) were quantified from N = 8/364 spindles from n = 5 control embryos; N = 36/324 spindles from n = 6 Set2RNAi embryos; N = 32/256 spindles from n = 4 Set2[E741Q]4 embryos; and N = 109/1608 spindles from n = 5 Set2[E741Q]10 NC 10–13 embryos. Statistical significance was determined by chi-square test relative to the control; ***, P < 0.001; ****, P < 0.0001. Scale bars: 10 μm.
To determine whether Set2 E741Q alters microtubule dynamics, we similarly assayed mitotic fidelity in 2 independent CRISPR mutant lines. Significantly more errant spindles were detected in Set2[E741Q]4 and Set2[E741Q]10 embryos relative to controls (N = 32/256 defective spindles from N = 4/4 Set2[E741Q]4 embryos, P < 0.0001 relative to control by chi-square test; N = 109/1608 defective spindles from N = 5/5 Set2[E741Q]10 embryos, P < 0.001 relative to control by chi-square test; Fig. 6b). Collectively, these data indicate that Set2 E741Q supports spindle integrity as well as H3K36 trimethylation.
Discussion
Herein, we describe a missense SNP in SETD2 that we identified in RCC cases. We also demonstrated the same SNP as a somatic mutation in RCC. There is a substantial ancestry disparity in the frequency of the minor G allele. While it is present in only 0.07% of the EA population, it is present in 21% of the AA population (Sherry et al. 2001). Analysis of available databases (All of Us Public Data Browser, gnomAD, others) demonstrates that the SNP rs58906143 is present in AA populations at the frequency of 10.1–25.6% (Supplementary Table 2). While our data hinted that the SNP is more common in cases than controls regardless of ancestry, this did not reach statistical significance.
Although acquired somatic mutations in SETD2 are noted in ∼10% of RCC cases, the E902Q substitution has not been previously described. In a cohort of 50 individuals whose tumors and/or ctDNA were sequenced using an RCC-specific gene panel, we found this exact mutation in 10/50, or 20%, of cases. The reason for this discrepancy between the large online databases of somatic tumor-associated mutations (COSMIC, TCGA) and our findings is not immediately clear. One possible explanation relates to approach. We limited our analysis to a small number of genes known to be relevant to RCC. This allows for deep and redundant sequencing (typically 1000- to 10,000-fold). In addition, we optimized our protocol to accurately detect mutations in the plasma (ctDNA) present in very low abundance (0.8% allele frequency) and have used strict criteria for calling positives (not present in WBC, bidirectional when data available, minimum of 40 counts in mutation). Finally, every mutation called by our automated pipeline (SureCall) has been verified by manually examining the sequences. For these reasons, we may have had a greater sensitivity in identifying the mutation, especially when it is present at low allele frequency.
The E902Q substitution is not located in one of the 5 canonical SETD2 functional domains, which cluster at the C-terminus (Fig. 1). However, we note another missense mutation within SETD2 (D837G) identified from TCGA RCC cases residing within the same structurally undefined region (Apweiler et al. 2000; Cancer Genome Atlas Research Network 2013; Jumper et al. 2021). Both mutations alter a negatively charged acidic residue (aspartic acid, D vs glutamic acid, E). Although unstructured, this N-terminal region could influence SETD2 functionality. Given the recent discovery of a second target of SETD2 methylation activity, namely αTub, future work will likewise contribute additional insights into SETD2 (Park et al. 2016).
Generation of the homologous E741Q mutation in Drosophila demonstrated that the mutant protein has diminished ability to trimethylate H3K36 and organize spindles. Our finding that Set2 E741Q embryos display mitotic spindle errors at rates comparable to Set2RNAi suggests the SETD2 function in modifying αTub is likely conserved in Drosophila Set2. These data are consistent with 2 models: Set2 E741Q may indirectly influence microtubule dynamics through its role in transcriptional regulation via histone modification; alternatively, Set2 E741Q may directly alter αTubK40me3 levels. Because heterochromatin is established later in Drosophila embryogenesis around NC 14 and is largely coupled with zygotic genome activation (Yuan and O'Farrell 2016; Shindo et al. 2022), we hypothesize that Set2 E741 functions directly in microtubule modification. However, future investigation is required to parse apart these models.
Our findings argue the Set2 E741Q mutation is a partial loss-of-function allele, which may genetically sensitize animals to second-site mutations that could exacerbate mitotic defects and embryonic viability. Importantly, SETD2 is mutated in scores of cancers, and its relevance is not restricted to RCC (Lu et al. 2021). One intriguing hypothesis is that SETD2 E902Q could sensitize individuals to RCC or other cancers. Additional studies are needed to test this idea. Establishment of a genetically tractable Drosophila model should propel these efforts.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the Emory Integrated Genomics Core (EIGC) shared resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. Stocks obtained from the Bloomington Drosophila Stock Center (NIH grant P40OD018537) and antibodies from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH, and maintained at the University of Iowa Department of Biology were used in this study. We thank Drs. Mitzi Kuroda (Harvard University) and Hiroshi Kimura (Tokyo Institute of Technology) for gifts of reagents. We would like to acknowledge Dr. Yan Sun (Emory) for ancestry determination and Ms. Taylor Hailstock for technical assistance.
Contributor Information
Jovan S Brockett, Department of Cell Biology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Tad Manalo, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Hala Zein-Sabatto, Department of Cell Biology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Jina Lee, Department of Cell Biology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Junnan Fang, Department of Cell Biology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Philip Chu, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Harry Feng, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Dattatraya Patil, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Priscilla Davidson, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Kenneth Ogan, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Viraj A Master, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
John G Pattaras, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA.
David L Roberts, Emory University Department of Medicine, Division of General Internal Medicine, Atlanta, GA 30322, USA.
Sharon H Bergquist, Emory University Department of Medicine, Division of General Internal Medicine, Atlanta, GA 30322, USA.
Matthew A Reyna, Department of Biomedical Informatics, Emory University School of Medicine, Atlanta, GA 30322, USA; Department of Pharmacology and Chemical Biology, Emory University School of Medicine, Atlanta, GA 30322, USA.
John A Petros, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA; Winship Cancer Institute, Emory University, Atlanta, GA 30322, USA.
Dorothy A Lerit, Department of Cell Biology, Emory University School of Medicine, Atlanta, GA 30322, USA; Winship Cancer Institute, Emory University, Atlanta, GA 30322, USA.
Rebecca S Arnold, Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA; Winship Cancer Institute, Emory University, Atlanta, GA 30322, USA.
Data availability
Drosophila strains are available upon request. Supplemental videos are available on Figshare: timelapse recordings Supplementary Video 1 (control), Supplementary Video 2 (Set2RNAi), Supplementary Video 3 (Set2[E741Q]4), and Supplementary Video 4 (Set2[E741Q]10) show cycling embryos expressing GFP-αTub (https://doi.org/10.6084/m9.figshare.20914090). Sequence and Genotyping data are deposited at the database of Genotypes and Phenotypes under study ID 53713 and accession phs003474.v1.p1 Samples were collected with informed consent under institutional review board (IRB) approved protocols (The Urological Satellite Specimen Bank at Emory University) in accordance with procedures approved by Emory University: IRB00055316, IRB00085068, and IRB00058903.
Supplemental material available at GENETICS online.
Funding
This work was supported by the following grants: U.S. Department of Defense grant W81XW1910859 (RSA and DAL), U.S. Department of Veterans Affairs grant 1 I01 BX003367-01 (JP), Evans County Cares Foundation Award (JP), National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 (MAR), The John and Mary Brock Discovery Fund (DR and SB), National Institutes of Health grants 5K12GM000680 (HZ-S), 5K99GM143517 (JF), and R01GM138544 (DAL). DAL is also supported by a Research Scholar Grant RSG-22-874157-01-CCB from the American Cancer Society. JP was also supported by the Woodruff Health Sciences Center and the Woodruff Foundation through the WHSC Synergy Awards program . The content is solely the responsibility of the authors and does not necessarily represent the official views of the Woodruff Foundation.
Literature cited
- Apweiler R, Attwood TK, Bairoch A, Bateman A, Birney E, Biswas M, Bucher P, Cerutti L, Corpet F, Croning MD, et al. 2000. InterPro–an integrated documentation resource for protein families, domains and functional sites. Bioinformatics. 16(12):1145–1150. doi: 10.1093/bioinformatics/16.12.1145. [DOI] [PubMed] [Google Scholar]
- 1000 Genomes Project Consortium; Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, et al. 2015. A global reference for human genetic variation. Nature. 526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Schneider R, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. 2005. Spatial distribution of di- and tri-methyl lysine 36 of histone H3 at active genes. J Biol Chem. 280(18):17732–17736. doi: 10.1074/jbc.M500796200. [DOI] [PubMed] [Google Scholar]
- Benner L, Castro EA, Whitworth C, Venken KJT, Yang H, Fang J, Oliver B, Cook KR, Lerit DA. 2019. Drosophila heterochromatin stabilization requires the zinc-finger protein small ovary. Genetics. 213(3):877–895. doi: 10.1534/genetics.119.302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Spradling AC. 2009. Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science. 323(5911):248–251. doi: 10.1126/science.1165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network . 2013. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 499(7456):43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duns G, van den Berg E, van Duivenbode I, Osinga J, Hollema H, Hofstra RM, Kok K. 2010. Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res. 70(11):4287–4291. doi: 10.1158/0008-5472.CAN-10-0120. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Sprenger F, Duronio RJ, Leopold P, O'Farrell PH. 1994. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 8(4):440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds JW, Mahadevan LC, Clayton AL. 2008. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 27(2):406–420. doi: 10.1038/sj.emboj.7601967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. 2021. Cancer statistics for the year 2020: an overview. Int J Cancer. 149(4):778–789 doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- Foe VE, Alberts BM. 1983. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J. Cell Sci. 61(1):31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. 2021. Highly accurate protein structure prediction with AlphaFold. Nature. 596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, et al. 2020. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns S, Mason FM, Rathmell WK, Park IY, Walker C, Verhey KJ, Cianfrocco MA. 2021. Molecular determinants for alpha-tubulin methylation by SETD2. J Biol Chem. 297(1):100898. doi: 10.1016/j.jbc.2021.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Hayashi-Takanaka Y, Goto Y, Takizawa N, Nozaki N. 2008. The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell Struct Funct. 33(1):61–73. doi: 10.1247/csf.07035. [DOI] [PubMed] [Google Scholar]
- Kizer KO, Phatnani HP, Shibata Y, Hall H, Greenleaf AL, Strahl BD. 2005. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol Cell Biol. 25(8):3305–3316. doi: 10.1128/MCB.25.8.3305-3316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larschan E, Alekseyenko AA, Gortchakov AA, Peng S, Li B, Yang P, Workman JL, Park PJ, Kuroda MI. 2007. MSL complex is attracted to genes marked by H3K36 trimethylation using a sequence-independent mechanism. Mol Cell. 28(1):121–133. doi: 10.1016/j.molcel.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. 2016. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 536(7616):285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerit DA, Gavis ER. 2011. Transport of germ plasm on astral microtubules directs germ cell development in Drosophila. Curr Biol. 21(6):439–448. doi: 10.1016/j.cub.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerit DA, Jordan HA, Poulton JS, Fagerstrom CJ, Galletta BJ, Peifer M, Rusan NM. 2015. Interphase centrosome organization by the PLP-Cnn scaffold is required for centrosome function. J. Cell Biol. 210(1):79–97. doi: 10.1083/jcb.201503117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Duns G, Westers H, Sijmons R, van den Berg A, Kok K. 2016. SETD2: an epigenetic modifier with tumor suppressor functionality. Oncotarget. 7(31):50719–50734. doi: 10.18632/oncotarget.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Zhao B, Liu M, Wu L, Li Y, Zhai Y, Shen X. 2021. Pan-cancer analysis of SETD2 mutation and its association with the efficacy of immunotherapy. NPJ Precis Oncol. 5(1):51. doi: 10.1038/s41698-021-00193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EP, Raff JW. 2007. Maintaining the proper connection between the centrioles and the pericentriolar matrix requires Drosophila centrosomin. J. Cell Biol. 178(5):725–732. doi: 10.1083/jcb.200704081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Hira S, Nakamura K, Nakamura S, Kimura H, Sato M, Kobayashi S. 2015. H3k36 trimethylation-mediated epigenetic regulation is activated by bam and promotes germ cell differentiation during early oogenesis in Drosophila. Biol Open. 4(2):119–124. doi: 10.1242/bio.201410850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman SB, Wunsch CD. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Obeng RC, Arnold RS, Ogan K, Master VA, Pattaras JG, Petros JA, Osunkoya AO. 2020. Molecular characteristics and markers of advanced clear cell renal cell carcinoma: pitfalls due to intratumoral heterogeneity and identification of genetic alterations associated with metastasis. Int J Urol. 27(9):790–797. doi: 10.1111/iju.14302. [DOI] [PubMed] [Google Scholar]
- Park IY, Powell RT, Tripathi DN, Dere R, Ho TH, Blasius TL, Chiang YC, Davis IJ, Fahey CC, Hacker KE, et al. 2016. Dual chromatin and cytoskeletal remodeling by SETD2. Cell. 166(4):950–962. doi: 10.1016/j.cell.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan L, Jin Y, Zhang H, Qiang W, Shekhtman E, Shao D, Revoe D, Villamarin R, Ivanchenko E, Kimura M, et al. 2020. ALFA: allele frequency aggregator (National Center for Biotechnology Information, U.S. National Library of Medicine). www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/).
- Piper MD, Partridge L. 2016. Protocols to study aging in Drosophila. Methods Mol Biol. 1478:291–302. doi: 10.1007/978-1-4939-6371-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Huang J, Tcheandjieu C, Huffman JE, Litkowski E, Liu C, Ho YA, Hunter-Zinck H, Zhao H, Marouli E, et al. 2022. A multi-population phenome-wide association study of genetically-predicted height in the Million Veteran Program. PLoS Genet. 18(6):e1010193. doi: 10.1371/journal.pgen.1010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, et al. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 406(6796):593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. 2001. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y, Brown MG, Amodeo AA. 2022. Versatile roles for histones in early development. Curr Opin Cell Biol. 75:102069. doi: 10.1016/j.ceb.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Wagle NS, Jemal A. 2023. Cancer statistics, 2023. CA Cancer J Clin. 73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- Stabell M, Larsson J, Aalen RB, Lambertsson A. 2007. Drosophila dset2 functions in H3-K36 methylation and is required for development. Biochem Biophys Res Commun. 359(3):784–789. doi: 10.1016/j.bbrc.2007.05.189. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Grant PA, Briggs SD, Sun ZW, Bone JR, Caldwell JA, Mollah S, Cook RG, Shabanowitz J, Hunt DF, et al. 2002. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol Cell Biol. 22(5):1298–1306. doi: 10.1128/MCB.22.5.1298-1306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W, Fogarty P, Theurkauf W. 1993. Mutations affecting the cytoskeletal organization of syncytial Drosophila embryos. Development. 118(4):1245–1254. doi: 10.1242/dev.118.4.1245. [DOI] [PubMed] [Google Scholar]
- Takada S, Kelkar A, Theurkauf WE. 2003. Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell. 113(1):87–99. doi: 10.1016/S0092-8674(03)00202-2. [DOI] [PubMed] [Google Scholar]
- Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E, et al. 2019. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47(D1):D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE. 1994. Immunofluorescence analysis of the cytoskeleton during oogenesis and early embryogenesis. Methods Cell Biol. 44:489–505. doi: 10.1016/S0091-679X(08)60928-0. [DOI] [PubMed] [Google Scholar]
- Wojcik GL, Graff M, Nishimura KK, Tao R, Haessler J, Gignoux CR, Highland HM, Patel YM, Sorokin EP, Avery CL, et al. 2019. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 570(7762):514–518. doi: 10.1038/s41586-019-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang S, Li M, Diao L, Pan X, Chen J, Zou W, Zhang X, Feng W, Bao L. 2021. alpha-TubK40me3 is required for neuronal polarization and migration by promoting microtubule formation. Nat Commun. 12(1):4113. doi: 10.1038/s41467-021-24376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, O'Farrell PH. 2016. TALE-light imaging reveals maternally guided, H3K9me2/3-independent emergence of functional heterochromatin in Drosophila embryos. Genes Dev. 30(5):579–593. doi: 10.1101/gad.272237.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Drosophila strains are available upon request. Supplemental videos are available on Figshare: timelapse recordings Supplementary Video 1 (control), Supplementary Video 2 (Set2RNAi), Supplementary Video 3 (Set2[E741Q]4), and Supplementary Video 4 (Set2[E741Q]10) show cycling embryos expressing GFP-αTub (https://doi.org/10.6084/m9.figshare.20914090). Sequence and Genotyping data are deposited at the database of Genotypes and Phenotypes under study ID 53713 and accession phs003474.v1.p1 Samples were collected with informed consent under institutional review board (IRB) approved protocols (The Urological Satellite Specimen Bank at Emory University) in accordance with procedures approved by Emory University: IRB00055316, IRB00085068, and IRB00058903.
Supplemental material available at GENETICS online.