ABSTRACT
Background: High-quality friendships have a positive impact on the mental health of young people with childhood adversity (CA). Social stress buffering, the phenomenon of a social partner attenuating acute stress responses, is a potential yet unexplored mechanism that may underlie this relationship.
Objective: This study examined whether perceived friendship quality was related to better mental health and lower neural stress response in young people with CA.
Method: A total of N = 102 young people (aged 16–26) with low to moderate CA were included in the study. We first investigated associations between friendship quality, mental health, and CA. In a representative subset (n = 62), we assessed neural stress responses using the Montreal Imaging Stress Task. In our sample, CA was best described along two dimensions resembling threat or deprivation like experiences. Hence, we investigated both cumulative and dimensional effects of CA.
Results: We found no support for social thinning after CA, meaning that the severity of CA (cumulative or dimensional) did not differentially impact friendship quality. High-quality friendships, on the other hand, were strongly associated with better mental health. Furthermore, acute stress increased state anxiety and enhanced neural activity in five frontolimbic brain regions, including the left hippocampus. We found weak support that threat experiences interacted with friendship quality to predict left hippocampal reactivity to stress. However, this effect did not survive multiple comparison correction.
Conclusion: The absence of social thinning in our sample may suggest that the risk of developing impoverished social networks is low for rather well-functioning young people with low to moderate CA. Regardless, our findings align with prior research, consistently showing a strong association between high-quality friendships and better mental health in young people with CA. Future research is needed to examine whether friendships aid neural stress responses in young people with childhood threat experiences.
KEYWORDS: Childhood adversity, threat experiences, hippocampus, neural stress mechanisms, friendship quality, young people
HIGHLIGHTS
Young people with childhood adversity underwent acute stress induction, eliciting frontolimbic reactivity.
High-quality friendships were strongly associated with better mental health.
Weak support for friendship stress buffering did not survive multiple comparison correction.
Abstract
Antecedentes: Amistades de alta calidad tienen un impacto positivo en la salud mental de las personas jóvenes con adversidad en la infancia (CA en su sigla en inglés). La amortiguacion del estrés social, fenómeno en el que un interlocutor social atenúa las respuestas de estrés agudo, es un mecanismo potencial que todavía no ha sido explorado que podría subyacer a esta relación.
Objetivo: Este estudio examinó si la calidad de la amistad percibida se relaciona con una mejor salud mental y una menor respuesta al estrés neuronal en jóvenes con CA.
Método: Se incluyeron en este estudio un total de N = 102 jóvenes (edades entre 16 y 26 años) con CA baja a moderada. Primero, investigamos las asociaciones entre calidad de la amistad, la salud mental y la CA. En una sub-muestra representativa (n = 62), evaluamos las respuestas al estrés neuronal usando la Tarea de Estrés de Imágenes de Montreal. En nuestra muestra, CA se describió mejor en dos dimensiones que se asemejan a experiencias similares a amenaza o privaciones. Por lo tanto, investigamos tanto los efectos acumulativos como dimensionales de los CA.
Resultados: No encontramos apoyo para una red social acotada o más estrecha después de CA, lo que significa que la severidad del CA (acumulativo o dimensional) no afectó de manera diferencial la calidad de la amistad. Las amistades de alta calidad, por otro lado, se asociaron fuertemente con mejor salud mental. Además, el estrés agudo aumentó el estado de ansiedad y mejoró la actividad neuronal en cinco regiones frontolímbicas del cerebro, incluido el hipocampo izquierdo. Encontramos un apoyo débil de que las experiencias de amenaza interactuaban con la calidad de la amistad para predecir la reactividad del hipocampo izquierdo al estrés. Sin embargo, este efecto no sobrevivió a la corrección de comparación múltiple.
Conclusión: La ausencia de una red social acotada o más estrecha en nuestra muestra podría sugerir que el riesgo de desarrollar redes sociales empobrecidas es bajo para jóvenes que funcionan bien y tienen una CA baja a moderada. Independientemente, nuestros hallazgos se alinean con investigaciones previas, mostrando consistentemente una asociación fuerte entre amistades de alta calidad y mejor salud mental en jóvenes con CA. Futuras investigaciones se necesitan para examinar si las amistades ayudan a las respuestas de estrés neuronal en jóvenes con experiencias de amenaza en la infancia.
PALABRAS CLAVE: Adversidad en la infancia, experiencia de amenaza, hipocampo, mecanismos de estrés neuronal, calidad de amistades, jóvenes
Abstract
背景:高质量的友谊对患有童年逆境(CA)的年轻人的心理健康有积极的影响。社会压力缓冲,即社会伙伴减弱急性压力反应的现象,是这种关系潜在但尚未探讨的机制。
目的:本研究探讨了有 CA 年轻人所感知的友谊质量是否与更好的心理健康和更低的神经应激反应有关。
方法:本研究共纳入102 名有低至中度 CA 的年轻人(16–26 岁)。我们首先考查了友谊质量、心理健康和 CA 之间的关联。在一个代表性子集(n = 62)中,我们使用蒙特利尔成像压力任务评估了神经压力反应。在我们的样本中,CA由两个维度被最佳描述,例如威胁或剥夺等经历。因此,我们研究了 CA 的累积效应和维度效应。
结果:我们没有发现对 CA 后社交变弱的支持,这意味着 CA 的严重程度(累积或维度)不会对友谊质量产生差异性影响。另一方面,高质量的友谊与更好的心理健康密切相关。此外,急性压力会增加状态焦虑,并增强五个额叶脑区域(包括左侧海马体)的神经活动。 我们发现威胁经历与友谊质量相互作用以预测左海马体对压力的反应的证据微弱。然而,这种效应未能通过多重比较校正。
结论:我们的样本中不存在社交变弱现象,这可能表明对于功能良好且 CA 低至中度的年轻人来说,发展贫困社交网络的风险较低。 无论如何,我们的研究结果与之前的研究一致,始终表明患有 CA 的年轻人的高质量友谊与更好的心理健康之间存在密切关系。 未来的研究需要检验友谊是否有助于有童年威胁经历的年轻人的神经压力反应。
关键词: 童年逆境;威胁经历;海马体;神经应激机制;友谊质量;年轻人
1. Introduction
Up to half of children and adolescents worldwide experience at least one form of childhood adversity (CA), such as abuse, neglect, bullying, or poverty (Bellis et al., 2014). Exposure to CA represents a deviation from the ‘expectable’ environment, which requires young people to adapt their psychological, social, and neurobiological functioning, ultimately putting them at greater risk for prolonged mental health problems (Cicchetti & Valentino, 2006; Clark et al., 2010; Kessler et al., 2010; McLaughlin, 2016). In fact, around a third of lifetime mood, anxiety, and substance use disorders can be attributed to CA (Green et al., 2010). Hence, investigating neurodevelopmental mechanisms that underlie mental health vulnerability and identifying protective factors that buffer these risk pathways is crucial for informing the development of effective psychosocial interventions.
Chronic or repeated exposure to CA has programming effects on key stress response systems, which can increase later-life mental health vulnerability (Lupien et al., 2009). For example, repeated activation of the hypothalamic-pituitary-adrenal (HPA) axis leads to elevated levels of stress hormones (glucocorticoids) in the body (McEwen, 2017). This neuroendocrine response to prolonged psychosocial stress may promote adaptive functioning (e.g. increased alertness) in the short-term to support survival in stressful environments. However, over time, sustained activation of this stress response system may be detrimental to the structure and function of stress-sensitive brain regions (Arnsten, 2009; Chen & Baram, 2016; Danese & McEwen, 2012). Frontolimbic brain regions, including the hippocampus, amygdala, and prefrontal cortex (PFC), may be particularly sensitive to stress in the context of chronic CA exposure due to their dense innervation with glucocorticoid receptors (Cohodes et al., 2021; Ioannidis et al., 2020; Tottenham & Sheridan, 2010).
Given the importance of the frontolimbic system for social information and emotional processing (Humphreys et al., 2016; McLaughlin et al., 2020), alterations to that system following CA may increase mental health vulnerability, as suggested by (McCrory et al., 2022). Their social transactional model of mental health vulnerability posits that through neurocognitive adaptations to high stress environments, young people with CA might become more likely to subsequently experience (interpersonal) stress (i.e. ‘stress generation’; [McCrory et al., 2019]) and attenuation in their support networks (i.e. ‘social thinning’; [Nevard et al., 2021; Sheikh et al., 2016]), contributing to greater mental health vulnerability.
Critically, social support is a key protective factor against the emergence of mental health problems in young people with CA (Li et al., 2022; Trickey et al., 2012). However, little is known about the underlying mechanisms of this relationship. Social stress buffering models suggest that the presence of a social partner can reduce physiological responses to acute psychosocial stress, measured by glucocorticoid blood levels (Gunnar, 2017; Sullivan & Perry, 2015). During childhood, caregiver support suppresses cortisol secretion to acute psychosocial stress (Hostinar et al., 2015), dampens amygdala reactivity, and promotes emotion regulation (Gee et al., 2014) in children without CA. In addition, previously institutionalised children with greater self-reported feelings of caregiver security exhibited reduced amygdala reactivity to caregiver cues, which was also predictive of a greater decrease in future anxiety symptoms (Callaghan et al., 2019). During adolescence, a unique time of social reorientation and increased sensitivity to peers (Cosme et al., 2022), friendship support takes on a more potent stress buffering role, capable of protecting against the emergence and progression of mental health problems following CA (van Harmelen et al., 2016, 2021). Preliminary evidence for friendship stress buffering has shown that the more time spent interacting with supportive friends was associated with diminished neurobiological stress responses (i.e. reduced cortisol, dorsal anterior cingulate cortex (dACC), and anterior insula reactivity) in young people without CA (Eisenberger et al., 2007; Masten et al., 2012). To date, only two studies have examined friendship stress buffering in young people with CA and reporting mixed results, for a systematic review see (Scheuplein & van Harmelen, 2022). Tang et al. (2021) showed that high-quality friendships were associated with improved sympathetic nervous system reactivity to social rejection feedback at age 12 and reduced peer problems at age 16 in early institutionalised young people. In contrast, no support for friendship stress buffering was found in a small community sample of well-functioning adolescents with low to moderate CA (Fritz et al., 2020). Therefore, it remains unclear whether high-quality friendships aid mental health and well-being in young people with CA through dampening neurobiological stress responses.
To investigate friendship stress buffering in young people with CA, it is crucial to clearly quantify CA experiences, whilst accounting for the fact that different types of CA often co-occur (Brown et al., 2019; Dong et al., 2004). On the one hand, the cumulative-risk approach assumes that discrete forms of CA have additive, but not distinct, effects on neurocognitive functioning, with more CA being associated with stronger effects. Hence, this prevailing approach combines the number of distinct types of adverse experiences into a cumulative risk score (Evans et al., 2013). This approach has been highly influential in public policy and clinical practice (Lacey & Minnis, 2020). On the other hand, dimensional models of adversity differentiate between experiences of threat/harshness (involving harm or threat of harm to oneself and others), deprivation (involving absence of expected cognitive and social stimulation), and unpredictability (involving spatial-temporal variation in threat) to identify mechanisms linking these partially distinct experiences of CA with unique neurodevelopmental and psychopathological consequences (Ellis et al., 2009; Humphreys & Zeanah, 2015; McLaughlin et al., 2021; Sheridan & McLaughlin, 2014). In line with this framework, Puetz et al. (2020) showed that different forms of childhood maltreatment (abuse, neglect, and their combination) were associated with differential neural processing of threat-related cues. Specifically, childhood abuse was associated with increased localised ventral amygdala reactivity to threat, whereas childhood neglect was associated with heightened reactivity in the dorsal amygdala and across spatially distributed frontoparietal brain networks. Notably, cumulative experiences of abuse and neglect were associated with hypoactivation in various higher- order cortical regions, in addition to the amygdala. Furthermore, a systematic review by (McLaughlin et al., 2019) investigated differential associations between threat and deprivation experiences and neural development. To summarise their key findings, threat experiences were found to influence frontolimbic neural networks involved in threat detection and emotion regulation (amygdala and medial prefrontal cortex (mPFC)), salience processing (insula, ACC), and various forms of learning and memory (hippocampus). Deprivation experiences, on the other hand, were found to influence frontoparietal circuits (dorsolateral prefrontal cortex (dlPFC) and superior parietal cortex) contributing to working memory and cognitive control. However, these neural patterns were not consistently observed across studies, highlighting the need for more neuroimaging research to establish consistent and replicable associations between brain alterations and different dimensions of CA.
The main goal of this study was to investigate whether perceived friendship quality was related to reduced neural stress responses in a sample of adolescents and young adults (aged 16–26) with low to moderate CA. In addition, we examined relations between CA, friendship quality, and mental health and well-being. To challenge neural stress responses affected by CA, we utilised the Montreal Imaging Stress Task (MIST) (Dedovic et al., 2005). The MIST is a well-validate acute psychosocial stress paradigm combining the stress-eliciting effects of high cognitive demands (solving math problems under time pressure) with negative social feedback (on screen and verbally via the experimenter), see review by (Noack et al., 2019). Previous studies that utilised the MIST reported stress-related activation in frontolimbic regions, including the hippocampus, amygdala, insula, mPFC, ACC, nucleus accumbens (NAc), and thalamus (Chung et al., 2016; Noack et al., 2019; Voges et al., 2022; Wheelock et al., 2016). Hence, we examined the neural correlates of friendship stress buffering in these regions of interest (ROIs).
Specifically, we examined three hypotheses. First, we hypothesised that more severe CA experiences would be associated with lower levels of perceived friendship quality (McCrory et al., 2022; Nevard et al., 2021; Sheikh et al., 2016) and that reduced friendship quality would be associated with worse mental health functioning (Fritz et al., 2020; van Harmelen et al., 2016, 2021) (hypothesis 1). Second, we expected that acute psychosocial stress would increase state anxiety (Chung et al., 2016; Zschucke et al., 2015) and increase neural activity in our ROIs (Noack et al., 2019) (hypothesis 2). Third, we expected that friendship quality would moderate the relationship between CA and neural stress responses. Specifically, we investigated whether higher friendship quality would be associated with reduced frontolimbic ROI reactivity to stress (Scheuplein & van Harmelen, 2022; Tang et al., 2021) (hypothesis 3). Finally, we examined these hypotheses with both a cumulative-risk approach and a dimensional approach. We expected that more CA experiences, or more severe threat experiences, would be associated with greater frontolimbic ROI reactivity to stress (McLaughlin et al., 2019).
2. Method
2.1. Resilience after individual stress exposure (RAISE) study
Data were drawn from the Resilience after Individual Stress Exposure (RAISE) study, a multilevel study of N = 102 young people aged 16–26 (Moreno-López et al., 2021). All RAISE participants retrospectively self-reported a history of CA, which was defined as exposure to any adverse life event experienced within the family environment before the age of 16. This included childhood maltreatment (e.g. emotional, sexual, or physical abuse, emotional or physical neglect) or intrafamily adversity (e.g. marital distress or conflict, parental mental health problems or parental alcohol dependence, violence, or aggressive behaviour) (Figure 1). Participants were recruited across Cambridgeshire, UK from the general population through flyers and via social media as well as from previous studies conducted at the Department of Psychiatry, University of Cambridge (NSPN 2400 Cohort; [Kiddle et al., 2018]). The RAISE study has received funding from the Royal Society in January 2018, ethical approval from the National Research Ethics Service and the NRES Committee East of England-Cambridge Central (REC reference: 18/EE/0388, IRAS project ID: 241765) in February 2019, and commenced in August 2019.
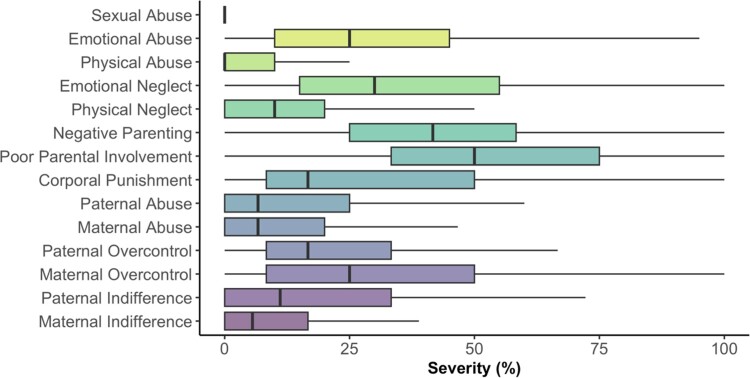
Figure 1.
Severity of childhood adversities in the baseline sample.
Note. Severity (in percent; x-axis) of individual childhood adversities (y-axis) retrospectively self-reported by all N = 102 young people, who participated during the first assessment timepoint (T1) of the RAISE study. Each boxplot displays the median severity (solid vertical line) and interquartile range. Based on established cut-off scores for the CTQ (Bernstein et al. 1994), this baseline sample can be characterised reporting low to moderate levels of CA. Summary statistics are provided in the supplementary Table A.1.1.
2.2. Participants
This study utilised data from the first two RAISE assessment timepoints. At timepoint 1, participants completed online questionnaires (N = 102; ‘Baseline sample’). Timepoint 2 was completed on average 1 month later and consisted of an in-unit visit at Addenbrooke’s Hospital in Cambridge, UK during which, among other measures, functional magnetic resonance imaging (fMRI) data was acquired (n = 62; ‘Neuroimaging sample’). At each timepoint, informed consent was obtained from the participant. A comprehensive description of the full study procedure as well as the inclusion and exclusion criteria has been previously published by (Moreno-López et al., 2021). To summarise, individuals were eligible to participate if they were aged between 16 and 26 years, able to speak, write, and understand English, had a body mass index (BMI) between 18.5 and 29.9 kg/m2, did not currently take medication (e.g. corticosteroids) likely to compromise the interpretation of our data, had no MRI contraindications, and self-reported CA experienced before the age of 16. All inclusion and exclusion criteria were assessed via telephone by a trained member of the study team to ensure that interested participants were eligible. Medication use and BMI were re-assessed by a trained research nurse at the day of scanning. Participants received a total of £150 upon completing all three study phases (please note that data from the third study phase was not included in the analysis for this study). If participants chose not to proceed after the first or second phase, they were partially reimbursed. The payment was distributed as follows: £10 for the initial completion of online questionnaires and three cognitive tasks, £100 for their attendance at Addenbrooke's Hospital, and £40 for completing the second set of online questionnaires. This study commenced in August 2019 and was terminated prematurely in March 2020, prompted by a University-wide closure of laboratory research activities and the redirection of clinical research facilities toward COVID-19 related studies. Hence, n = 42 participants who completed the baseline assessment could not be assessed at timepoint 2. However, key characteristics (e.g. age, gender, CA experiences, or friendship quality) are comparable between the neuroimaging sample and baseline and the participants who could not complete the study as a consequence of the COVID-19 pandemic (Table A.1.1).
2.3. Baseline assessments (T1)
At baseline, participants received an email containing an online link to remotely complete self-report questionnaires assessing past CA experiences as well as current (past two to four weeks) mental health, well-being, and friendship quality. Specifically, CA was assessed with the Short-Form of the Childhood Trauma Questionnaire (CTQ-SF; [Bernstein et al., 2003]), the Measure of Parental Style Questionnaire (MOPS; [Parker et al., 1997]), and the Alabama Parenting Questionnaire (APQ; [Frick, 1991]). Mental health and well-being (in the following referred to as psychosocial functioning) was assessed with the Mood and Feelings Questionnaire (MFQ; [Angold & Costello, 1987]), Revised Children’s Manifest Anxiety Scale (RCMAS; [Reynolds & Richmond, 1978]), the Leyton Obsessional Inventory-Child Version (LOI-CV; [Bamber et al., 2002]), the Behavioral Checklist (BCL; [van Harmelen et al., 2017]), the Rosenberg Self-Esteem Scale (SES; [Rosenberg, 1965]), the Kessler Psychological Distress Scale (K10; [Kessler et al., 2002]), the Warwick-Edinburgh Mental Well-Being Scale (WEMWBS; [Tennant et al., 2007]), and the Drugs and Self Injury Questionnaire (DASI; [Wilkinson et al., 2018]). Friendship quality was assessed with the Cambridge Friendship Questionnaire (CFQ; [van Harmelen et al., 2017]). Across all questionnaires, higher scores reflect more severe CA experiences, better psychosocial functioning, and greater perceived friendship quality. A detailed description of all questionnaires is provided in the supplementary information (section B). Given that we recruited adolescents and young adults aged 16–26 (Mage = 22.24 at baseline), we chose these measures to ensure that all questionnaires (incl. instructions and items) were accessible and age-appropriate for the entire sample (Demkowicz et al., 2020), which is also in line with similar approaches utilised in previous large-scale longitudinal cohort studies assessing young people aged 14–24 (Goodyer et al., 2010; Kiddle et al., 2018).
2.4. In-unit assessments (T2)
During the in-unit visit, participants completed the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI-II; [Wechsler, 2011]) from which age-normed IQ scores were derived. IQ scores ranged from 78 to 138 (MIQ = 116.09, SD = 10.18). Furthermore, the Edinburgh Handedness Inventory (EHI; [Oldfield, 1971]) indicated that 91% of participants preferred using the right hand for more complex manual tasks. Furthermore, state anxiety was assessed with the State-Trait Anxiety Inventory (STAI; [Spielberger & Vagg, 1984]) before and after participants completed the MIST in the MRI scanner.
2.4.1. fMRI stress paradigm
The Montreal Imaging Stress Task (MIST) is a well-validated and widely used acute psychosocial stress paradigm for fMRI (Berretz et al., 2021; Chung et al., 2016; Corr et al., 2021; Dedovic et al., 2005, 2009; Noack et al., 2019; Pruessner et al., 2008). This computerised mental arithmetic task with an artificially induced failure component was presented as a block design across two imaging runs. Each run lasted 11 min and consisted of a stress, control, and rest condition (Figure 2). The order of these conditions was counterbalanced across participants to avoid order effects.
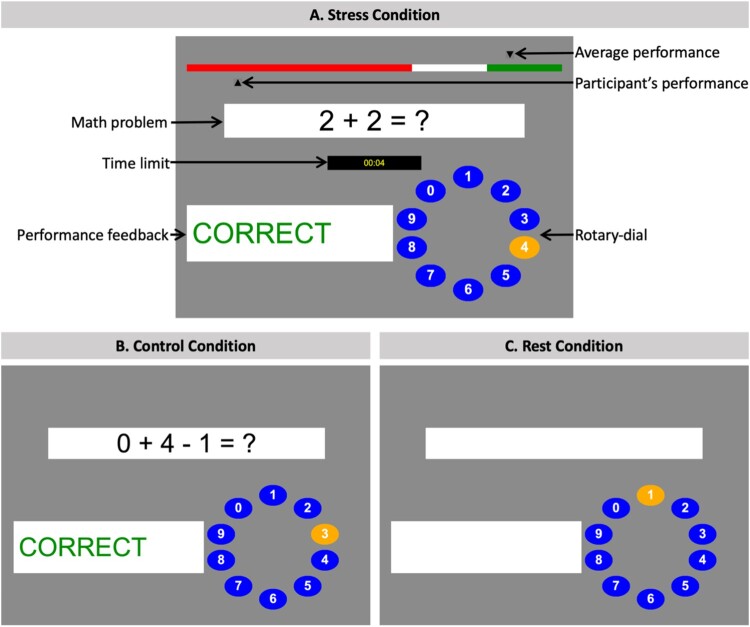
Figure 2.
Graphical user interfaces of the Montreal Imaging Stress Task.
Note. (A) Stress condition: from top to bottom, the figure shows the performance bar displaying the average performance of previous participants (artificially set to 80%) as well as the participant’s current performance. Below, participants were presented with math problems of varying difficulty whilst being shown the titrated time limit, they had to provide a response. A response was submitted via the rotary-dial (answer choices between 0 and 9). Finally, participants received trial-by-trial on screen performance feedback (‘correct’ in green, ‘error’ in red, or ‘timeout’ in yellow). (B) Control condition: participants answered math problems of the same difficulty level and received trial-by-trial performance feedback (‘correct’, ‘error’). However, no time constraints were enforced, the performance bar was not displayed, and participants were instructed that their performance would not be recorded. (C) Rest condition: participants were presented with the empty task interface and asked to keep their eyes open and not press buttons until the next math problem would appear.
During the 5 min stress condition (Figure 2A), participants were asked to answer math problems of varying difficulty under time constraints whilst receiving trial-by-trial on screen performance feedback (‘correct’ in green, ‘error’ in red, or ‘timeout’ in yellow). To answer, participants were provided a button box and instructed to navigate left or right on a rotary-dial to the correct digit (between 0 and 9). In addition, a performance bar at the top of the screen continuously displayed the ‘average’ performance of previous participants (artificially set to 80%) as well as the participant’s current performance. Participants were instructed to attain or surpass the average performance of their peers. To induce a high failure rate, the participant’s response time limit got adjusted throughout the task to enforce a range of approximately 20% to 45% correct responses (Dedovic et al., 2005). Specifically, participants were given 10% less time after three consecutive correct responses and 10% more time after three consecutive incorrect or timeout responses. To further induce psychosocial stress, participants were presented with a 5 sec on screen summary of their current performance and were reminded that their ‘performance should be close to or better than the average performance’. This summary was presented at five timepoints during the stress condition. In addition, participants received scripted negative verbal feedback in between runs from a member of the study team saying: ‘Your performance is below average. In order for your data to be used, your performance should be close to or better than the average performance. Please try as hard as you can next round’.
During the 5 min control condition (Figure 2B), participants answered math problems of the same difficulty level and received trial-by-trial performance feedback (‘correct’ in green, ‘error’ in red). However, no time constraints were enforced, the performance bar (including the ‘average’ peer performance) was not displayed, and participants were instructed that their performance would not be recorded.
During the 1 min rest condition (Figure 2C), participants were presented with the empty task interface and asked to keep their eyes open and not press buttons until the next math problem would appear.
The MIST took approximately 35 min to complete including approximately 5 min of practice outside the MRI scanner to familiarise participants with each condition. For this study, we used an adapted version of the MIST originally programmed by (Borchert, 2019) for Millisecond Software, LLC (openly available at: https://www.millisecond.com/download/library/montrealstresstest).
2.5. Imaging procedures
2.5.1. fMRI data acquisition
fMRI was conducted on a Siemens 3T Magnetom Prisma Fit whole body MRI scanner (Siemens Healthcare GmbH, Erlangen, Germany) with a 32-channel head coil. Blood oxygen level-dependent (BOLD) data were collected using a T2*-weighted transversal echo planar imaging (EPI) sequence with interleaved slice acquisition, covering the entire brain (repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 78°, number of slices = 34, slice thickness = 3 mm, slice gap = 0.3 mm, voxel size = 3 × 3 × 3 mm3, field of view (FOV) = 192 × 192 mm2, in-plane resolution = 64 × 64). To obtain a 3D structural scan, high-resolution sagittal T1-weighted images were acquired using a magnetisation prepared-rapid gradient echo (MPRAGE) sequence (TR = 2000 ms, TE = 2.98 ms, flip angle = 9°, number of slices = 176, slice thickness = 1 mm, slice gap = 0.5 mm, voxel size = 1 × 1 × 1 mm3, FOV = 256 × 256 mm2, in-plane resolution = 256 × 256).
2.5.2. fMRI preprocessing and data analysis
Preprocessing of the imaging data was performed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB (version R2020a; MathWorks) following standard procedures. To summarise, images were realigned to the mean image of the scan run using a 6-parameter rigid body spatial transformation, spatially normalised to the standard stereotactic space of the Montreal Neurological Institute (MNI) template, resampled to 3 mm isotropic voxels, and smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. In addition, framewise displacement (FD) was computed based on the head motion parameter and used as quality checks (Power et al., 2012). As recommended by (Schwarz et al., 2020), participants with a MFD > 0.5 mm or more than 20% volumes with FD > 0.5 mm in any of the two runs were excluded from subsequent analyses. Based on this rule and through visual inspection, n = 2 participants were excluded, leaving a total neuroimaging sample of n = 60 (Table A.1.1).
For the first-level analysis, we defined a general linear model (GLM) for each subject and each condition of the MIST (convolved with the canonical hemodynamic response function (HRF) of SPM12). Six head motion parameters from the realignment step were included as covariates. A high-pass filter with a cut-off frequency of 1/262 Hz and an autoregressive model of the first order were applied. To identify regions showing greater activation (i.e. greater mean BOLD signal) during the stress condition compared to the control condition, we computed stress > control first-level contrasts for each participant. This contrast allowed for investigating the effect of acute psychosocial stress on brain activation whilst controlling for activation changes induced by mental arithmetic.
For the second-level analysis, single-subject contrast maps were entered into random effects analyses (one-sample t-test). Based on our a-priori hypotheses, ROI analyses were performed using bilateral masks for the hippocampus, amygdala, insula, mPFC, ACC, NAc, and thalamus. ROI analyses were conducted using the pipeline implemented in the Wake Forest University (WFU) PickAtlas SPM12 toolbox (version 3.0.5; (Maldjian et al., 2004, 2003); https://www.nitrc.org/projects/wfu_pickatlas/). Specifically, these ROIs were defined using the PickAtlas GUI and resliced to match smoothing. Given that the anatomical region of the mPFC is less well defined, this ROI mask was based on the anatomical location of both dorsal and ventral mPFC (including the ACC; Brodmann areas (BA): 9, 10, 11, 24, 25, 32) (Moreno-López et al., 2020; Passingham & Wise, 2012; van Harmelen et al., 2013). All other ROIs were based on the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). ROI results were familywise error (FWE) corrected (pFWE < .05) using voxel-level statistics. For all ROIs that showed a significant main effect of task, we extracted individual beta weights by averaging across all activated voxels in the cluster containing the ROI peak (Tong et al., 2016). We applied no restriction for the minimum cluster size. To comprehensively examine neural activation outside our ROIs, we additionally conducted follow-up exploratory whole-brain analyses (pFWE < .05) using the same stress > control contrast, k > 25 voxels.
2.6. Principal component analysis
Principal component analyses (PCAs) with oblique rotation were used to explore differential dimensions of CA experiences and to capture the range of psychosocial outcomes in our sample. Specifically, we computed principal component (PC) scores, weighted by their explained variance for CA and psychosocial functioning, respectively. The PCA for CA revealed a two-component solution so that CA could be delineated along two dimensions resembling threat and deprivation experiences. Those dimensional scores were subsequently combined into a cumulative CA index, with a higher index indicating more severe CA (Figure 3A). Specifically, this PCA was conducted on standardised individual total scores of the Measure of Parental Style Questionnaire (MOPS; measure of maternal and paternal abuse, indifference, and overcontrol), the Short-Form of the Childhood Trauma Questionnaire (CTQ-SF; measure of physical abuse, emotional abuse, physical neglect, and emotional neglect), and the Alabama Parenting Questionnaire (APQ; measure of corporal punishment, poor parental involvement, and negative parenting). The PCA for psychosocial functioning revealed a three-component solution, and we summed the weighted PC1, PC2, and PC3 scores to compute a cumulative psychosocial functioning index, with a higher index indicating better mental health and well-being (Figure 3B). This PCA was conducted on standardised individual total scores of the Warwick-Edinburgh Mental Well-Being Scale (WEMWBS; measure of mental well-being), the Revised Children’s Manifest Anxiety Scale (RCMAS; measure of physiological anxiety, worry/oversensitivity, and social concerns/concentration), the Mood and Feelings Questionnaire (MFQ; measure of depressive symptoms); the Rosenberg Self-Esteem Scale (SES; measure of self-esteem); the Kessler Psychological Distress Scale (K10; measure of psychological distress); the Leyton Obsessional Inventory-Child Version (LOI-CV; measure of compulsions, obsessions, and cleanliness), and the Behavioral Checklist (BCL; measure of behavioural problems). Please note that all results hold when only using the weighted PC1 score for psychosocial functioning. A similar method has been employed by (Anand et al., 2019) and a detailed description of our analyses as well as a summary of the PC scores and their associations can be found in our supplementary information (section E).
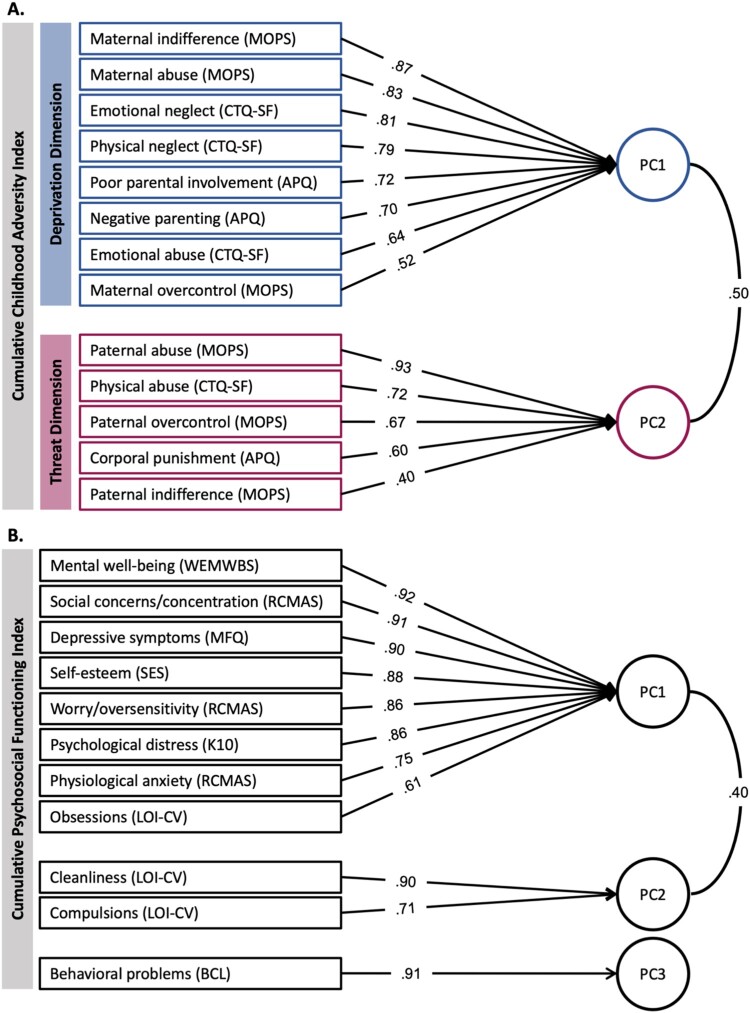
Figure 3.
Principal component analysis loading matrices for childhood adversity and psychosocial functioning.
Note. Two PCAs with oblique rotation were conducted on individual scores of (A) CA measures and (B) psychosocial functioning measures. The PCA for CA resulted in two principal components (PCs) which were further divided into a deprivation dimension (blue; PC1 explaining 37% of variance) and a threat dimension (red; PC2 explaining 21% of variance). To account for the contributions of both PCs, we weighted the scores for each PC by their explained variance and subsequently summed these scores to compute a single index of total severity experienced (cumulative CA index). The PCA for psychosocial functioning resulted in three PCs (PC1 explained 55% of variance; PC2 explained 15%; PC3 explained 10%). To compute a cumulative psychosocial functioning index, we summed the weighted PC1, PC2, and PC3 scores. Factor loadings of each measure are displayed on the arrows.
2.7. Statistical analyses
First, we analysed behavioural data collected at baseline (T1; N = 102). Specifically, we examined associations between friendship quality and CA through linear regression models. We ran separate models for the cumulative CA index (derived through summing the weighted PC1 and PC2 scores) as well as the weighted deprivation (PC1) and threat dimensions (PC2). In addition, we examined associations between friendship quality and the cumulative psychosocial functioning index (hypothesis 1). All models included age at the time of assessment and gender identity as covariates. To handle missing questionnaire data, we derived sum scores from scales with ≥15 items if 85% or more of the items were answered. For scales with less than 15 items, a sum score was only derived if 100% of the items were answered. This resulted in 2.45% of missing data.
Second, we analysed data collected during the in-unit assessment (T2; n = 60). We used a paired t-test to examine individual mean differences in state anxiety before and after completing the MIST in the MRI scanner. Afterwards, we investigated overall task effects in our predefined ROIs (hypothesis 2) and then examined associations between CA and friendship quality on stress-induced significant ROI reactivity during stress (> control) trials of the MIST (hypothesis 3). We ran separate moderated multiple regression models for the cumulative CA index, the deprivation, and threat dimensions. All multiple regression models included age at the time of scanning and gender identity as covariates. As our stressor comprised of a timed arithmetic test, we further added IQ as a covariate to all models. Furthermore, friendship quality scores were mean centred to align the scaling of the predictor variables and thereby enhance interpretation of the multiple regression results (Iacobucci et al., 2016).
All statistical analyses outlined above were run in R version 3.6.3 (R Core Team, 2022). The PCAs were performed using the psych R package (version 2.2.9; [Revelle, 2022]) and mean imputations to replace missing values were performed using the mice R package (version 3.15.0; [van Buuren & Groothuis-Oudshoorn, 2011]). Regression models were run using the stats R package (version 3.6.3). Partial Cohen’s f-squared ( ) and Cohen’s d (d) effect size estimates are reported for all relevant tests. Significance was set at p < .05 throughout all analyses unless stated otherwise and all tests were Bonferroni corrected for multiple comparisons (# of models tested). In addition, we used the Median Absolute Deviation (MAD) method (i.e. median plus or minus 3 times the MAD; [Leys et al., 2013]) in combination with the Rosner’s test (EnvStats R package version 2.7.0; [Millard, 2013]) to detect and exclude potential outliers. Moreover, to visualise significant interactions, we plotted model estimated marginal means using the sjPlot R package (version 2.8.14; [Lüdecke, 2023]) alongside 95% confidence intervals. Specifically, we explored how the relationship between CA and stress-induced ROI reactivity changed as a function of low and high friendship quality (−1SD, +1SD). For statistical power considerations, please refer to the supplementary information (section F).
3. Results
3.1. Behavioural results (T1; N = 102)
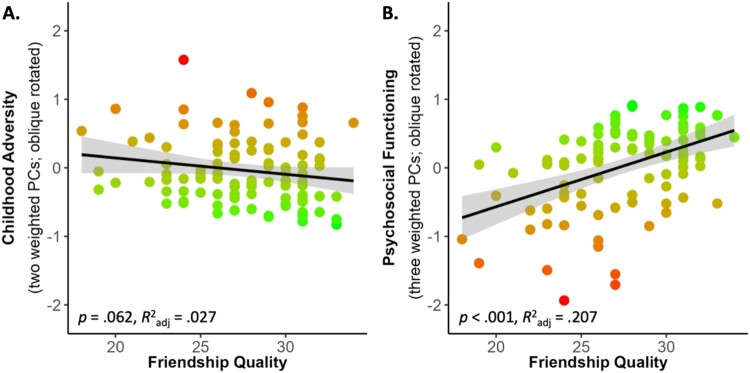
First, we did not observe that participants with more severe retrospectively self-reported CA experiences self-reported lower friendship quality, β = −0.19, SE = .01, t97 = −1.89, p = .062 (Figure 4A). Next, we observed that models specifying either deprivation or threat experiences as a predictor showed a better model fit compared to a model specifying cumulative CA (see supplementary information for full details). However, none of these dimensional models significantly predicted differences in friendship quality (deprivation experiences: β = −0.16, SE = .01, t97 = −1.57, p = .120; threat experiences: β = −0.17, SE = .01, t97 = −1.65, p = .103). Second, we found that greater subjectively perceived friendship quality was significantly related to better psychosocial functioning in young people with CA, β = 0.44, SE = .02, t97 = 4.87, p < .001, = .245, R2adj = .207 (Figure 4B). Finally, we observed no significant associations between CA (including cumulative index, threat, or deprivation experiences) and psychosocial functioning, p’s > .235. Please see our supplementary information for the full model output, descriptive statistics, and correlations between the study variables (sections G-H).
Figure 4.
Associations between friendship quality and (A) Childhood adversity and (B) Psychosocial functioning.
Note. Participants with greater subjectively perceived friendship quality (A) did not retrospectively self-reported less negative CA (p = .062) but (B) showed better psychosocial functioning (p < .001). Index scores of CA comprise two weighted principal components (PCs) and index scores of psychosocial functioning comprise three weighted PCs, both oblique rotated. Both y-axes represent factor scores with M = 0 and SD = 1. Brighter shading (green) of individual data points represents (A) less severe CA and (B) better psychosocial functioning on each graph respectively. The black lines show the best-fitting linear regression lines, and the shaded regions around them represent the 95% confidence intervals.
3.2. Neuroimaging results (T2; n = 60)
3.2.1. State anxiety before and after acute psychosocial stress
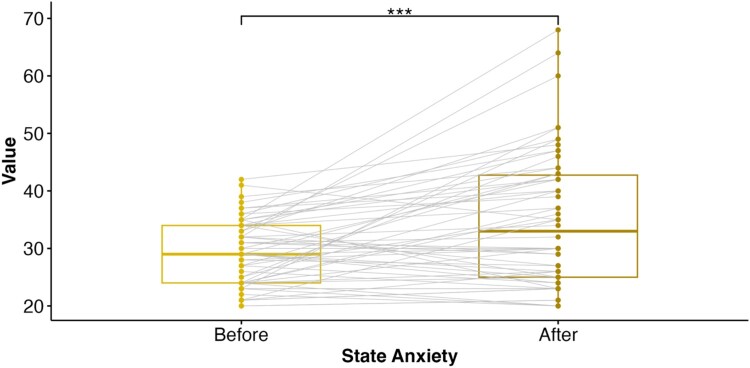
We observed a significant increase in self-reported state anxiety upon completion of the MIST (Mbefore = 29.19, SD = 5.75; Mafter = 34.88, SD = 11.57), t57 = −4.33, p < .001, d = .568, suggesting that our task successfully induced subjective emotional stress (Figure 5).
Figure 5.
State anxiety increased after acute psychosocial stress.
Note. Participants exhibited greater self-reported state anxiety after completing the Montreal Imaging Stress Task (MIST) compared to state anxiety levels before the MIST (p < .001). The boxplot displays the median (Mdn, solid vertical line) and interquartile range (IQR) before (Mdn = 29.00, IQR = 10) and after (Mdn = 33.00, IQR = 17.75) completing the MIST. The points represent individual datapoints with the grey lines connecting paired observations. *** p < .001.
3.2.2. Brain responses to acute psychosocial stress
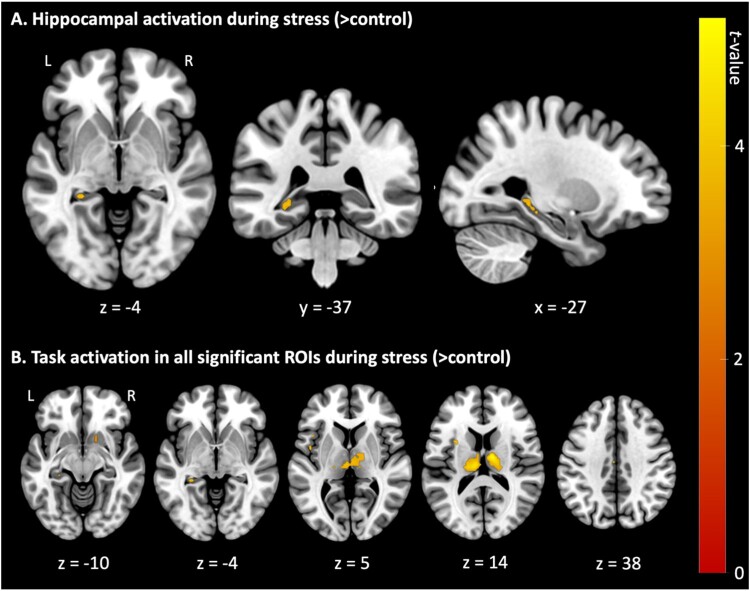
First, we investigated the main effect of stress (> control) in our predefined ROIs (hippocampus, amygdala, insula, mPFC (ACC), NAc, and thalamus) using familywise error (FWE) correction (pFWE < .05) at the voxel level. This analysis identified significant activation in the left hippocampus (t57 = 4.24, pFWE = .007; MNI coordinates: −27, −37, −4), bilateral insula (t57 = 4.07, pFWE = .020; MNI coordinates: −42, 14, 5), left mPFC (ACC) (t57 = 4.35, pFWE = .043; MNI coordinates: −3, 16, 38), right NAc (t57 = 3.09, pFWE = .016; MNI coordinates: 15, 11, −10), and bilateral thalamus (t57 = 5.22, pFWE < .001; MNI coordinates: 12, −10, 14). All significant task-related ROI clusters are summarised in Table 1 and visualised in Figure 6 below. Whole-brain analyses (pFWE < .05) revealed no significant activation outside our predefined ROIs.
Table 1:
ROIs activated during stress (> control) trials of the Montreal Imaging Stress Task.
| Region | Side | MNI Coordinates | Cluster Size | t | pFWE (Peak) | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | z | |||||
| Hippocampus | L | −27 | −37 | −4 | 8 | 4.24 | 3.93 | .007 |
| Insula | L | −42 | 14 | 5 | 2 | 4.07 | 3.80 | .020 |
| L | −30 | 5 | 14 | 4 | 3.98 | 3.73 | .026 | |
| R | 45 | 11 | 2 | 1 | 3.97 | 3.71 | .027 | |
| L | −45 | −1 | 5 | 4 | 3.88 | 3.64 | .035 | |
| L | −33 | −16 | 8 | 1 | 3.76 | 3.54 | .048 | |
| mPFC (ACC) | L | −3 | −16 | 38 | 1 | 4.35 | 4.03 | .043 |
| NAc | R | 15 | 11 | −10 | 4 | 3.09 | 2.96 | .016 |
| Thalamus | R | 12 | −10 | 14 | 195 | 5.22 | 4.70 | <.001 |
| L | −9 | −19 | 14 | 4.80 | 4.38 | .001 | ||
| L | −18 | −22 | 14 | 4.61 | 4.23 | .002 | ||
Note. All reported statistics are significant at pFWE < .05, voxel-level corrected for the ROI. All ROIs were bilaterally defined using the WFU PickAtlas Tool (version 3.0.5; [Maldjian et al., 2003]) and based on the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). Given that the anatomical region of the mPFC is less well defined the ROI mask was based on the anatomical location of both dorsal and ventral mPFC (including the ACC; Brodmann areas (BA): 9, 10, 11, 24, 25, 32) (Moreno-López et al., 2020; Passingham & Wise, 2012; van Harmelen et al., 2013). ACC = anterior cingulate cortex; mPFC = medial prefrontal cortex; NAc = nucleus accumbens. L = left; R = right.
Figure 6.
Overview of neural activation during acute psychosocial stress (> control).
Note. Displayed are t-values of neural activation (pFWE < .05) during stress (> control) trials of the Montreal Imaging Stress Task. Results are presented (A) centred at the left hippocampus region of interest (MNI coordinates: x = −27, y = −37, z = −4) and (B) as axial slices with corresponding z-coordinates. L = left; R = right.
3.2.3. Moderation effect of friendship quality
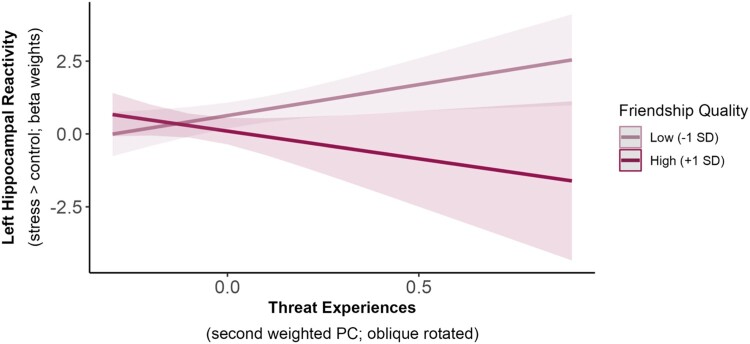
To examine whether perceived friendship quality was related to lower neural stress responses, we ran three separate linear regression models for each of our five predefined ROIs. Specifically, we examined the interaction between friendship quality and cumulative CA, deprivation, or threat experiences. These analyses revealed only a significant threat experiences x friendship quality interaction on left hippocampal reactivity, β = −0.33, SE = .26, t46 = −2.26, p = .029, = .111, R2adj = .142 (Figure 7). However, this effect did not survive correction for multiple comparisons (pBonf = .145; corrected for five ROI comparisons). Age had a significant effect on left hippocampal reactivity across all analyses, with older participants showing increased left hippocampal reactivity. No other main effects or interactions were observed in any of our analyses (p’s > .050).
Figure 7.
Exploratory average marginal effects in the interaction of threat experiences and friendship quality on left hippocampal reactivity to acute stress.
Note. Friendship quality had a weak moderating effect on the relationship between threat experiences and left hippocampal reactivity to acute stress (p = .029), such that hippocampal reactivity increased with more negative threat experiences in participants reporting low friendship quality. However, this effect did not survive correction for multiple comparisons (pBonf = .145; corrected for five ROI comparisons). The lines show the estimated marginal means of threat experiences (x-axis; second weighted PC) on left hippocampal reactivity (y-axis; beta weights) at different values of friendship quality (−1SD = 24.31; +1SD = 31.05) with a pointwise 95% confidence interval, derived from a multiple linear regression model.
4. Discussion
In this study, we examined whether perceived friendship quality was related to better mental health and well-being (N = 102) and lower neural stress responses using the Montreal Imaging Stress Task (n = 62) in young people (aged 16–26) with low to moderate CA. In addition, we examined the relation between CA and friendship quality. A principal component analysis revealed two dimensions of CA resembling threat or deprivation like experiences. Hence, we investigated both cumulative and dimension specific effects of CA (Evans et al., 2013; Sheridan & McLaughlin, 2014). Contrary to the social transactional model of mental health vulnerability (McCrory et al., 2022), we found no support for social thinning after CA, meaning that the severity of CA (neither cumulative nor dimension specific) did not differentially impact friendship quality. Higher friendship quality, on the other hand, was strongly associated with better psychosocial functioning. Furthermore, we found that experimentally induced acute stress increased state anxiety and enhanced neural activity in five frontolimbic regions (left hippocampus, bilateral insula, left mPFC (ACC), right NAc, and bilateral thalamus). Finally, we found weak support that threat experiences interacted with friendship quality to predict left hippocampal reactivity to acute stress. However, this effect did not survive correction for multiple comparisons. Therefore, future research is needed to examine whether friendships aid neural responses to acute stress in young people with childhood threat experiences.
Despite the prominence of research suggesting that CA can lead to impoverished social networks (Horan & Widom, 2015; McCrory et al., 2022; Nevard et al., 2021; Sheikh et al., 2016; Sperry & Widom, 2013), we observed that neither conceptualisation of CA (i.e. cumulative, deprivation, or threat) was associated with lower friendship quality. Our findings are in fact aligned with previous longitudinal studies by (Miller et al., 2014; van Harmelen et al., 2016) who showed that CA was not directly associated with poor friendships in healthy community samples. In addition, (Fritz et al., 2020) even showed that CA predicted higher friendship quality at ages 14 and 18. The absence of social thinning in our sample may suggest that the risk of developing impoverished social networks is low for rather well-functioning young people with low to moderate CA. This assumption is further supported by our finding that neither conceptualisation of CA was related to subsequent psychosocial functioning. Furthermore, we showed that higher friendship quality was strongly associated with better psychosocial functioning. This is in line with previous research showing that social support provided by friends, family, or significant others is related to better mental health and well-being in samples with CA (Jaffee, 2017; Lagdon et al., 2021; Salazar et al., 2011; van Harmelen et al., 2016, 2021; Vranceanu et al., 2007).
Next, we found that high-quality friendships aided left hippocampal reactivity to acute stress in young people with childhood threat experiences. While this interaction effect did not survive stringent correction for multiple comparisons, we recognise the value in cautiously aligning our uncorrected findings with previous research. For example, recent work by (Tang et al., 2021) showed that low, but not high, levels of friendship quality facilitated blunted sympathetic nervous system reactivity to social rejection feedback, linking early institutionalisation (i.e. severe deprivation experiences) with later-life peer problems. In contrast, (Fritz et al., 2020) utilised a cumulative-risk approach to quantify CA and found that friendship support at ages 14 or 17 was not associated with neural responses to social rejection at age 18. Consequently, our results align with previous findings that have shown friendship stress buffering through dimensional, but not cumulative, approaches, despite some divergence regarding the specific dimensions investigated. However, it is worth noting that (Tang et al., 2021) did not formally examine different dimensions of early experiences in their sample.
Given the established association between past threat experiences and hippocampal neurodevelopment, our uncorrected findings regarding friendship stress buffering on hippocampal functioning are particularly interesting. The hippocampus is a subcortical region which develops mainly in the first two years of life, and is vital for learning, memory, spatial navigation, and emotional processing (Bird & Burgess, 2008; Phelps, 2004). The hippocampus also plays an important role in inhibiting HPA axis activity in response to elevated blood glucocorticoid levels (Lupien et al., 2009). Due to its dense innervation with glucocorticoid receptors, the hippocampus is particularly sensitive to chronic or repeated stress exposure. Both animal and human studies have shown that early onset and increased severity of CA, specifically threat exposure, was associated with structural and functional alterations in the hippocampus, which in turn was identified as a risk factor for later-life psychopathology (Chen et al., 2008; Cohodes et al., 2021). For example, reductions in hippocampal volume were consistently observed in children and adolescents with past threat experiences, which partially mediated the relationship between threat exposure and internalising (Weissman et al., 2020) and externalising problems (Hanson et al., 2015), whilst also being associated with reduced friendship support (Malhi et al., 2020). Furthermore, hippocampal hyperreactivity to acute stress has been reported in young adults with cumulative CA (Seo et al., 2014) and middle-aged adults with emotional maltreatment (Leicht-Deobald et al., 2018). Interestingly, such hippocampal hyperreactivity to acute stress was associated with greater adverse health symptoms (Seo et al., 2014). In other words, reductions in hippocampal volume and functioning may act as a potential mechanism of stress vulnerability in young people with CA. In line with this claim, CA has been linked with a greater sensitivity towards peer rejection (van Harmelen et al., 2014) and a greater likelihood of experiencing interpersonal stress (Benedini et al., 2016; Handley et al., 2019; van Harmelen et al., 2016; Widom et al., 2014). Through this process, CA experiences are thought to reduce an individual’s likelihood to form and maintain long-lasting, high-quality relationships (Labella et al., 2018; McLafferty et al., 2018). Again, an effect we did not observe in the current study, despite other studies reporting impoverished social networks in individuals with CA (Horan & Widom, 2015; Nevard et al., 2021; Sperry & Widom, 2013). Regardless, our findings, as well as those from other studies, consistently demonstrate a strong association between high-quality friendships and better mental health outcomes in young people with CA.
Our findings are considered in the context of important limitations. First, the current study design prohibits causal inferences. Future large-scale, longitudinal, prospective, and genetically sensitive studies are needed to draw conclusions about the causal impact of CA on neurocognitive and social functioning and the relationship to mental health vulnerability (Danese & Lewis, 2022; McCrory et al., 2022). Second, the data collection period of this study was cut short due to re-allocation of the clinical research facilities in Cambridge, UK during the COVID-19 pandemic, resulting in a small neuroimaging sample (n = 62). Although a retrospective power analysis confirmed that the sample size was sufficient to detect large effects, future research is needed to validate and extend our findings. Despite previous research indicating that stress can induce laterality changes in the hippocampus (Riem et al., 2015), we refrained from interpreting our laterality findings as these might be driven by our stringent significance threshold and reduced sample size. Third, the value of dimensional approaches for conceptualising CA and identifying mechanisms shaping developmental outcomes is actively being debated (McLaughlin et al., 2021; Pollak & Smith, 2021). The current study suggests that continuously assessing the severity of different CA dimensions may be helpful for specifying putative neural mechanisms that potentially increase mental health vulnerability (McLaughlin et al., 2019; Puetz et al., 2020). In addition, future work should consider the developmental timing and chronicity of exposure to holistically understand the detrimental impact CA can have on the developing brain and consequently on neurocognitive and social functioning. Similarly, future work should account for differential friendship dimensions, such as intimacy, loyalty, frequency of engagement, or network size, as well as differences in stress paradigms with regards to type, intensity, and duration of acute stress, to gain a more nuanced mechanistic understanding about friendship stress buffering after CA. It is worth noting that our sample self-reported on average high levels of friendship quality suggesting a well-functioning group of young people. Nevertheless, current individual characteristics, such as mental health vulnerabilities, may have biased the reporting of friendship quality, in that relationships may be perceived as more negative (Baldwin & Degli Esposti, 2021; Colman et al., 2016). However, this concern seems negligible given that we successfully replicated previous longitudinal findings showing a strong link between high-quality friendships and better mental health in samples with low to moderate CA (van Harmelen et al., 2016, 2017, 2021). Furthermore, in our sample, we found no association between CA and psychosocial functioning, which is at odds with robust associations reported in the literature (Humphreys et al., 2016; McCrory et al., 2019; Shackman & Pollak, 2014; Sheikh et al., 2016). It is plausible that the remote assessment of CA and psychosocial functioning in our study may have introduced some limitations to the validity of these measures in our particular sample. However, remote psychosocial functioning assessments have demonstrated adequate psychometric properties (van Ballegooijen et al., 2016) and strong internal consistency (Brock et al., 2012) in previous studies. Furthermore, our remote assessment of the MFQ at T1 exhibited a moderate correlation with the MFQ assessment we conducted in the laboratory at T2 (r = .69, p < .001). Additionally, all our questionnaires demonstrated acceptable to excellent internal consistency at baseline, and we successfully replicated the above mentioned large-scale longitudinal findings (van Harmelen et al., 2017, 2021). Given these considerations, it is possible that the absence of a relationship between CA and psychosocial functioning in our sample can be attributed to the fact that our sample consisted of relatively well-functioning young people who reported only low to moderate CA.
In conclusion, we showed that young people with more severe CA did not self-report lower friendship quality. However, higher friendship quality was strongly associated with better psychosocial functioning. We found only weak support that threat experiences interacted with friendship quality to predict left hippocampal reactivity to acute stress. However, this effect did not survive correction for multiple comparisons and therefore requires replication in larger ideally longitudinal samples. Hence, future research is needed to examine whether friendships aid neural responses to acute stress in young people with childhood threat experiences.
Supplementary Material
Acknowledgments
The authors would like to thank Alicia Smith for their support with recruitment and data processing, the research nurses at the Cambridge Clinical Research Facility (Addenbrooke’s Hospital) for their contributions as well as all members of the RAISE study team. Finally, the authors would like to thank all adolescents and young adults who participated in the study.
Funding Statement
The Resilience after Individual Stress Exposure (RAISE) study has been funded by two grants from the Royal Society to ALvH [grant number RGF\EA\180029 & RGF\R1\ 180064]. This work was further supported by a Royal Society Dorothy Hodgkin fellowship for ALvH [grant number DH150176] and a Wolfe Health fellowship for LML. MK and ALvH were supported by the Social Resilience and Security program at Leiden University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
CRediT author statement
Maximilian König: Conceptualisation, Methodology, Validation, Formal analysis, Data curation, Visualisation, Writing – original draft. Oksana Berhe: Methodology, Validation, Writing – review & editing. Konstantinos Ioannidis: Investigation, Writing – review & editing. Sofia Orellana: Methodology, Writing – review & editing. Eugenia Davidson: Methodology, Writing – review & editing. Muzaffer Kaser: Investigation, Writing – review & editing. Laura Moreno-López: Conceptualisation, Investigation, Project administration, Writing – review & editing. Anne-Laura van Harmelen: Conceptualisation, Investigation, Methodology, Supervision, Funding acquisition, Writing – review & editing.
Declaration of competing interests
The authors have no competing interests to declare that are relevant to the content of this article. KI receives a stipend for editorial work from Elsevier.
Protocol paper
Moreno-López, L., Sallie, S. N., Ioannidis, K., Kaser, M., Schueler, K., Askelund, A. D., … & van Harmelen, A. L. (2021). RAISE study protocol: a cross-sectional, multilevel, neurobiological study of resilience after individual stress exposure. BMJ open, 11(1), e040394. doi: 10.1136/bmjopen-2020-040394.
Data availability statement
All scripts and pseudonymized data necessary to reproduce the findings of this study are publicly available on OSF at https://osf.io/mdfhw/?view_only=f3c85e8f608843469d23cb347d01fcdf (DOI 10.17605/OSF.IO/MDFHW) and upon publication will be made available on DataverseNL. For all significant ROIs, group-level statistical maps displaying t-values of neural activation (pFWE < .05) during stress (>control) trials of the MIST have been uploaded to NeuroVault (https://neurovault.org/collections/AXXZBAGI/).
References
- Anand, K. J. S., Rigdon, J., Rovnaghi, C. R., Qin, F., Tembulkar, S., Bush, N., LeWinn, K., Tylavsky, F. A., Davis, R., Barr, D. A., & Gotlib, I. H. (2019). Measuring socioeconomic adversity in early life. Acta Paediatrica, 108(7), 1267–1277. 10.1111/apa.14715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold, A., & Costello, E. J. (1987). Mood and feelings questionnaire (MFQ). Developmental Epidemiology Program. [Google Scholar]
- Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin, J. R., & Degli Esposti, M. (2021). Triangulating evidence on the role of perceived versus objective experiences of childhood adversity in psychopathology. JCPP Advances, 1(1). 10.1111/jcv2.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber, D., Tamplin, A., Park, R. J., Kyte, Z. A., & Goodyer, I. M. (2002). Development of a short leyton obsessional inventory for children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 41(10), 1246–1252. 10.1097/00004583-200210000-00015 [DOI] [PubMed] [Google Scholar]
- Bellis, M. A., Hughes, K., Leckenby, N., Perkins, C., & Lowey, H. (2014). National household survey of adverse childhood experiences and their relationship with resilience to health-harming behaviors in England. BMC Medicine, 12(1), 72. 10.1186/1741-7015-12-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedini, K. M., Fagan, A. A., & Gibson, C. L. (2016). The cycle of victimization: The relationship between childhood maltreatment and adolescent peer victimization. Child Abuse & Neglect, 59, 111–121. 10.1016/j.chiabu.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Bernstein, D. P., Fink, L., Handelsman, L., & Foote, J. (1994). Childhood Trauma Questionnaire (CTQ) [Database record]. APA PsycTests. 10.1037/t02080-000. [DOI]
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., Stokes, J., Handelsman, L., Medrano, M., Desmond, D., & Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Berretz, G., Packheiser, J., Kumsta, R., Wolf, O. T., & Ocklenburg, S. (2021). The brain under stress—A systematic review and activation likelihood estimation meta-analysis of changes in BOLD signal associated with acute stress exposure. Neuroscience & Biobehavioral Reviews, 124, 89–99. 10.1016/j.neubiorev.2021.01.001 [DOI] [PubMed] [Google Scholar]
- Bird, C. M., & Burgess, N. (2008). The hippocampus and memory: Insights from spatial processing. Nature Reviews Neuroscience, 9(3), 182–194. 10.1038/nrn2335 [DOI] [PubMed] [Google Scholar]
- Borchert, K. (2019). Inquisit Montreal Imaging Stress Task. https://www.millisecond.com/download/library/montrealstresstest.
- Brock, R. L., Barry, R. A., Lawrence, E., Dey, J., & Rolffs, J. (2012). Internet administration of paper-and-pencil questionnaires used in couple research: Assessing psychometric equivalence. Assessment, 19(2), 226–242. 10.1177/1073191110382850 [DOI] [PubMed] [Google Scholar]
- Brown, S. M., Rienks, S., McCrae, J. S., & Watamura, S. E. (2019). The co-occurrence of adverse childhood experiences among children investigated for child maltreatment: A latent class analysis. Child Abuse & Neglect, 87, 18–27. 10.1016/j.chiabu.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan, B. L., Gee, D. G., Gabard-Durnam, L., Telzer, E. H., Humphreys, K. L., Goff, B., Shapiro, M., Flannery, J., Lumian, D. S., Fareri, D. S., Caldera, C., & Tottenham, N. (2019). Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: An examination across 3 years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(7), 664–671. 10.1016/j.bpsc.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., & Baram, T. Z. (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology, 41(1), 197–206. 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Dubé, C. M., Rice, C. J., & Baram, T. Z. (2008). Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. The Journal of Neuroscience, 28(11), 2903–2911. 10.1523/JNEUROSCI.0225-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K. C., Springer, I., Kogler, L., Turetsky, B., Freiherr, J., & Derntl, B. (2016). The influence of androstadienone during psychosocial stress is modulated by gender, trait anxiety and subjective stress: An fMRI study. Psychoneuroendocrinology, 68, 126–139. 10.1016/j.psyneuen.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Cicchetti, D., & Valentino, K. (2006). An ecological-transactional perspective on child maltreatment: Failure of the average expectable environment and its influence on child development. In Cicchetti D. (Ed.), Developmental psychopathology: Risk, disorder, and adaptation (Vol. 3, pp. 129–201). John Wiley & Sons, Inc. xvi. https://psycnet.apa.org/fulltext/2006-03609-004.pdf. [Google Scholar]
- Clark, C., Caldwell, T., Power, C., & Stansfeld, S. A. (2010). Does the influence of childhood adversity on psychopathology persist across the lifecourse? A 45-year prospective epidemiologic study. Annals of Epidemiology, 20(5), 385–394. 10.1016/j.annepidem.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Cohodes, E. M., Kitt, E. R., Baskin-Sommers, A., & Gee, D. G. (2021). Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Developmental Psychobiology, 63(2), 153–172. 10.1002/dev.21969 [DOI] [PubMed] [Google Scholar]
- Colman, I., Kingsbury, M., Garad, Y., Zeng, Y., Naicker, K., Patten, S., Jones, P. B., Wild, T. C., & Thompson, A. H. (2016). Consistency in adult reporting of adverse childhood experiences. Psychological Medicine, 46(3), 543–549. 10.1017/S0033291715002032 [DOI] [PubMed] [Google Scholar]
- Corr, R., Pelletier-Baldelli, A., Glier, S., Bizzell, J., Campbell, A., & Belger, A. (2021). Neural mechanisms of acute stress and trait anxiety in adolescents. NeuroImage: Clinical, 29, 102543. 10.1016/j.nicl.2020.102543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme, D., Flournoy, J. C., Livingston, J. L., Lieberman, M. D., Dapretto, M., & Pfeifer, J. H. (2022). Testing the adolescent social reorientation model during self and other evaluation using hierarchical growth curve modeling with parcellated fMRI data. Developmental Cognitive Neuroscience, 54, 101089. 10.1016/j.dcn.2022.101089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A., & Lewis, S. J. (2022). New directions in research on childhood adversity. The British Journal of Psychiatry, 220(3), 107–108. 10.1192/bjp.2021.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A., & McEwen, B. S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106(1), 29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Dedovic, K., Renwick, R., Mahani, N. K., Engert, V., Lupien, S. J., & Pruessner, J. C. (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry & Neuroscience: JPN, 30(5), 319–325. https://www.ncbi.nlm.nih.gov/pubmed/16151536. [PMC free article] [PubMed] [Google Scholar]
- Dedovic, K., Rexroth, M., Wolff, E., Duchesne, A., Scherling, C., Beaudry, T., Lue, S. D., Lord, C., Engert, V., & Pruessner, J. C. (2009). Neural correlates of processing stressful information: An event-related fMRI study. Brain Research, 1293, 49–60. 10.1016/j.brainres.2009.06.044 [DOI] [PubMed] [Google Scholar]
- Demkowicz, O., Ritchie, B., Ashworth, E., Tait, N., Miles, H., Panayiotou, M., Patalay, P., Burrell, K., & Deighton, J. (2020). Mental health questionnaire research with children and young people in schools: Recommendations for good practice. Evidence Based Practice Unit.
- Dong, M., Anda, R. F., Felitti, V. J., & Dube, S. R. (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect. 10.1016/j.chiabu.2004.01.008 [DOI] [PubMed]
- Eisenberger, N. I., Taylor, S. E., Gable, S. L., Hilmert, C. J., & Lieberman, M. D. (2007). Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage, 35(4), 1601–1612. 10.1016/j.neuroimage.2007.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, B. J., Figueredo, A. J., Brumbach, B. H., & Schlomer, G. L. (2009). Fundamental dimensions of environmental risk. Human Nature, 20(2), 204–268. 10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- Evans, G. W., Li, D., & Whipple, S. S. (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342–1396. 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Frick, P. J. (1991). The Alabama parenting questionnaire. Unpublished Rating Scale, University of Alabama.
- Fritz, J., Stretton, J., Askelund, A. D., Schweizer, S., Walsh, N. D., Elzinga, B. M., Goodyer, I. M., Wilkinson, P. O., & van Harmelen, A.-L. (2020). Mood and neural responses to social rejection do not seem to be altered in resilient adolescents with a history of adversity. Development and Psychopathology, 32(2), 411–423. 10.1017/S0954579419000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, D. G., Gabard-Durnam, L., Telzer, E. H., Humphreys, K. L., Goff, B., Shapiro, M., Flannery, J., Lumian, D. S., Fareri, D. S., Caldera, C., & Tottenham, N. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25(11), 2067–2078. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer, I. M., Croudace, T., Dunn, V., Herbert, J., & Jones, P. B. (2010). Cohort profile: Risk patterns and processes for psychopathology emerging during adolescence: The ROOTS project. International Journal of Epidemiology, 39(2), 361–369. 10.1093/ije/dyp173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. G., McLaughlin, K. A., Berglund, P. A., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., & Kessler, R. C. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar, M. R. (2017). Social buffering of stress in development: A career perspective. Perspectives on Psychological Science, 12(3), 355–373. 10.1177/1745691616680612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley, E. D., Russotti, J., Rogosch, F. A., & Cicchetti, D. (2019). Developmental cascades from child maltreatment to negative friend and romantic interactions in emerging adulthood. Development and Psychopathology, 31(5), 1649–1659. 10.1017/S095457941900124X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L., Nacewicz, B. M., Sutterer, M. J., Cayo, A. A., Schaefer, S. M., Rudolph, K. D., Shirtcliff, E. A., Pollak, S. D., & Davidson, R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan, J. M., & Widom, C. S. (2015). From childhood maltreatment to allostatic load in adulthood: The role of social support. Child Maltreatment, 20(4), 229–239. 10.1177/1077559515597063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar, C. E., Johnson, A. E., & Gunnar, M. R. (2015). Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science, 18(2), 281–297. 10.1111/desc.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, K. L., Kircanski, K., Colich, N. L., & Gotlib, I. H. (2016). Attentional avoidance of fearful facial expressions following early life stress is associated with impaired social functioning. Journal of Child Psychology and Psychiatry, 57(10), 1174–1182. 10.1111/jcpp.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, K. L., & Zeanah, C. H. (2015). Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology, 40(1), 154–170. 10.1038/npp.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci, D., Schneider, M. J., Popovich, D. L., & Bakamitsos, G. A. (2016). Mean centering helps alleviate “micro” but not “macro” multicollinearity. Behavior Research Methods, 48(4), 1308–1317. 10.3758/s13428-015-0624-x [DOI] [PubMed] [Google Scholar]
- Ioannidis, K., Askelund, A. D., Kievit, R. A., & van Harmelen, A.-L. (2020). The complex neurobiology of resilient functioning after childhood maltreatment. BMC Medicine, 18(1), 32. 10.1186/s12916-020-1490-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee, S. R. (2017). Child maltreatment and risk for psychopathology in childhood and adulthood. Annual Review of Clinical Psychology, 13(1), 525–551. 10.1146/annurev-clinpsy-032816-045005 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C., Andrews, G., Colpe, L. J., Hiripi, E., Mroczek, D. K., Normand, S. L. T., Walters, E. E., & Zaslavsky, A. M. (2002). Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine, 32(6), 959–976. 10.1017/S0033291702006074 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C., McLaughlin, K. A., Green, J. G., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., Aguilar-Gaxiola, S., Alhamzawi, A. O., Alonso, J., Angermeyer, M., Benjet, C., Bromet, E., Chatterji, S., de Girolamo, G., Demyttenaere, K., Fayyad, J., Florescu, S., Gal, G., Gureje, O., … Williams, D. R. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. British Journal of Psychiatry, 197(5), 378–385. 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiddle, B., Inkster, B., Prabhu, G., Moutoussis, M., Whitaker, K. J., Bullmore, E. T., Dolan, R. J., Fonagy, P., Goodyer, I. M., & Jones, P. B. (2018). Cohort profile: The NSPN 2400 Cohort: A developmental sample supporting the Wellcome Trust NeuroScience in psychiatry network. International Journal of Epidemiology, 47(1), 18–19g. 10.1093/ije/dyx117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labella, M. H., Johnson, W. F., Martin, J., Ruiz, S. K., Shankman, J. L., Englund, M. M., Collins, W. A., Roisman, G. I., & Simpson, J. A. (2018). Multiple dimensions of childhood abuse and neglect prospectively predict poorer adult romantic functioning. Personality and Social Psychology Bulletin, 44(2), 238–251. 10.1177/0146167217736049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey, R. E., & Minnis, H. (2020). Practitioner review: Twenty years of research with adverse childhood experience scores–advantages, disadvantages and applications to practice. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 10.1111/jcpp.13135 [DOI] [PubMed] [Google Scholar]
- Lagdon, S., Ross, J., Robinson, M., Contractor, A. A., Charak, R., & Armour, C. (2021). Assessing the mediating role of social support in childhood maltreatment and psychopathology among college students in Northern Ireland. Journal of Interpersonal Violence, 36(3–4), NP2112–2136NP. 10.1177/0886260518755489 [DOI] [PubMed] [Google Scholar]
- Leicht-Deobald, U., Bruch, H., Bönke, L., Stevense, A., Fan, Y., Bajbouj, M., & Grimm, S. (2018). Work-related social support modulates effects of early life stress on limbic reactivity during stress. Brain Imaging and Behavior, 12(5), 1405–1418. 10.1007/s11682-017-9810-z [DOI] [PubMed] [Google Scholar]
- Leys, C., Ley, C., Klein, O., Bernard, P., & Licata, L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49(4), 764–766. 10.1016/j.jesp.2013.03.013 [DOI] [Google Scholar]
- Li, M., O’Donnell, K. J., Caron, J., Meaney, M. J., Kobor, M., D’Arcy, C., Su, Y., Liu, A., & Meng, X. (2022). To what extent do social support and coping strategies mediate the relation between childhood maltreatment and major depressive disorder: A longitudinal community-based cohort. Development and Psychopathology, 1–12. 10.1017/S0954579422000918 [DOI] [PubMed] [Google Scholar]
- Lüdecke, D. (2023). sjPlot: Data visualization for statistics in social science (2.8.14) [Computer software]. https://CRAN.R-project.org/package=sjPlot.
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Maldjian, J. A., Laurienti, P. J., & Burdette, J. H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. 10.1016/j.neuroimage.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Malhi, G. S., Das, P., Outhred, T., Dobson-Stone, C., Bell, E., Gessler, D., Bryant, R., & Mannie, Z. (2020). Interactions of OXTR rs53576 and emotional trauma on hippocampal volumes and perceived social support in adolescent girls. Psychoneuroendocrinology, 115, 104635. 10.1016/j.psyneuen.2020.104635 [DOI] [PubMed] [Google Scholar]
- Masten, C. L., Telzer, E. H., Fuligni, A. J., Lieberman, M. D., & Eisenberger, N. I. (2012). Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience, 7(1), 106–114. 10.1093/scan/nsq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory, E. J., Foulkes, L., & Viding, E. (2022). Social thinning and stress generation after childhood maltreatment: A neurocognitive social transactional model of psychiatric vulnerability. The Lancet Psychiatry, 9(10), 828–837. 10.1016/S2215-0366(22)00202-4 [DOI] [PubMed] [Google Scholar]
- McCrory, E. J., Ogle, J. R., Gerin, M. I., & Viding, E. (2019). Neurocognitive adaptation and mental health vulnerability following maltreatment: The role of social functioning. Child Maltreatment, 24(4), 435–451. 10.1177/1077559519830524 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. 10.1177/2470547017692328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLafferty, M., O’Neill, S., Armour, C., Murphy, S., & Bunting, B. (2018). The mediating role of various types of social networks on psychopathology following adverse childhood experiences. Journal of Affective Disorders, 238, 547–553. 10.1016/j.jad.2018.06.020 [DOI] [PubMed] [Google Scholar]
- McLaughlin, K. A. (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 45(3), 361–382. 10.1080/15374416.2015.1110823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A., Colich, N. L., Rodman, A. M., & Weissman, D. G. (2020). Mechanisms linking childhood trauma exposure and psychopathology: A transdiagnostic model of risk and resilience. BMC Medicine, 18(1), 96. 10.1186/s12916-020-01561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A., Sheridan, M. A., Humphreys, K. L., Belsky, J., & Ellis, B. J. (2021). The value of dimensional models of early experience: Thinking clearly about concepts and categories. Perspectives on Psychological Science, 16(6), 1463–1472. 10.1177/1745691621992346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A., Weissman, D., & Bitrán, D. (2019). Childhood adversity and neural development: A systematic review. Annual Review of Developmental Psychology, 1(1), 277–312. 10.1146/annurev-devpsych-121318-084950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard, S. P. (2013). EnvStats: An R package for environmental statistics. Comprehensive R Archive Network (CRAN). https://cran.r-project.org/web/packages/EnvStats/index.html.
- Miller, A. B., Adams, L. M., Esposito-Smythers, C., Thompson, R., & Proctor, L. J. (2014). Parents and friendships: A longitudinal examination of interpersonal mediators of the relationship between child maltreatment and suicidal ideation. Psychiatry Research, 220(3), 998–1006. 10.1016/j.psychres.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López, L., Ioannidis, K., Askelund, A. D., Smith, A. J., Schueler, K., & van Harmelen, A.-L. (2020). The resilient emotional brain: A scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 392–402. 10.1016/j.bpsc.2019.12.008 [DOI] [PubMed] [Google Scholar]
- Moreno-López, L., Sallie, S. N., Ioannidis, K., Kaser, M., Schueler, K., Askelund, A. D., Turner, L., van Harmelen, A.-L., & RAISE Consortium . (2021). RAISE study protocol: A cross-sectional, multilevel, neurobiological study of resilience after individual stress exposure. BMJ Open, 11(1), e040394. 10.1136/bmjopen-2020-040394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevard, I., Green, C., Bell, V., Gellatly, J., Brooks, H., & Bee, P. (2021). Conceptualising the social networks of vulnerable children and young people: A systematic review and narrative synthesis. Social Psychiatry and Psychiatric Epidemiology, 56(2), 169–182. 10.1007/s00127-020-01968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack, H., Nolte, L., Nieratschker, V., Habel, U., & Derntl, B. (2019). Imaging stress: An overview of stress induction methods in the MR scanner. Journal of Neural Transmission, 126(9), 1187–1202. 10.1007/s00702-018-01965-y [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Parker, G., Roussos, J., Hadzi-Pavlovic, D., Mitchell, P., Wilhelm, K., & Austin, M. P. (1997). The development of a refined measure of dysfunctional parenting and assessment of its relevance in patients with affective disorders. Psychological Medicine, 27(5), 1193–1203. 10.1017/S003329179700545X [DOI] [PubMed] [Google Scholar]
- Passingham, R. E., & Wise, S. P. (2012). The neurobiology of the prefrontal cortex: Anatomy, evolution, and the origin of insight. OUP Oxford. 10.1093/acprof:osobl/9780199552917.001.0001 [DOI] [Google Scholar]
- Phelps, E. A. (2004). Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology, 14(2), 198–202. 10.1016/j.conb.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Pollak, S. D., & Smith, K. E. (2021). Thinking clearly about biology and childhood adversity: Next steps for continued progress. Perspectives on Psychological Science, 16(6), 1473–1477. 10.1177/17456916211031539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner, J. C., Dedovic, K., Khalili-Mahani, N., Engert, V., Pruessner, M., Buss, C., Renwick, R., Dagher, A., Meaney, M. J., & Lupien, S. (2008). Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry, 63(2), 234–240. 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Puetz, V. B., Viding, E., Gerin, M. I., Pingault, J. B., Sethi, A., Knodt, A. R., Radtke, S. R., Brigidi, B. D., Hariri, A. R., & McCrory, E. (2020). Investigating patterns of neural response associated with childhood abuse v. childhood neglect. Psychological Medicine, 50(8), 1398–1407. 10.1017/S003329171900134X [DOI] [PubMed] [Google Scholar]
- R Core Team . (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/.
- Revelle, W. (2022). Procedures for psychological, psychometric, and personality research (2.2.9) [Computer software]. https://cran.r-project.org/web/packages/psych/psych.pdf.
- Reynolds, C. R., & Richmond, B. O. (1978). What I think and feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology, 6(2), 271–280. 10.1007/BF00919131 [DOI] [PubMed] [Google Scholar]
- Riem, M. M. E., Alink, L. R. A., Out, D., Van Ijzendoorn, M. H., & Bakermans-Kranenburg, M. J. (2015). Beating the brain about abuse: Empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Development and Psychopathology, 27(2), 507–520. https://psycnet.apa.org/doi/ 10.1017/S0954579415000127 [DOI] [PubMed] [Google Scholar]
- Rosenberg, M. (1965). Society and the adolescent self-image. Princeton University Press. https://www.jstor.org/stable/j.ctt183pjjh. [Google Scholar]
- Salazar, A. M., Keller, T. E., & Courtney, M. E. (2011). Understanding social support’s role in the relationship between maltreatment and depression in youth with foster care experience. Child Maltreatment, 16(2), 102–113. 10.1177/1077559511402985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuplein, M., & van Harmelen, A.-L. (2022). The importance of friendships in reducing brain responses to stress in adolescents exposed to childhood adversity: A pre-registered systematic review. Current Opinion in Psychology, 45, 101310. 10.1016/j.copsyc.2022.101310 [DOI] [PubMed] [Google Scholar]
- Schwarz, K., Moessnang, C., Schweiger, J. I., Baumeister, S., Plichta, M. M., Brandeis, D., Banaschewski, T., Wackerhagen, C., Erk, S., Walter, H., Tost, H., & Meyer-Lindenberg, A. (2020). Transdiagnostic prediction of affective, cognitive, and social function through brain reward anticipation in schizophrenia, bipolar disorder, major depression, and autism spectrum diagnoses. Schizophrenia Bulletin, 46(3), 592–602. 10.1093/schbul/sbz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, D., Tsou, K. A., Ansell, E. B., Potenza, M. N., & Sinha, R. (2014). Cumulative adversity sensitizes neural response to acute stress: Association with health symptoms. Neuropsychopharmacology, 39(3), 670–680. 10.1038/npp.2013.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, J. E., & Pollak, S. D. (2014). Impact of physical maltreatment on the regulation of negative affect and aggression. Development and Psychopathology, 26(4pt1), 1021–1033. 10.1017/S0954579414000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, M. A., Abelsen, B., & Olsen, J. A. (2016). Clarifying associations between childhood adversity, social support, behavioral factors, and mental health, health, and well-being in adulthood: A population-based study. Frontiers in Psychology, 7, 727. 10.3389/fpsyg.2016.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, M. A., & McLaughlin, K. A. (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–585. 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry, D. M., & Widom, C. S. (2013). Child abuse and neglect, social support, and psychopathology in adulthood: A prospective investigation. Child Abuse & Neglect, 37(6), 415–425. 10.1016/j.chiabu.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D., & Vagg, P. R. (1984). Psychometric properties of the STAI: A reply to Ramanaiah, Franzen, and Schill. Journal of Personality Assessment, 48(1), 95–97. 10.1207/s15327752jpa4801_16 [DOI] [PubMed] [Google Scholar]
- Sullivan, R. M., & Perry, R. E. (2015). Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Social Neuroscience, 10(5), 500–511. 10.1080/17470919.2015.1087425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, A., McLaughlin, K. A., Sheridan, M. A., Nelson, C. A., Zeanah, C. H., & Fox, N. A. (2021). Autonomic reactivity to social rejection, peer difficulties, and the buffering effects of adolescent friendships following early psychosocial deprivation. Emotion, 22(2), 318–330. 10.1037/emo0001016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant, R., Hiller, L., Fishwick, R., Platt, S., Joseph, S., Weich, S., Parkinson, J., Secker, J., & Stewart-Brown, S. (2007). The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): Development and UK validation. Health and Quality of Life Outcomes, 5(1), 63. 10.1186/1477-7525-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Y., Chen, Q., Nichols, T. E., Rasetti, R., Callicott, J. H., Berman, K. F., Weinberger, D. R., & Mattay, V. S. (2016). Seeking optimal Region-Of-Interest (ROI) single-value summary measures for fMRI studies in imaging genetics. PLoS One, 11(3), e0151391. 10.1371/journal.pone.0151391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham, N., & Sheridan, M. (2010). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey, D., Siddaway, A. P., Meiser-Stedman, R., Serpell, L., & Field, A. P. (2012). A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clinical Psychology Review, 32(2), 122–138. 10.1016/j.cpr.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- van Ballegooijen, W., Riper, H., Cuijpers, P., van Oppen, P., & Smit, J. H. (2016). Validation of online psychometric instruments for common mental health disorders: A systematic review. BMC Psychiatry, 16(1), 1–12. 10.1186/s12888-015-0706-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren, S., & Groothuis-Oudshoorn, K. (2011). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45(3), 1–67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- van Harmelen, A.-L., Blakemore, S. J., Goodyer, I. M., & Kievit, R. A. (2021). The interplay between adolescent friendship quality and resilient functioning following childhood and adolescent adversity. Adversity and Resilience Science, 2(1), 37–50. 10.1007/s42844-020-00027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen, A.-L., Gibson, J. L., St Clair, M. C., Owens, M., Brodbeck, J., Dunn, V., Lewis, G., Croudace, T., Jones, P. B., Kievit, R. A., & Goodyer, I. M. (2016). Friendships and family support reduce subsequent depressive symptoms in at-risk adolescents. PLoS One, 11(5), e0153715. 10.1371/journal.pone.0153715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen, A.-L., Hauber, K., Moor, B. G., Spinhoven, P., Boon, A. E., Crone, E. A., & Elzinga, B. M. (2014). Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS One, 9(1), e85107. 10.1371/journal.pone.0085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen, A.-L., Kievit, R. A., Ioannidis, K., Neufeld, S., Jones, P. B., Bullmore, E., Dolan, R., NSPN Consortium, Fonagy, P., & Goodyer, I. (2017). Adolescent friendships predict later resilient functioning across psychosocial domains in a healthy community cohort. Psychological Medicine, 47(13), 2312–2322. 10.1017/S0033291717000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen, A.-L., van Tol, M.-J., Demenescu, L. R., van der Wee, N. J. A., Veltman, D. J., Aleman, A., van Buchem, M. A., Spinhoven, P., Penninx, B. W. J. H., & Elzinga, B. M. (2013). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 8(4), 362–369. 10.1093/scan/nss007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges, J. F., Müller-Pinzler, L., Neis, M., Luebber, F., Lange, T., Hundt, J. E., Kasten, M., Krämer, U. M., Krach, S., & Rademacher, L. (2022). Association of stress-related neural activity and baseline interleukin-6 plasma levels in healthy adults. Stress, 25(1), 267–275. 10.1080/10253890.2022.2094704 [DOI] [PubMed] [Google Scholar]
- Vranceanu, A.-M., Hobfoll, S. E., & Johnson, R. J. (2007). Child multi-type maltreatment and associated depression and PTSD symptoms: The role of social support and stress. Child Abuse & Neglect, 31(1), 71–84. 10.1016/j.chiabu.2006.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2011). Wechsler abbreviated scale of intelligence–second edition (WASI-II) [Database record]. In PsycTESTS dataset. American Psychological Association (APA). 10.1037/t15171-000 [DOI] [Google Scholar]
- Weissman, D. G., Lambert, H. K., Rodman, A. M., Peverill, M., Sheridan, M. A., & McLaughlin, K. A. (2020). Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depression and Anxiety, 37(9), 916–925. 10.1002/da.23062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock, M. D., Harnett, N. G., Wood, K. H., Orem, T. R., Granger, D. A., Mrug, S., & Knight, D. C. (2016). Prefrontal cortex activity is associated with biobehavioral components of the stress response. Frontiers in Human Neuroscience, 10, 583. 10.3389/fnhum.2016.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom, C. S., Czaja, S., & Dutton, M. A. (2014). Child abuse and neglect and intimate partner violence victimization and perpetration: A prospective investigation. Child Abuse & Neglect, 38(4), 650–663. 10.1016/j.chiabu.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, P. O., Qiu, T., Neufeld, S., Jones, P. B., & Goodyer, I. M. (2018). Sporadic and recurrent non-suicidal self-injury before age 14 and incident onset of psychiatric disorders by 17 years: Prospective cohort study. The British Journal of Psychiatry, 212(4), 222–226. 10.1192/bjp.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschucke, E., Renneberg, B., Dimeo, F., Wüstenberg, T., & Ströhle, A. (2015). The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology, 51, 414–425. 10.1016/j.psyneuen.2014.10.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All scripts and pseudonymized data necessary to reproduce the findings of this study are publicly available on OSF at https://osf.io/mdfhw/?view_only=f3c85e8f608843469d23cb347d01fcdf (DOI 10.17605/OSF.IO/MDFHW) and upon publication will be made available on DataverseNL. For all significant ROIs, group-level statistical maps displaying t-values of neural activation (pFWE < .05) during stress (>control) trials of the MIST have been uploaded to NeuroVault (https://neurovault.org/collections/AXXZBAGI/).