Abstract
For productive replication of human immunodeficiency virus type 1 (HIV-1) in host cells, the viral genome-encoded transactivator Tat and several cellular transcription factors are required for efficient viral gene transcription. However, it remains unclear how the viral genome initiates transcription before Tat is transcribed or when Tat is at suboptimal levels. Here, we utilized the human T-cell leukemia type 1 Tax protein as a molecular tool to investigate the mechanism of viral gene transcription that initiates the early phase of infection of HIV-1. Tax alone does not significantly increase the activity of HIV-1 long terminal repeat (LTR) in T lymphocytes, but it markedly enhanced the replication of an infectious HIV-1 provirus with a truncated nef gene. This enhancement is preferentially mediated by the cooperation of Tax and Tat which is dependent on TAR and duplicated κB cis elements of the HIV-1 LTR as well as the NF-κB activation domain of Tax. Furthermore, phorbol myristate acetate and membrane-targeted HIV-1 Nef also enhanced the LTR activity in the presence of Tat in the TAR- and κB cis element-dependent manner. These data suggest that activated NF-κB can functionally interact with a suboptimal amount of Tat and the HIV-1 LTR for efficient initiation of viral gene transcription.
Replication of human immunodeficiency virus type 1 (HIV-1) in T lymphocytes is modulated by HIV-1 early regulatory proteins such as Tat, Rev, and Nef (9, 26). Tat and Rev are viral nuclear proteins that bind to the RNA products of HIV-1 transcription and are essential for HIV-1 gene expression at transcriptional and posttranscriptional levels. Nef is required for virus growth in resting T lymphocytes and monocytes, and it enhances viral replication in cell culture and in animals infected by a simian counterpart of HIV-1 (18, 22, 33). In addition to these early viral proteins, cellular factors play critical roles in the regulation of HIV-1 replication. HIV-1 gene expression is enhanced in activated primary and transformed T cells or monocytes (17, 21, 24, 30). This enhancement is perhaps related to the intrinsic features of the interleukin 2 (IL-2) enhancerlike region in the HIV-1 5′ long terminal repeat (LTR) that controls viral gene expression (35, 36). The HIV-1 LTR is conceptually divided into three major regions: modulatory, core, and TAR (named TAR for transactivation responsive) (12, 16). The modulatory region contains numerous cis-acting sequences for the binding of transcriptional factors including NF-κB, NF-AT, and AP-1. The core region contains a basal promoter including three copies of Sp1 elements and a TATA box. The TAR sequence is unique for the primate lentiviruses, and its RNA serves as the binding site for Tat (12, 16).
Among the cis-acting sequences located in the modulatory region of the LTR, two highly conserved consecutive copies of κB elements at nucleotides −104 to −81 are critical for HIV-1 replication in T cells (12, 16). These duplicated κB sequences are preserved in clinical HIV-1 isolates as well as in tissue culture-adapted viral strains. A single κB cis element is found in HIV-2 and simian immunodeficiency virus (SIV). The activity of the κB element is achieved by the binding of an activated transcriptional factor NF-κB translocated from the cytoplasm to the nucleus. NF-κB can be activated following HIV-1 infection (12, 16) and in T lymphocytes and macrophages as well as by certain exogenous factors including certain cytokines and heterologous viral proteins such as human T-cell leukemia virus type 1 (HTLV-1) Tax (13, 28). A persistent activation of NF-κB is observed during HIV-1 infection and is possibly mediated by Tat and Nef (4, 10).
Although it has been shown that NF-κB activation is important for HIV-1 growth in primary T cells, the virus can replicate in a κB element-independent manner (15, 20, 27). In addition, NF-κB or Tax, when acting as a single factor, may not be sufficient for the activation of the HIV-1 LTR (7, 11, 30, 32, 37). Still, it remains unknown how the viral genome initiates early transcription of the viral genes immediately after viral entry when Tat is at a suboptimal level. To study the molecular mechanism of HIV-1 gene transcription at this very early stage of infection, we established an HIV-1 replication reporter system that imitates the viral early transcription by utilizing HTLV-1 Tax as a molecular tool. We show that HTLV-I Tax alone is a weak activator of the HIV-1 LTR, but it can dramatically upregulate HIV-1 gene transcription in cooperation with a suboptimal amount of HIV-1 Tat. The marked enhancement of HIV-1 replication by Tax is mediated through a functional cooperation between NF-κB and Tat. Further, membrane-associated Nef can also stimulate HIV-1 LTR in the presence of suboptimal levels of Tat, presumably through activation of NF-κB.
Tax enhances HIV-1 replication in Jurkat T lymphocytes.
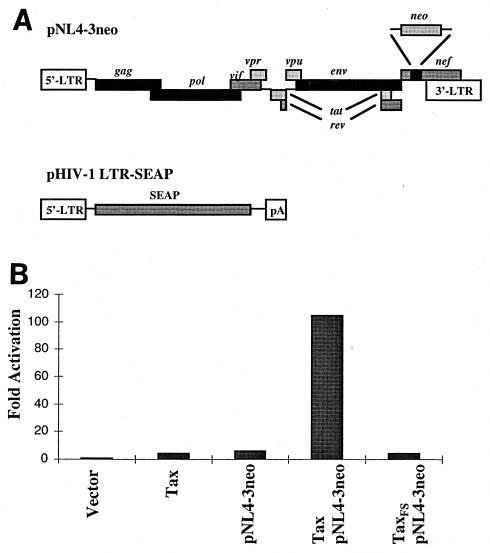
Since the effect of Tax on transcriptional regulation of the HIV-1 LTR activity was previously determined in a virus-free, HIV-1 LTR reporter system, this approach may underestimate the influence of Tax on HIV-1 replication. To determine how Tax influences HIV-1 replication at the transcriptional level and to examine possible cooperation of Tax and HIV-1 viral proteins, an HIV-1 replication reporter system was developed. This system includes Tax, an HIV-1 LTR-SEAP reporter, and HIV-1 infectious clone with a truncated nef gene as well as human Jurkat T cells. An infectious molecular clone of pNL4-3 was reconstructed by inserting a neo gene into the nef locus to generate the pNL4-3neo variant (depicted in Fig. 1A). Transfection of pNL4-3neo into Jurkat T-antigen (TAg) cells produces infectious viral particles in transfected cell culture medium, and the kinetics of HIV-1 replication can be assessed by cotransfection with pHIV-1 LTR-SEAP reporter.
FIG. 1.
Tax enhances replication of the HIV-1 provirus with a truncated nef gene in human T lymphocytes. (A) Schematic representation of the truncated-nef infectious provirus of HIV-1 and the HIV-1 LTR-SEAP reporter. The truncated-nef HIV-1 provirus (pNL4-3neo) is derived from pNL4-3 in which a neo gene fragment was inserted into the XhoI site at the nef locus of pNL4-3 at amino acid position 35. The nef gene is truncated and encodes only the first N-terminal 35 amino acids of the Nef protein. The complete LTR of HIV-1 was derived from pNL4-3 and was constructed by PCR with Pfu DNA polymerase. The SEAP reporter gene (14) is linked to the full-length LTR. (B) Flag-tagged wild-type Tax (Flag-Tax) and frameshift mutant (Flag-TaxFS) were constructed in a phEFneo vector in which the human elongation factor promoter controls the expression of Tax. Tax (1 μg) or TaxFS (1 μg) was cotransfected with or without pNL4-3neo (0.1 μg), together with pHIV-1 LTR-SEAP (0.2 μg) into Jurkat TAg cells by using DMRIE-C liposome transfection reagent. SEAP activity was quantified by using chemiluminescent substrate. The basal activity of the LTR was set at 1. The data are representative of at least two independent experiments.
The cell-free supernatant from the transfected cell cultures was collected for secreted alkaline phosphatase (SEAP) activity analysis. As shown in Fig. 1B, there was an approximately 23-fold increase in SEAP activity in the Jurkat T lymphocytes cotransfected with pNL4-3neo and Flag-Tax compared to the activity induced by cotransfection of pNL4-3neo and the frameshift mutant of Tax (Flag-TaxFS). However, Tax alone induced the HIV-1 LTR activity only about fivefold over the basal activity of the LTR, as quantified by SEAP activity analysis. These results suggest that Tax can alter the activity of the HIV-1 LTR, perhaps in synergy with one of the viral proteins encoded by the HIV-1 genome.
Tax cooperates with Tat to enhance HIV-1 gene transcription.
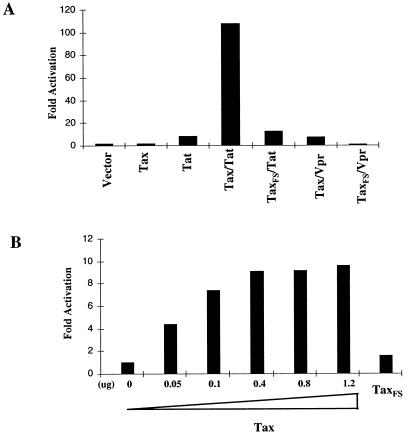
Two potential candidates of the HIV-1 proteins that could cooperate with Tax to enhance the transcriptional activity of the HIV-1 LTR are Tat and Vpr, since both Tat and Vpr are transactivators of the HIV-1 LTR. To examine this possibility, a transient cotransfection of Tax and Tat or Vpr together with the LTR-SEAP reporter into Jurkat cells was performed. Tax increased the LTR activity about 10-fold over that of TaxFS in the presence of Tat (Fig. 2A). This cooperative effect was dose dependent, using various amounts of Tax plasmid within the range 0.05 to 0.4 μg (Fig. 2B). The SEAP activity was saturated when large amounts of Tax plasmid DNA were used for transfection (Fig. 2B). This synergistic activation induced by Tax and Tat was best observed when Tat was expressed at low levels, since overexpression of Tat can saturate the HIV-1 LTR activity and therefore might mask the potential synergistic effect mediated by Tax. Under optimal condition settings, increases in the HIV-1 LTR activity induced by Tax in the presence of a low-level Tat of up to 40-fold were observed (see Fig. 5B). In contrast, Tax did not exhibit any cooperative effect on transactivation of the HIV-1 LTR with Vpr (Fig. 2A). Although it was shown that cotransfection of Tax and Vpr increased the HIV-1 LTR activity about fivefold over that mediated by TaxFS plus Vpr, this simply reflects the effect mediated by Tax alone. These results suggest that Tax enhances the HIV-1 LTR activity specifically in cooperation with the HIV-1 transactivator Tat.
FIG. 2.
Tax cooperates with Tat to enhance HIV-1 LTR activity. (A) The transient cotransfection was performed with LTR-SEAP (0.4 μg), Flag-Tax (1 μg), Tat (0.05 μg), and Vpr (0.1 μg) either alone or in combination as indicated in the figure. The DNA amount used for transfection was standardized to a total of 2 μg with phEFneo vector. The data are representative of two independent experiments. (B) The dose-response activation to the LTR by costimulation of Jurkat T cells with Tax and Tat was measured by cotransfection of Tat (0.1 μg), LTR-SEAP (0.4 μg), and various amounts of Tax plasmid as indicated in the figure.
FIG. 5.
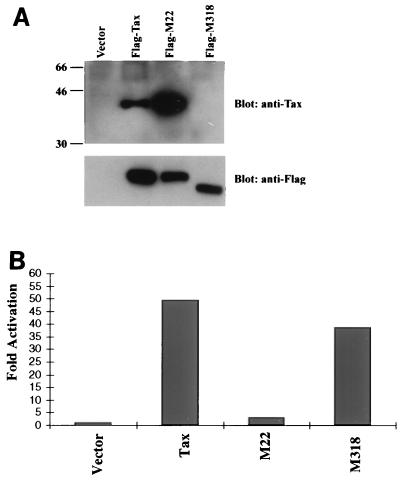
The NF-κB activation domain of Tax is required for the cooperative transactivation of the HIV-1 LTR. (A) Flag-tagged Tax mutants with mutations in the NF-κB activation domain (M22) and in the transcriptional activation domain (M318). M22 contains amino acid substitutions Ser130 Ala131 for Thr130 Leu131 within the NF-κB activation domain of the Tax protein. M318 has a carboxyl-terminal deletion in the transcriptional activation domain of Tax. Expression of Tax was detected by immunoblotting analysis. The Tax constructs were transiently transfected into 293T cells by using Lipofectamine reagent. The cellular extract prepared from the transfected cells was directly analyzed by immunoblotting with anti-HTLV-I Tax or with anti-Flag. (B) The wild-type Tax or the Tax mutants including M22, M318, and TaxFS (1 μg/each) were cotransfected with the LTR-SEAP reporter plasmid (0.2 μg) and Tat (0.1 μg) into Jurkat T cells. The SEAP assay was performed as described above. The data are representative of at least three independent experiments.
The cooperation of Tax and Tat is dependent on the TAR and cis κB elements.
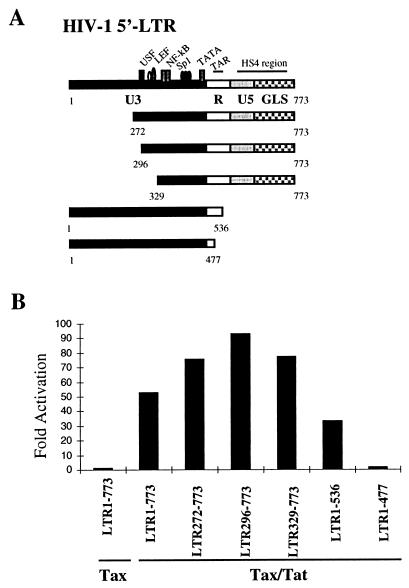
To assess requirement of the cis elements within the HIV-1 LTR for Tax-induced cooperative effect, a subset of the HIV-1 LTR mutants were generated by sequential 5′- and 3′-end deletions of the cis-acting sequences of the LTR (depicted in Fig. 3A). Deletion of upstream sequences, including several cis elements for the binding of transcriptional factors NF-AT, USF, Ets, and LEF, did not impair the cooperative activity (Fig. 3B). Deletion of the HS4 region led to a slight decrease of the SEAP activity, but this LTR construct was still activated by the combination of Tax and Tat with an increased SEAP activity of at least 30-fold over that induced by Tax alone. However, deletion of the TAR element in the LTR abolished the cooperative activity (Fig. 3B). The requirement of the TAR element for the Tax’s transactivation activity indicates that Tat is essential for the synergistic activation of the HIV-1 LTR through specific binding to the TAR element.
FIG. 3.
The cooperative transactivation activity of Tax requires the TAR element. (A) Schematic representation of the wild-type HIV-1 LTR and the mutants with various cis element deletions. The nucleotide acid number is based on the numbering of the complete sequence of pNL4-3 derived from GenBank. LTR272-773 flanks a full-length promoter, but the upstream cis sequences immediately before the USF element were deleted. LTR296-773 covers the complete promoter region with the upstream sequence deletion immediately after the USF site. LTR329-773 contains the entire promoter but the upstream region immediately before the duplicated κB sites was deleted. LTR1-536 covers the entire promoter and enhancer, but the cis elements immediately after the TAR sequence and HS4 region were deleted. LTR1-477 (LTRΔTAR) includes the complete upstream modulatory region and the core region but the TAR sequence was deleted. The wild-type LTR and all LTR mutants were linked to the SEAP gene. (B) The transfection of Jurkat TAg cells was performed using Tax (0.6 μg) and Tat (0.1 μg) as well as various LTR constructs (0.2 μg/each) as indicated in the figure. The data are representative of two independent experiments.
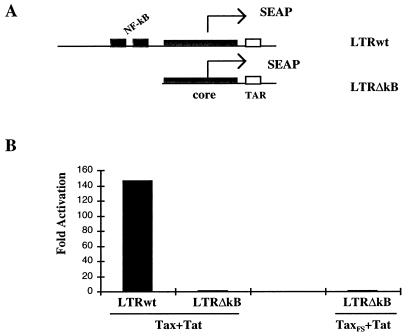
To determine if Tax mediates the cooperative effect through activation of NF-κB, an LTR reporter with the κB site deleted (LTRΔκB) was constructed by deleting the upstream enhancer including two copies of the κB elements (depicted in Fig. 4A). The activity of LTRΔκB was 145-fold less than that of the wild-type LTR in Jurkat TAg cells transfected with both the Tax and Tat plasmids (Fig. 4B). These results suggest that the cooperative activation of the HIV-1 LTR mediated by Tax plus Tat requires a minimal promoter of the HIV-1 LTR in which at least two copies of κB element and the TAR sequence are essential.
FIG. 4.
Tax’s transactivation to the HIV-1 LTR is κB element dependent. (A) Structures of wild-type LTR (LTRwt) and LTRΔκB. The wild-type HIV-1 LTR contains a complete upstream modulatory region as well as the core region and the TAR sequence. LTRΔκB was constructed by deleting all upstream modulatory region including the duplicated κB elements, but the core and the TAR regions are reserved. Both LTR fragments were linked to the SEAP gene. (B) Tax or TaxFS and Tat together with the wild-type LTR (LTRwt) or LTRΔκB reporter plasmid were transiently cotransfected into Jurkat TAg cells. The SEAP activity was quantified 48 h after transfection. The data shown below represent two independent experiments.
The NF-κB activation domain of Tax is required for the cooperative activity.
As shown above, the κB cis element is required for Tax-mediated cooperative activity. This suggests that the nuclear translocated NF-κB activated by the Tax protein cooperates with Tat to enhance the HIV-1 LTR activity. To further test this possibility, we generated Tax mutants M22 and M318 with specific mutations in two distinct functional domains. Tax contains three identified domains required for its multiple functions. An N-terminal nuclear localization signal is essential for all of Tax’s functions. The NF-κB activation domain of Tax is also located at its N terminus and is necessary for the activation of NF-κB (31). Tax’s transcriptional activation domain is at its carboxyl terminus flanking amino acids from 289 to 322 (29). To generate the Tax variant (M22) with mutations in the NF-κB activation domain, we substituted Ser130 Ala131 for Thr130 Leu131 of Tax to generate Flag-tagged M22 (Flag-M22), in which the mutations at these two amino acid positions abrogate Tax’s ability to activate NF-κB (31). The M318 variant (Flag-M318) with deletion of the transcriptional activation domain at the carboxyl terminus of Tax that contains the N-terminal 318 amino acids was also constructed. The wild-type Tax and its variants were expressed at comparable levels and were detected by a specific antibody reacting to the carboxyl terminus of Tax or by anti-Flag (Fig. 5A). Flag-M318 was not detected by anti-Tax antibody probably due to lack of the carboxyl terminus of Tax but was detected using anti-Flag epitope antibody (Fig. 5A). As shown in Fig. 5B, the Flag-M22 variant with mutations within the NF-κB activation domain abrogated the ability to activate the HIV-1 LTR in cooperation with Tat, whereas the Flag-M318 variant with mutations in the transcriptional activation domain retained the ability to activate the HIV-1 LTR in cooperation with Tat. These results provide further evidence that the cooperative transactivation activity of Tax to the HIV-1 LTR is mediated through interaction of activated NF-κB and Tat.
T-cell mitogens cooperate with Tat, not with Tax, for HIV-1 gene expression.
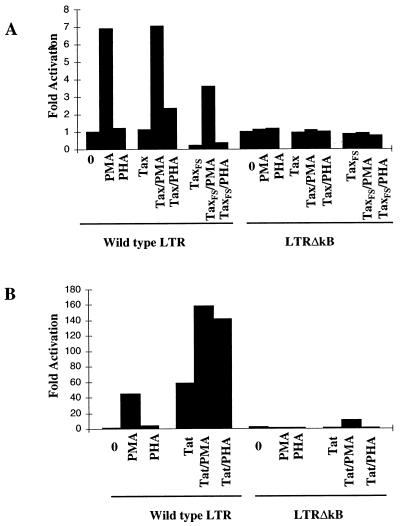
To assess the effects of the transcriptional regulation of the HIV-1 LTR mediated by the cooperation of NF-κB and Tat or between transcriptional factors in more detail, we examined the potential cooperation of Tax or Tat with T-cell mitogens including phorbol myristate acetate (PMA) and phytohemagglutinin (PHA) on the activation of the HIV-1 LTR. PMA is an activator of protein kinase C and consequently activates NF-κB. PHA induction imitates T-lymphocyte activation events mediated through T-cell receptor stimulation and stimulates T-cell activation in Ca2+-dependent signaling pathways that leads to activation of NF-AT (8). Stimulation of Jurkat TAg cells by PMA resulted in 10- to 40-fold increases of the HIV-1 LTR activity over that of the unstimulated cells (Fig. 6). PHA alone induced an increase in the LTR activity of less than threefold over the basal activity of the LTR (Fig. 6). Stimulation of Jurkat T cells with the combination of PMA and PHA did not synergistically increase the LTR activity (data not shown). To determine if Tax cooperates with T-lymphocyte activation mitogens on the activation of the HIV-1 LTR activity, Tax together with the LTR-SEAP reporter was transiently cotransfected into Jurkat T cells, and the transfected cells were further stimulated by either PHA or PMA. As shown in Fig. 6A, the LTR activity induced by a combination of Tax and PMA was equal to that noted with PMA stimulation alone. The combination of Tax and PHA resulted in a slight increase of the LTR activity over that of either PHA or the Tax protein alone (Fig. 6A).
FIG. 6.
T-cell mitogens cooperate with Tat, but not Tax, to enhance the HIV-1 LTR activity in the TAR- or κB element-dependent manner. (A) Tax has no cooperative effect with either PMA or PHA. The LTR-SEAP was cotransfected with or without Tax into Jurkat TAg cells. Twenty-four hours after transfection, the transfected cells were left unstimulated or stimulated with either PMA (50 ng/ml) or PHA (2 μg/ml) for another 24 h. The SEAP activity was quantified as described above. (B) PMA or PHA synergistically activates the HIV-1 LTR in the presence of Tat. The experiment was performed as described above except that the Tat plasmid (0.1 μg) was used to replace Tax. The data are representative of two independent experiments.
In contrast, a synergistic effect on the transactivation of the HIV-1 LTR was seen in the Jurkat TAg cells stimulated with a combination of PMA and Tat or PHA and Tat (Fig. 6B). Deletion of the κB element in the HIV-1 LTR not only abolished the SEAP activity induced by either PMA or PHA alone but also negated the Tat-induced activation of the HIV-1 LTR (Fig. 6B). Interestingly, costimulation of the LTRΔκB-SEAP-transfected cells with Tat plus PMA increased SEAP activity about 10-fold over that induced by either Tat or PMA alone (Fig. 6B), suggesting that a cis element in the LTR other than the duplicated κB sites is responsive to the cooperative activity mediated by a combination of PMA and Tat. Taken together, these results indicate that the T-cell mitogens PMA and PHA can enhance the HIV-1 LTR activity in cooperation with Tat in a TAR- and cis κB element-dependent manner and Tax has no significant effect in cooperation with either PHA or PMA on HIV-1 gene transcription.
Membrane-targeted HIV-1 Nef enhances the HIV-1 activity in the presence of Tat.
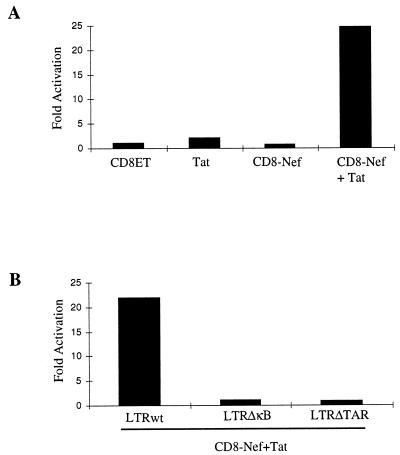
As described above, HTLV-I Tax enhanced the replication of an infectious provirus with the truncated nef gene (pNL4-3neo), suggesting that Nef might utilize a similar mechanism to Tax for the enhancement of HIV-1 replication. We tested a membrane-targeted HIV-1 Nef (CD8-Nef) to examine its activity on viral gene transcription, since the chimeric protein has been reported to spontaneously activate Jurkat T cells and led to a constitutive activation of NF-κB when targeted to the cell membrane (4). The CD8-Nef protein alone had no detectable transactivation activity for the HIV-1 LTR in Jurkat TAg cells, but the Nef protein increased SEAP activity some 24-fold greater than that noted using the truncated CD8 molecule in the presence of Tat (Fig. 7A). This cooperative transactivation activity requires the duplicated κB cis elements and the TAR sequence, since LTRΔκB and the LTRΔTAR both showed markedly diminished activities when induced by a combination of CD8-Nef and Tat (Fig. 7B). This suggests that Nef enhances HIV-1 gene expression, probably through modification of the activity of the transcription factor NF-κB.
FIG. 7.
Membrane-targeted Nef activates HIV-1 LTR in cooperation with Tat in Jurkat T cells. (A) The CD8-Nef chimera was described previously (4). The truncated CD8 molecule (CD8ET) that encodes only extracellular and transmembrane domains was used as a control. CD8-Nef (1 μg) or CD8-ET (1 μg) was cotransfected with or without Tat (0.05 μg) together with the wild-type LTR (LTRwt)-SEAP reporter vector (0.2 μg) into Jurkat TAg cells. The SEAP activity was measured 48 h after transfection. (B) The cooperative effect of the CD8-Nef requires the κB and TAR elements. The transient cotransfection was performed by using CD8-Nef and Tat together with the LTRwt-SEAP, LTRΔκB-SEAP, or LTRΔTAR-SEAP in Jurkat cells. The data are representative of two independent experiments.
The present work indicates that deletion of the duplicated κB binding sequences in the modulatory region significantly impaired the ability of the LTR to respond to PMA, PHA, and Tax as well as to Nef. Although LTRΔκB contains an intact TAR element, its activity is not significantly enhanced by the Tat protein alone in Jurkat T cells. These results, which are consistent with a previous report by Berkhout et al. (5), indicate that the κB elements are required for the full activity of the HIV-1 LTR and also are critical to Tat responsiveness. The importance of the κB cis sequences appears to be dependent on the basal activity of NF-κB in a particular cell line. It has been recently shown that in cells with a constitutively high level of nuclear NF-κB, the wild-type HIV-1 replicates at a rate 20-fold higher than a mutant virus with the κB element deleted (6). In contrast, in cells with a low basal activity of NF-κB, the wild-type and ΔκB viruses multiply at equal rates (6). In addition, the ΔκB SIV exhibits efficient replication in CEMx174 cells and peripheral blood mononuclear cells (PBMCs) at rates similar to those of the wild-type SIV (15). These results, however, are not inconsistent with the hypothesis that the κB elements in both HIV-1 LTR and SIV LTR are critical for virus replication. Both factors, the κB elements in the HIV-1 LTR and the activated NF-κB protein, are required for the full activity of the NF-κB–κB DNA complex. Thus, it is reasonable to assume that wild-type HIV-1 can replicate in the absence of NF-κB but will multiply more efficiently in cells with activated NF-κB. This property enables both cellular and exogenous factors to affect virus replication kinetics in vivo.
In general, the transcription initiation of cellular or viral genes is achieved through structural and functional interactions among a number of transcriptional factors. NF-κB can synergize the HIV-1 LTR activity with other transcriptional factors such as Sp1 or Ets through direct interaction (3). Early studies showed that the HIV-1 LTR is activated by Tat and can be dramatically enhanced in PHA- and PMA-stimulated T cells (24). It has been recently shown that NF-κB can interact with NF-AT or with Tat to mediate synergistic activation of the LTR (19). By analyzing the cis elements of the LTR, we show in the present work that the synergistic activation mediated by PMA, PHA, or Tax in combination with Tat absolutely requires either the κB sites or the TAR element in human T lymphocytes. Mutagenesis studies indicate that HTLV-I Tax’s NF-κB activation domain is essential for this cooperative activity and suggest that the cooperation of Tax and Tat is mediated by a functional interaction of NF-κB and Tat. Interestingly, the activity of LTRΔκB is not enhanced by either Tat or PMA; however, costimulation with PMA plus Tat resulted in an increase in the LTRΔκB activity of about 10-fold. There is an alternate κB site located at the initiator region of the LTR for binding of NF-κB homodimer (23) that can respond to the PMA-plus-Tat stimulation but not to either alone, indicating that the cooperation of NF-κB and Tat is potent. The activated NF-κB appears to cooperate with suboptimal levels of Tat to activate the HIV-1 LTR. Although synergistic activation of the HIV-1 LTR mediated by transcriptional factors has been reported by some groups (19), we found that the functional cooperation of NF-κB or NF-AT with suboptimal levels of Tat is possibly the more important in initiating viral gene transcription during the early postintegration of HIV-1 infection.
As indicated, the κB cis sequences are highly conserved among HIV-1 isolates. Thus, the kinetics of viral replication in vivo are significantly affected by cellular factors. HIV-1 infection alone induces a persistent activation of NF-κB that is probably mediated by Tat and Nef (4, 10). Tat may cooperate with itself to form a positive-feedback autoloop to enhance HIV-1 replication. However, immediately postintegration of the proviral DNA, Tat is expressed at very low, presumably suboptimal levels. Thus, viral and cellular factors, such as Tax, or cytokines, such as tumor necrosis factor alpha and IL-1, could amplify the activity of Tat through activation of NF-κB and may play a critical role in enhanced HIV-1 replication during the early stages of HIV-1 infection.
HIV-1 has the ability to replicate in quiescent PBMCs, and this ability is dependent on a functional nef gene (22, 33). With respect to the viral mRNA transcription pattern, Tat is expressed at a very low level within 6 h postinfection and may not be sufficient for initiating viral gene transcription through the HIV-1 LTR. On the other hand, Nef is abundantly expressed (26). Nef could amplify Tat-mediated activation during the early phase of infection through regulation of the activity of a cellular factor, for example, NF-κB. In this respect, Nef may be essential for a permissive acute infection in the host. This feature may explain why the nef gene with the premature stop codon of SIVmac239 virus can revert to the full-length open reading frame in vivo because of a strong requirement of the functional nef gene for the virus to establish an acute infection (18). Finally, our data suggest that Nef’s ability to upregulate the viral replication can be replaced by NF-κB activators, such as Tax. This is consistent with the finding that both HIV-1 and SIV Nef proteins enhanced SIV replication in an IL-2-dependent rhesus macaque T cells in the absence of exogenous IL-2 and increased the production of IL-2 (1). This function of Nef could be replaced by an oncogenic protein v-Ras but not by c-Ras (1), suggesting that Nef may potentiate a Ras signaling pathway. Future studies might focus on how Nef mediates the infection of HIV-1 in quiescent PBMCs and how this function is linked to interaction between Nef and cellular factors.
Acknowledgments
We thank Celicia Cheng-Mayer (Aaron Diamond AIDS Center, New York, N.Y.) for PCMV/CD8-Nef and Gerald Crabtree (Stanford University School of Medicine, Palo Alto, Calif.) for Jurkat TAg cells. We also thank K. Nagata for pMAXRHneo/I and M plasmids.
This work is supported in part by Carter-Wallace AIDS Fellowship.
REFERENCES
- 1.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 3.Bassuk A G, Anandappa R T, Leiden J M. Physical interactions between Ets and NF-κB/NFAT proteins play an important role in their cooperative activation of the human immunodeficiency virus enhancer in T cells. J Virol. 1997;71:3563–3573. doi: 10.1128/jvi.71.5.3563-3573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 5.Berkhout B, Gatignol A, Rabson A B, Jeang K T. TAR-independent activation of the HIV-1 LTR: evidence that tat requires specific regions of the promoter. Cell. 1990;62:757–767. doi: 10.1016/0092-8674(90)90120-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen B K, Feinberg M B, Baltimore D. The κB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J Virol. 1997;71:5495–5504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conant K, Atwood W J, Traub R, Tornatore C, Major E O. An increase in p50/65 NF-κB binding to the HIV-1 LTR is not sufficient to increase viral expression in the primary human astrocytes. Virology. 1994;205:586–590. doi: 10.1006/viro.1994.1685. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree G R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 9.Cullen B R. Regulation of human immunodeficiency virus replication. Annu Rev Microbiol. 1991;45:219–250. doi: 10.1146/annurev.mi.45.100191.001251. [DOI] [PubMed] [Google Scholar]
- 10.Demarchi F, d’Adda di Fagagna F, Falaschi A, Giacca M. Activation of transcription factor NF-κB by the Tat protein of human immunodeficiency virus type 1. J Virol. 1996;70:4427–4437. doi: 10.1128/jvi.70.7.4427-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doppler C, Schalasta G, Amtmann E, Sauer G. Binding of NF-κB to the HIV-1 LTR is not sufficient to induce HIV-1 LTR activity. AIDS Res Hum Retroviruses. 1992;8:245–252. doi: 10.1089/aid.1992.8.245. [DOI] [PubMed] [Google Scholar]
- 12.Garcia J A, Gaynor R B. The human immunodeficiency virus type-1 long terminal repeat and its role in gene expression. Prog Nucleic Acid Res Mol Biol. 1994;49:157–196. doi: 10.1016/s0079-6603(08)60050-1. [DOI] [PubMed] [Google Scholar]
- 13.Good L, Sun S C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type I Tax involves degradation of IκBβ. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henthorn P, Zervos P, Raducha M, Harris H, Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci USA. 1988;85:6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilyinskii P O, Desrosiers R C. Efficient transcription and replication of simian immunodeficiency virus in the absence of NF-κB and Sp1 binding elements. J Virol. 1996;70:3118–3126. doi: 10.1128/jvi.70.5.3118-3126.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Scheidereit C, Roeder R G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci USA. 1988;85:4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita S, Su L, Amano M, Timmerman L A, Kaneshima H, Nolan G P. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 20.Leonard J, Parrott C, Buckler-White A J, Turner W, Ross E K, Martin M A, Rabson A B. The NF-κB binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J Virol. 1989;63:4919–4924. doi: 10.1128/jvi.63.11.4919-4924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markovitz D M, Hannibal M, Perez V L, Gauntt C, Folks T M, Nabel G J. Differential regulation of human immunodeficiency viruses (HIVs): a specific regulatory element in HIV-2 responds to stimulation of the T-cell antigen receptor. Proc Natl Acad Sci USA. 1990;87:9098–9102. doi: 10.1073/pnas.87.23.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montano M A, Kripke K, Norina C D, Achacoso P, Herzenberg L A, Roy A L, Nolan G P. NF-κB homodimer binding within the HIV-1 initiator region and interactions with TFII-I. Proc Natl Acad Sci USA. 1996;93:12376–12381. doi: 10.1073/pnas.93.22.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1990;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 25.Poli G, Kinter A, Justement J S, Kehrl J H, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratner L, Niederman T M. Nef. Curr Top Microbiol Immunol. 1995;193:169–208. doi: 10.1007/978-3-642-78929-8_10. [DOI] [PubMed] [Google Scholar]
- 27.Ross E K, Buckler-White A J, Rabson A B, Englund G, Martin M A. Contribution of NF-κB and Sp1 binding motifs to the replicative capacity of human immunodeficiency virus type 1: distinct patterns of viral growth are determined by T-cell types. J Virol. 1991;65:4350–4358. doi: 10.1128/jvi.65.8.4350-4358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer S L, Hiscott J, Pitha P M. Differential regulation of the HIV-1 LTR by specific NF-kappa B subunits in HSV-1-infected cells. Virology. 1996;224:214–223. doi: 10.1006/viro.1996.0523. [DOI] [PubMed] [Google Scholar]
- 29.Semmes O J, Jeang K T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siekevitz M, Josephs S F, Dukovich M, Peffer N, Wong-Staal F, Greene W C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987;238:1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- 31.Smith M R, Greene W C. Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology. 1992;187:316–320. doi: 10.1016/0042-6822(92)90320-o. [DOI] [PubMed] [Google Scholar]
- 32.Sodroski J, Rosen C A. Trans-acting transcriptional regulation of human T-cell leukemia virus type I long terminal repeat. Science. 1985;238:1575–1578. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- 33.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzan M, Salaun D, Neuveut C, Spire B, Hirsch I, Le Bouteiller P, Querat G, Sire J. Induction of NF-κB during monocyte differentiation by HIV type 1 infection. J Immunol. 1991;146:377–383. [PubMed] [Google Scholar]
- 35.Tong S S, Luciw P A, Peterlin B M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci USA. 1987;84:6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ton-Starksen S E, Luciew P A, Peterlin B M. Signaling through the T lymphocyte surface protein, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J Immunol. 1989;142:702–709. [PubMed] [Google Scholar]
- 37.Zack J A, Cann A J, Lugo J P, Chen I S. HIV-1 production from infected peripheral blood T cells after HTLV-I induced mitogenic stimulation. Science. 1988;240:1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]