Abstract
Interleukin-12 (IL-12), a key cytokine in immune regulation, has an important role in activating the cell-mediated immune response in infectious diseases. Recently, a dichotomy between IL-12 and IL-10 regarding progression of a variety diseases has emerged. IL-12 activates type 1 cytokine production and has an antagonistic effect on type 2 cytokines. Here, by using quantitative competitive PCR, we show that peripheral blood mononuclear cells from bovine leukemia virus-infected animals in the alymphocytotic stage of disease express an increased amount of IL-12 p40 mRNA. In contrast, IL-12 p40 mRNA expression by cells from animals with late-stage disease, termed persistent lymphocytosis, was significantly decreased compared to that by normal and alymphocytotic animals. Interestingly, IL-12 p40 mRNA was also detected in tumor-bearing animals. IL-12 p40 expression occurred only in monocytes/macrophages, not B or T lymphocytes. The present study combined with previous findings suggest that IL-12 in bovine leukemia virus-infected animals may regulate production of other cytokines such as gamma interferon and IL-10 and the progression of bovine leukosis in animals that develop more advanced disease such as a persistent lymphocytosis of B cells or B-cell lymphosarcoma.
Bovine leukemia virus (BLV), closely related to human T-cell leukemia virus type 1, is a type C retrovirus infecting bovine B cells and leads to development of enzootic bovine leukosis (20). Fewer than 5% of infected animals develop malignant lymphosarcoma (12), while 30% of infected animals progress to persistent lymphocytosis (PL animals), in which nonneoplastic B cells proliferate and leukocyte counts may exceed 10,000/mm3 (22). However, most infected animals remain in the alymphocytotic (AL) stage. Despite the often long duration for disease transition, the mechanisms for progression are as yet unknown. Previously, we determined that cytokine profiles of BLV-infected animals differ depending on the stage of disease (29). Type 1 cytokines, interleukin-2 (IL-2) and gamma interferon (IFN-γ), were expressed in high amounts in AL animals, while the type 2 cytokine IL-10 increased in PL animals. This finding suggests that the transition in cytokines may be a contributing factor to disease progression. Cytokine imbalance may contribute to disease progression in human immunodeficiency virus (HIV) infection as well as autoimmune diseases and cancers (8, 33). To examine the polarization of cytokine production in BLV infection, we tested the quantity of IL-12 in animals in different stages of disease. IL-12 is a heterodimer comprised of two unrelated chains, p40 and p35, and is produced by several different types of cells, including dendritic cells, macrophages, neutrophils, keratinocytes, Langerhans cells, and B cells (23, 34). T cells, natural killer (NK) cells, and B cells are affected by IL-12. IL-12 has proinflammatory and immunoregulatory effects on T helper (Th) cell responses inducing T-cell differentiation and optimal proliferation and cytokine production in mature Th1 cells and inhibiting production of type 2 cytokines. Also, IL-12 can initiate development of Th1 cells because only Th1 cells possess the IL-12 receptor β2 subunit (30, 32). In this study, we demonstrate that IL-12 p40 quantitatively increases in AL animals but significantly decreases in PL animals and that monocytes/macrophages are the only detectable source of IL-12 production.
Determination of disease stage and IL-12 p40 production.
Adult female Holstein cattle, 2 to 12 years of age, were assigned to three groups according to their disease stage. Five normal, three AL, three PL, and three tumor-bearing animals were used for reverse transcriptase PCR (RT-PCR) of IL-12 p40 mRNA. Heparinized blood was obtained from the jugular vein, and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (7). BLV-infected animals were identified by enzyme-linked immunosorbent assay by using sera of cattle with BLV antigen-coated microplates (14). PL animals were distinguished by cell counts and changes in B-cell numbers determined by flow cytometry. PL animals had more than 5,500 PBMCs/mm3 and 55 to 80% B cells, while AL and normal animals had 20 to 30% B cells (Table 1).
TABLE 1.
Determination of disease stage of animal
| Animal type | BLV antigena | PBMCs/mm3b | % B cells | % MΦc |
|---|---|---|---|---|
| Normal | − | ≤2,500 | 20–30 | 10–14 |
| AL | + | ≤2,500 | 20–30 | 10–12 |
| PL | + | 5,500–15,000 | 55–80 | 7–12 |
| Tumor bearing | + | 1,000–45,000 | NDd | ND |
BLV seropositivity (+) or -negativity (−) was determined by enzyme-linked immunosorbent assay.
Measured by flow cytometry by using mouse anti-bovine IgM.
MΦ, macrophages.
ND, not defined.
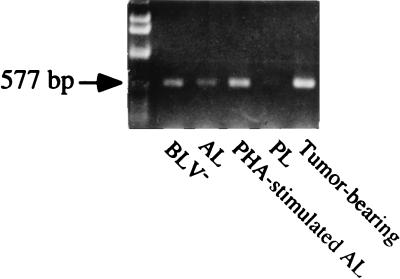
Cytoplasmic lysates from freshly isolated PBMCs were obtained by adding 200 μl of chloroform, and total RNA was prepared by using TRI reagent (MRC, Cincinnati, Ohio) as described in the manufacturer’s protocol. Concentration of purified total RNA was determined by spectrophotometer. Total mRNA was isolated from PBMCs of animals in different disease stages by using magnetic isolation. PCR was performed in a DNA thermocycler (Perkin-Elmer, Norwalk, Conn.) for 33 to 38 cycles consisting of 45 s at 94°C for denaturation, 1 min at 60 or 62°C for annealing, and 1 min at 72°C for polymerization. Each PCR mixture contained 1.25 U of Taq polymerase, 1.5 mM MgCl2, 0.8 mM deoxynucleoside triphosphates, 1 μM primers, template, and 10× thermobuffer (500 mM KCl, 100 mM Tris-HCl [pH 9.0], 1% Triton X-100). Primers were designed with Oligo 5.0 software (National Bioscience, Plymouth, Minn.) and were based on GenBank sequence information (Table 2). Primers for IL-12 p40 were based on the bovine IL-12 p40 cDNA sequence (37). To produce a plasmid containing IL-12 p40, PCR products from phytohemagglutinin (5 μg/ml; Sigma, St. Louis, Mo.)- or concanavalin A (10 μg/ml; Sigma)-stimulated PBMCs were cloned into the TA cloning vector pCR2.1 (Invitrogen, San Diego, Calif.) as recommended by the manufacturer. Plasmids were purified from transformed Escherichia coli by use of the Wizard miniprep system (Promega, Madison, Wis.) and then screened for an insert by EcoRI digestion followed by agarose gel electrophoresis. Dideoxy sequencing of positive clones was performed with T7 and M13 sequencing primers followed by separation on a Sequagel-6 6% sequencing gel (National Diagnostics, Atlanta, Ga.). β-Actin (5′-ACCAACTGGGACATGGAG, 3′-GCATTTGCGGTGGACAATGGA; 890-bp product) (11) and IL-12 p40 (5′-TGAGGCAAAGGATTATTCTG, 3′-AGATGCCCATTCACTCCAGA; 577-bp product) primers (37) were used for amplification. Amplified products were analyzed by 1% agarose gel electrophoresis. Samples from RT reaction mixtures without Moloney murine leukemia virus RT and mixtures without cDNA template were used in PCR assays as controls for amplification of contaminated DNA fragments. IL-12 p40 mRNA expression by animals in AL, PL, and tumor-bearing stages was qualitatively detected by RT-PCR. The clear bands of IL-12 p40 were detected in noncultured cells from normal, AL, and tumor-bearing animals. In contrast, only a trace amount of IL-12 p40 was detected in animals in the PL stage of disease (Fig. 1).
TABLE 2.
Primer sequences
| Primer | Sequence (5′→3′) | Product size (bp)
|
Refer- ence | |
|---|---|---|---|---|
| Standard | Mimic | |||
| β-actin | ||||
| 5′ | ACCAACTGGGACATGGAG | 890 | 525 | 11 |
| 3′ | GCATTTGCGGTGGACAATGGA | |||
| IL-12 p40 | ||||
| 5′ | TGAGGCAAAGGATTATTCTG | 577 | 380 | 34 |
| 3′ | AGATGCCCATTCACTCCAGA | |||
FIG. 1.
Representative bands of IL-12 p40 qualitatively expressed by PBMCs of BLV-infected animals with different stages of disease and of a normal (BLV−) animal. The IL-12 p40 band, 577 bp, was generated from total RNA by using RT-PCR. All products were analyzed on a 1% agarose gel stained by ethidium bromide.
Quantification of IL-12 p40.
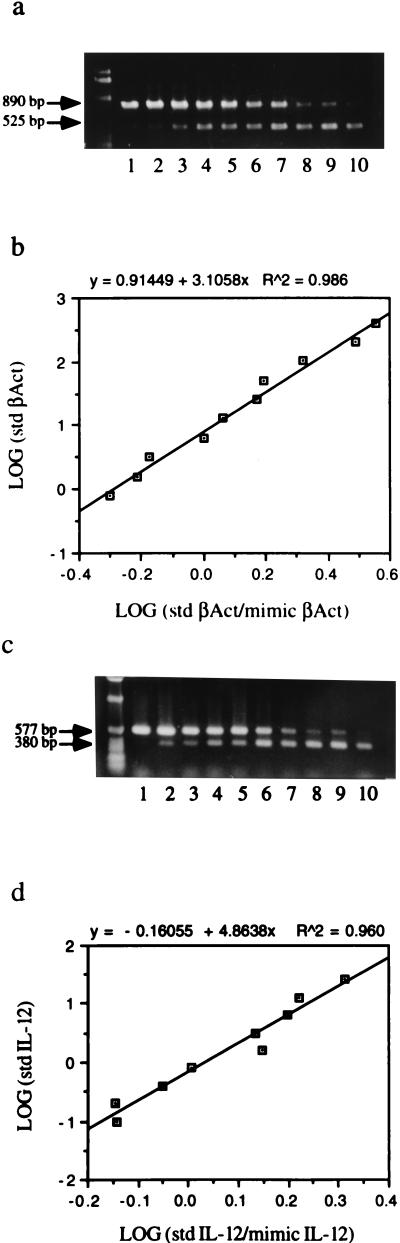
To verify the precise differences in IL-12 p40 levels produced by animals in different disease stages, IL-12 p40 mRNA was quantified by using mimics. IL-12 p40 mimic (380 bp) and β-actin mimic (525 bp) were generated by nonspecific PCR amplification. IL-12 p40 and β-actin quantitation were optimized by using different amounts of standard template plasmid and a fixed amount of mimic plasmids in each tube (35). In β-actin standard reaction mixtures, serial twofold dilutions of standard templates from 410 fmol to 800 amol were amplified competitively with 1 fmol mimic template (Fig. 2a and b). In the IL-12 standard reaction mixtures, serial twofold dilutions of the IL-12 templates from 26 fmol to 50 amol were amplified with 5 fmol mimic template (Fig. 2c and d). On the basis of band ratios, a standard graph was generated and r2 values of the standard reaction mixture were more than 0.950, confirming that the amount of total RNA paralleled the ratio of total RNA to IL-12 p40 mimic (data not shown). Since the concentration of isolated mRNA was below the limit of detection by spectrophotometry, the concentration of β-actin was measured for each animal and used as an internal control for IL-12 p40 mRNA production. Isolated mRNA was diluted at least 100 times for β-actin quantitation to compensate for the difference between β-actin and IL-12 p40 mRNA concentrations.
FIG. 2.
Standard reaction of β-actin (a and b) and IL-12 p40 (c and d). Standard (std) DNA, 410 fmol to 800 amol for β-actin (βAct) and 26 fmol to 50 amol for IL-12 p40, was amplified with 1 or 5 fmol of mimic DNA for β-actin or IL-12 p40, respectively. The product sizes are as follows: standard β-actin, 890 bp; mimic β-actin, 525 bp; standard IL-12 p40, 577 bp; mimic IL-12 p40, 380 bp. All products were analyzed on a 1% agarose gel stained by ethidium bromide. Band density was measured by densitometry by using the NIH Image 1.59 program, and the standard graph was generated on the basis of density ratios.
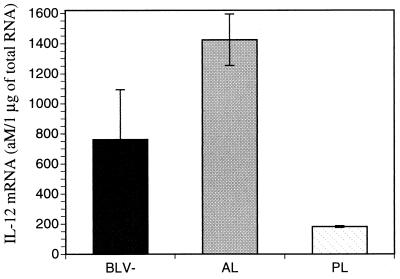
Interestingly, expression of IL-12 p40 mRNA increased in AL animals but decreased in PL animals relative to that in BLV-seronegative animals (Fig. 3). AL animals expressed 4 to 5 times more IL-12 p40 mRNA and PL animals expressed 7 to 20 times less IL-12 p40 mRNA than normal animals. Despite apparent differences, β-actin production may be changed by viral antigens. To confirm that differences in IL-12 p40 mRNA expression in PL animals was not influenced by a change in β-actin mRNA expression, total RNA was isolated and the amount of IL-12 p40 mRNA per microgram of total RNA was quantitated. Again, AL animals expressed approximately twice the amount of IL-12 p40 mRNA than normal animals did, while PL animals produced four times less IL-12 p40 mRNA per microgram of total RNA than normal animals did (Fig. 4). Thus, IL-12 p40 mRNA production by AL animals increased and that by PL animals decreased relative to that by normal animals, when measurements of either mRNA or total RNA were used for comparison.
FIG. 3.
Representative QC-PCR bands of β-actin and IL-12 p40 produced by BLV-infected animals with different stages of disease and by a normal (BLV−) animal. (a) Isolated mRNA was amplified by using a fixed amount of mimic DNA. (b) The concentration of IL-12 p40 mRNA (in attomoles per picomole of β-actin) was calculated. Standard error bars are shown.
FIG. 4.
Concentrations of IL-12 p40 mRNA (in attomoles per microgram of total RNA) expressed by BLV-infected animals with different stages of disease and by normal (BLV−) animals. An equal amount, 0.5 μg, of total RNA was amplified by using a fixed concentration of mimic IL-12 p40. Three to five animals were used for each disease stage. Standard error bars are shown.
IL-12 is produced only by monocytes/macrophages.
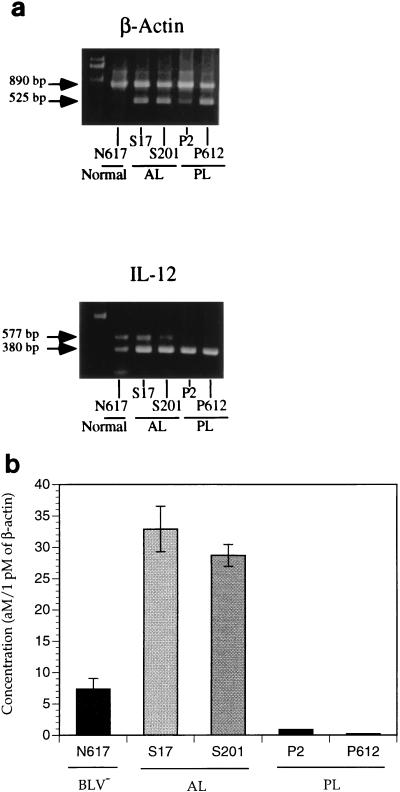
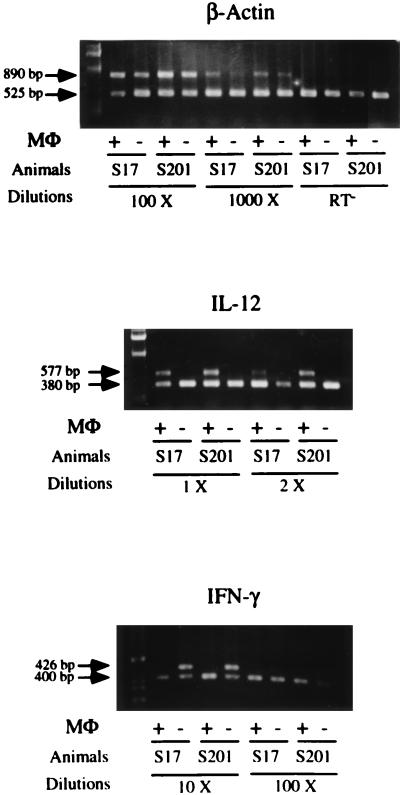
To determine the cell type producing IL-12 p40, monocytes/macrophages were isolated from PBMCs of AL animals by using mouse anti-bovine CD14 antibodies (CAM36A; Veterinary Medical Research and Development, Inc., Pullman, Wash.) and goat anti-mouse immunoglobulin G (IgG)-coated magnetic beads (Dynal, Lake Success, N.Y.) and confirmed by esterase staining (24). More than 90% of the CD14 positively selected cells exhibited dark brown α-naphthyl acetate staining, while the negatively sorted cell population stained yellow. THP-1 (ATCC TIB202), a human monocyte cell line, was used as a positive control and Daudi (ATCC CCL 213), a human B-cell line, was used as the negative control. By using RT-PCR, IL-12 p40 mRNA expression was detected only in the positively sorted monocytes/macrophages, not in the negatively selected cells (Fig. 5). We also determined that IFN-γ mRNA was detected in negatively isolated cells (Fig. 5), consisting predominantly of T and B lymphocytes as determined by flow cytometry (data not shown). However, IFN-γ mRNA was not expressed by positively sorted cells, while β-actin was constitutively expressed from both positively and negatively sorted cells. Thus, increased IL-12 p40 mRNA expression in PBMCs of AL animals is from monocytes/macrophages.
FIG. 5.
IL-12 p40 mRNA expression by sorted monocytes/macrophages. Monocytes/macrophages were separated by use of magnetic beads. Mouse anti-bovine CD14 antibody was used, followed by goat anti-mouse IgG-coated magnetic beads. Sorted monocytes/macrophages were confirmed by esterase staining. β-actin was used to confirm the RT-PCR results and that a similar product was evident in different animals and isolated cell populations. IFN-γ transcription was used to confirm the separation of T cells from the monocyte/macrophage population. MΦ, macrophages.
The results presented here demonstrate that IL-12 p40 mRNA was produced at relatively high concentrations by freshly isolated PBMCs of BLV-infected animals in the AL disease stage and at significantly reduced levels in PL animals. Biological activity of IL-12 is derived from the heterodimer p70, composed of p40 and p35, and the homodimer p40 could be an antagonist for IL-12 activity, binding the IL-12 receptor in the murine system (15). However, p35 IL-12 mRNA is constitutively expressed by nonhemapoietic tissues as well as lymphoid cells, while p40 IL-12 mRNA expression is regulated upon immune stimulation and limited to lymphoid cells (19, 37). Therefore, even if IL-12 p40 is a nonfunctional subunit, the changes in IL-12 p40 mRNA expression may implicate the functional differences in BLV infection. Previously, we reported that IL-10 mRNA expression increased with progression to the PL disease stage and that IL-2 and IFN-γ were reduced in PL and tumor-bearing animals (29). Together, our previous studies and present findings indicate that the type 1 immune response prevails in animals with the AL stage of disease and the cytokine profile converts to a type 2 response in animals with the more progressed PL stage of disease. Therefore, the polarity of the cytokine pattern is closely related to disease progression in BLV infection.
Whether the change in cytokine profile from type 1 to type 2 is a cause or result of BLV disease progression is presently unknown; however, this cytokine profile shift may be initiated by several mechanisms. First, the change in cytokine polarity may result directly from cytokines produced by viral infection or viral load. Type 1 cytokines activate T cells, and activated T cells may be eliminated by programmed cell death; continuous T-cell death may exhaust a type 1 immune response (10, 36). Second, the amount of viral antigen may lead to cytokine polarization. Low antigen concentration can reportedly trigger type 1 cytokine production, while high amounts of antigen cause a type 2 response (3, 31). In support of this mechanism, BLV antigen is transcribed in greater amounts in PL animals than in AL animals (16). Third, particular regions of a peptide sequence or the availability of a viral protein may stimulate different type of cytokines. For example, HIV tat (26) and gp120 (2) proteins stimulate IL-10 production, while CKS-17, a consensus transmembrane domain of several retroviruses, inhibits type 1 cytokines and IL-12 production (17, 18). Similarly, BLV also encodes tax and gp51 proteins, which are homologous to HIV tat and gp120, while BLV gp30 has a motif similar to that of CKS-17 (17).
Presently, no BLV antigen that preferentially stimulates type 2 cytokine production is known. However, evidence exists that naturally occurring variants of other viruses, including HIV, can antagonize cytotoxic T-cell responses (5, 21). Selected mutant viral antigens can alter the binding affinity of peptides to major histocompatibility complex molecules or recognition by T-cell receptors (9). Since BLV infection can persist for years in an animal, accumulation of proteins with altered peptides possessing different binding affinities to major histocompatibility complex molecules or T-cell receptors is possible. In fact, variants of gp51 have been reported (27), reenforcing this possibility. Infected animals with minimal virus production and minimal antigenic variation would maintain high-affinity ligand interactions promoting type 1 cytokines in the AL stage. In contrast, once sufficient variants with low-affinity peptides are generated, the T-cell response would be altered, with production of type 2 cytokines with disease progression to the PL stage.
We also found that IL-12 p40 mRNA was expressed by PBMCs from tumor-bearing animals. IL-12 expression was detected in AIDS-related lymphoma B-cell lines, and the antagonistic effect of IL-10 on IL-12 does not apply to these B cells because they are tumor cells (4). Thus, despite high expression of IL-10, IL-12 p40 was also produced by BLV-infected tumor-bearing animals. However, further research was not possible due to the death of the tumor-bearing animals. Host immune response with differing cytokine expression may influence viral replication and virus control by the host. For example, γδ T cells from AL animals have more efficient cytotoxic-T-lymphocyte activity than those from PL animals (25). High titers of virus exist in the PL stage, supporting the inability of PL animals to effectively control BLV infection. IL-12 is an important cytokine for promoting cytotoxic-T-lymphocyte activity and regulating the cell-mediated immune response. In contrast, an increased level of IL-10 in HIV infection suppressed HIV replication by monocytes/macrophages (1, 6), while IL-12 enhanced HIV replication in PBMCs (13). Therefore, decreased IL-12 and increased IL-10 in PL animals may further inhibit BLV replication. We have observed that IL-10 significantly inhibits BLV tax and pol expression (28). Further studies to examine the in vitro effects of other cytokines such as IL-2 and IL-12 on viral replication would aid in understanding the mechanism of cytokine polarization in BLV infection. The cytokine patterns in BLV infection and IL-12 expression appear important in the regulation of disease progression.
Acknowledgments
We thank Kathy L. O’Reilly for providing a blood sample from a PL animal and Yeon-Soo Han for help with photography.
This work was supported by National Cancer Institute grant R01 CA59127, BARD 95-34339-2556, and the College of Agricultural and Life Sciences.
REFERENCES
- 1.Akridge R E, Oyafuso L K, Reed S G. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–5789. [PubMed] [Google Scholar]
- 2.Ameglio F, Capobianchi M R, Castilletti C, Cordiali Fei P, Fais S, Trento E, Dianzani F. Recombinant gp120 induces IL-10 in resting peripheral blood mononuclear cells; correlation with the induction of other cytokines. Clin Exp Immunol. 1994;95:455–458. doi: 10.1111/j.1365-2249.1994.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancroft A J, Else K J, Grencis R K. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur J Immunol. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin D, Venkatanarayanan S, Kubin M, Klein J L, Alexandrina S, Holliday J, Trinchieri G. IL-12 expression in AIDS-related lymphoma B cells lines. J Immunol. 1996;156:1626–1637. [PubMed] [Google Scholar]
- 5.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 6.Borghi P, Fantuzzi L, Varano B, Gessani S, Puddu P, Conti L, Capobianchi M R, Ameglio F, Belardelli F. Induction of interleukin-10 by human immunodeficiency virus type 1 and its gp120 protein in human monocytes/macrophages. J Virol. 1995;69:1284–1287. doi: 10.1128/jvi.69.2.1284-1287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S-H, Splitter G A. Induction of MHC unrestricted cytolytic CD4+ T cells against virally infected target cells by cross-linking CD4 molecules. J Immunol. 1994;153:3874–3881. [PubMed] [Google Scholar]
- 8.Clerici M, Shearer G M. Th1→Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 9.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell response: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 10.Copeland K F, Heeney J L. T helper cell activation and human retroviral pathogenesis. Microbiol Rev. 1996;60:722–742. doi: 10.1128/mr.60.4.722-742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covert J C, Splitter G A. Detection of cytokine transcriptional profiles from peripheral blood mononuclear cells and CD4+ lymphocytes by reverse transcriptase polymerase chain reaction. Vet Immunol Immunopathol. 1995;49:39–50. doi: 10.1016/0165-2427(95)05451-b. [DOI] [PubMed] [Google Scholar]
- 12.Esteban E N, Thorn R M, Ferrer J F. Characterization of the blood lymphocyte population in cattle infected with bovine leukemia virus. Cancer Res. 1985;45:3225–3230. [PubMed] [Google Scholar]
- 13.Foli A, Saville M W, Baseler M W, Yarchoan R. Effects of the Th1 and Th2 stimulatory cytokines interleukin-12 and interleukin-4 on human immunodeficiency virus replication. Blood. 1995;85L:2114–2123. [PubMed] [Google Scholar]
- 14.Gao Y, Daley M J, Splitter G A. BHV-1 glycoprotein 1 and recombinant interleukin 1 beta efficiently elicit mucosal IgA response. Vaccine. 1995;13:871–877. doi: 10.1016/0264-410x(94)00004-7. [DOI] [PubMed] [Google Scholar]
- 15.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 16.Haas L, Divers T, Casey J W. Bovine leukemia virus gene expression in vivo. J Virol. 1992;66:6223–6225. doi: 10.1128/jvi.66.10.6223-6225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haraguchi S, Good R A, Day N K. Immunosuppressive retroviral peptides: cAMP and cytokine patterns. Immunol Today. 1995;16:595–603. doi: 10.1016/0167-5699(95)80083-2. [DOI] [PubMed] [Google Scholar]
- 18.Haraguchi S, Good R A, James-Yarish M, Cianciolo G J, Day N K. Induction of intracellular cAMP by a synthetic retroviral envelope peptide: a possible mechanism of immunopathogenesis in retroviral infections. Proc Natl Acad Sci USA. 1995;92:5568–5571. doi: 10.1073/pnas.92.12.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzel F P, Rerko R M, Ling P, Hakimi J, Schoenhaut D S. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kettmann R, Portelle D, Mammerickx M, Cleuter Y, Dekegel D, Galoux M, Ghysdael J, Burny A, Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci USA. 1976;73:1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A, Giangrande P, Phillips R E, McMichael A J. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 22.Koyama H, Nakanishi H, Kajikawa O, Yoshikawa H, Tsubaki S, Yoshikawa T, Saito H. T and B lymphocytes in persistently lymphocytotic and leukemic cattle. Jpn J Vet Sci. 1983;45:471–475. doi: 10.1292/jvms1939.45.471. [DOI] [PubMed] [Google Scholar]
- 23.Lamont A G, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 24.Li C Y, Lam K W, Yam L T. Esterase in human leukocytes. J Histochem Cytochem. 1973;21:1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg, P., and G. A. Splitter. Unpublished data.
- 26.Masood R, Lunardi-Iskandar Y, Moudgil T, Zhang Y, Law R E, Huang C L, Puri R K, Levine A M, Gill P S. IL-10 inhibits HIV-1 replication and is induced by tat. Biochem Biophys Res Commun. 1994;202:374–383. doi: 10.1006/bbrc.1994.1938. [DOI] [PubMed] [Google Scholar]
- 27.Portetelle D, Couez D, Bruck C, Kettmann R, Mammerickx M, Van der Maaten M, Brasseur R, Burny A. Antigenic variants of bovine leukemia virus (BLV) are defined by amino acid substitutions in the NH2 part of the envelope glycoprotein gp51. Virology. 1989;169:27–33. doi: 10.1016/0042-6822(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 28.Pyeon, D., and G. A. Splitter. Unpublished data.
- 29.Pyeon D, O’Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky D H, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarzotti M, Robbins D S, Hoffman P M. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 32.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomar R H. Breaking the asymptomatic phase of HIV-1 infection. J Clin Lab Anal. 1994;8:116–119. doi: 10.1002/jcla.1860080210. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 35.Tsai S J, Wiltbank M C. Quantification of mRNA using competitive RT-PCR with standard curve methodology. Biotechniques. 1996;21:862–866. doi: 10.2144/96215st04. [DOI] [PubMed] [Google Scholar]
- 36.Varadhachary A S, Perdow S N, Hu C, Ramanarayanan M, Salgame P. Differential ability of T cell subsets to undergo activation-induced cell death. Proc Natl Acad Sci USA. 1997;94:5778–5783. doi: 10.1073/pnas.94.11.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarlenga D S, Canals A, Aschenbrenner R A, Gasbarre L C. Enzymatic amplification and molecular cloning of cDNA encoding the small and large subunits of bovine interleukin 12. Biochim Biophys Acta. 1995;1270:215–217. doi: 10.1016/0925-4439(95)00042-3. [DOI] [PubMed] [Google Scholar]