ABSTRACT
Background
We assessed the relative vaccine effectiveness (rVE) of high‐dose quadrivalent influenza vaccine (QIV‐HD) versus standard‐dose quadrivalent influenza vaccine (QIV‐SD) in preventing respiratory or cardiovascular hospitalizations in older adults.
Methods
FinFluHD was a phase 3b/4 modified double‐blind, randomized pragmatic trial. Enrolment of 121,000 adults ≥65 years was planned over three influenza seasons (October to December 2019–2021). Participants received a single injection of QIV‐HD or QIV‐SD. The primary endpoint was first occurrence of an unscheduled acute respiratory or cardiovascular hospitalization (ICD‐10 primary discharge J/I codes), from ≥14 days post‐vaccination until May 31. The study was terminated after one season due to COVID‐19; follow‐up data for 2019–2020 are presented.
Results
33,093 participants were vaccinated (QIV‐HD, n = 16,549; QIV‐SD, n = 16,544); 529 respiratory or cardiovascular hospitalizations (QIV‐HD, n = 257; QIV‐SD, n = 272) were recorded. The rVE of QIV‐HD versus QIV‐SD to prevent respiratory/cardiovascular hospitalizations was 5.5% (95% CI, −12.4 to 20.7). When prevention of respiratory and cardiovascular hospitalizations were considered separately, rVE estimates of QIV‐HD versus QIV‐SD were 5.4% (95% CI, −28.0 to 30.1) and 7.1% (95% CI, −15.0 to 25.0), respectively. Serious adverse reactions were <0.01% in both groups.
Conclusions
Despite insufficient statistical power due to the impact of COVID‐19, rVE point estimates demonstrated a trend toward a benefit of QIV‐HD over QIV‐SD. QIV‐HD was associated with lower respiratory or cardiovascular hospitalization rates than QIV‐SD, with a comparable safety profile. Adequately powered studies conducted over multiple influenza seasons are needed to determine statistical significance of QIV‐HD compared with QIV‐SD against preventing respiratory and cardiovascular hospitalizations.
Trial Registration: ClinicalTrials.gov number: NCT04137887
Keywords: cardiovascular hospitalization, high‐dose quadrivalent influenza vaccine, pragmatic vaccine trial, respiratory hospitalization
1. Introduction
Influenza is an acute infectious viral disease that is associated with considerable morbidity and mortality, particularly among older individuals [1]. In addition to influenza infections, secondary respiratory and cardiovascular complications contribute to disease burden [1]. Influenza infection can be a trigger of major adverse cardiovascular events (MACE), such as myocardial infarction [2, 3, 4, 5, 6] and thromboembolic stroke [4, 5, 7]. A recent community surveillance study also suggests a link between influenza infection and risk of heart failure [8].
While inactivated influenza vaccines have been an effective means of prophylaxis since the 1940s, lower antibody responses and protection are observed among individuals aged ≥65 years [9, 10]. High‐dose (HD) vaccines containing four times the hemagglutinin (HA) dose per strain than standard‐dose (SD) vaccines were developed for enhanced protection in this specific population [11]. In a multicenter, double‐blind, randomized controlled trial (RCT) of 31,989 participants aged ≥65 years, a high‐dose trivalent influenza vaccine (TIV‐HD) demonstrated 24.2% greater relative efficacy than standard‐dose trivalent influenza vaccine (TIV‐SD) in preventing influenza‐like illness [11]. An additional analysis of this trial suggested that TIV‐HD provided 17.7% greater relative efficacy than TIV‐SD in preventing serious cardiovascular and respiratory events [12]. This and various other studies support the benefit of TIV‐HD over TIV‐SD for a broad range of endpoints, including influenza infection, hospitalizations due to influenza‐like illness, pneumonia, cardiovascular events, all‐cause deaths, and post‐influenza death [11, 12, 13, 14, 15, 16]. A quadrivalent influenza vaccine (QIV) containing an additional influenza B strain lineage was subsequently introduced to provide broader antigenic coverage than trivalent formulations [17]. In addition to QIV as a standard‐dose (QIV‐SD), a high‐dose (QIV‐HD) formulation was developed for improved protection in older individuals [18].
There is a paucity of high‐quality evidence supporting the benefit of influenza vaccination in preventing secondary cardiovascular or respiratory complications. Studies are often compromised by healthy vaccinee bias, confounding by indication, or inadequate statistical power to assess cardiovascular events [19, 20, 21]. Randomized study designs reduce the impact of these biases, enabling assessment of cardiovascular events as a primary outcome.
Here, we present a pragmatic trial assessing the relative vaccine effectiveness (rVE) of QIV‐HD versus QIV‐SD in preventing cardiovascular and/or respiratory hospitalizations in a large cohort of adults aged ≥65 years, using a combination of RCT and real‐world data [18].
2. Methods
2.1. Study Design
The Finnish Influenza High‐Dose (FinFluHD) vaccine trial was a phase 3b/4 modified double‐blind, randomized trial with registry‐based follow‐up, conducted by the Finnish Institute for Health and Welfare (THL). The study design is described in detail elsewhere [18]. Briefly, adults aged ≥65 years were to be enrolled over three influenza seasons, between October and December in 2019, 2020, and 2021, at 41 health stations in Finland. However, the COVID‐19 pandemic created significant challenges to study conduct, and the study was terminated after one active season. Thus, we present data for the 2019–2020 season only. Effectiveness follow‐up (first occurrences of cardiovascular or respiratory inpatient hospitalizations) was approximately 7 months (up to May 31 the following year) and safety follow‐up (serious adverse reactions [SARs] and adverse events of special interest [AESIs]) was approximately 10 months (up to August 31 the following year). Follow‐up data were collected from multiple Finnish health registries for all study participants by THL investigators using the unique personal identity codes (PIC) used by all residents in Finland. THL then compiled an analysis database with pseudonymized individual level data.
Written informed consent was obtained from participants before enrollment. Study conduct was consistent with standards established by the Declaration of Helsinki, compliant with the International Conference on Harmonization guidelines for Good Clinical Practice, and with all national and European regulations and directives. The study and subsequent amendments were approved by an independent ethics committee and the Finnish Medicines Agency, Fimea.
2.2. Study Population
Adults aged ≥65 years were enrolled. Participants were excluded if they were participating in another clinical trial at the time of enrollment (or within 28 days preceding vaccination) or planned to participate in another vaccine or drug clinical trial during the study period, were vaccinated against influenza within 6 months prior to study start, or had known hypersensitivity to any vaccine components or a history of a life‐threatening reaction to the vaccines used or to another vaccine containing any of the same substances.
2.3. Vaccine Interventions
Participants received a single dose of the investigational vaccine (QIV‐HD [Efluelda®, Sanofi, in the European Union (EU); Fluzone® High‐Dose, Sanofi, in the USA]), or the control vaccine (QIV‐SD [Vaxigrip Tetra®, Sanofi]) via intramuscular injection. At study initiation in October 2019, QIV‐HD was not licensed in Finland [18] but was subsequently granted marketing authorization in June 2020 [22]. QIV‐SD is the standard‐of‐care and is included in Finland's national influenza vaccination program for adults aged ≥65 years, with 50% national coverage [23]. Both are inactivated, split‐virion vaccines containing four hemagglutinin (HA) strains recommended by the World Health Organization (WHO) and EU for the 2019–2020 Northern Hemisphere influenza season: influenza A virus subtype H1N1, influenza A virus subtypeH3N2, influenza B virus (Victoria Lineage), and influenza B virus (Yamagata Lineage) strains. QIV‐SD contains 15 μg of each HA strain component, while QIV‐HD contains four‐times this quantity (60 μg of each component). Both vaccines are egg‐based products.
2.4. Randomization and Blinding
Participants were randomized 1:1 to either QIV‐HD or QIV‐SD using a scratchable randomization list provided by the study sponsor. This study had a modified double‐blind design: The vaccine was administered by an unblinded administrator but participants, investigators, and healthcare professionals (HCPs) in healthcare centers, hospitals, outpatient care, and the THL were blinded to vaccine assignment to avoid reporting and evaluation biases. Unblinded THL study nurses monitored study procedures at the vaccination sites.
2.5. Outcomes
Respiratory and cardiovascular outcomes were identified using International Classification of Diseases tenth revision (ICD‐10) discharge “J” (respiratory) and “I” (cardiovascular) codes from the Finnish hospital discharge register, as described previously [18]. Codes I16, I75, and I76 were originally planned for inclusion owing to a high probability of causal association with influenza [18] but were excluded as they were not referenced in ICD‐10 Finland nor in ICD‐10 WHO 2019.
The primary study objective was to demonstrate superior rVE of QIV‐HD versus QIV‐SD for the prevention of respiratory or cardiovascular hospitalizations among participants aged ≥65 years. As a corresponding primary endpoint, first occurrences of an unscheduled acute respiratory or cardiovascular inpatient hospitalization, defined by primary ICD‐10 discharge codes between ≥14 days post‐vaccination until May 31 of the year following the vaccination, were recorded. This interval corresponded to the timings of previous influenza epidemic periods.
Secondary objectives included assessment of rVE of QIV‐HD versus QIV‐SD with regard to prevention of inpatient hospitalization for selected respiratory or cardiovascular causes (using primary/secondary discharge diagnoses and primary/secondary admission diagnoses), death (all‐cause or respiratory or cardiovascular causes), hospital emergency room visits (not resulting in hospitalization), and primary care visits to a physician. The rVE of QIV‐HD versus QIV‐SD with regard to prevention of MACE, including ischemic heart diseases (I20–I25 codes), myocardial infarction (I21–I23 codes), unstable angina (I20 and I25 codes), and cerebral infarction (I63 code, including thrombotic or embolic events, excluding hemorrhagic events), was assessed as a secondary objective. The rVE of QIV‐HD versus QIV‐SD was determined for respiratory or cardiovascular hospitalizations, after exclusion of secondary discharge events related to COVID‐19 (U07.1 or U07.02 codes). An additional secondary objective was to assess the rVE of QIV‐HD versus QIV‐SD by age group and specific comorbidities.
Electronically recorded serious adverse events (eSAEs) were reported by investigators using an electronic case report form. All SARs, AESIs, fatal cases, and non‐fatal SAEs were assessed using the Medical Dictionary for Regulatory Activities (MedDRA) system organ class (SOC)/preferred term (PT) classification. SARs and AESIs assessed by a HCP to be related to the vaccine, were reported to THL from inclusion until August 2020. Fatal SAEs and AESIs were collected by THL from Finnish health registers until August 2020, up to 10 months after vaccination. AESIs included onset of Guillain‐Barré syndrome (GBS), encephalitis/myelitis (including transverse myelitis), Bell's palsy, optic neuritis, and brachial neuritis. All other SAEs (unless included in the primary endpoint) were collected by the THL from the hospital discharge register (HILMO) up to 6 months post‐vaccination and were reported separately.
A post hoc analysis was undertaken of respiratory and cardiovascular hospitalizations, with follow‐up limited to influenza circulation until the start of the COVID‐19 pandemic in Finland in March 2020 (Week 52/2019 to Week 11/2020) [24, 25].
2.6. Statistical Methods
The analysis was performed on all participants who received a QIV‐HD or QIV‐SD vaccination. Statistical analysis was carried out according to the actual vaccine each participant received, rather than the pre‐specified analysis groups.
The rVE of QIV‐HD to QIV‐SD was estimated for the primary endpoint:
where CQIV‐HD and CQIV‐SD are numbers of respiratory and cardiovascular hospitalization cases meeting the primary endpoint definition in the QIV‐HD and QIV‐SD groups, respectively. NQIV‐HD and NQIV‐SD are the numbers of participants in the QIV‐HD and QIV‐SD groups, respectively.
Confidence intervals (CIs) for rVE were calculated by an exact method, assuming binomial distribution of the number of cases in the QIV‐HD group conditional on the total number of cases in both groups. Superiority of QIV‐HD effectiveness over QIV‐SD was demonstrated if the CI lower bound for the rVE was >0%. Safety endpoints were summarized per vaccine group, with 95% CI calculated using the Clopper–Pearson method. A sample size of approximately 121,000 participants was planned over three influenza seasons to provide ≥2200 evaluable participants with ≥1 cardiovascular and/or respiratory hospitalization. This would provide approximately 90% power (by exact method) to conclude on the primary objective under the following assumptions: the true rVE of QIV‐HD over QIV‐SD is 13% against prevention of cardiovascular and respiratory hospitalization; 0.025 one‐sided type I error; rVE lower bound >0%; 1:1 allocation ratio between groups [11, 14, 15, 26]. The rVE of 13% was assumed based on rVE estimates of HD versus SD trivalent vaccines against cardiorespiratory‐associated hospitalizations in studies published by Young‐Xu et al. [14] (18% [95% CI, 15.0 to 21.0]) and Lee et al. [15] (18.2% [95% CI, 6.8 to 28.1]). Owing to the COVID‐19 pandemic, the number of respiratory or cardiovascular events was smaller than expected, and rVE values were underpowered. Thus, effectiveness statistics are largely descriptive and limited to the 2019–2020 season only.
3. Results
3.1. Participants
Enrollment and data collection were disrupted during the 2020–2021 and 2021–2022 influenza seasons due to the COVID‐19 pandemic, leading to premature study termination. Only data for the 2019–2020 influenza season and subsequent follow‐up until August 31, 2020 (last visit, last subject) were available. Of 33,096 participants enrolled during this season, one was not randomized or vaccinated and two were randomized but not vaccinated (Figure 1). Of the remaining 33,093 randomized and vaccinated participants, 21 in the QIV‐HD group inadvertently received QIV‐SD and 21 in the QIV‐SD group inadvertently received QIV‐HD; therefore, analysis was carried out by actual vaccine received (QIV‐HD, n = 16,549; QIV‐SD, n = 16,544). During follow‐up, three participants (QIV‐HD, n = 2; QIV‐SD, n = 1) discontinued due to voluntary withdrawal.
FIGURE 1.
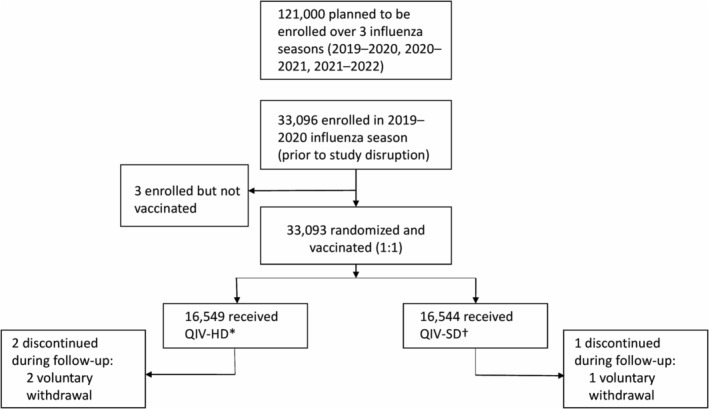
Trial participant flow. *Includes n = 21 that inadvertently received QIV‐HD after randomization to QIV‐SD. †Includes n = 21 that inadvertently received QIV‐SD after randomization to QIV‐HD. Effectiveness follow‐up was approximately 7 months (up to May 31 the following year) and safety follow‐up was approximately 10 months (up to August 31 the following year). QIV‐HD, high‐dose quadrivalent vaccine; QIV‐SD, standard‐dose quadrivalent vaccine.
Baseline demographic characteristics of vaccinated participants were well‐balanced between the groups (Table 1). The mean age of participants was 72.5 (SD 5.6) years and 34.7% had at least one registered comorbidity. Race and ethnicity data were not collected. Proportions of participants with a previous influenza vaccination within the last 5 years were similar between QIV‐HD and QIV‐SD groups: 88.8% and 88.6%, respectively.
TABLE 1.
Demographic and baseline characteristics.
| QIV‐HD (n = 16,549) | QIV‐SD (n = 16,544) | All (n = 33,093) | |
|---|---|---|---|
| Demographic characteristics | |||
| Sex, n (%) | |||
| Male | 8276 (50.01) | 8303 (50.19) | 16,579 (50.10) |
| Female | 8273 (49.99) | 8241 (49.81) | 16,514 (49.90) |
| Age in years | |||
| Mean (SD) | 72.6 (5.7) | 72.5 (5.6) | 72.5 (5.6) |
| Median (IQR) | 72.0 (68–76) | 72.0 (68–76) | 72.0 (68–76) |
| Range | 64–98 | 63–98 | 63–98 |
| Age in categories, n (%) | |||
| <65 years | 1 (< 0.01) | 2 (0.01) | 3 (< 0.01) |
| 65 to 69 years | 5754 (34.77) | 5734 (34.66) | 11,488 (34.71) |
| 70 to 74 years | 5656 (34.18) | 5681 (34.34) | 11,337 (34.26) |
| 75 years and above | 5138 (31.05) | 5127 (30.99) | 10,265 (31.02) |
| 85 years and above | 635 (3.84) | 628 (3.80) | 1263 (3.82) |
| Baseline characteristics | |||
| Previous vaccination status, n (%) | |||
| ≥1 influenza vaccination in the last 5 years | 14,688 (88.75) | 14,660 (88.61) | 29,348 (88.68) |
| Influenza vaccination in the previous 2018–2019 season prior to FinFluHD study | 12,675 (76.59) | 12,628 (76.33) | 25,303 (76.46) |
| ≥1 pneumococcal vaccination in the last 5 years | 4142 (25.03) | 4110 (24.84) | 8252 (24.94) |
| Specific comorbidities | |||
| No specific comorbidity (I to V) a | 10,738 (64.89) | 10,884 (65.79) | 21,622 (65.34) |
| At least one comorbidity (I to V) | 5811 (35.11) | 5660 (34.21) | 11,471 (34.66) |
| (I) Diabetes | 2572 (15.54) | 2506 (15.15) | 5078 (15.34) |
| (II) Coronary artery disease | 1612 (9.74) | 1547 (9.35) | 3159 (9.55) |
| (III) Chronic heart insufficiency | 169 (1.02) | 160 (0.97) | 329 (0.99) |
| (IV) Cardiac arrhythmia | 1420 (8.58) | 1328 (8.03) | 2748 (8.30) |
| (V) Chronic lung disease | 1687 (10.19) | 1680 (10.15) | 3367 (10.17) |
| Cardiovascular history (II to IV) | 2774 (16.76) | 2648 (16.01) | 5422 (16.38) |
Based on registry data collection.
Abbreviations: IQR, interquartile range; QIV‐HD, high‐dose quadrivalent vaccine; QIV‐SD, standard‐dose quadrivalent vaccine; SD, standard deviation.
3.1.1. rVE for Unscheduled Respiratory or Cardiovascular Hospitalizations Based on Primary Discharge Codes
Unscheduled respiratory or cardiovascular hospitalizations, based on primary discharge codes, were recorded for 529 participants (QIV‐HD, n = 257; QIV‐SD, n = 272). The rVE of QIV‐HD for prevention of such events versus QIV‐SD was 5.5% (95% CI, −12.4 to 20.7) (Figure 2). For prevention of respiratory hospitalizations alone (J codes), the rVE of QIV‐HD versus QIV‐SD was 5.4% (95% CI, −28.0 to 30.1). For prevention of cardiovascular hospitalizations alone (I codes), the rVE of QIV‐HD versus QIV‐SD was 7.1% (95% CI, −15.0 to 25.0). QIV‐HD was associated with a lower number of hospitalization events linked to MACE overall versus QIV‐SD, with a rVE of 24.0% (95% CI, −4.2 to 44.7) (Figure 2); this was particularly evident for ischemic heart diseases, with a rVE for prevention of related hospitalizations of 32.4% (95% CI, 0.2 to 54.5) (Figure 2).
FIGURE 2.
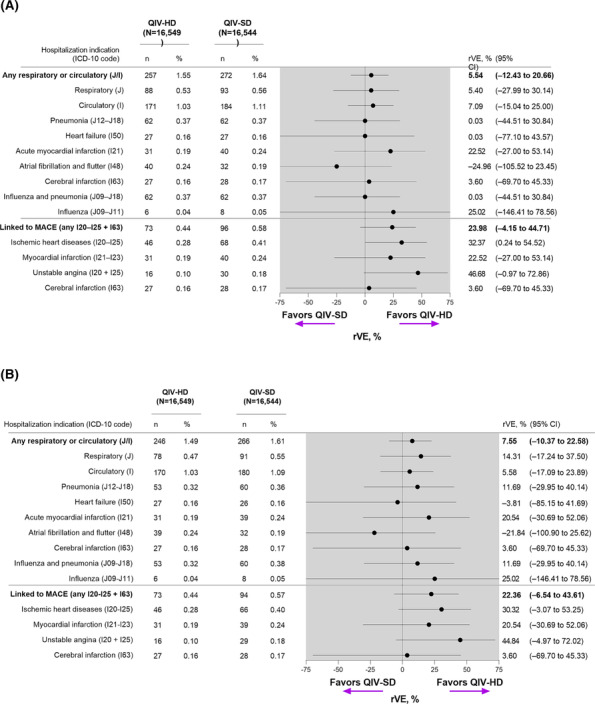
Relative vaccine effectiveness of QIV‐HD versus QIV‐SD for prevention of unscheduled respiratory or cardiovascular hospitalizations (A) primary discharge codes and (B) excluding cases linked to COVID‐19. In panel B, cases were based on primary discharge codes after exclusion of secondary discharge codes linked to COVID‐19. CI, confidence interval; ICD‐10, International Classification of Diseases tenth revision; MACE, major acute cardiovascular events; QIV‐HD, high‐dose quadrivalent vaccine; QIV‐SD, standard‐dose quadrivalent vaccine; rVE, relative vaccine effectiveness.
QIV‐HD was consistently associated with lower respiratory and cardiovascular hospitalization rates versus QIV‐SD from approximately 60 days post‐vaccination (Figure S1). In the post‐hoc analysis, with follow‐up time restricted to the influenza circulation in Finland during the COVID‐19 pandemic (Week 52/2019 to Week 11/2020), 348 respiratory or cardiovascular hospitalizations (QIV‐HD, n = 162; QIV‐SD, n = 186) were recorded.
3.1.2. rVE for Unscheduled Respiratory or Cardiovascular Hospitalizations After Excluding Events Associated With COVID‐19
COVID‐19‐associated events were reported in 17 participants (QIV‐HD, n = 11; QIV‐SD, n = 6). After excluding these patients, the rVE (QIV‐HD vs. QIV‐SD) for prevention of respiratory or cardiovascular hospitalizations was 7.6% (95% CI, −10.4 to 22.6); 14.3% (95% CI, −17.2 to 37.5) for respiratory hospitalizations alone, and 5.6% (95% CI, −17.1 to 23.9) for cardiovascular hospitalizations alone (Figure 2). The overall rVE for hospitalizations related to MACE was 22.4% (95% CI, −6.5 to 43.6) and the overall rVE for ischemic heart disease was 30.3% (95% CI, −3.1 to 53.3) (Figure 2).
3.1.3. rVE for Respiratory or Cardiovascular Hospital Emergency Room Visits
QIV‐HD and QIV‐SD showed comparable effectiveness for prevention of emergency room visits, with 250 participants in the QIV‐HD group undergoing 311 visits, and 252 participants in the QIV‐SD group undergoing 326 visits. The rVE was 2.1% (95% CI, −31.3 to 27.0) for respiratory‐related visits and 0.0% (95% CI, −25.4 to 20.3) for cardiovascular‐related visits (Figure S2).
3.1.4. rVE for Respiratory or Cardiovascular Primary Care Visits to a Physician
The number of visits to a physician in a primary care setting relating to acute respiratory or cardiovascular indications were 479 visits for 412 participants in the QIV‐HD group and 497 visits for 398 participants in the QIV‐SD group. The rVE for respiratory‐related visits was 2.1% (95% CI, −15.8 to 17.3), and the rVE for cardiovascular‐related visits was −14.1% (95% CI, −47.1 to 11.3) (Figure S3).
3.1.5. rVE for Unscheduled Respiratory or Cardiovascular Hospitalizations by Age Group, Previous Vaccination Status, and Comorbidity Status
QIV‐HD was favored over QIV‐SD for prevention of respiratory and cardiovascular hospitalizations in participants aged 65–74 years, with an overall rVE of 20.5% (95% CI, −1.7 to 38.0) (Figure 3). When considered separately, the rVE of QIV‐HD versus QIV‐SD for respiratory events was 12.5% (95% CI, −35.3 to 43.6), and the rVE for cardiovascular events was 25.7% (95% CI, −0.6 to 45.3). For older age groups, no trend was observed for greater effectiveness or QIV‐HD versus QIV‐SD, potentially due to progressively diminishing sample sizes.
FIGURE 3.
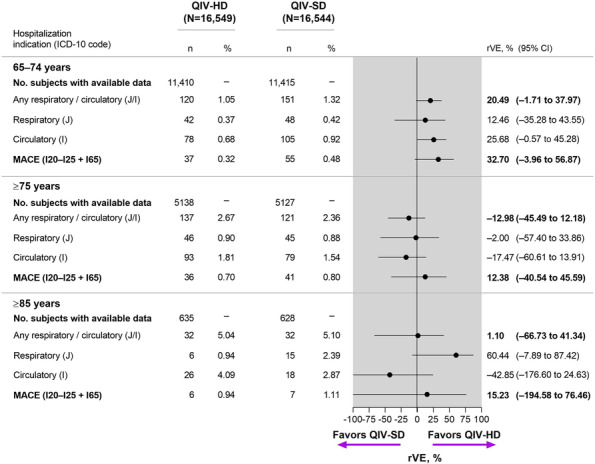
Relative Vaccine Effectiveness for Unscheduled Respiratory or Cardiovascular Hospitalizations, by Age Group. CI, confidence interval; ICD‐10, International Classification of Diseases tenth revision; MACE, major acute cardiovascular events; QIV‐HD, high‐dose quadrivalent vaccine; QIV‐SD, standard‐dose quadrivalent vaccine; rVE, relative vaccine effectiveness.
Nearly 90% of the participants had received at least one influenza vaccine in the previous 5 years. Therefore, rVE estimates for this subgroup were similar to those for the overall study population (Figure S4). Estimates for rVE are less reliable for participants who received a pneumococcal vaccine in the previous 5 years, owing to small numbers of hospitalizations.
The rVE estimates varied by comorbidity status and tended to be higher for participants with comorbidities than for those with no specific comorbidity (Figure S5).
3.2. Safety
Proportions of participants with eSAEs (including SARs, AESIs, deaths, and other SAEs) were similar in the QIV‐HD and QIV‐SD groups (n = 109 [0.66%] and n = 98 [0.59%], respectively) (Table 2). There were two SARs that were considered related to the study intervention: one participant in the QIV‐SD group (Bell's palsy), who subsequently recovered, and one participant in the QIV‐HD group (hypersensitivity vasculitis), who subsequently recovered with sequelae. Frequencies of SARs considered to be related to the vaccine were low (<0.01%) in both groups.
TABLE 2.
Adverse events based on MedDRA.
| Adverse event | QIV‐HD (n = 16,549) n (%) | QIV‐SD (n = 16,544) n (%) |
|---|---|---|
| eSAE | 109 (0.66) | 98 (0.59) |
| SAR (PT) | 1 (<0.01) | 1 (<0.01) |
| Bell's palsy | 0 | 1 (<0.01) |
| Hypersensitivity vasculitis | 1 (<0.01) | |
| AESIs (PT) | 4 (0.02) | 6 (0.04) |
| Bell's palsy | 3 (0.02) | 6 (0.04) |
| Guillain–Barré syndrome | 1 (<0.01) | 0 |
| Fatal SAEs (SOC and PT a ) | 102 (0.62) | 92 (0.56) |
| Cardiac disorders | 23 (0.14) | 15 (0.09) |
| Coronary artery disease a | 13 (0.08) | 2 (0.01) |
| Gastrointestinal disorders | 4 (0.02) | 2 (0.01) |
| General disorders and administration site conditions | 2 (0.01) | 3 (0.02) |
| Hepatobiliary disorders | 1 (<0.01) | 1 (<0.01) |
| Infections and infestations | 9 (0.05) | 16 (0.10) |
| Injury, poisoning, and procedural complications | 4 (0.02) | 6 (0.04) |
| Metabolism and nutrition disorders | 2 (0.01) | 0 |
| Neoplasms (benign, malignant, and unspecified) | 35 (0.21) | 31 (0.19) |
| Nervous system disorders | 11 (0.07) | 5 (0.03) |
| Psychiatric disorders | 1 (<0.01) | 1 (<0.01) |
| Renal and urinary disorders | 1 (<0.01) | 1 (<0.01) |
| Respiratory, thoracic, and mediastinal disorders | 6 (0.04) | 4 (0.02) |
| Social circumstances | 0 | 1 (<0.01) |
| Vascular disorders | 3 (0.02) | 6 (0.04) |
| Arteriosclerosis a | 1 (<0.01) | 4 (0.02) |
| Other eSAEs (PT) b | 2 (0.01) | 0 |
| Atrial flutter | 1 (<0.01) | 0 |
| Gastroenteritis | 1 (<0.01) | 0 |
Deaths attributed to coronary artery disease and arteriosclerosis have been included as MedDRA PTs.
Other eSAEs were reported by the HCP or the participants, but not considered related to the study vaccination by the investigators or the sponsor. All other SAEs (except those included in the primary endpoint) were collected by THL from the Finnish health registers up to 6 months after vaccination.
Abbreviations: AESI, adverse event of special interest; eSAE, serious adverse event entered in electronic Case Report Form; MedDRA, Medical Dictionary for Regulatory Activities; n, number of participants reporting an adverse event; PT, preferred term; QIV‐HD, high‐dose quadrivalent vaccine; QIV‐SD, standard‐dose quadrivalent.
AESIs were reported in four participants in the QIV‐HD group (Bell's palsy, n = 3; GBS, n = 1) and six in the QIV‐SD group (Bell's palsy, n = 6) (Table 2). All AESIs were considered medically significant. Excluding the two SARs reported above, all AESIs were considered not related to the study intervention, and all were resolved by the end of the study. There were no AESIs that led to study termination.
Fatal SAEs were reported in 102 (0.62%) participants in the QIV‐HD group and 92 (0.56%) participants in the QIV‐SD group (Table 2). Cardiac disorders were reported for 23 (0.1%) participants in the QIV‐HD group and 15 (0.1%) participants in the QIV‐SD group; these include deaths related to coronary artery disease (CAD) in 13 participants in the QIV‐HD group and two participants in the QIV‐SD group. Deaths related to the vascular disorder arteriosclerosis were reported in one participant in the QIV‐HD group and four participants in the QIV‐SD group.
Two other SAEs, not classified as SARs, AESIs, or fatal SAEs, were reported by HCPs as potentially related to the vaccination but determined to be unrelated by the investigator: atrial flutter in one participant and gastroenteritis in one participant. There were no AEs that led to study withdrawal.
4. Discussion
FinFluHD was the first pragmatic registry‐based RCT to assess prevention of respiratory and cardiovascular hospitalizations following influenza vaccination. However, this study was terminated after one influenza season, as the COVID‐19 pandemic impacted study design and influenza epidemiology. Thus, the number of participants vaccinated was less than one‐third of the targeted sample size, with a consequentially low number of primary outcome cases.
Despite the COVID‐19‐related disruption, QIV‐HD was associated with lower respiratory and cardiovascular hospitalization rates than QIV‐SD. Point estimates for rVE consistently supported the benefit of QIV‐HD over QIV‐SD in >30,000 participants. However, findings were statistically inconclusive on the primary endpoint; the rVE (QIV‐HD vs. QIV‐SD) for prevention of respiratory and cardiovascular hospitalizations did not reach statistical significance. This could potentially be due to small sample sizes and limited number of hospitalizations. Adequately powered studies with a greater number of participants, conducted over multiple influenza seasons, are needed to determine statistically significant benefits of QIV‐HD compared with QIV‐SD on respiratory and cardiovascular hospitalizations. I this study, QIV‐HD showed markedly greater effectiveness than QIV‐SD in preventing MACE‐related hospitalizations, with rVE estimates >20%.
Following the first confirmed COVID‐19 case in Finland in late January 2020 [24], recorded cases rose in early March as testing capacity increased [24, 25]. Thus, alongside influenza, SARS‐CoV‐2 circulation may have been a competing cause of hospitalization outcomes during our study. Indeed, rVE estimates were slightly higher after excluding COVID‐19‐linked secondary discharge codes (Figure 3). Additional hospitalizations with undetected COVID‐19 may have occurred early in the pandemic, when there were limited diagnostic resources, although SARS‐CoV‐2 seroprevalence remained low [27]. In mid‐March 2020, Finland introduced extensive social restrictions, after which influenza circulation declined rapidly (Figure S6) [25, 28]. Influenza vaccination was still anticipated to impact influenza circulation, since circulating influenza A and B strains in 2019–2020 corresponded to those in the QIV, and vaccine effectiveness estimates from a Finnish cohort study were high in children (68%–71%) and moderate in the elderly (29%) [29].
No major safety signals were observed, in line with post‐marketing surveillance data of QIV [30]. The imbalance in cardiac deaths (CAD‐related deaths [a cardiac SOC] were higher with QIV‐HD, whereas arteriosclerosis‐related deaths [a vascular SOC]) were marginally higher with QIV‐SD) may be partially due to MedDRA SOC/PT coding of cardiovascular indications. Arteriosclerosis is etiologically related to CAD, despite different SOC coding. We speculate that QIV‐HD is not able to prevent events caused by advanced CAD. The imbalance may be further compounded by small sample sizes and baseline cardiovascular variability. Conversely, QIV‐HD seemed to prevent CAD‐related hospitalizations, and emergency room and primary care visits, especially those linked to MACE.
This study has a number of limitations. Study endpoints corresponded only to the 2019–2020 influenza season, resulting in a limited sample size and insufficient statistical power; therefore, results should be interpreted with caution. The COVID‐19 pandemic likely contributed to respiratory and cardiovascular hospitalizations and diluted the number of influenza‐related cases. Moreover, effectiveness endpoints were inpatient hospitalization events based on routine reporting, with no adjudication. Generally, pragmatic trials have various strengths, including feasibility of a large sample size and evaluation of effectiveness in a real‐world setting, with randomization providing a high degree of internal validity [31]. The feasibility of using a pragmatic trial design for assessing influenza vaccination is supported by the DANFLU‐1 trial (NCT05048589), which was conducted during the 2021–2022 influenza season in Denmark [24, 32]. Furthermore, history of laboratory‐confirmed influenza illness prior to the study was not collected. However, reporting of this data is not systematic, so these data were not informative enough for consideration in this study.
In conclusion, despite insufficient statistical power owing to the impact of COVID‐19, these results suggest a clinical benefit of QIV‐HD over QIV‐SD for prevention of respiratory and cardiovascular hospitalizations, comparable safety profiles, and are aligned with trivalent HD influenza vaccine studies [12, 25, 28, 33, 34, 35]. FinFluHD demonstrated the feasibility of a pre‐licensure pragmatic randomized trial with follow‐up data from registries, and as such will help the design of more robust pragmatic trials for assessing influenza vaccination effectiveness.
Author Contributions
Arto A. Palmu: Conceptualization (equal); Formal analysis (equal); Writing – original draft (equal); Writing – review and editing (equal). Stephanie Pepin: Conceptualization (equal); Formal analysis (equal); Writing – original draft (equal); Writing – review and editing (equal). Ritva K. Syrjänen: Conceptualization (equal); Formal analysis (equal); Writing – original draft (equal); Writing – review and editing (equal). Karine Mari: Writing – original draft (equal); Writing – review and editing (equal). Tamala Mallett Moore: Formal analysis (equal); Writing – original draft (equal); Writing – review and editing (equal). Jukka Jokinen: Conceptualization (equal); Writing – original draft (equal); Writing – review and editing (equal). Heta Nieminen: Formal analysis (equal); Writing – original draft (equal); Writing – review and editing (equal). Terhi Kilpi: Conceptualization (equal); Writing – original draft (equal); Writing – review and editing (equal). Sandrine I. Samson: Conceptualization (equal); Writing – original draft (equal); Writing – review and editing (equal). Iris De Bruijn: Conceptualization (equal); Writing – original draft (equal); Writing – review and editing (equal).
Disclosure
The authors thank all participants, investigators and staff at Helsinki, Tampere, Espoo, Vantaa, Turku, Oulu, Jyväskylä, Lahti, Kymsote, Hämeenlinna, Pori, Nokia, and Kangasala health care centers, and staff at the Finnish Institute for Health and Welfare (THL) involved in study conduct, especially Esa Ruokokoski for data management, Elina Isosaari for quality management, Päivi Siren and Maila Kyrölä for coordination of clinical activities. Authors also thank Anu Soininen and Maarit Inkeri (Sanofi) for their input on study preparation and conduct. Editorial assistance for manuscript preparation was provided by Sherwin Barretto, PhD., and Juliette Gray, PhD., of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Sanofi.
Ethics Statement
Study conduct was consistent with standards established by the Declaration of Helsinki, compliant with the International Conference on Harmonization guidelines for Good Clinical Practice, and with all national and European regulations and directives. The study and subsequent amendments were approved by an independent ethics committee and the Finnish Medicines Agency, Fimea.
Conflicts of Interest
SP, KM, TMM, SS, and IDB are employees of Sanofi and hold stock in the company. AAP, JJ, RKS, HN, and TK are investigators at the Finnish Institute for Health and Welfare (THL), which received research funding from Sanofi for the current study and from GlaxoSmithKline SA, and Pfizer, Inc. outside of the submitted work. AAP, RKS, HN are currently employed by FVR – Finnish Vaccine Research, which conducts vaccine research funded by a number of vaccine manufacturers, including Sanofi, GlaxoSmithKline SA, Pfizer, Inc., MSD, Moderna, and Janssen.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/irv.13270.
Supporting information
Figure S1. Kaplan–Meier Curve for Respiratory or Hospitalizations, based on Primary Discharge Codes.
Figure S2. rVE for Respiratory or Cardiovascular Hospital Emergency Room Visits.
Figure S3. rVE for Respiratory or Cardiovascular Acute Primary Care Visits to Physician.
Figure 4. rVE for Respiratory or Cardiovascular Hospitalization, by Previous Vaccination Status.
Figure S5. rVE for Respiratory or Cardiovascular Hospitalization, by Comorbidity.
Figure S6. Influenza A and B, and SARS‐CoV‐2 infections in all age groups, and influenza and COVID‐19 hospitalizations in adults aged ≥65 years in Finland, August 2019–August 20201,2.
Funding: This work was funded by Sanofi.
Data Availability Statement
Qualified researchers may request access to patient‐level data and related documents, including, for example, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
References
- 1. Talbot H. K., “Influenza in Older Adults,” Infectious Disease Clinics of North America 31, no. 4 (2017): 757–766, 10.1016/j.idc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 2. Warren‐Gash C., Bhaskaran K., Hayward A., et al., “Circulating Influenza Virus, Climatic Factors, and Acute Myocardial Infarction: A Time Series Study in England and Wales and Hong Kong,” The Journal of Infectious Diseases 203, no. 12 (2011): 1710–1718, 10.1093/infdis/jir171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwong J. C., Schwartz K. L., Campitelli M. A., et al., “Acute Myocardial Infarction After Laboratory‐Confirmed Influenza Infection,” The New England Journal of Medicine 378, no. 4 (2018): 345–353, 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 4. Smeeth L., Thomas S. L., Hall A. J., Hubbard R., Farrington P., and Vallance P., “Risk of Myocardial Infarction and Stroke After Acute Infection or Vaccination,” The New England Journal of Medicine 351, no. 25 (2004): 2611–2618, 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 5. Blackburn R., Zhao H., Pebody R., Hayward A., and Warren‐Gash C., “Laboratory‐Confirmed Respiratory Infections as Predictors of Hospital Admission for Myocardial Infarction and Stroke: Time‐Series Analysis of English Data for 2004–2015,” Clinical Infectious Diseases 67, no. 1 (2018): 8–17, 10.1093/cid/cix1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warren‐Gash C., Smeeth L., and Hayward A. C., “Influenza as a Trigger for Acute Myocardial Infarction or Death From Cardiovascular Disease: A Systematic Review,” The Lancet Infectious Diseases 9, no. 10 (2009): 601–610, 10.1016/s1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 7. Boehme A. K., Luna J., Kulick E. R., Kamel H., and Elkind M. S. V., “Influenza‐Like Illness as a Trigger for Ischemic Stroke,” Annals of Clinical Translational Neurology 5, no. 4 (2018): 456–463, 10.1002/acn3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kytömaa S., Hegde S., Claggett B., et al., “Association of Influenza‐Like Illness Activity With Hospitalizations for Heart Failure: The Atherosclerosis Risk in Communities Study,” JAMA Cardiology 4, no. 4 (2019): 363–369, 10.1001/jamacardio.2019.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim Y. H., Hong K. J., Kim H., and Nam J. H., “Influenza Vaccines: Past, Present, and Future,” Reviews in Medical Virology 32, no. 1 (2022): e2243, 10.1002/rmv.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodwin K., Viboud C., and Simonsen L., “Antibody Response to Influenza Vaccination in the Elderly: A Quantitative Review,” Vaccine 24, no. 8 (2006): 1159–1169, 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 11. DiazGranados C. A., Dunning A. J., Kimmel M., et al., “Efficacy of High‐Dose Versus Standard‐Dose Influenza Vaccine in Older Adults,” The New England Journal of Medicine 371, no. 7 (2014): 635–645, 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 12. DiazGranados C. A., Robertson C. A., Talbot H. K., Landolfi V., Dunning A. J., and Greenberg D. P., “Prevention of Serious Events in Adults 65 Years of Age or Older: A Comparison Between High‐Dose and Standard‐Dose Inactivated Influenza Vaccines,” Vaccine 33, no. 38 (2015): 4988–4993, 10.1016/j.vaccine.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 13. Falsey A. R., Treanor J. J., Tornieporth N., Capellan J., and Gorse G. J., “Randomized, Double‐Blind Controlled Phase 3 Trial Comparing the Immunogenicity of High‐Dose and Standard‐Dose Influenza Vaccine in Adults 65 Years of Age and Older,” The Journal of Infectious Diseases 200, no. 2 (2009): 172–180, 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 14. Young‐Xu Y., Snider J. T., van Aalst R., et al., “Analysis of Relative Effectiveness of High‐Dose Versus Standard‐Dose Influenza Vaccines Using an Instrumental Variable Method,” Vaccine 37, no. 11 (2019): 1484–1490, 10.1016/j.vaccine.2019.01.063. [DOI] [PubMed] [Google Scholar]
- 15. Lee J. K. H., Lam G. K. L., Shin T., et al., “Efficacy and Effectiveness of High‐Dose Versus Standard‐Dose Influenza Vaccination for Older Adults: A Systematic Review and Meta‐Analysis,” Expert Review of Vaccines 17, no. 5 (2018): 435–443, 10.1080/14760584.2018.1471989. [DOI] [PubMed] [Google Scholar]
- 16. Lee J. K. H., Lam G. K. L., Shin T., Samson S. I., Greenberg D. P., and Chit A., “Efficacy and Effectiveness of High‐Dose Influenza Vaccine in Older Adults by Circulating Strain and Antigenic Match: An Updated Systematic Review and Meta‐Analysis,” Vaccine 39, no. Suppl 1 (2021): A24–a35, 10.1016/j.vaccine.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 17. Sanofi Pasteur . Summary of Product Characteristics. Vaxigrip Tetra® 2019. Available at: https://www.medicines.org.uk/emc/product/666/smpc [Last accessed: Apr 2023].
- 18. Hollingsworth R., Palmu A., Pepin S., et al., “Effectiveness of the Quadrivalent High‐Dose Influenza Vaccine for Prevention of Cardiovascular and Respiratory Events in People Aged 65 Years and Above: Rationale and Design of a Real‐World Pragmatic Randomized Clinical Trial,” American Heart Journal 237 (2021): 54–61, 10.1016/j.ahj.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 19. Jackson L. A., Jackson M. L., Nelson J. C., Neuzil K. M., and Weiss N. S., “Evidence of Bias in Estimates of Influenza Vaccine Effectiveness in Seniors,” International Journal of Epidemiology 35, no. 2 (2006): 337–344, 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 20. Remschmidt C., Wichmann O., and Harder T., “Frequency and Impact of Confounding by Indication and Healthy Vaccinee Bias in Observational Studies Assessing Influenza Vaccine Effectiveness: A Systematic Review,” BMC Infectious Diseases 15 (2015): 429, 10.1186/s12879-015-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clar C., Oseni Z., Flowers N., Keshtkar‐Jahromi M., and Rees K., “Influenza Vaccines for Preventing Cardiovascular Disease,” Cochrane Database of Systematic Reviews 2015, no. 5 (2015): Cd005050, 10.1002/14651858.CD005050.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alvarez F. P., Chevalier P., Borms M., et al., “Cost‐Effectiveness of Influenza Vaccination With a High Dose Quadrivalent Vaccine of the Elderly Population in Belgium, Finland, and Portugal,” Journal of Medical Economics 26, no. 1 (2023): 710–719, 10.1080/13696998.2023.2194193. [DOI] [PubMed] [Google Scholar]
- 23. THL . Rokotuskattavuus Atlas. Accessed 26 May, 2022. https://www.thl.fi/roko/vaccreg/atlas/public/atlas.html?show=influenza.
- 24. Johansen N. D., Modin D., Nealon J., et al., “A Pragmatic Randomized Feasibility Trial of Influenza Vaccines,” NEJM Evidence 2, no. 2 (2023): EVIDoa2200206, 10.1056/EVIDoa2200206. [DOI] [PubMed] [Google Scholar]
- 25. Gravenstein S., Davidson H. E., Taljaard M., et al., “Comparative Effectiveness of High‐Dose Versus Standard‐Dose Influenza Vaccination on Numbers of US Nursing Home Residents Admitted to Hospital: A Cluster‐Randomised Trial,” The Lancet Respiratory Medicine 5, no. 9 (2017): 738–746, 10.1016/s2213-2600(17)30235-7. [DOI] [PubMed] [Google Scholar]
- 26. Gravenstein S., Davidson H. E., Mcconeghy K., et al., “996. A Cluster‐Randomized Trial of Adjuvanted Trivalent Influenza Vaccine vs. Standard Dose in US Nursing Homes,” Open Forum Infectious Diseases 5, no. suppl_1 (2018): S296–S296, 10.1093/ofid/ofy210.833. [DOI] [PubMed] [Google Scholar]
- 27. Solastie A., Nieminen T., Ekström N., et al., “Changes in SARS‐CoV‐2 Seroprevalence and Population Immunity in Finland, 2020‐2022,” Emerging Microbes & Infections 12, no. 2 (2023): 2222849, 10.1080/22221751.2023.2222849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shay D. K., Chillarige Y., Kelman J., et al., “Comparative Effectiveness of High‐Dose Versus Standard‐Dose Influenza Vaccines Among US Medicare Beneficiaries in Preventing Postinfluenza Deaths During 2012–2013 and 2013–2014,” The Journal of Infectious Diseases 215, no. 4 (2017): 510–517, 10.1093/infdis/jiw641. [DOI] [PubMed] [Google Scholar]
- 29. Stuurman A. L., Biccler J., Carmona A., et al., “Brand‐Specific Influenza Vaccine Effectiveness Estimates During 2019/20 Season in Europe: Results From the DRIVE EU Study Platform,” Vaccine 39, no. 29 (2021): 3964–3973, 10.1016/j.vaccine.2021.05.059. [DOI] [PubMed] [Google Scholar]
- 30. Gandhi‐Banga S., Wague S., Shrestha A., et al., “Enhanced Passive Safety Surveillance of High‐Dose and Standard‐Dose Quadrivalent Inactivated Split‐Virion Influenza Vaccines in Germany and Finland During the Influenza Season 2021/22,” Influenza and Other Respiratory Viruses 17, no. 1 (2023): e13071, 10.1111/irv.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. James S., Rao S. V., and Granger C. B., “Registry‐Based Randomized Clinical Trials: A New Clinical Trial Paradigm,” Nature Reviews. Cardiology 12, no. 5 (2015): 312–316, 10.1038/nrcardio.2015.33. [DOI] [PubMed] [Google Scholar]
- 32. Johansen N. D., Modin D., Nealon J., et al., “Feasibility of Randomizing Danish Citizens Aged 65–79 Years to High‐Dose Quadrivalent Influenza Vaccine vs. Standard‐Dose Quadrivalent Influenza Vaccine in a Pragmatic Registry‐Based Setting: Rationale and Design of the DANFLU‐1 Trial,” Pilot and Feasibility Studies 8, no. 1 (2022): –87, 10.1186/s40814-022-01044-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gravenstein S., Davidson H. E., Han L. F., et al., “Feasibility of a Cluster‐Randomized Influenza Vaccination Trial in U.S. Nursing Homes: Lessons Learned,” Human Vaccines & Immunotherapeutics 14, no. 3 (2018): 736–743, 10.1080/21645515.2017.1398872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richardson D. M., Medvedeva E. L., Roberts C. B., and Linkin D. R., “Comparative Effectiveness of High‐Dose Versus Standard‐Dose Influenza Vaccination in Community‐Dwelling Veterans,” Clinical Infectious Diseases 61, no. 2 (2015): 171–176, 10.1093/cid/civ261. [DOI] [PubMed] [Google Scholar]
- 35. Robison S. G. and Thomas A. R., “Assessing the Effectiveness of High‐Dose Influenza Vaccine in Preventing Hospitalization Among Seniors, and Observations on the Limitations of Effectiveness Study Design,” Vaccine 36, no. 45 (2018): 6683–6687, 10.1016/j.vaccine.2018.09.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier Curve for Respiratory or Hospitalizations, based on Primary Discharge Codes.
Figure S2. rVE for Respiratory or Cardiovascular Hospital Emergency Room Visits.
Figure S3. rVE for Respiratory or Cardiovascular Acute Primary Care Visits to Physician.
Figure 4. rVE for Respiratory or Cardiovascular Hospitalization, by Previous Vaccination Status.
Figure S5. rVE for Respiratory or Cardiovascular Hospitalization, by Comorbidity.
Figure S6. Influenza A and B, and SARS‐CoV‐2 infections in all age groups, and influenza and COVID‐19 hospitalizations in adults aged ≥65 years in Finland, August 2019–August 20201,2.
Data Availability Statement
Qualified researchers may request access to patient‐level data and related documents, including, for example, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.


