Summary
Background
Ultra-processed foods (UPFs) are emerging as a risk factor for colorectal cancer (CRC), yet how post-diagnostic UPF intake may impact CRC prognosis remains unexplored.
Methods
Data collected from food frequency questionnaires were used to estimate intakes of total UPFs and UPF subgroups (serving/d) at least 6 months but less than 4 years post-diagnosis among 2498 patients diagnosed with stages I–III CRC within the Nurses’ Health Study and Health Professionals Follow-up Study during 1980–2016. Hazard ratios (HR) and 95% confidence intervals (CIs) of all-cause, CRC- and cardiovascular disease (CVD)-specific mortality in association with UPF consumption were estimated using an inverse probability weighted multivariable Cox proportional hazards regression model, adjusted for confounders.
Findings
The mean (SD) age of patients at diagnosis was 68.5 (9.4) years. A total of 1661 deaths were documented, including 321 from CRC and 335 from CVD. Compared to those in the lowest quintile (median = 3.6 servings/d), patients in the highest quintile (median = 10 servings/d) of post-diagnostic UPF intake had higher CVD mortality (HR = 1.65, 95% CI = 1.13–2.40) but not CRC or all-cause mortality. Among UPF subgroups, higher consumption of fats/condiments/sauces was associated with a higher risk of CVD-specific mortality (highest vs. lowest quintile of intake, HR = 1.96, 95% CI = 1.41–2.73), and higher intake of ice cream/sherbet was associated with an increased risk of CRC-specific mortality (highest vs. lowest quintile, HR = 1.86, 95% CI: 1.33–2.61). No statistically significant association was found between UPF subgroups and overall mortality.
Interpretation
Higher post-diagnostic intake of total UPFs and fats/condiments/sauces in CRC survivors is associated with higher CVD mortality, and higher ice cream/sherbet intake is linked to higher CRC mortality.
Funding
US National Institutes of Health and the American Cancer Society.
Keywords: Post-diagnostic dietary intake, Colorectal cancer survivorship, Patient care, Longitudinal analysis
Research in context.
Evidence before this study
We searched PubMed, Embase, and Web of Science on November 14th, 2023, using terms including “ultra-processed food” “colorectal cancer”, “survival”, “prognosis”, and “mortality”. We found only one cohort study reporting a positive association of pre-diagnostic ultra-processed food (UPF) intake with colorectal cancer (CRC)-specific mortality among stage I-II CRC patients. However, dietary assessment was performed only at a single time point before cancer diagnosis, and lifestyle factors after diagnosis were not controlled. We did not identify previous publications reporting the associations between post-diagnostic UPF intake and CRC prognosis.
Added value of this study
Using data from 2498 stages I-III CRC patients within the Nurses’ Health Study and Health Professionals Follow-up Study, we found that compared to patients in the lowest quintile of post-diagnostic UPF intake (median = 3.6 servings/d), those in the highest quintile (median = 10 servings/d) had a 65% increased cardiovascular disease (CVD) mortality risk. Furthermore, higher consumption of fats/condiments/sauces correlated with an increased CVD mortality risk, while increased ice cream/sherbet consumption with a higher CRC-specific mortality risk.
Implications of all the available evidence
Study findings highlight the need for probing UPF-CRC survival mechanisms and crafting effective strategies to reduce UPF intake, thereby lowering CVD- and CRC-specific mortality among CRC patients.
Introduction
Colorectal cancer (CRC) remains a leading cause of cancer deaths in the US, with an estimated 53,000 deaths in 2023.1 The number of CRC survivors has surpassed 1.5 million and continues to rise, owing to improved early detection and treatment.2 CRC prognosis appears to be worse among patients with low physical activity, smoking, and underweight.3 Moreover, emerging evidence suggests that diet is a modifiable factor that can prevent recurrence, comorbidities, and premature deaths in CRC.4 Cancer survivors are often motivated to make dietary changes to improve survival and overall health.5 However, limited evidence on post-diagnostic diet and CRC survival has led to dietary recommendations primarily based on studies of CRC incidence.6 Therefore, comprehending how dietary factors may impact cancer survival is urgently needed to guide dietary recommendations for long-term CRC survivorship.
Ultra-processed foods (UPFs) contribute to nearly 60% of Americans’ daily calories, with an upward trend over the past two decades.7 A recent prospective cohort study reported that a high UPF intake was associated with a 28% higher risk of developing CRC among 36,341 men.8 Pre-diagnostic intake of UPF was also suggested to increase the risk of CRC-specific mortality among stage I-II CRC patients.9 Meanwhile, several cohort studies conducted in the US and Europe reported positive associations between UPF consumption and all-cause mortality.10, 11, 12, 13, 14, 15, 16 Studies in the UK Biobank and Moli-sani have also linked UPF intake to increased cardiovascular disease (CVD) incidence and mortality.12,16 Major UPF categories, including refined carbohydrates,17 sugar-sweetened beverages,18 and processed meats,19 have been associated with worse CRC survival, possibly due to their high glycemic load and inflammatory potential, along with related metabolites that can promote tumor proliferation and metastasis (e.g., trimethylamine-N-oxide (TMAO), hydrogen sulfide).20 Cumulative intake of carcinogens (e.g., N-nitroso compounds, heterocyclic amines) from food processing may cause genomic mutations associated with CRC and enhance tumor metastasis.21
Collectively, the evidence suggests that UPFs may negatively affect CRC survival, yet there is a lack of well-designed longitudinal studies to evaluate this. To address this gap, we analyzed data from two large prospective cohorts – the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) – to investigate whether high consumption of total UPFs and major UPF subgroups after CRC diagnosis is associated with increased mortality due to CRC, CVDs, and all causes.
Methods
Study participants
This analysis included participants from two large US prospective cohorts. As previously described,22,23 the Nurses’ Health Study (NHS) enrolled 121 700 female registered nurses aged 35–55 years in 1976 and the Health Professionals Follow-up Study (HPFS) enrolled 51 529 male healthcare professionals aged 40–75 years in 1986. All participants completed a questionnaire at baseline and were mailed follow-up questionnaires biennially to update their lifestyle, disease status, and clinical information. Diet was assessed using a validated food frequency questionnaire (FFQ) every four years. The average follow-up rates were greater than 90% in both cohorts. Based on the availability of dietary data, we considered 1980 and 1986 as the baseline for NHS and HPFS, respectively.
Assessment of CRC cases
During each biennial follow-up, participants reported any CRC diagnosis in the previous 2 years and provided consent for medical record acquisition. Study physicians, blinded to exposure data, reviewed medical records to confirm CRC diagnosis and record the disease stage, histologic findings, and tumor location. Between baseline and December 2016, we documented 3379 CRC cases among NHS participants and 1518 among HPFS participants (Supplementary Fig. S1). We excluded individuals who were diagnosed at the time of death, had post-diagnostic diet assessed within 6 months or more than four years after diagnosis, or had missing pre- or post-diagnosis dietary data. Patients with stage IV CRC were also excluded mainly because very few of them returned the post-diagnosis questionnaire due to their relatively short survival time. Finally, we included 2498 cases with stage I to III CRC in this analysis, comprising 1764 cases from NHS and 734 cases from HPFS.
Dietary assessments
In NHS and HPFS, participants reported foods and drinks that they had typically consumed over the past year using FFQs with ∼130 food items. The FFQs captured the frequency of food consumption in nine categories, ranging from “never/less than 1 per month” to “more than 6 per day” with standard portion sizes. Based on the reported frequency and portion size, we estimated daily UPF consumption using the Nova classification system.24
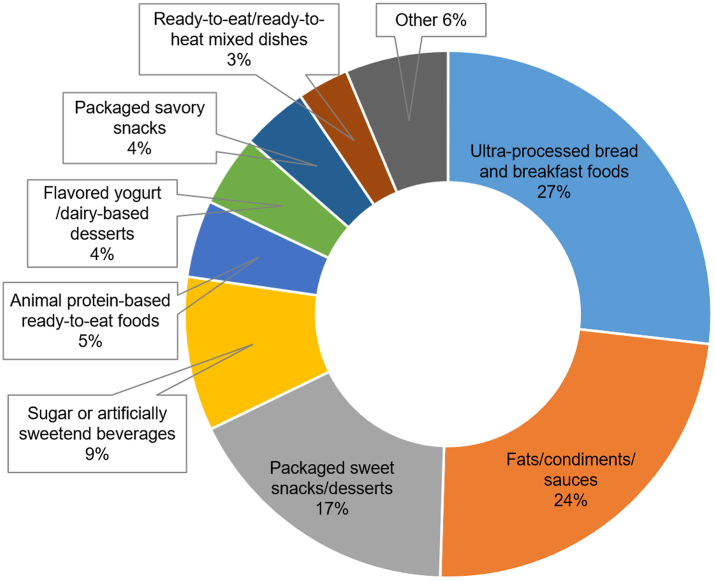
The Nova classifies foods into four groups based on the extent of industrial processing: unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and ultra-processed foods.24 The methods of categorizing UPFs using the FFQ data from NHS and HPFS have been described previously.25 UPFs were further classified into mutually exclusive subgroups (Fig. 1), including ultra-processed bread and breakfast foods, fats/condiments/sauces, packaged sweet snacks/desserts, sugar or artificially sweetened beverages, animal protein-based ready-to-eat foods, flavored yogurt/dairy-based desserts, packaged savory snacks, and ready-to-eat/ready-to-heat mixed dishes, to evaluate whether the association between UPF intake and mortality was driven by a specific UPF subgroup.26 The false discovery rate (FDR) method with a 0.05 threshold was applied to account for multiple comparisons.
Fig. 1.
The proportion of each ultra-processed food subgroup in total ultra-processed food consumption after a colorectal cancer diagnosis.
We estimated the pre-diagnostic UPF intake using the last FFQ reported before CRC diagnosis, and post-diagnostic intake using the first FFQ collected at least 6 months to avoid the active treatment period. The median time between the assessment of pre-diagnostic diet and CRC diagnosis was 2.2 years (interquartile range (IQR): 1.2–3.2 years), and the median time between CRC diagnosis and the assessment of post-diagnostic diet was 2.4 years (IQR: 1.4–3.4 years). Changes in total UPF intake were computed using post-diagnostic UPF intake minus pre-diagnostic UPF intake.
Assessment of deaths
Deaths were identified from state records, the National Death Index, next of kin, and the postal system in response to the follow-up questionnaires. The cause of death was identified from death certificates or medical records reviewed by study physicians. We modeled CRC- and CVD-specific mortality as the primary outcomes and all-cause mortality as the secondary outcome.
Statistics
Person-time of follow-up for each participant was calculated from the age in months at the return date of the post-diagnostic FFQ until the age at the death date, loss to follow-up, or end of follow-up (December 31, 2019), whichever came first. We used a Cox proportional hazards model with time since diagnosis as the time scale to estimate the hazard ratio (HR) and 95% confidence interval (CI) for mortality associated with UPF intake, accounting for left truncation due to the variation between patients in the timing of dietary assessment after CRC diagnosis.27 We applied inverse probability weighting (IPW) in models to reduce potential selection bias arising from the exclusion of patients who had missing dietary data, as previously described.28 The proportional hazards assumption was tested via a likelihood ratio test comparing the models with and without the product terms of UPF intake and follow-up time.
Because the carcinogenic effect of UPFs may go beyond the calories they present, we quantified UPF intake as servings per day (servings/d) and adjusted for total energy using the residual method. We assessed the association between UPF intake in quintiles and CRC survival. Trend tests were conducted using the median of each quintile of UPF intake as a continuous variable. The multivariable model adjusted for potential confounders, including age at diagnosis, sex (women and men), cancer stage (I, II, III, and unspecified), calendar year of diagnosis, tumor grade of differentiation (well differentiated, moderately differentiated, poorly differentiated, and unspecified), CRC subsite (proximal colon, distal colon, rectum, and unspecified), family history of CRC, pre-diagnostic UPF intake, and post-diagnostic covariates including alcohol intake (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), current smoking (yes or no), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), body mass index (BMI, <23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 metabolic equivalent of task (MET)-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), and regular use of aspirin and other nonsteroidal anti-inflammatory drugs (≥2 tablets per week). In a sensitivity analysis, we performed a competing risk survival analysis by estimating the sub-distribution HR for cause-specific mortality, which quantified the risk of each mortality endpoint while taking into account the competing risk of mortality from other causes.29
Considering that dietary fiber from whole grains can be beneficial for CRC survival,30 in a sensitivity analysis, we removed whole-grain bread and breakfast cereals from total UPFs. To examine potential confounding by diet quality and other dietary factors, we additionally adjusted for diet quality measured by the Alternative Healthy Eating Index (AHEI) and dietary intake of total fiber, total sugars, marine omega-3 fatty acid, folate, calcium, and vitamin D in two sensitivity analyses, respectively.
We conducted stratified analyses according to tumor subsites (proximal colon, distal colon, and rectum) and cancer stage (stages I, II, and III) to evaluate whether the associations may differ across cancer sites or stages. We also performed stratified analyses according to sex and the median year of CRC diagnosis (the year 1999). P for heterogeneity was evaluated using the likelihood ratio test. Additionally, we evaluated the association of pre-diagnostic UPF intake and the change in UPF intake with mortality outcomes in secondary analyses. All analyses were performed in SAS (version 9.4). Statistical significance was considered at the α = 0.05 level.
Ethics
The study protocol was approved by the institutional review boards (IRBs) of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required (IRB Protocol 10372). The IRBs allowed participants’ completion of questionnaires to be considered as implied consent.
Role of the funding source
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Results
Characteristics of CRC patients
Among 2498 patients with stage I to III CRC, the mean (SD) age at diagnosis was 68.5 (9.4) years. The median (IQR) intake of total UPFs after CRC diagnosis was 6.0 (4.6–7.8) servings/d. The top 3 major UPF subgroups include ultra-processed bread and breakfast foods (27%), fats/condiments/sauces (24%), and packaged sweet snacks/desserts (17%) (Fig. 1). Pre- and post-diagnostic intakes of total UPFs were modestly correlated (Spearman correlation coefficient r = 0.60) (Supplementary Table S1). In a subset of patients who provided chemotherapy data, we found that the post-diagnostic UPF consumption was similar between those who received chemotherapy (n = 270) and those who did not (n = 359) (median [IQR]: 5.9 [4.5–7.9] vs. 5.8 [4.4–7.8] servings/d).
Patients with higher post-diagnostic UPF consumption tended to have a higher BMI, more pack-years of smoking, less alcohol consumption, poorer diet quality, and lower intake of dietary fiber, calcium, vitamin D, and marine omega-3 fatty acids (Table 1).
Table 1.
Age-standardized characteristics of colorectal cancer patients at diagnosis according to quintiles of ultra-processed food intake (n = 2498).a
| Quintile 1 |
Quintile 2 |
Quintile 3 |
Quintile 4 |
Quintile 5 |
|
|---|---|---|---|---|---|
| (n = 499) | (n = 501) | (n = 495) | (n = 503) | (n = 500) | |
| Male, % | 30 | 29 | 29 | 29 | 30 |
| Age, years | 68.1 (9.3) | 68.4 (10.1) | 68.4 (9.7) | 69.1 (8.9) | 69.7 (8.9) |
| Body mass index, kg/m2 | 25.4 (4.9) | 25.7 (4.3) | 26.2 (4.4) | 26.6 (5.2) | 27.4 (4.9) |
| Physical activity, MET-hours/week | 20.6 (27.1) | 18.6 (22.7) | 18.2 (23.3) | 15.9 (21.1) | 17.1 (23.6) |
| Pack-years of smoking | 13.8 (20.1) | 14.6 (21.1) | 18.1 (24.2) | 16.2 (22.1) | 21.1 (24.3) |
| Current smokers, % | 7 | 6 | 7 | 6 | 5 |
| Regular use of aspirin, % | 46 | 49 | 53 | 58 | 53 |
| Alternative Healthy Eating Index | 57.7 (11.2) | 54.8 (11.3) | 52.6 (11.3) | 52.1 (10.5) | 49.9 (11.2) |
| Food and nutrient intake | |||||
| Total fiber, g/day | 22.9 (7.5) | 20.9 (5.8) | 20.5 (6.1) | 20.6 (6.1) | 19.7 (6.8) |
| Total folate, μg/day | 685 (345) | 662 (295) | 639 (313) | 676 (344) | 652 (356) |
| Total sugar, g/day | 102 (31) | 103 (27) | 104 (30) | 103 (31) | 101 (32) |
| Calcium, mg/day | 1227 (603) | 1235 (562) | 1155 (563) | 1200 (572) | 1145 (543) |
| Vitamin D, IU/day | 608 (490) | 569 (423) | 551 (476) | 580 (473) | 522 (443) |
| Marine ω-3 PUFAs, mg/day | 365 (369) | 310 (313) | 272 (287) | 289 (318) | 221 (224) |
| Alcohol, g/day | 10.0 (14.7) | 8.7 (13.5) | 6.6 (10.0) | 6.8 (11.6) | 4.7 (9.6) |
| Ultra-processed foodsb | 3.4 (0.8) | 4.9 (0.5) | 6.0 (0.5) | 7.4 (0.6) | 10.3 (2) |
| Bread and breakfast foodsb | 1.1 (0.6) | 1.4 (0.7) | 1.7 (0.8) | 2.1 (1.1) | 2.4 (1.3) |
| Fats/condiments/saucesb | 0.8 (0.6) | 1.1 (0.6) | 1.4 (0.8) | 1.8 (1) | 2.6 (1.6) |
| Packaged sweet snacks/dessertsb | 0.6 (0.5) | 0.9 (0.6) | 1.1 (0.8) | 1.3 (1) | 1.6 (1.4) |
| Sugar or artificially sweetened beveragesb | 0.2 (0.4) | 0.4 (0.5) | 0.6 (0.6) | 0.7 (0.9) | 1.3 (1.6) |
| Animal protein-based ready-to-eat foodsb | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) | 0.4 (0.5) |
| Flavored yogurt/dairy-based dessertsb | 0.3 (0.3) | 0.3 (0.3) | 0.4 (0.3) | 0.3 (0.5) | 0.4 (0.4) |
| Packaged savory snacksb | 0.2 (0.2) | 0.2 (0.2) | 0.3 (0.4) | 0.4 (0.6) | 0.5 (0.9) |
| Ready-to-eat/ready-to-heat mixed dishesb | 0.1 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.1 (0.1) | 0.2 (0.2) |
| Other ultra-processed foodsb | 0 (0.1) | 0.1 (0.3) | 0.2 (0.5) | 0.4 (0.9) | 0.9 (1.4) |
| Cancer subsite, % | |||||
| Proximal colon | 43 | 50 | 43 | 43 | 45 |
| Distal colon | 28 | 26 | 35 | 32 | 27 |
| Rectum | 24 | 19 | 18 | 21 | 23 |
| Unspecified | 5 | 4 | 4 | 4 | 4 |
| Cancer differentiation, % | |||||
| Well differentiated | 15 | 12 | 15 | 14 | 14 |
| Moderately differentiated | 61 | 59 | 56 | 61 | 58 |
| Poorly differentiated | 10 | 16 | 13 | 11 | 14 |
| Unspecified | 14 | 13 | 16 | 14 | 14 |
| Cancer stage, % | |||||
| I | 33 | 31 | 35 | 34 | 30 |
| II | 32 | 32 | 29 | 32 | 33 |
| III | 24 | 26 | 24 | 21 | 24 |
| Unspecified | 11 | 11 | 13 | 13 | 14 |
Quintiles are created in women and men separately. Means are calculated for continuous variables. All variables are age-standardized except age.
Energy-adjusted servings per day.
Post-diagnostic intake of total and UPF subgroups and mortality
During a median follow-up of 11.0 years, we documented 1661 deaths, including 321 due to CRC (19.3%), 335 due to CVD (20.2%), and 1005 primarily due to cancers other than CRC, dementia, respiratory disease, and various diseases of other systems. Post-diagnostic total UPF intake was associated with higher CVD-specific mortality in the age-, sex-, and stage-adjusted model (Q5 vs. Q1: HR = 1.80, 95% CI: 1.31–2.47), and the association remained significant after multivariable adjustment (HR = 1.65, 95% CI: 1.13–2.40) (Table 2). Competing risk survival analysis for cause-specific mortality produced essentially the same results (Supplementary Table S2). Not adjusting for BMI or further adjusting for diet quality and other dietary factors did not essentially change the estimates (Supplementary Table S3). The association between total UPF intake and CVD-specific mortality slightly weakened after removing whole grains from total UPFs (HR = 1.46, 95% CI: 1.03–2.07). Total UPF intake was not associated with CRC-specific or overall mortality.
Table 2.
Association of post-diagnostic consumption of ultra-processed foods with mortality among colorectal cancer patients (n = 2498).a
| Ultra-processed Food Intake, Post-diagnosis of Colorectal Cancer |
P for trend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Total UPF Intake, median (IQR), serving/d | 3.6 (2.9–3.9) | 4.9 (4.5–5.2) | 6.0 (5.6–6.3) | 7.4 (6.9–7.8) | 10.0 (9.0–11.2) | |
| CRC-specific mortality | ||||||
| No. of events (n = 321) | 60 | 72 | 70 | 60 | 59 | |
| Model 1, HR (95% CI)b | Reference | 1.07 (0.79, 1.44) | 1.18 (0.87, 1.59) | 1.14 (0.84, 1.55) | 0.96 (0.70, 1.31) | 0.75 |
| Model 2, HR (95% CI)c | Reference | 0.99 (0.73, 1.36) | 1.12 (0.82, 1.53) | 0.93 (0.66, 1.31) | 0.82 (0.57, 1.18) | 0.20 |
| CVD-specific mortality | ||||||
| No. of events (n = 335) | 47 | 69 | 52 | 79 | 88 | |
| Model 1, HR (95% CI)b | Reference | 1.44 (1.03, 2.00) | 1.07 (0.75, 1.53) | 1.57 (1.13, 2.19) | 1.80 (1.31, 2.47) | 0.0002 |
| Model 2, HR (95% CI)c | Reference | 1.47 (1.05, 2.06) | 1.01 (0.70, 1.46) | 1.60 (1.13, 2.27) | 1.65 (1.13, 2.40) | 0.01 |
| All-cause mortality | ||||||
| No. of events (n = 1661) | 310 | 329 | 308 | 358 | 356 | |
| Model 1, HR (95% CI)b | Reference | 0.98 (0.85, 1.12) | 0.91 (0.79, 1.05) | 1.03 (0.89, 1.18) | 1.08 (0.94, 1.24) | 0.14 |
| Model 2, HR (95% CI)c | Reference | 0.99 (0.86, 1.14) | 0.87 (0.75, 1.01) | 0.97 (0.84, 1.12) | 0.95 (0.81, 1.11) | 0.58 |
Post-diagnostic intake was assessed at least 6 months after diagnosis to minimize the influence of active treatment.
Model 1: Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated in Cox proportional hazards regression model after adjusting for age at diagnosis (continuous), sex, and cancer stage (I, II, III, and unspecified).
Model 2: Model 1+ further adjusted for pre-diagnostic consumption of ultra-processed foods (continuous), year of diagnosis (continuous), tumor grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum and unspecified), post-diagnostic alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), current smoking (yes or no), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), and regular use of aspirin and other nonsteroidal anti-inflammatory drugs (≥2 tablets per week).
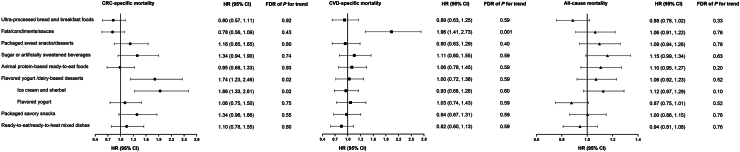
Among UPF subgroups, higher consumption of fats/condiments/sauces was associated with increased CVD-specific mortality (Q5 vs. Q1: multivariable HR = 1.96, 95% CI: 1.41–2.73; FDR = 0.001) (Fig. 2). Increased CRC-specific mortality was associated with a higher intake of flavored yogurt/dairy-based desserts, specifically ice cream/sherbet (HR = 1.86, 95% CI: 1.33–2.61; FDR = 0.02). No significant association was found between UPF subgroups and overall mortality.
Fig. 2.
Association of post-diagnostic consumption of ultra-processed food subgroups with mortality among colorectal cancer (CRC) patients. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated in the Cox proportional hazards regression model after adjusting for age at diagnosis (continuous), sex, and cancer stage (I, II, III, and unspecified), pre-diagnostic consumption of ultra-processed foods (continuous), year of diagnosis (continuous), tumor grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum and unspecified), post-diagnostic alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), current smoking (yes or no), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), body mass index (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 metabolic equivalent of task (MET)-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), and regular use of aspirin and other nonsteroidal anti-inflammatory drugs (≥2 tablets per week). The false discovery rate (FDR) method with a 0.05 threshold was applied to account for multiple comparisons. HRs and 95% CIs (highest vs. lowest quintile) were plotted with solid symbols representing point estimates of HRs and whiskers indicating 95% CIs. Abbreviation: CVD, cardiovascular disease.
No significant heterogeneity was detected for the association between total UPF intake and mortality across tumor subsites or stages (P for heterogeneity > 0.05) (Supplementary Tables S4 and S5). We also found that the associations were consistent according to sex (Supplementary Table S6) and year of CRC diagnosis (Supplementary Table S7) (P for heterogeneity > 0.05).
Change in total UPF intake after diagnosis and mortality
Patients who reduced their UPF intake after diagnosis with a median reduction of 2.7 servings/d from levels before diagnosis had lower CVD-specific mortality (Q1 vs. Q3: multivariable HR = 0.65, 95% CI: 0.45–0.92) (Supplementary Table S8). No statistically significant association was found between increased UPF intake and mortality. Pre-diagnostic intake of total UPFs was not associated with any mortality outcome (Supplementary Table S9).
Discussion
This study is among the first to investigate the association of post-diagnostic UPF intake with mortality in CRC survivors. Compared to lower intake (median: 3.6 servings/d), higher post-diagnostic UPF intake (median: 10 servings/d) was associated with a 65% higher risk for CVD mortality but not CRC-specific or all-cause mortality, adjusting for potential confounding. Among UPF subgroups, fats/condiments/sauces were linked to increased CVD-specific mortality and ice cream/sherbet were associated with higher CRC-specific mortality.
CVD is one major cause of death among CRC patients, possibly due to shared risk factors and treatment-related cardiotoxicity. An analysis of nearly 840 000 CRC patients diagnosed between 1975 and 2016 in the US found that CVD is the most common non-cancer cause, accounting for 20.3% of all deaths among CRC patients,31 aligning with our findings. Meta-analyzed data have shown that metabolic dysregulation (e.g., obesity, diabetes, hypertension, and hyperlipidemia) and lifestyle factors (e.g., diet, smoking, alcohol consumption, and physical activity) are shared risk factors for CVD and CRC.32 Prior studies showed that chemotherapy and other CRC treatments can induce cardiotoxicity, leading to higher CVD risks.33 Consequently, prevention of CVD should become an important target in follow-up care for CRC patients and is worth a collective effort in the cardio-oncology community.34
It remains unknown whether our observed association between UPF intake and CVD mortality is specific to CRC patients. Studies in the general population have reported contradicting results. In the Framingham Offspring Study with 3000 CVD-free subjects, each additional daily UPF serving raised CVD mortality risk by 9%35; and a prior study of 22 475 adults from the Moli-sani cohort found a 58% higher CVD mortality associated with high UPF consumption (over 15% in total food weight/d).16 Nevertheless, null findings were reported in the US NHANES III10 and a systematic review.36 The inconsistency may be partly explained by the differences in study populations, sample sizes, follow-up time, and UPF compositions. Therefore, further investigation is needed to reconcile the existing findings in the general population.
Prior studies suggest that high UPF intake may increase CRC risk,8,26 although a UK biobank study showed no association between UPF intake and CRC-specific mortality among cancer-free individuals.37 This aligns with our results, indicating that among CRC patients UPFs may act more on systemic pathways to influence CVD-specific mortality rather than tumor recurrence or metastasis. Future clinical trials are warranted to investigate whether reducing UPF intake could mitigate the burden of cardiometabolic diseases and preventing premature death caused by CVDs among CRC patients.
We found that prognostic associations varied among UPF subgroups, possibly due to heterogeneity in biological effects across these food categories. Prior studies on food additives indicate that artificial sweeteners and emulsifiers, often found in fats/condiments/sauces and ice cream, may increase CVD risk and promote CRC progression. A large cohort study found one unit increment (log10 scale in mg/d) of artificial sweetener intake was associated with a 9% higher CVD risk.38 A metabolomics analysis with a pilot intervention suggested erythritol, a commonly used sugar substitute, was strongly associated with CVD incidence, partly by enhancing thrombosis.39 Artificial sweeteners can potentially alter gut microbiota, increasing the risk of atherosclerosis, hypertension, heart failure, obesity, and type 2 diabetes.40 Functional evidence suggests that stabilizers and thickening agents in ice cream and sherbet, particularly xanthan gum and carrageenan, could promote gut dysbiosis and increase the expression of microbiota-derived pro-inflammatory molecules,41,42 which may affect CRC prognosis.
Growing evidence underscores the role of diet in cancer survivorship, yet defining an optimal post-cancer diet remains unresolved. Our study indicates the need to delve beyond nutrient profiles and examine the effect of food processing. Although some progress has been made, our understanding of UPFs is incomplete, necessitating further research to fully grasp their health impact and mechanisms. Clarifying the UPF-cancer relationship would guide effective interventions for at-risk and general populations. Future nutrition education and evidence-based innovations (e.g., Food-is-Medicine intervention), involving healthcare providers or community-based programs, can enhance cancer survivors’ nutritional understanding and provide tailored nutrition advice for patients and caregivers. UPF-targeted food labeling policies may also be prioritized as these are cost-effective strategies to reduce intake, improve food quality, and alleviate disease and economic burdens by reshaping consumer behaviors and stimulating industry reformulations.43,44
The study strengths include the prospective design, detailed pre- and post-diagnostic diet and lifestyle data collection, standardized medical record review for CRC and deaths, and long-term follow-up. Detailed covariates collected in parallel with UPF intake allowed for rigorous control for confounding.
Some limitations are worth noting. First, detailed treatment data are often unavailable. However, since standardized adjuvant treatment correlated with the disease stage, the potential confounding by treatment was minimized through adjustment for stage. Moreover, as participants were healthcare professionals, discrepancies in treatment access were likely minimal. Second, data on cancer recurrences were unavailable. Nevertheless, because the median survival for metastatic CRC was approximately 10–12 months, CRC-specific mortality should be a reasonable surrogate for cancer-specific outcomes. Third, UPF classification relied on FFQ data, which did not cover the full spectrum of UPFs or provide information on the levels of processing. However, given the prospective design, misclassification was likely non-differential and would have biased the association estimates toward the null. Fourth, potential survival bias should be acknowledged. To address this, we applied inverse probability weighting, a statistical technique that involves assigning different weights to participants based on their likelihood of being included in the study, thereby accounting for potential differential probabilities of inclusion in the study. Finally, as an observational study, residual confounding cannot be excluded and thus limits our ability for causal inference.
In conclusion, our findings indicate that higher consumption of total UPF after CRC diagnosis is associated with an increased risk of CVD-specific mortality. The prognostic associations varied greatly among UPF subgroups, possibly due to the heterogeneity in biological effects across these food categories.
Contributors
Study concept and design: DH, MD, MS, FFZ. Methodology: DH, MD, LW, KW, ZF, FFZ, MS. Acquisition, curation, analysis, validation, visualization, and interpretation of data: DH, MD. Acquisition and curation of data: NK, SRL, EMS, QS. Interpretation of data: DH, MD, ATC, FBH, JAM, DM, SO, QS, JBW, FFZ, MS. Drafting of the manuscript: MD, DH, MS. Critical revision of the manuscript for important intellectual content: All authors. Funding acquisition: MS, ATC, MD, DH. Administrative, technical, or material support, and study supervision: MS, FFZ. DH, MD, and MS accessed and verified the underlying data. MS and FFZ were responsible for the decision to submit the manuscript, had full access to all of the data in the study, and took responsibility for the integrity of the data. All authors read and approved the final version of the manuscript.
Data sharing statement
Because of participant confidentiality and privacy concerns, data are available upon reasonable written request. According to standard controlled access procedure, applications to use NHS/HPFS resources will be reviewed by our External Collaborators Committee for scientific aims, evaluation of the fit of the data for the proposed methodology, and verification that the proposed use meets the guidelines of the Ethics and Governance Framework and the consent that was provided by the participants. Investigators wishing to use NHS/HPFS data are asked to submit a brief description of the proposed project (go to https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/ for details).
Declaration of interests
NK serves as a consultant for the Pan American Health Organization and Resolve to Save Lives. JM serves as a consultant on the scientific advisory board for Merck. DM reports research funding from the National Institutes of Health, Gates Foundation, Kaiser Permanent Fund, National Association of Chain Drug Stores Foundation, and Rockefeller Foundation; consulting fees from Acasti Pharma; participating on scientific advisory boards of start-up companies focused on innovations for health including Beren Therapeutics Brightseed, Calibrate, Elysium Health, Filtricine, HumanCo, Instacart Health, January Inc., Season Health, Validation Institute, Perfect Day, and Tiny Organics; and chapter royalties from UpToDate. All of the above is outside the submitted work. No other relationships or activities could appear to have influenced the submitted work.
Acknowledgements
We thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. This work was supported by grant MRSG-17-220-01-NEC from the American Cancer Society (MS), grants UM1 CA186107, P01 CA87969, U01 CA176726, U01 CA167552, U01 CA261961, R01CA263776 (MS), R35 CA253185, and 1F99CA274714 (MD) from the National Institutes of Health, grant BK20230005 (DH) from the Outstanding Youth Fund of Jiangsu Natural Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102572.
Contributor Information
Fang Fang Zhang, Email: fang_fang.zhang@tufts.edu.
Mingyang Song, Email: mis911@mail.harvard.edu.
Appendix ASupplementary data
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . American Cancer Society; Atlanta: 2020. Colorectal cancer facts & figures 2020-2022. [Google Scholar]
- 3.van Zutphen M., Kampman E., Giovannucci E.L., van Duijnhoven F.J.B. Lifestyle after colorectal cancer diagnosis in relation to survival and recurrence: a review of the literature. Curr Colorectal Cancer Rep. 2017;13(5):370–401. doi: 10.1007/s11888-017-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song M., Chan A.T. The potential role of exercise and nutrition in harnessing the immune system to improve colorectal cancer survival. Gastroenterology. 2018;155(3):596–600. doi: 10.1053/j.gastro.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock C.L., Doyle C., Demark-Wahnefried W., et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 6.El-Shami K., Oeffinger K.C., Erb N.L., et al. American cancer society colorectal cancer survivorship care guidelines. CA Cancer J Clin. 2015;65(6):428–455. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul F., Parekh N., Martinez-Steele E., Monteiro C.A., Chang V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr. 2022;115(1):211–221. doi: 10.1093/ajcn/nqab305. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Du M., Wang K., et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. BMJ. 2022;378 doi: 10.1136/bmj-2021-068921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pu J.Y., Xu W., Zhu Q., et al. Prediagnosis ultra-processed food consumption and prognosis of patients with colorectal, lung, prostate, or breast cancer: a large prospective multicenter study. Front Nutr. 2023;10 doi: 10.3389/fnut.2023.1258242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H., Hu E.A., Rebholz C.M. Ultra-processed food intake and mortality in the USA: results from the third national health and nutrition examination survey (NHANES III, 1988-1994) Public Health Nutr. 2019;22(10):1777–1785. doi: 10.1017/S1368980018003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlich M.J., Sabaté J., Mashchak A., et al. Ultra-processed food intake and animal-based food intake and mortality in the Adventist Health Study-2. Am J Clin Nutr. 2022;115(6):1589–1601. doi: 10.1093/ajcn/nqac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Chu J., Hu W., et al. Associations of ultra-processed food consumption with cardiovascular disease and all-cause mortality: UK Biobank. Eur J Public Health. 2022;32(5):779–785. doi: 10.1093/eurpub/ckac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnabel L., Kesse-Guyot E., Allès B., et al. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med. 2019;179(4):490–498. doi: 10.1001/jamainternmed.2018.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rico-Campà A., Martínez-González M.A., Alvarez-Alvarez I., et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. doi: 10.1136/bmj.l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco-Rojo R., Sandoval-Insausti H., López-Garcia E., et al. Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc. 2019;94(11):2178–2188. doi: 10.1016/j.mayocp.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Bonaccio M., Di Castelnuovo A., Costanzo S., et al. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am J Clin Nutr. 2021;113(2):446–455. doi: 10.1093/ajcn/nqaa299. [DOI] [PubMed] [Google Scholar]
- 17.Song M., Wu K., Meyerhardt J.A., et al. Low-carbohydrate diet score and macronutrient intake in relation to survival after colorectal cancer diagnosis. JNCI Cancer Spectr. 2018;2(4) doi: 10.1093/jncics/pky077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoltick E.S., Smith-Warner S.A., Yuan C., et al. Sugar-sweetened beverage, artificially sweetened beverage and sugar intake and colorectal cancer survival. Br J Cancer. 2021;125(7):1016–1024. doi: 10.1038/s41416-021-01487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr P.R., Jansen L., Walter V., et al. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am J Clin Nutr. 2016;103(1):192–200. doi: 10.3945/ajcn.115.121145. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt D.R., Patel R., Kirsch D.G., Lewis C.A., Vander Heiden M.G., Locasale J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021;71(4):333–358. doi: 10.3322/caac.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Cancer Research Fund/American Institute for Cancer Research . 2018. Continuous Update Project Expert Report 2018. Preservation and processing of foods and the risk of cancer. [Google Scholar]
- 22.Rimm E.B., Giovannucci E.L., Willett W.C., et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 23.Bao Y., Bertoia M.L., Lenart E.B., et al. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health. 2016;106(9):1573–1581. doi: 10.2105/AJPH.2016.303338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monteiro C.A., Cannon G., Moubarac J.C., Levy R.B., Louzada M.L.C., Jaime P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khandpur N., Rossato S., Drouin-Chartier J.P., et al. Categorising ultra-processed foods in large-scale cohort studies: evidence from the nurses’ health studies, the health professionals follow-up study, and the growing up today study. J Nutr Sci. 2021;10:e77. doi: 10.1017/jns.2021.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hang D., Wang L., Fang Z., et al. Ultra-processed food consumption and risk of colorectal cancer precursors: results from 3 prospective cohorts. J Natl Cancer Inst. 2023;115(2):155–164. doi: 10.1093/jnci/djac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cain K.C., Harlow S.D., Little R.J., et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol. 2011;173(9):1078–1084. doi: 10.1093/aje/kwq481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seaman S.R., White I.R. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 29.Mirizzi A., Aballay L.R., Misciagna G., et al. Modified WCRF/AICR score and all-cause, digestive system, cardiovascular, cancer and other-cause-related mortality: a competing risk analysis of two cohort studies conducted in southern Italy. Nutrients. 2021;13(11):4002. doi: 10.3390/nu13114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song M., Wu K., Meyerhardt J.A., et al. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018;4(1):71–79. doi: 10.1001/jamaoncol.2017.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J., Zheng Y., Wang H., et al. Cause of death among patients with colorectal cancer: a population-based study in the United States. Aging (Albany NY) 2020;12(22):22927–22948. doi: 10.18632/aging.104022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moslehi J.J. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 34.Hang D., Knudsen M.D., Song M. Moving toward personalized colorectal cancer follow-up care. JAMA Oncol. 2024;10(1):29–31. doi: 10.1001/jamaoncol.2023.5072. [DOI] [PubMed] [Google Scholar]
- 35.Juul F., Vaidean G., Lin Y., Deierlein A.L., Parekh N. Ultra-processed foods and incident cardiovascular disease in the Framingham offspring study. J Am Coll Cardiol. 2021;77(12):1520–1531. doi: 10.1016/j.jacc.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Chen X., Zhang Z., Yang H., et al. Consumption of ultra-processed foods and health outcomes: a systematic review of epidemiological studies. Nutr J. 2020;19(1):86. doi: 10.1186/s12937-020-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang K., Gunter M.J., Rauber F., et al. Ultra-processed food consumption, cancer risk and cancer mortality: a large-scale prospective analysis within the UK Biobank. EClinicalMedicine. 2023;56 doi: 10.1016/j.eclinm.2023.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debras C., Chazelas E., Sellem L., et al. Artificial sweeteners and risk of cardiovascular diseases: results from the prospective NutriNet-Santé cohort. BMJ. 2022;378 doi: 10.1136/bmj-2022-071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witkowski M., Nemet I., Alamri H., et al. The artificial sweetener erythritol and cardiovascular event risk. Nat Med. 2023;29(3):710–718. doi: 10.1038/s41591-023-02223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Ojeda F.J., Plaza-Díaz J., Sáez-Lara M.J., Gil A. Effects of sweeteners on the gut microbiota: a review of experimental studies and clinical trials. Adv Nutr. 2019;10(suppl_1):S31–S48. doi: 10.1093/advances/nmy037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrowski M.P., La Rosa S.L., Kunath B.J., et al. Mechanistic insights into consumption of the food additive xanthan gum by the human gut microbiota. Nat Microbiol. 2022;7(4):556–569. doi: 10.1038/s41564-022-01093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naimi S., Viennois E., Gewirtz A.T., Chassaing B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome. 2021;9(1):66. doi: 10.1186/s40168-020-00996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shangguan S., Afshin A., Shulkin M., et al. A meta-analysis of food labeling effects on consumer diet behaviors and industry practices. Am J Prev Med. 2019;56(2):300–314. doi: 10.1016/j.amepre.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du M., Griecci C.F., Cudhea F.F., et al. Cost-effectiveness analysis of nutrition facts added-sugar labeling and obesity-associated cancer rates in the US. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.