Abstract
Background
The alpha-2 adrenergic agonist dexmedetomidine induces EEG patterns resembling those of non-rapid eye movement (NREM) sleep. Fulfilment of slow wave sleep (SWS) homeostatic needs would address the assumption that dexmedetomidine induces functional biomimetic sleep states.
Methods
In-home sleep EEG recordings were obtained from 13 healthy participants before and after dexmedetomidine sedation. Dexmedetomidine target-controlled infusions and closed-loop acoustic stimulation were implemented to induce and enhance EEG slow waves, respectively. EEG recordings during sedation and sleep were staged using modified American Academy of Sleep Medicine criteria. Slow wave activity (EEG power from 0.5 to 4 Hz) was computed for NREM stage 2 (N2) and NREM stage 3 (N3/SWS) epochs, with the aggregate partitioned into quintiles by time. The first slow wave activity quintile served as a surrogate for slow wave pressure, and the difference between the first and fifth quintiles as a measure of slow wave pressure dissipation.
Results
Compared with pre-sedation sleep, post-sedation sleep showed reduced N3 duration (mean difference of −17.1 min, 95% confidence interval −30.0 to −8.2, P=0.015). Dissipation of slow wave pressure was reduced (P=0.02). Changes in combined durations of N2 and N3 between pre- and post-sedation sleep correlated with total dexmedetomidine dose, (r=−0.61, P=0.03).
Conclusions
Daytime dexmedetomidine sedation and closed-loop acoustic stimulation targeting EEG slow waves reduced N3/SWS duration and measures of slow wave pressure dissipation on the post-sedation night in healthy young adults. Thus, the paired intervention induces sleep-like states that fulfil certain homeostatic NREM sleep needs in healthy young adults.
Clinical trial registration
ClinicalTrials.gov NCT04206059.
Keywords: closed-loop acoustic stimulation, dexmedetomidine, electroencephalography, sedation, sleep, slow waves
Dexmedetomidine is a commonly used sedative in perioperative and critical care settings. It is notable for inducing behavioural states resembling natural sleep, characterised by an intact respiratory drive, rousability to voice and touch, and dreaming.1,2 Its safety profile and similarities to natural sleep have led to its use as a sedative in hospital,3 and there is mounting interest in the use of dexmedetomidine in ambulatory settings.4, 5, 6
Converging lines of evidence suggest that dexmedetomidine induces biomimetic non-rapid eye movement (NREM) sleep, but it remains unclear whether this induced state fulfils homeostatic NREM sleep needs.7,8 Murine mechanistic studies have revealed that dexmedetomidine suppresses the release of norepinephrine from the locus coeruleus via alpha-2 agonism.9 The loss of noradrenergic tone from the locus coeruleus to the ventral lateral preoptic nucleus of the hypothalamus then enables inhibition of arousal centres in the midbrain and pons in much the same way that NREM sleep is initiated.10 Sedation and unconscious states induced by dexmedetomidine appear to be distinct,11 with galanin neurones in the preoptic nucleus important in pathways for both inducing sedation and regulating sleep homeostasis.12 Clinical studies have reinforced the view that dexmedetomidine engages endogenous sleep circuits; human EEG during dexmedetomidine sedation shows a dose-dependent expression of markers resembling those used to identify stage 2 NREM (N2) sleep—spindles and K-complexes, with a progressive shift through theta (4–8 Hz) to the slow-delta (0.5–4 Hz) oscillations.13, 14, 15, 16 The predominance of the latter mimics stage 3 NREM (N3) sleep, also known as slow wave sleep (SWS),17 primarily studied for its role in memory consolidation and regulation of important physiological processes.18, 19, 20, 21, 22
Despite this body of evidence characterising the circuit mechanisms and EEG signatures of dexmedetomidine sedation, less is known about its effects on natural sleep after drug redistribution, elimination, and metabolism. In rats, dexmedetomidine disrupts the sleep/wake cycle and increases EEG slow wave activity (SWA, 0.5–4 Hz) associated with slow-delta oscillations of N2 and N3 sleep.23 Several clinical studies have examined longitudinal sleep architecture in relation to the timing of dexmedetomidine infusions, but all have taken place in the setting of critical illness or surgery.24,25 From a clinical perspective, the possibility that dexmedetomidine induces natural sleep may benefit critically ill patients, given the current lack of strong evidence for its utility during mechanical ventilation compared with other non-benzodiazepine sedatives.26
The effects of dexmedetomidine could be enhanced through a noninvasive neuromodulation technique known as closed-loop acoustic stimulation (CLAS).27 CLAS entails the delivery of sound in response to real-time changes in the EEG. Precise pairing of CLAS with EEG sleep slow waves leads to SWS enhancement, with associated cognitive and physiological benefits.18, 22,28 It remains unknown whether dexmedetomidine sedation alone, or with CLAS, could impact subsequent sleep structure.
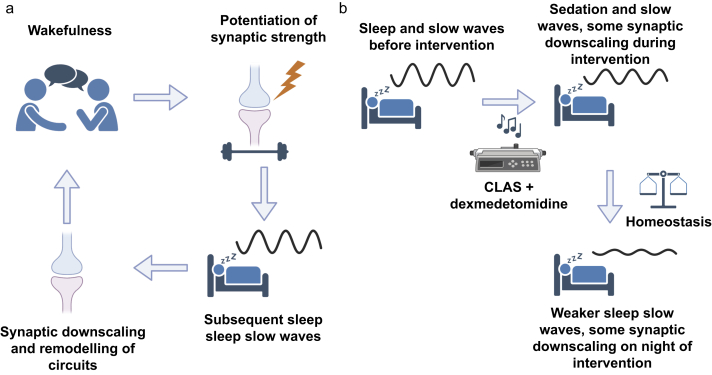
While many aspects of N3 sleep, such as duration, undergo homeostatic regulation,29 there have been no reports addressing SWA or sleep pressure in healthy human volunteers recently sedated with dexmedetomidine with CLAS. One theory of the role of sleep pressure and SWA homeostasis is the synaptic homeostasis hypothesis30 (Fig. 1a), where wakefulness leads to a potentiation of synapses that is reflected the following night by increases in sleep SWA. This sleep SWA, a measure of sleep pressure, typically dissipates during overnight sleep as homeostatic processes converge. In contrast, daytime sleep rich in SWA would dissipate sleep pressure, as reflected by a reduction in SWA on the subsequent night of sleep. Thus, if dexmedetomidine and CLAS induce biomimetic NREM and SWA-rich sleep, the hypothesis would predict that post-sedation sleep would have lower measures of SWA compared with pre-sedation nights (Fig. 1b).
Fig 1.
Testing the hypothesis of slow wave sleep homeostasis after dexmedetomidine sedation. (a) The synaptic homeostasis hypothesis (SHY), where wakefulness potentiates synaptic strength, and subsequent sleep SWA reflects synaptic downscaling and remodelling of circuits. (b) The hypothesis predicts that promotion of daytime sleep SWA because of the intervention will result in reduced sleep SWA on the subsequent night, thus maintaining sleep homeostasis. CLAS, closed-loop acoustic stimulation; SWA, slow wave activity. Created in BioRender.
Here we address these gaps by testing the following three hypotheses: 1. daytime dexmedetomidine sedation and CLAS targeting EEG slow-delta waves would alter post-sedation overnight sleep macrostructure; 2. such infusions with CLAS would reduce post-sedation measures of SWS homeostasis; and 3. measures of dexmedetomidine dosing or states induced during sedation and CLAS would account for these changes.
Methods
Study design and participants
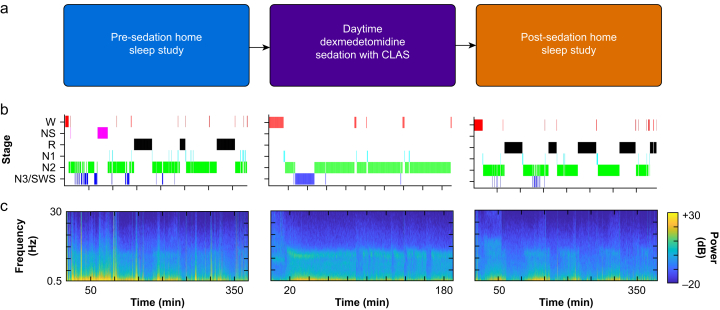
Fifteen healthy adults were recruited as part of the Closed-Loop Acoustic Stimulation during Sedation with Dexmedetomidine (CLASS-D) trial, a prospective, within-subject, crossover, controlled, interventional trial. CLASS-D was approved by the Human Research Protection Office (HRPO) at Washington University School of Medicine in St. Louis (#2019007086) and registered on ClinicalTrials.gov (NCT04206059) before study initiation. Interval changes in sleep structure after dexmedetomidine sedation was a pre-specified outcome in the published protocol (Fig. 2a).31
Fig 2.
Study overview with corresponding EEG hypnograms and spectrograms from a single participant. (a) Participants utilised a wireless wearable EEG device to record their pre- and post-sedation sleep at home, and high-density EEG recordings were obtained during daytime dexmedetomidine sedation with CLAS. (b, c) Representative EEG hypnograms and corresponding spectrograms from a single participant at each phase of the study. CLAS, closed-loop acoustic stimulation; N1, non-rapid eye movement (NREM) stage 1; N2, NREM stage 2; N3/SWS, NREM stage 3/slow wave sleep; NS, non-scoreable epoch; R, rapid eye movement (REM) sleep; W, wakefulness.
Study participants were recruited using posted fliers, internet advertisements, and university volunteer databases. Written informed consent was obtained from all participants before study procedures. Volunteers fulfilled inclusion criteria if able to provide informed consent, were between the ages of 18 and 40 yr, and were classified as American Society of Anesthesiologists (ASA) physical status 1–2 by a board-certified anaesthesiologist. Exclusion criteria were diagnosed sleep disorders, psychiatric disorders, hearing disorders, pregnancy or active nursing, habitually short sleeper of <6 h/night, use of psychoactive medication, recreational drugs or nicotine, body mass index (BMI) >30, neck circumference >40 cm, heat pain tolerance threshold >50ºC, and average heart rate <40 beats min−1 during N3 sleep, as monitored using a Dreem headband (Dreem, New York, NY, USA). Participants were allowed to consume caffeine throughout the study. They were instructed to adhere to standard ASA fasting guidelines on the morning of the sedation session and to refrain from post-sedation daytime napping. Compliance to daytime nap avoidance on the day of sedation was confirmed by all participants at study follow-up.
Target-controlled dexmedetomidine sedation and recovery
Study procedures were performed at the Washington University Medical Center after eligibility and fasting status were confirmed. ASA standard monitoring equipment, suction, airway equipment, and a dedicated board-certified anaesthesiologist were present, in accordance with ASA practice guidelines for anaesthesia care. Two peripheral i.v. catheters were placed in the upper extremities.
An open-loop target-controlled infusion (TCI) protocol was used to precisely initiate and maintain dexmedetomidine sedation based on participant age, sex, height, and weight. Rugloop II software (Demed, Temse, Belgium), and a computer-controlled infusion pump (Harvard Apparatus 22; Harvard Apparatus, South Natick, MA, USA) was used to administer i.v. dexmedetomidine (concentration of 16 μg ml−1), according to the 2015 Hannivort pharmacokinetic model.32 For all participants, the TCI protocol began with targeting an effect-site concentration of 1.5 ng ml−1. Once this level was reached, the concentration was escalated in increments of 0.5 ng ml−1 (maximum of 4 ng ml−1) until two endpoints were met: loss of responsiveness and induction of EEG large amplitude slow waves (>40 μV amplitude, 0.5–4 Hz frequency). Loss of responsiveness was assessed using a validated behavioural task: participants were asked to squeeze a hand dynamometer (Vernier, Beaverton, OR, USA) during inspiration and release it during expiration.33,34 A participant was considered unresponsive after failing the task for five successive breaths. Over the following 100–200 min, CLAS of EEG slow-delta waves and thermal arousal thresholds were evaluated according to the protocol using a crossover design, where each participant was exposed to both in-phase and anti-phase CLAS.31 Dexmedetomidine plasma concentrations were measured from blood sampled at steady-state, before and after CLAS.31 Dexmedetomidine was discontinued while participants were monitored for return of responsiveness. Once participants were considered responsive (performing the behavioural task correctly for five consecutive breaths), EEG acquisition was stopped. Participants were discharged after achieving a modified Aldrete scale of 10 and meeting standard institutional post-anaesthesia discharge criteria.
Sleep EEG recordings and staging
The Dreem headband was utilised by participants to record their pre- and post-sedation sleep data through in-home unattended studies. This wireless wearable device has previously been validated against polysomnography (PSG) for a broad age range,35 and has been implemented in the perioperative period to assess baseline sleep with a single night of recording before surgery.36 The Dreem headband records five domains of physiological signals. EEG are acquired via five dry electrodes: F7, F8, Fpz, O1, and O2. Head position, head motion, and respiratory effort are tracked via accelerometry in three perpendicular axes. Heart rate is detected via near-red infrared pulse plethysmography.
Up to two pre-sedation and two post-sedation overnight sleep EEG recordings were obtained from each participant. Comparative analyses utilised one pre-sedation sleep recording and one post-sedation sleep recording taken on the night of sedation. In instances where two pre-sedation sleep recordings were obtained, the sleep recording with the lowest percent non-scorable time was used (Supplementary Table S1). Records underwent 0.3–30 Hz bandpass filtering and were outputted as European Data Format files. Dreem recordings were scored in 30-s epochs by a registered polysomnographic technologist in Philips G3 software (Philips, Amsterdam, Netherlands), using modified American Academy of Sleep Medicine criteria.37,38 Epochs were denoted as non-scorable if they did not fulfil staging criteria for wakefulness (W), NREM (N1, N2, N3), and REM sleep. Evaluation of manual sleep staging revealed substantial agreement with the manufacturer's autoscoring algorithm (percent agreement was 79% for sleep classification and wake and sleep; pair-wise Cohen's Kappa of 0.72). Total sleep time (TST) was identified from the timing of the first and last sleep epochs. Two participants were excluded because of lack of usable post-sedation EEG data.
Sedation EEG recordings and staging
High-density EEG recordings were acquired during dexmedetomidine sedation, using 64-electrode EEG GSN caps (MagStim, Whitland, UK), a Net Amps 400 amplifier, and Net Station Software Version 5.0 (MagStim). Elefix conducting paste (Nihon Kohden, Tokyo, Japan) was inserted into GSN cap electrodes, with efforts to minimise impedance below 50 kOhm. Recordings were taken at 500 Hz sampling rate and underwent bandpass filtering (0.3–30 Hz) in EEGLAB39 as with the Dreem recordings. Virtual channels that matched preferred polysomnographic EEG derivations (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1) were created after re-referencing to sensors most closely matching corresponding 10–20 locations. After export to European Data Format, standard American Academy of Sleep Medicine scoring criteria and Philips G3 software were used in staging the NREM-like states. TST during sedation was noted from the first and last epoch scored as sleep. Analysed epochs encompassed the entire sedation period containing epochs of in-phase CLAS, anti-phase CLAS, and no CLAS. Supplemental analyses separated epochs of sedation into those with dexmedetomidine only from those paired with CLAS.
SWA measures
Sleep EEG data were recorded at a sampling rate of 250 Hz and underwent 0.1–50 Hz band-pass filtering using EEGLAB.39 We then applied a band-stop filter between 0.1 and 0.6 Hz to remove the respiratory artifact. Spectral analyses were performed using the Chronux toolbox,40 based on 5-s non-overlapping time windows, a time-bandwidth product of 3, and five tapers. Five-second windows with a maximum absolute EEG value of >250 μV were excluded for further analysis. To further remove EEG artifacts from sleep recordings, outlier detection based on the three standard deviation rule was applied to SWA measures. Custom-written scripts in MATLAB (MathWorks Inc., Natick, MA, USA) were used to quantify SWA as the average 0.5–4 Hz frontal EEG (F7–F8) power per minute for each 30-s epoch of either N2 or N3 sleep.
Sleep slow wave pressure and dissipation
Slow wave pressure measures were calculated using quintile analysis to capture changes in SWA across a night of sleep for each recording. In quintile analysis, SWA measures during N2 and N3 epochs for a given recording were sorted based on their sequential order throughout the night and divided into five equal segments denoted as quintiles. The average SWA for each quintile was subsequently calculated for each of these quintiles.41 SWA of the first quintile (Q1) reflects sleep slow wave pressure,42 while the SWA of the first quintile minus the SWA of the last quintile (Q1−Q5) represents dissipation of sleep slow wave pressure.41
Sleep slow wave energy
Sleep slow wave energy (SWE) has been a useful measure for evaluating cumulative changes in sleep slow wave expression over the night of sleep.43,44 SWE was calculated as cumulative SWA across each epoch of N2N3 sleep per sleep recording. We calculated SWE over the first 90 min of N2N3 sleep to minimise the impact of TST on this measure. We then normalised SWE measures to facilitate comparisons between pre- and post-sedation data across all participants. For this, we divided SWE measures by the maximum SWE measure across the pre- and post-sedation recordings per participant. In this way, the SWE could be compared across all participants on a uniform scale.
Statistical analysis
One-sample Kolmogorov–Smirnov tests were used to determine normality of all continuous variables. Paired t-tests were used to compare normally distributed sleep macrostructure measures before and after sedation, using a two-stage Benjamini, Krieger, and Yeuktieli false-discovery rate procedure. Wilcoxon signed-rank tests were utilised to test for paired differences in medians for sleep slow wave pressure and sleep slow wave pressure dissipation. A binomial test was used to determine whether the number of subjects with higher SWE for pre-sedation than SWE for post-sedation differed from chance expectation. All statistical tests conducted in this study were two-sided, with an alpha of 0.05. Statistical analyses were conducted in GraphPad Prism version 9.5.1 (GraphPad Software Inc., San Diego, CA, USA) and MATLAB (MathWorks Inc.). Sample size calculations were based on the main study outcomes of enhancement of EEG slow waves between CLAS conditions and not on the secondary outcomes of this manuscript.31
Results
Participant characteristics
Participant characteristics, sedation variables, and data quality metrics are provided (Supplementary Table S1). The mean (standard deviation, sd) age of the 13 healthy participants (seven females) was 26.8 (5.6) yr old. The mean (sd) duration of dexmedetomidine infusion was 156.1 (38.4) min. While the protocol for CLAS was terminated early for two participants because of restlessness, their total dexmedetomidine doses (440 and 306.1 μg) and weight-adjusted dose (6.1 and 4.8 μg kg−1) resembled those of the 11 remaining participants for both absolute mean (sd) 459.5 (108) μg and weight-adjusted dose 6.3 (1.4) μg kg−1. These two participants were retained in primary analyses of change in sleep structure but excluded from secondary analyses of sedation variables. Overall, mean (sd) absolute and weight-adjusted dexmedetomidine infused per participant was 446.2 (107.4) μg and 6.2 (1.4) μg kg−1, respectively. Dexmedetomidine targeted effect-site concentrations that were required to induce slow-delta EEG oscillations and achieve a state of unresponsiveness ranged from 2 to 3 ng ml−1.
Dexmedetomidine sedation with CLAS targeting EEG slow-delta waves reduced duration of subsequent overnight N3 sleep
Representative hypnograms from a single participant showed differences in N3 during pre-sedation vs post-sedation overnight sleep (Fig. 2b). As expected in pre-sedation sleep, there were N3 cycles at the beginning of the night with more REM at the end of the night (Fig. 2b, left panel). During dexmedetomidine sedation with CLAS, the targeting of EEG slow waves resulted in N2 and N3 sleep without REM or sleep cycling (Fig. 2b, middle panel). Arousals with N1 and W in the middle of sedation occurred at the times of noxious thermal stimulation. During post-sedation sleep, fewer N3 epochs were observed but sleep cycling and REM were observed (Fig. 2b, right panel). Corresponding time-frequency spectrograms show SWA (intense colours at 0.5–4 Hz frequency band) during N2N3 sleep (Fig. 2c).
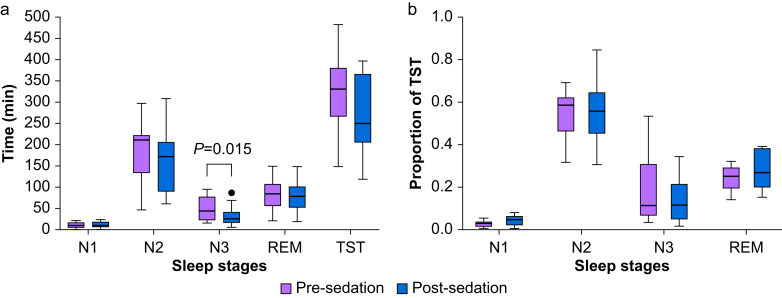
The effects of dexmedetomidine sedation on sleep macrostructure were then quantified (Fig. 3a). Compared with pre-sedation N3 sleep, the duration of N3 sleep on the first night after dexmedetomidine sedation was attenuated (mean difference of −17.1 min, 95% confidence interval [CI] −30.0 to −8.2 min, P=0.015, paired t-test). By contrast, no significant changes were observed for durations of N1 (mean difference −1.6 min, 95% CI −0.7 to 3.8 min, P=0.27), N2 (mean difference of −25.3 min, 95% CI −57.1 to 6.4 min, P=0.27), REM (mean difference −1.9 min, 95% CI −27.5 to 23.7 min, P=0.90), or TST (mean difference 48.1 min 95% CI −99.7 to 3.5 min, P=0.26).
Fig 3.
Dexmedetomidine sedation dosed to induce EEG slow wave reduced the duration for subsequent overnight N3 sleep. (a) Boxplots comparing sleep stages pre- and post-sedation. Duration of N3 sleep was significantly lower post-vs pre-sedation (paired t-test, P=0.015). (b) As a proportion of TST, a modest reduction for N3 sleep with a corresponding modest increase in N1 sleep was observed, although this did not meet statistical significance. REM, rapid eye movement; TST, total sleep time.
Changes in the percentage of TST spent in each sleep stage were also evaluated by subtracting values of pre-sedation sleep from post-sedation sleep (Fig. 3b). A modest reduction for N3 was observed (mean difference −4.84%, 95% CI −8.6% to −1.1%, P=0.06), with a corresponding modest increase in N1 (mean difference of 1.43%, 95% CI 0.34% to 2.52%, P=0.06). There were no significant differences in the percentage of TST for N2 (mean difference −1.0%, 95% CI −3.0% to 5.1%, P=0.65) or REM (mean difference −3.84%, 95% CI −9.9% to 2.1%, P=0.34).
Dexmedetomidine sedation with CLAS reduced rate of SWA dissipation on subsequent night of sleep
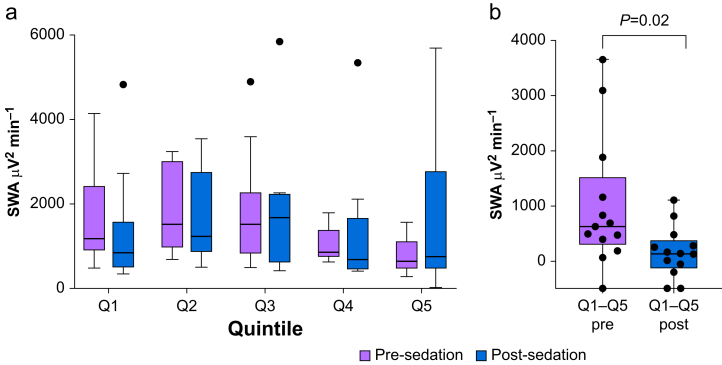
We assessed sleep slow wave pressure and sleep slow wave pressure dissipation after dexmedetomidine sedation with CLAS using quintile analysis. Figure 4a includes a boxplot comparing sleep SWA measures in different quintiles comparing pre- and post-sedation. Sleep slow wave pressure (SWA Q1) was not significantly different (Wilcoxon signed-rank test, P=0.27, with median (inter-quartile range, IQR) of 1180.60 (1511.73) μV2 min−1 and 840.69 (875.43) μV2 min−1 for pre- and post-sedation, respectively). However, the rate of sleep slow wave pressure dissipation (SWA Q1–Q5, Fig. 4b) was less during post-sedation sleep (Wilcoxon signed-rank test, P=0.02, with median (IQR) of 636.68 (990.09) μV2 min−1 and 141.32 (428.45) μV2 min−1 for pre- and post-sedation, respectively). Thus, while there were no statistical differences in sleep slow wave pressure during the first quintile of sleep, the rate of dissipation of sleep slow wave pressure was reduced after daytime dexmedetomidine sedation and CLAS targeting EEG slow waves.
Fig 4.
Dexmedetomidine sedation dosed to induce EEG slow waves alters rate of dissipation of SWA. (a) Boxplot comparing sleep SWA measures in different quintiles comparing pre- and post-sedation. Black dots represent individual data points falling outside of the upper fence of the Tukey box plots. Sleep pressure measured as SWA in the first quintile was not significantly different between pre- and post-sedation (Wilcoxon signed-rank test, P=0.27). (b) Comparison of SWA dissipation across the entire night expressed as the first quintile (Q1) minus the last quintile (Q5) for pre- and post-sedation. SWA dissipation was significantly reduced post-sedation compared with pre-sedation (Wilcoxon signed-rank test, P=0.02). SWA, slow wave activity.
Dexmedetomidine sedation with CLAS altered trajectories of SWE on subsequent night of sleep
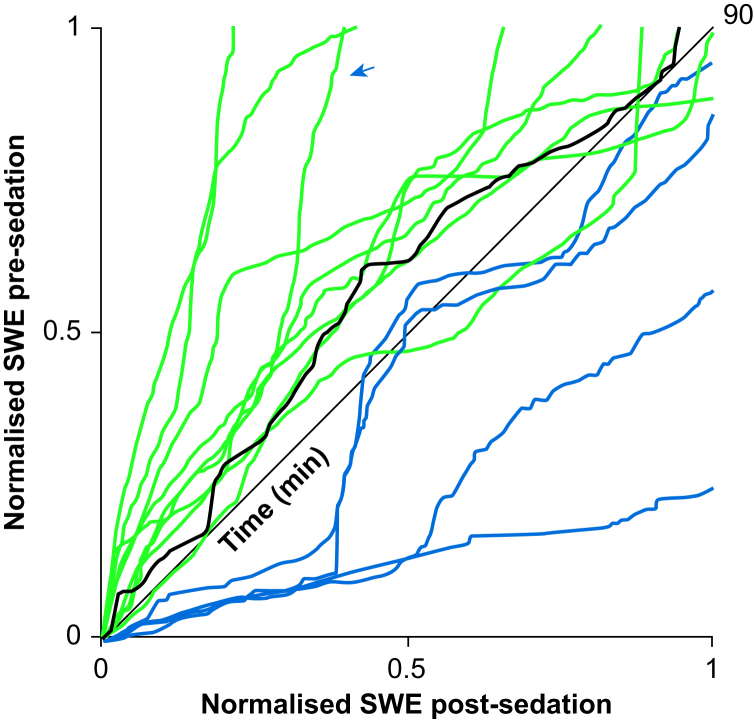
To further compare temporal changes in SWA pre- and post-sedation, we evaluated the trajectories of sleep SWE over the first 90 min of N2N3 sleep (Fig. 5). Each line represents the SWA trajectory for a single participant over the course of time, while the heavy black line represents the median across the population. For most participants (green), SWE pre-sedation was greater than SWE post-sedation for the majority of the 90-min period. For fewer participants (blue), SWE pre-sedation was less than that of post-sedation for the majority of time. An arrow denotes the SWE trajectory for the participant whose data are also shown in Fig. 2b and c. SWE at the end of the first 90 min of N2 and N3 was not significantly different between paired measurements (Wilcoxon signed-rank test, P=0.50, with median (IQR) of 1.00 (0.12) and 0.94 (0.40) for pre- and post-sedation, respectively). Therefore, over the course of the first 90 min of overnight N2N3 sleep, SWE was generally greater before than after dexmedetomidine sedation with CLAS targeting EEG slow waves, but did not differ by the end of this interval.
Fig 5.
Dexmedetomidine sedation and its association to changes in sleep slow wave energy (SWE). Normalised sleep SWE trajectory for post- vs pre-sedation during the first 90 min of non-rapid eye movement (NREM) N2 and N3 sleep. Each line represents the SWE trajectory for a single participant or the median across the population (heavy black). Green: participants where SWE for pre-sedation sleep was greater than SWE for post-sedation for the majority of time. Blue: participants where SWE for post-sedation was greater than SWE for pre-sedation for the majority of time. An arrow denotes the SWE trajectory for the same participant also shown in Fig 1.
Changes in sleep macrostructure related to total dexmedetomidine dose
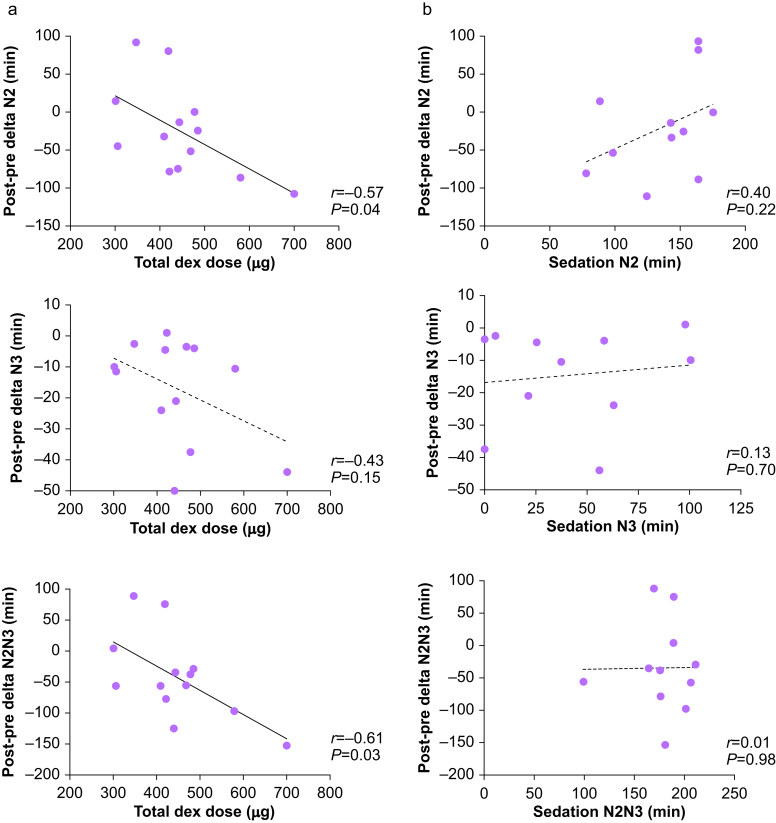
Given that dexmedetomidine induces EEG slow waves in a dose-dependent manner,45 we hypothesised that the observed reduction in post-sedation NREM sleep would relate to either the total dexmedetomidine dose or duration of the sleep-like state induced during dexmedetomidine sedation with CLAS. We evaluated whether total dexmedetomidine dose (μg) during sedation with CLAS correlated with change in NREM duration post-vs pre-sedation (Fig. 6a). Total dexmedetomidine dose negatively correlated with post-sedation changes in N2 duration (r=−0.57, P=0.04) and combined total duration of N2 and N3 (r=−0.61, P=0.03). A similar trend was observed with N3 sleep that did not meet statistical significance (r=−0.43, P=0.15). Duration of infusion also negatively correlated with post-sedation changes in N2 duration (r=−0.62, P=0.04) and combined total duration of N2 and N3 (r=−0.63, P=0.04), and a similar trend was also observed for N3 sleep that did not meet statistical significance (r=−0.27, P=0.42) (Supplementary Fig. S1). To summarise, higher daytime doses of dexmedetomidine translated to a greater decrease in NREM sleep the following night. Contrary to our expectations, duration of the N2- and N3-like states during sedation were not correlated with changes in sleep stage duration (all P>0.05, Fig. 6b). Furthermore, supplementary analyses separating dexmedetomidine sedation epochs from those paired with CLAS were unremarkable (Supplementary Fig. S2). No associations were observed between measured plasma concentrations collected at steady-state and changes in sleep stage duration (all P>0.05, data not shown). Thus, the changes in sleep architecture after dexmedetomidine and CLAS correlated with the total administered dose rather than the duration of states resembling N2 and N3 induced during dexmedetomidine sedation and CLAS.
Fig 6.
Total drug dose but not sleep stage duration during dexmedetomidine sedation with CLAS targeting EEG slow waves accounts for changes in sleep measures. (a) Scatter plots and Pearson correlations evaluating associations between the change in N2, N3, or N2N3 sleep duration pre- and post-sedation with total dexmedetomidine dose, where N2N3 refers to the sum of both stages. (b) Scatterplots and Pearson correlations evaluating associations between the change in N2, N3, or N2N3 sleep pre- and post-sedation (min) with duration of N2, N3, or N2N3 sleep during dexmedetomidine sedation (min). CLAS, closed-loop acoustic stimulation; SWA, slow wave activity; SWS, slow wave sleep.
Discussion
Distinct from other sedative hypnotic agents, dexmedetomidine sedation induces an arousal state that electrophysiologically resembles that of natural sleep.7,8 Our work demonstrates that dexmedetomidine sedation paired with CLAS targeting EEG slow waves fulfils the homeostatic need for SWS, as shown by a post-sedation reduction in N3 sleep duration. While we did not observe a post-sedation reduction in sleep pressure, as estimated by an observed difference in SWA Q1 during pre-sedation vs post-sedation sleep, we did observe a less rapid dissipation of SWA during the evening after dexmedetomidine sedation and CLAS. Furthermore, the changes in sleep macrostructure correlated with total dexmedetomidine dose. These findings suggest that the biomimetic sleep state induced by dexmedetomidine sedation and CLAS not only neurophysiologically resembles natural sleep, but more importantly, addresses homeostatic sleep needs at the macrostructural (N3) and microstructural (SWA) levels.
Our findings are important for perioperative medicine. There is recent interest in dexmedetomidine as a sleep aid both in the context of delirium and in the ambulatory setting. Restorative sleep promotion along with the presumed cognitive benefits are in line with previous and current investigations aimed at examining whether prophylactic i.v. dexmedetomidine decreases the incidence of delirium in post-surgical and critically ill older patients.46,47 Oral dexmedetomidine as a sleep aid in the ambulatory setting induces a similar neurophysiological sleep state.5 Our work reinforces support for the use of dexmedetomidine as a sleep aid, given its relatively unique properties in promoting a restorative, biomimetic sleep state. Future studies are warranted to analogously investigate the restorative nature of sleep enhanced by oral dexmedetomidine and CLAS.
The effects of midday sleep or sedation on night-time sleep pressure and cognitive function deserve further discussion. Daytime napping studies have commonly shown a reduction in slow wave pressure dissipation on the subsequent night of sleep.48, 49, 50 In this study, analogous reduction in time spent in N3, less rapid dissipation of SWA, and lower post-sedation SWE all support that dexmedetomidine and CLAS induce a biomimetic state that addresses homeostatic sleep pressure. While we anticipated that there would be a negative correlation between total duration of sleep stages during sedation and the interval change in N2N3 durations, this was not observed. Thus, there is a possible uncoupling between states induced during dexmedetomidine sedation paired with CLAS and subsequent sleep. Instead, there appears to be a dose-dependent response on the change in sleep structure following this paired intervention. The subsequent reduction in N3 sleep and SWA contrast with the effects of subanaesthetic ketamine51 and propofol,52,53 which both appear to enhance human N3 sleep after i.v. administration. These disparate effects may be related to both differences in molecular targets and effects of sleep homeostatic circuitry. In addition to sleep homeostasis, daytime naps have also been shown to improve long-term memory both in the laboratory and real-world settings.54 In this study, we did not assess effects on cognitive function. Whether these findings recapitulate the specific benefits of daytime naps vs overall improved sleep satiation is less clear. We do note that our findings contrast with those reported in rodent models,23 which may be related to important species-specific differences, dosing differences, or the confound of concurrent CLAS.
There are several limitations of this study. We emphasise that our sample size was small and was not based on a power calculation for these sleep outcomes. Only young healthy adults were enrolled, and future work is needed to generalise findings to children, older adults, or those in clinical settings. Nevertheless, similar logistically complex and labour-intensive studies have used comparable sample sizes.41 Caffeine intake was not accounted for in this investigation. As this study was nested in a larger clinical trial evaluating CLAS, our results should be interpreted with caution for extending to dexmedetomidine alone, as separate control infusion sessions without CLAS were not undertaken. Thus, it remains possible that these observations are driven by CLAS, despite supplementary analyses showing no relationship between changes in sleep macrostructure when only sedation paired with CLAS are analysed. In addition, we would not have expected total dexmedetomidine dose (a potent intervention over a long duration) to correlate with changes in sleep measures if CLAS (a transient and intermittent intervention) were the dominant contributor. Nevertheless, future studies are needed to discriminate the relative contributions of dexmedetomidine and CLAS. Because of the design of an individualised dexmedetomidine dosing strategy, in terms of total dose and length of infusion, variations in residual drug effects could have arisen. Future studies are also needed to relate daytime SWS measures during dexmedetomidine sedation to cognitive function on the subsequent day to evaluate for additional physiological effects. Finally, it is not known whether a nocturnal dexmedetomidine sedation session would similarly alter subsequent sleep structure and cognitive function.
This study also has several strengths. We examined natural sleep before and after daytime dexmedetomidine sedation paired with CLAS in the absence of confounding variables, such as additional anaesthetics, sedating agents, surgery, and critical illness. The ability to record sleep across multiple nights was afforded through the Dreem headband that bypasses logistical constraints of polysomnography by allowing for unattended in-home acquisition of sleep EEG records.36 We also classified the high-density EEG data to evaluate whether EEG SWA and the sleep-like states during dexmedetomidine sedation with CLAS accounted for any subsequent alteration in sleep structure. TCI allowed for controlled escalation and maintenance of targeted plasma drug concentration and revealed that dexmedetomidine sedation predicted the change in post-sedation sleep in a dose-dependent manner.
These findings may also be clinically relevant; daytime dexmedetomidine sedation with CLAS as carried out in this study could be useful for procedural sedation or for treating hyperactive delirium in the critically ill. The use of CLAS may potentially reduce dexmedetomidine exposure by enhancing a state of SWS with high thresholds for noxious stimulation. This potential strategy would be particularly advantageous in scenarios where rapid ‘wakeups’ are crucial alongside clinical sedation for daytime procedures. Additionally, it may prove beneficial in critical care settings where the use of dexmedetomidine is occasionally hindered by side-effects, such as bradycardia and blood pressure shifts.
In conclusion, daytime dexmedetomidine sedation with CLAS to target EEG slow waves reduced N3 sleep/SWS duration and slow wave dissipation on the post-sedation night. Thus, the pairing of dexmedetomidine and CLAS can induce sleep-like states that fulfil homeostatic NREM sleep needs in healthy young adults.
Author’s contributions
Study conception and design, and drafting of the manuscript: all authors
Data acquisition: SKS, MMK, RLR, CSG, BJAP
Data analysis and interpretation: SKS, MMK, RLR, ECL, CSG, BJAP
Acquisition of funding: CSG, BJAP
Critical review and approval of final version of the manuscript: all authors
Declarations of interest
ENB was involved in the start-up company, Neuradia, that is investigating drugs to induce wake up from anaesthesia and a start-up company that is developing systems for control of physiological states during anaesthesia. ENB has pending patents US20200187853A1 and US20190374158A1 and receives royalties from Masimo for pending patent US16373498 and issued patent US10299720. ENB holds founding interests in PASCALL, a start-up developing physiological monitoring systems. The interests of ENB were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. CSG, ENB, and BJAP report pending patent US 17/128,845. The other authors declare that they have no conflicts of interest.
Funding
McDonnell Center for Systems Neuroscience at Washington University in St. Louis, Washington University Department of Anesthesiology, International Anesthesia Research Society (SKS); National Institutes of Health (R01AG057901 to BJAP, U01MH128483 to BJAP, K01MH128663 to MK, P01GM118269 and R01NS123120 to ENB). This work was also supported by JPB Foundation; the Picower Institute of Learning and Memory; George J. Elbaum (MIT '59, SM '63, PhD '67), Mimi Jensen, Diane B. Green (MIT, SM '78), Mendel Rosenblum, and Bill Swanson, annual donors to the Anesthesia Initiative Fund (ENB).
Acknowledgements
The authors would like to thank the research participants for their efforts and staff support at the Washington University Clinical Translational Research Unit. Research efforts of Thomas Nguyen, Orlandrea Hyche, Matthew Robeck, Alyssa Labonte, and Anhthi Luong are kindly acknowledged. The authors also express gratitude to Michael Avidan and Brendan Lucey for providing research expertise. We thank Uwe Christians for assistance with plasma dexmedetomidine drug level measurements.
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2024.100276.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weerink M.A.S., Struys M., Hannivoort L.N., Barends C.R.M., Absalom A.R., Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radek L., Kallionpaa R.E., Karvonen M., et al. Dreaming and awareness during dexmedetomidine- and propofol-induced unresponsiveness. Br J Anaesth. 2018;121:260–269. doi: 10.1016/j.bja.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Chen K., Lu Z., Xin Y.C., Cai Y., Chen Y., Pan S.M. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev. 2015;1:CD010269. doi: 10.1002/14651858.CD010269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An J.-X., Williams J.P., Fang Q.-W., et al. Feasibility of patient-controlled sleep with dexmedetomidine in treating chronic intractable insomnia. Nat Sci Sleep. 2020;12:1033–1042. doi: 10.2147/NSS.S262991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamadia S., Hobbs L., Marota S., et al. Oral dexmedetomidine promotes non-rapid eye movement stage 2 sleep in humans. Anesthesiology. 2020;133:1234–1243. doi: 10.1097/ALN.0000000000003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X.H., Cui F., Zhang C., et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. 2016;125:979–991. doi: 10.1097/ALN.0000000000001325. [DOI] [PubMed] [Google Scholar]
- 7.Akeju O., Hobbs L.E., Gao L., et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin Neurophysiol. 2018;129:69–78. doi: 10.1016/j.clinph.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramaswamy S.M., Weerink M.A.S., Struys M., Nagaraj S.B. Dexmedetomidine-induced deep sedation mimics non-rapid eye movement stage 3 sleep: large-scale validation using machine learning. Sleep. 2021;44:zsaa167. doi: 10.1093/sleep/zsaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson L.E., Lu J., Guo T., Saper C.B., Franks N.P., Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Saper C.B., Fuller P.M., Pedersen N.P., Lu J., Scammell T.E. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z., Ferretti V., Guntan I., et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of alpha2 adrenergic agonists. Nat Neurosci. 2015;18:553–561. doi: 10.1038/nn.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y., Miracca G., Yu X., et al. Galanin neurons unite sleep homeostasis and alpha2-adrenergic sedation. Curr Biol. 2019;29 doi: 10.1016/j.cub.2019.07.087. 3315–22.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sleigh J.W., Vacas S., Flexman A.M., Talke P.O. Electroencephalographic arousal patterns under dexmedetomidine sedation. Anesth Analg. 2018;127:951–959. doi: 10.1213/ANE.0000000000003590. [DOI] [PubMed] [Google Scholar]
- 14.Guldenmund P., Vanhaudenhuyse A., Sanders R.D., et al. Brain functional connectivity differentiates dexmedetomidine from propofol and natural sleep. Br J Anaesth. 2017;119:674–684. doi: 10.1093/bja/aex257. [DOI] [PubMed] [Google Scholar]
- 15.Huupponen E., Maksimow A., Lapinlampi P., et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–294. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheinin A., Kallionpaa R.E., Li D., et al. Differentiating drug-related and state-related effects of dexmedetomidine and propofol on the electroencephalogram. Anesthesiology. 2018;129:22–36. doi: 10.1097/ALN.0000000000002192. [DOI] [PubMed] [Google Scholar]
- 17.Akeju O., Kim S.-E., Vazquez R., et al. Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Molina G., Tsoneva T., Jasko J., et al. Closed-loop system to enhance slow-wave activity. J Neural Eng. 2018;15 doi: 10.1088/1741-2552/aae18f. [DOI] [PubMed] [Google Scholar]
- 19.Besedovsky L., Ngo H.V., Dimitrov S., Gassenmaier C., Lehmann R., Born J. Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nat Commun. 2017;8:1984. doi: 10.1038/s41467-017-02170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leminen M.M., Virkkala J., Saure E., et al. Enhanced memory consolidation via automatic sound stimulation during non-REM sleep. Sleep. 2017;40:zsx003. doi: 10.1093/sleep/zsx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papalambros N.A., Santostasi G., Malkani R.G., et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci. 2017;11:109. doi: 10.3389/fnhum.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimaldi D., Papalambros N.A., Reid K.J., et al. Strengthening sleep-autonomic interaction via acoustic enhancement of slow oscillations. Sleep. 2019;42:zsz036. doi: 10.1093/sleep/zsz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrity A.G., Botta S., Lazar S.B., et al. Dexmedetomidine-induced sedation does not mimic the neurobehavioral phenotypes of sleep in Sprague Dawley rat. Sleep. 2015;38:73–84. doi: 10.5665/sleep.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexopoulou C., Kondili E., Diamantaki E., et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121:801–807. doi: 10.1097/ALN.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 25.Song B., Li Y., Teng X., Li X., Yang Y., Zhu J. The effect of intraoperative use of dexmedetomidine during the daytime operation vs the nighttime operation on postoperative sleep quality and pain under general anesthesia. Nat Sci Sleep. 2019;11:207–215. doi: 10.2147/NSS.S225041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlin J.W., Skrobik Y., Gelinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46 doi: 10.1097/CCM.0000000000003299. e825–e73. [DOI] [PubMed] [Google Scholar]
- 27.Ngo H.V., Martinetz T., Born J., Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Schreglmann S.R., Wang D., Peach R.L., et al. Non-invasive suppression of essential tremor via phase-locked disruption of its temporal coherence. Nat Commun. 2021;12:363. doi: 10.1038/s41467-020-20581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirelli C., Huber R., Gopalakrishnan A., Southard T.L., Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tononi G., Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Guay C.S., Labonte A.K., Montana M.C., et al. Closed-Loop Acoustic Stimulation during Sedation with Dexmedetomidine (CLASS-D): protocol for a within-subject, crossover, controlled, interventional trial with healthy volunteers. Nat Sci Sleep. 2021;13:303–313. doi: 10.2147/NSS.S293160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannivoort L.N., Eleveld D.J., Proost J.H., et al. Development of an optimized pharmacokinetic model of dexmedetomidine using target-controlled infusion in healthy volunteers. Anesthesiology. 2015;123:357–367. doi: 10.1097/ALN.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 33.Prerau M.J., Hartnack K.E., Obregon-Henao G., et al. Tracking the sleep onset process: an empirical model of behavioral and physiological dynamics. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guay C.S., Hight D., Gupta G., et al. Breathe-squeeze: pharmacodynamics of a stimulus-free behavioural paradigm to track conscious states during sedation. Br J Anaesth. 2023;130:557–566. doi: 10.1016/j.bja.2023.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Arnal P.J., Thorey V., Debellemaniere E., et al. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep. 2020;43:zsaa097. doi: 10.1093/sleep/zsaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kafashan M.M., Hyche O., Nguyen T., et al. Perioperative sleep in geriatric cardiac surgical patients: a feasibility study using a wireless wearable device. Br J Anaesth. 2021;126 doi: 10.1016/j.bja.2021.02.018. e205–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith S.K., Nguyen T., Labonte A.K., et al. Protocol for the Prognosticating Delirium Recovery Outcomes Using Wakefulness and Sleep Electroencephalography (P-DROWS-E) study: a prospective observational study of delirium in elderly cardiac surgical patients. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucey B.P., McLeland J.S., Toedebusch C.D., et al. Comparison of a single-channel EEG sleep study to polysomnography. J Sleep Res. 2016;25:625–635. doi: 10.1111/jsr.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Mitra P.P., Bokil H. Oxford University Press; New York: 2008. Observed brain dynamics. [Google Scholar]
- 41.Landsness E.C., Goldstein M.R., Peterson M.J., Tononi G., Benca R.M. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45:1019–1026. doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leemburg S., Vyazovskiy V.V., Olcese U., Bassetti C.L., Tononi G., Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks S., Van Dongen H.P., Maislin G., Dinges D.F. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–1026. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plante D.T., Goldstein M.R., Cook J.D., et al. Effects of partial sleep deprivation on slow waves during non-rapid eye movement sleep: a high density EEG investigation. Clin Neurophysiol. 2016;127:1436–1444. doi: 10.1016/j.clinph.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebert T.J., Hall J.E., Barney J.A., Uhrich T.D., Colinco M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Shehabi Y., Howe B.D., Bellomo R., et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380:2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 47.Qu J.Z., Mueller A., McKay T.B., et al. Nighttime dexmedetomidine for delirium prevention in non-mechanically ventilated patients after cardiac surgery (MINDDS): a single-centre, parallel-arm, randomised, placebo-controlled superiority trial. EClinicalMedicine. 2023;56 doi: 10.1016/j.eclinm.2022.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knoblauch V., Kräuchi K., Renz C., Wirz-Justice A., Cajochen C. Homeostatic control of slow-wave and spindle frequency activity during human sleep: effect of differential sleep pressure and brain topography. Cereb Cortex. 2002;12:1092–1100. doi: 10.1093/cercor/12.10.1092. [DOI] [PubMed] [Google Scholar]
- 49.Ong J.L., Lo J.C., Gooley J.J., Chee M.W.L. EEG Changes Accompanying successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2017;40:zsx030. doi: 10.1093/sleep/zsx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarokh L., Van Reen E., Achermann P., Carskadon M.A. Naps not as effective as a night of sleep at dissipating sleep pressure. J Sleep Res. 2021;30 doi: 10.1111/jsr.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncan W.C., Sarasso S., Ferrarelli F., et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Z., Jiang X., Li W., Gao D., Li X., Liu J. Propofol-induced sleep: efficacy and safety in patients with refractory chronic primary insomnia. Cell Biochem Biophys. 2011;60:161–166. doi: 10.1007/s12013-010-9135-7. [DOI] [PubMed] [Google Scholar]
- 53.Rios R.L., Kafashan M., Hyche O., et al. Targeting slow wave sleep deficiency in late-life depression: a case series with propofol. Am J Geriatr Psychiatry. 2023;31:643–652. doi: 10.1016/j.jagp.2023.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cousins J.N., Leong R.L.F., Jamaluddin S.A., Ng A.S.C., Ong J.L., Chee M.W.L. Splitting sleep between the night and a daytime nap reduces homeostatic sleep pressure and enhances long-term memory. Sci Rep. 2021;11:5275. doi: 10.1038/s41598-021-84625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.