Abstract
Decentralized clinical trials (DCTs) consist of off-site trial-related procedures referred to as decentralized elements. We aimed to provide an overview of the landscape of DCTs by comparing regulatory guidance reports and analyzing decentralized elements from clinical trial registries. Two guidance reports on DCTs published by the U.S. Food and Drug Administration and the European Medicines Agencies were summarized and analyzed. Both guidance publications commonly emphasized an assessment of the appropriateness of decentralized elements along 2 axes: patient safety and data integrity. DCT cases were identified from ClinicalTrials.gov by searching with 6 keywords: decentralized, remote, mobile, digital, virtual, and hybrid. Cases where the keyword was used in a non-DCT context, such as digital flexor tendon, were excluded by means of natural language processing. A total of 4,874 trials were identified as DCT cases, with annual increases, especially after 2020. The most common keywords were ‘mobile’ and ‘digital’ (36.2% and 24.8%, respectively). Interventions in the DCT cases were analyzed by means of a network analysis. Behavioral and technological tokens were frequently combined, such as ‘rehabilitation’ and ‘app.’ Drugs were used in only 1.8% of the DCT cases. Of these, most drugs had been approved previously (96.8%) and were in oral formulation (67.2%). Most of the DCT cases identified in this study involved simple interventions and low-risk drugs. These characteristics were in accordance with the common recommendations in the DCT guidance publications.
Keywords: Clinical Trial, Remote Consultation, Mobile Applications, Guideline, Natural Language Processing
INTRODUCTION
Decentralized clinical trials (DCTs) involve decentralized elements, trial-related procedures performed outside of trial centers. Depending on the extent and how decentralized elements are implemented, a DCT can range from a hybrid DCT to a fully DCT [1]. Conventional clinical trials also include several decentralized elements, such as telephone-based monitoring [2]. However, fully DCTs became available with the widespread use of digital technologies [3].
The DCT has many advantages in terms of accessibility to clinical trials. The long distance from patients’ homes to the trial center has been a barrier preventing patients from participate in clinical trials [4,5]. However, decentralized elements allow participants to maintain their daily lives during the trial [6]. In addition, researchers can recruit participants from diverse backgrounds and obtain real-world data more quickly and cost-effectively [7,8]. These benefits are in line with the recent paradigm of patient-centric clinical trials, emphasizing diversity, equity and inclusion [9].
In response, regulatory agencies have issued guidance on DCTs. Public authorities such as the Danish Medicines Agency [10] or the Swiss Agency for Therapeutic Products (Swissmedic) [11] published guidance on DCTs in 2021. The European Medicines Agency (EMA) issued a recommendation paper in 2022 [12]. The U.S. Food and Drug Administration (FDA) [1] and Clinical Research Malaysia [13] published guidance in 2023. Recommendations from industry were also published; the quality-by-design manual for DCT by the Association of Clinical Research Organizations [14] influenced the EMA guidance [12].
However, the landscape of DCTs has not been suitably evaluated. First, it is difficult to identify DCT cases due to the broad range of terms used. The term ‘decentralized’ has only recently been standardized, with virtual trials [15], remote trials [16], and digital trials [17] being used previously. Due to the lack of a unified keyword or a typical method of searching for DCTs, the number of DCTs is only vaguely estimated.
Additionally, a systematic analysis of decentralized elements is difficult due to the heterogeneous DCT guidance. To date, different recommendation papers have emphasized various aspects of decentralized elements. In order to categorize and analyze them structurally, commonly highlighted recommendations should be analyzed. However, to the best of our knowledge, there is little research on these issues.
Hence, we sought to provide an overview of the landscape of DCTs by comparing the regulatory guidance and analyzing the decentralized elements from clinical trial registries. We attempted to evaluate how decentralized elements have been implemented in real-world trials and whether they were in accordance with the DCT guidance.
METHODS
Comparison of DCT guidance publications
We compared 2 regulatory guidance publications on DCTs: ‘Decentralized Clinical Trials for Drugs, Biological Products, and Devices’ published by the FDA [1] and ‘Recommendation Paper on Decentralized Elements in Clinical Trials’ issued by the EMA [12].
We summarized the 2 guidance publications into topic sentences and grouped them according to the following 3 categories: 1) assessing the appropriateness of using decentralized elements, 2) additional roles and responsibilities in DCTs, and 3) implementation strategies tailored to decentralized elements. In each category, we matched the topic sentences in sequence between the 2 guidance publications to find commonalities. The similarities and differences between the guidance were listed in tables as 3 categories. In addition, we set the commonly suggested recommendations as criteria to be used to evaluate whether the decentralized elements were in consistent with the DCT guidance.
Identification of DCT cases
We searched for DCT cases in the ClinicalTrials.gov registry that were initiated before October of 2023. We selected the following 6 keywords commonly used in DCTs in the intervention/treatment field of the registry: decentralized, remote, mobile, digital, virtual and hybrid. We excluded results where these keywords were used in a non-DCT context. In other words, if a keyword was used in conjunction with words from the pre-defined exception list, the case was excluded. Examples of the keywords used in a non-DCT context were as follows: remote ischemic preconditioning, intraoperative mobile gamma camera, dorsal digital flap, volumetric virtual histology intravascular ultrasound, and hybrid immunotherapy.
The exception list was determined by means of natural language processing [18,19]. The non-DCT context was initially manually explored in the preliminary set. This set was randomly sampled at 10% from the results of the keyword-based searches. The sentences in the preliminary set were tokenized after removing stopwords and special characters. Three tokens before and after the keyword were collected with a unique trial identifier (ClinicalTrials.gov NCT number). Tokens used in more than 3 trials in a non-DCT context were included on the exception list. Finally, the remaining cases (90%) were inspected as to whether they contained any keywords from the exception list. All procedures were performed using the nltk library in Python version 3.10.7 (Python Software Foundation, Wilmington, DE, USA).
Interventions used in the identified DCT cases
A network analysis was conducted to analyze the types of interventions used in the identified DCT cases [20,21]. We visualized how tokens, meaningful units of text, were connected within a sentence. The nodes represented tokens and the edges connected the tokens reflecting co-occurrences. The size of each node was proportional to the frequency of the corresponding token. The thresholds for displaying connections among the tokens in the figure were adjusted to avoid over-complexity. The process was performed using the tidytext, tidyr, widyr, tidygraph libraries in R version 4.3.1 (R Foundation, Vienna, Austria) and with the nltk library in Python version 3.10.7 (Python Software Foundation).
Interventions used in the identified DCT cases were analyzed according to the registry’s own classification system of 8 categories: behavioral, device, diagnostic test, dietary supplement, drug, genetic, procedural, and other. If an identified DCT case included interventions from more than one category, the interventions were counted separately. DCT cases with an intervention classified as ‘drug’ were defined as drug-related DCT cases and were subjected to an additional analysis. The drug-related DCT cases were descriptively summarized for the indication, name, and the route of administration of the drugs.
RESULTS
Comparison of DCT guidance publications
Both the FDA and the EMA commonly emphasized that the appropriateness of the decentralized element should be assessed along 2 axes: patient safety and data integrity. First, both regulatory agencies recommended assessing the impact of decentralized elements on patient safety based on the type of the trial, the complexity of the elements performed, and the safety information about the drug. Second, they also recommended that strategies be put in place to reduce potential errors that could compromise data integrity, such as data variability between investigators. The FDA specifically emphasized that the same non-inferiority margin in DCT as in the conventional trial may not be reasonable. The EMA additionally highlighted the importance of balancing the burdens of decentralized elements (Table 1).
Table 1. Criteria for assessing the appropriateness of using decentralized elements (FDA vs. EMA).
| Categories | Attributes | FDA | EMA | ||
|---|---|---|---|---|---|
| Patients’ safety | Similarities | [Considerations for the use of decentralized elements] | |||
| - Consider the type of clinical trial, the trial population, the disease being treated, the condition of the trial participant, the type of medicinal product, the clarity of its safety profile, its developmental stage, and the complexity of the procedures. | |||||
| [Considerations for off-site drug administration] | |||||
| - Consider the route of administration, whether an observation period is required, the need for emergency plans, the preparation of the drug administration, its stability, the storage conditions, the robustness of the shipping system, the ability to self-medicate, the shelf life and the return plan for unused drugs. | |||||
| Differences | [Considerations for procedures at home] | [Considerations for the use of decentralized element] | |||
| - Only if the procedures do not cause an additional risk to trial participants or to the reliability of the data assessment. | - Weigh the burden of the decentralized elements transferred to the participants or investigators against the potential benefits. | ||||
| [Considerations for telehealth visits] | [Considerations for the use of electronic methods] | ||||
| - If the drug does not pose a significant risk to the participants and adverse events can be properly assessed in such a setting. | - Consider the burdens and potential benefits, the vulnerability of the trial population, the complexity of the trial design and the risks associated with the trial-specific interventions. | ||||
| [Hybrid DCT as an alternative to a fully DCT] | |||||
| - Drug administration at trial sites with remote follow-up. | |||||
| - Specify when a telehealth visit is appropriate and when a participant should be seen in person. | |||||
| Data integrity | Similarities | [Potential error due to a decentralized element] | |||
| - Variability of the data depending on the performer. | |||||
| [Strategies for data integrity] | |||||
| - Present an overview of the data flow in the protocol or data management plan. | |||||
| Differences | [Strategies for data integrity: quality control] | [Potential error due to decentralized element] | |||
| - Regularly review the data entered by delegated personnel using methods tailored to its criticality and complexity. | - Exclusion of digitally illiterate people. | ||||
| [Degree of data integrity] | - Missing data. | ||||
| - A non-inferiority margin may not be reasonable to be considered as equal to an on-site trial. | - Sample handling and storage. | ||||
| - Complex data from multiple systems. | |||||
| - Malfunction of the digital tool. | |||||
| - Disrupted decentralized procedure. | |||||
| [Strategies for data integrity] | |||||
| - Make a contingency plan about decentralized elements. | |||||
| - Involve the stakeholders in advance. | |||||
| [Degree of data integrity] | |||||
| - Meet the same expectations as for an on-site trial for an authorization trial. | |||||
FDA, Food and Drug Administration; EMA, European Medicines Agency; DCT, decentralized clinical trial.
The 2 regulatory authorities commonly defined 5 new roles of in relation to DCTs: local personnel, local laboratory, shipping system, software and regulation. Also, they commonly described newly added DCT-specific responsibilities for investigators, sponsors and participants. The FDA additionally addressed the delegation of local personnel and the centralization of shipping. The EMA described more practical details about the education of subjects and the shipping procedures of the drugs (Supplementary Table 1).
The 2 guidance publications also commonly described implementation strategies tailored to decentralized elements. For example, the 2 authorities recommended risk-based data monitoring in general. In particular, for safety data, a clear description about managing actions by whom was commonly recommended. Also, effective communication between stakeholders was emphasized by both authorities. Additionally, the EMA provided detailed strategies for remote consent interviews (Supplementary Table 2).
Overall trends of the identified DCT cases
A total of 15,474 cases were searched using the 6 DCT-specific keywords, and 4,874 DCT cases were identified. The exception list consisted of 153 tokens, of which 78 (51.0%) were for the keyword ‘digital’ (Supplementary Table 3). For example, the term ‘digital,’ which referred to anatomical terms for fingers and the digitization of imaging or surgical equipment, was determined as a non-DCT context.
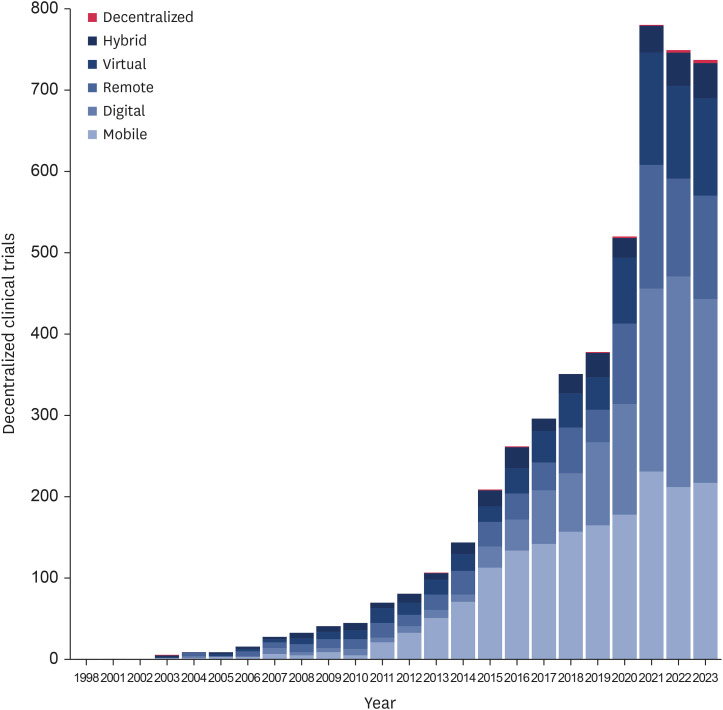
The annual number of DCT cases increased gradually. The first DCT case was identified in 1998 (ClinicalTrials.gov No.: NCT02235389). Since the outbreak of the coronavirus disease 2019 pandemic in 2020, more than 500 DCTs were conducted annually (Figure 1). The most common keywords in the DCT cases were ‘mobile’ 1,762 (36.2%) and ‘digital’ 1,211 (24.8%) (Table 2). The term ‘decentralized’ appeared in 15 cases (0.3%) and the first case was found in 2003 (ClinicalTrials.gov No.: NCT00124085). No DCT case had more than one keyword at the same time.
Figure 1. Identified decentralized clinical trial cases depicted by years and keywords.
Table 2. Identified DCT cases by 6 keywords.
| Keyword | DCT cases |
|---|---|
| Mobile | 1,762 (36.2) |
| Digital | 1,211 (24.8) |
| Remote | 821 (16.8) |
| Virtual | 736 (15.1) |
| Hybrid | 329 (6.8) |
| Decentralized | 15 (0.3) |
| Total | 4,874 (100) |
All data are expressed as a number (proportion %).
DCT, decentralized clinical trial.
Interventions used in the identified DCT cases
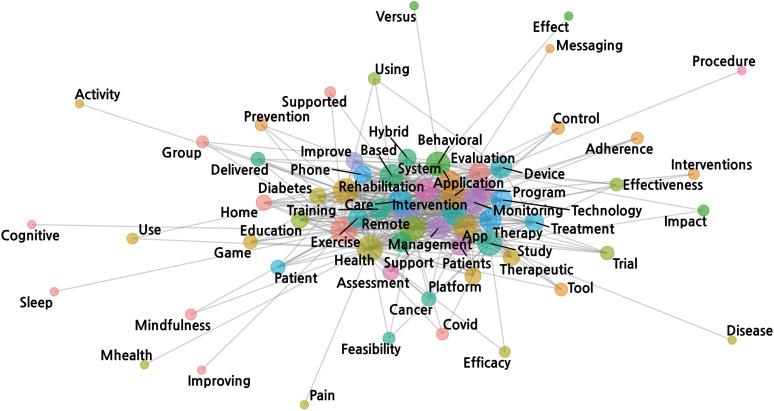
The network analysis demonstrated that the following tokens were frequently described in the interventions in the DCT cases: rehabilitation, exercise, education, monitoring, prevention and training. These words were combined with technology-related words such as app/application, system, platform, and game (Figure 2). In addition, according to the registry’s own classification system, 3,152 interventions were classified as ‘behavioral’ (41.0%), whereas only 143 interventions were classified as ‘drug’ (1.8%) (Table 3).
Figure 2. Network analysis by tokenizing each sentence in the intervention field in the decentralized clinical trial cases. The size of the nodes (circle) indicates the frequency of the tokens. The color of the nodes indicates the group of associations. The edges (grey solid lines) reflect the co-occurrences of the tokens. Fifteen or more used tokens are shown to avoid over-complexity. The 6 keywords are not labelled due to lack of space.
Table 3. Interventions in the identified DCT cases according to the registry’s own classifications.
| Intervention/Treatment | Interventions* | DCT cases† |
|---|---|---|
| Behavioral | 3,152 (41.0) | 1,950 (37.6) |
| Other | 2,337 (30.4) | 1,606 (31.0 |
| Device | 1,516 (19.7) | 1,178 (22.7) |
| Procedure | 351 (4.6) | 219 (4.2) |
| Diagnostic test | 175 (2.3) | 122 (2.4) |
| Drug | 143 (1.8) | 94 (1.8) |
| Dietary supplement | 14 (0.2) | 9 (0.2) |
| Genetic | 3 (0.0) | 3 (0.1) |
| Total | 7,691 (100) | 5,181 (100) |
All data are expressed as a number (proportion %).
DCT, decentralized clinical trial.
*If a trial included interventions from more than one intervention, the researchers allowed for duplicates and counted them all.
†If a trial included interventions from more than one category, the researchers allowed for duplicates and counted them all.
Characteristics of drug-related DCT cases
Among the 94 drug-related DCT cases excluding duplicates, there were 36 indications (Table 4). The most common indication was smoking cessation, with 22 DCT cases involving nicotine replacement therapy, bupropion and varenicline. This was followed by 8 DCT cases for diabetes using metformin and insulin, 7 DCT cases for asthma using albuterol and fluticasone, and 6 DCT cases for human immunodeficiency virus prophylaxis using emtricitabine. There were also 2 DCT cases for spinal cord injury using buspirone and testosterone (ClinicalTrials.gov No.: NCT03576001, NCT04458324) [22].
Table 4. Indications targeted by drug-related DCTs.
| Indications | DCT cases | |
|---|---|---|
| Psychiatry | ||
| Smoking cessation | 2 (23.7) | |
| Schizophrenia | 3 (3.2) | |
| Attention deficit hyperactivity disorder | 3 (3.2) | |
| Major depressive disorder | 3 (3.2) | |
| Drug abuse | 2 (2.2) | |
| Alcohol withdrawal | 1 (1.1) | |
| Cannabis use disorder | 1 (1.1) | |
| Anxiety disorder | 1 (1.1) | |
| Sleep disorder | 1 (1.1) | |
| Endocrinology | ||
| Diabetes | 8 (8.6) | |
| Dyslipidemia | 1 (1.1) | |
| Overweight | 1 (1.1) | |
| Post-menopausal osteoporosis | 1 (1.1) | |
| Infection | ||
| Human immunodeficiency virus prophylaxis | 6 (6.5) | |
| COVID-19 | 5 (5.4) | |
| Malaria chemoprevention | 1 (1.1) | |
| Malaria infection | 1 (1.1) | |
| Pulmonology | ||
| Asthma | 7 (7.5) | |
| Chronic obstructive pulmonary disease | 3 (3.2) | |
| Oncology | ||
| Breast cancer | 2 (2.2) | |
| Cancer (various) | 2 (2.2) | |
| Sickle cell anemia | 1 (1.1) | |
| Cardiology | ||
| Hypertension | 2 (2.2) | |
| Arrhythmia | 1 (1.1) | |
| Myocardial infarction | 1 (1.1) | |
| Neurology | ||
| Spinal cord injury | 2 (2.2) | |
| Cerebral palsy | 1 (1.1) | |
| Essential tremor | 1 (1.1) | |
| Dentistry | ||
| Cavity protection | 2 (2.2) | |
| Gingivitis | 1 (1.1) | |
| Gastroenterology | ||
| Bowel prep | 1 (1.1) | |
| Liver cirrhosis | 1 (1.1) | |
| Rheumatology | ||
| Raynaud’s disease | 1 (1.1) | |
| Dermatology | ||
| Rosacea | 1 (1.1) | |
| Ophthalmology | ||
| Dry eye | 1 (1.1) | |
| Pediatrics | ||
| Respiratory syncytial virus infection | 1 (1.1) | |
All data are expressed as a number (proportion %).
DCT, decentralized clinical trial; COVID-19, coronavirus disease 2019.
Most of the drug-related DCT cases were in phase III trials with previously approved drugs (96.8%). Three cases involved investigational drugs, and all of them were evaluated in phase II trials (ClinicalTrials.gov No.: NCT04423757, NCT05419869, NCT04068792). Six cases were phase I trials, but all of them were repurposing studies with previously approved drugs (ClinicalTrials.gov No.: NCT02018263, NCT02932215, NCT03804840, NCT04525755, NCT05268497, NCT05962632) (Supplementary Table 4).
The most common routes of drug administration were oral (67.2%) and inhalation (12.1%) (Table 5). In only one trial, a paramedic administered intravenous alteplase in an ambulance to a patient for acute myocardial infarction with remote electrocardiogram monitoring (ClinicalTrials.gov No.: NCT02225389) (Supplementary Table 4).
Table 5. Route of administration of the drugs used in drug-related DCTs.
| Route of administration | DCT cases* |
|---|---|
| Oral | 78 (67.2) |
| Inhalation | 14 (12.1) |
| Subcutaneous | 7 (6.0) |
| Topical | 7 (6.0) |
| Transdermal | 5 (4.3) |
| Intramuscular | 3 (2.6) |
| Iontophoresis | 1 (0.9) |
| Intravenous | 1 (0.9) |
| Total | 116 (100) |
All data are expressed as a number (proportion %).
DCT, decentralized clinical trial.
*If a trial used more than one drugs, the researchers allowed for duplicates and counted them all.
DISCUSSION
The FDA and EMA commonly emphasized the need to assess the appropriateness of decentralized elements in terms of patient safety and data integrity. These 2 axes were the main criteria for assessing whether the decentralized elements were in accordance with the guidance. In addition, they suggested newly added roles and responsibilities in relation to DCTs. Also, they both addressed implementation strategies tailored to the decentralized elements.
We found that the keyword ‘decentralized’ was not suitable for searching DCT cases in the clinical trial registry. Instead, ‘remote,’ ‘virtual’ and ‘hybrid’ were more commonly used because they implied the meaning of off-site procedures. Other words such as ‘electronic’ or ‘computer’ were initially considered as candidates. However, these words were not chosen as keywords because doing so led to an excessive number of non-DCT context searches. A limitation of the study is that 6 keywords may not be sufficient to cover all DCT cases.
The decentralized elements used in the identified DCT cases were consistent with the patient safety and data integrity. In the identified DCT cases, most interventions showed low risk and were easy to implement [23,24]. The indications for the drug-related DCT cases were mainly chronic or prophylactic conditions that did not require urgent management [25,26]. The routes of administration of the drugs used in drug-related DCT cases were mostly available for self-administration.
There are still concerns that patient safety or data integrity could be compromised due to decentralized elements [27,28]. Security, compliance with local regulations, and delegation of the study personnel are also critical for DCTs. However, such information was limited in the registry. Further investigations of the technological robustness or capabilities of local personnel are needed.
In conclusion, the number of the DCT cases identified in this study has increased. With the benefit of off-site procedures, DCTs are in line with the current trend of patient-centered clinical trials [29,30]. DCT expands the access of clinical trials to subjects with the limited mobility [22]. Most of the decentralized elements in the identified DCTs used low-risk and simple interventions. These characteristics were consistent with patient safety and data integrity, which are commonly recommended by the FDA and EMA.
Footnotes
Funding: This research was supported by a grant (22113MFDS497) from the Ministry of Food and Drug Safety in 2024.
Reviewer: This article was reviewed by peer experts who are not TCP editors.
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
- Conceptualization: Park J.
- Data curation: Park J.
- Formal analysis: Park J.
- Funding acquisition: Yu KS.
- Investigation: Park J.
- Methodology: Park J.
- Project administration: Park J.
- Resources: Yu KS.
- Software: Park J.
- Supervision: Yu KS, Huh KY, Chung WK.
- Validation: Yu KS, Huh KY, Chung WK.
- Visualization: Park J.
- Writing - original draft: Park J.
- Writing - review & editing: Yu KS, Huh KY, Chung WK.
SUPPLEMENTARY MATERIALS
Additional roles and responsibilities in DCTs (FDA vs. EMA)
Implementation strategies tailored to the decentralized elements (FDA vs. EMA)
Exception list by keyword
The list of drug-related DCT cases
References
- 1.U.S. Food and Drug Administration (FDA) Decentralized clinical trials for drugs, biological products, and devices guidance for industry, investigators, and other stakeholders [Internet] [Accessed December 21, 2023]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/decentralized-clinical-trials-drugs-biological-products-and-devices .
- 2.Cassel GH, Ferris FL., 3rd Site visits in a multicenter ophthalmic clinical trial. Control Clin Trials. 1984;5:251–262. doi: 10.1016/0197-2456(84)90029-1. [DOI] [PubMed] [Google Scholar]
- 3.Orri M, Lipset CH, Jacobs BP, Costello AJ, Cummings SR. Web-based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials. 2014;38:190–197. doi: 10.1016/j.cct.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Lara PN, Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Leira EC, Viscoli CM, Polgreen LA, Gorman M, Kernan WN The IRIS Trial Investigators. Distance from home to research center: a barrier to in-person visits but not treatment adherence in a stroke trial. Neuroepidemiology. 2018;50:137–143. doi: 10.1159/000486315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks MA. Core concept: in the wake of COVID-19, decentralized clinical trials move to center stage. Proc Natl Acad Sci U S A. 2021;118:e2119097118. doi: 10.1073/pnas.2119097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelsey MD, Patrick-Lake B, Abdulai R, Broedl UC, Brown A, Cohn E, et al. Inclusion and diversity in clinical trials: actionable steps to drive lasting change. Contemp Clin Trials. 2022;116:106740. doi: 10.1016/j.cct.2022.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMasi JA, Smith Z, Oakley-Girvan I, Mackinnon A, Costello M, Tenaerts P, et al. Assessing the financial value of decentralized clinical trials. Ther Innov Regul Sci. 2023;57:209–219. doi: 10.1007/s43441-022-00454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washington V, Franklin JB, Huang ES, Mega JL, Abernethy AP. Diversity, equity, and inclusion in clinical research: a path toward precision health for everyone. Clin Pharmacol Ther. 2023;113:575–584. doi: 10.1002/cpt.2804. [DOI] [PubMed] [Google Scholar]
- 10.Danish Medicines Agency (DKMA) The Danish Medicines Agency’s guidance on the implementation of decentralised elements in clinical trials with medicinal products [Internet] [Accessed December 21, 2023]. https://laegemiddelstyrelsen.dk/en/news/2021/guidance-on-the-implementation-of-decentralised-elements-in-clinical-trials-with-medicinal-products-is-now-available/~/media/5A96356760ED408CBFA9F85784543B53.ashx .
- 11.Swissmedic. Position paper on decentralised clinical trials (DCTs) with medicinal products in Switzerland [Internet] [Accessed December 21, 2023]. https://www.swissmedic.ch/dam/swissmedic/fr/dokumente/bewilligungen/klv/positionspapier-dct.pdf.download.pdf/DCT_EN_.pdf .
- 12.European Medicines Agencies (EMA) Recommendation paper on decentralised elements in clinical trials [Internet] [Accessed December 21, 2023]. https://health.ec.europa.eu/system/files/2023-03/mp_decentralised-elements_clinical-trials_rec_en.pdf .
- 13.Clinical Research Malaysia (CRM) Malaysia decentralised clinical trial (DCT) guidance document [Internet] [Accessed December 21, 2023]. https://nmrr.gov.my/storage/media/content/content-attachments/91baf52d-9412-4930-8bc9-2260729c2999/Malaysian-DCT-Guidance-Document.pdf .
- 14.Association of Clinical Research Organizations (ACRO) Bringing the trial to the patient: a quality-by-design manual for decentralized clinical trials [Internet] [Accessed December 21, 2023]. https://www.acrohealth.org/dctqbdmanual/
- 15.Alemayehu D, Hemmings R, Natarajan K, Roychoudhury S. Perspectives on virtual (remote) clinical trials as the “New Normal” to accelerate drug development. Clin Pharmacol Ther. 2022;111:373–381. doi: 10.1002/cpt.2248. [DOI] [PubMed] [Google Scholar]
- 16.Arean PA, Hallgren KA, Jordan JT, Gazzaley A, Atkins DC, Heagerty PJ, et al. The use and effectiveness of mobile apps for depression: results from a fully remote clinical trial. J Med Internet Res. 2016;18:e330. doi: 10.2196/jmir.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angeletti F, Chatzigiannakis I, Vitaletti A. The role of blockchain and IoT in recruiting participants for digital clinical trials; Proceedings of 2017 25th International Conference on Software, Telecommunications and Computer Networks (SoftCOM); 2017 September 21–23; Split, Croatia. New York (NY): IEEE; 2017. pp. 1–5. [Google Scholar]
- 18.Farkiya A, Saini P, Sinha S, Desai S. Natural language processing using NLTK and wordNet. Int J Comput Sci Inf Technol. 2015;6:5465–5469. [Google Scholar]
- 19.Wang M, Hu F. The application of NLTK library for python natural language processing in corpus research. Theory Pract Lang Stud. 2021;11:1041–1049. [Google Scholar]
- 20.Ahmed MM, Tazyeen S. Biological networks in human health and disease. Singapore: Springer; 2023. Role of R in biological network analysis; pp. 91–110. [Google Scholar]
- 21.Srivastava H, Ferrell D, Popescu GV. NetSeekR: a network analysis pipeline for RNA-Seq time series data. BMC Bioinformatics. 2022;23:54. doi: 10.1186/s12859-021-04554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guha L, Kumar H. Drug repurposing for spinal cord injury: progress towards therapeutic intervention for primary factors and secondary complications. Pharmaceut Med. 2023;37:463–490. doi: 10.1007/s40290-023-00499-3. [DOI] [PubMed] [Google Scholar]
- 23.Sysko R, Bibeau J, Boyar A, Costello K, Michaelides A, Mitchell ES, et al. A 2.5-year weight management program using Noom Health: protocol for a randomized controlled trial. JMIR Res Protoc. 2022;11:e37541. doi: 10.2196/37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chrisman SP, Bollinger BJ, Mendoza JA, Palermo TM, Zhou C, Brooks MA, et al. Mobile Subthreshold Exercise Program (MSTEP) for concussion: study protocol for a randomized controlled trial. Trials. 2022;23:355. doi: 10.1186/s13063-022-06239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruse GR, Joyce A, Yu L, Park ER, Neil J, Chang Y, et al. A pilot adaptive trial of text messages, mailed nicotine replacement therapy, and telephone coaching among primary care patients who smoke. J Subst Use Addict Treat. 2023;145:208930. doi: 10.1016/j.josat.2022.208930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spears CA, Mhende J, Hawkins C, Do VV, Hayat MJ, Eriksen MP, et al. Mindfulness-based smoking cessation delivered through telehealth and text messaging for low-income smokers: protocol for a randomized controlled trial. JMIR Res Protoc. 2022;11:e35688. doi: 10.2196/35688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbar S, Coiera E, Magrabi F. Safety concerns with consumer-facing mobile health applications and their consequences: a scoping review. J Am Med Inform Assoc. 2020;27:330–340. doi: 10.1093/jamia/ocz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh KY, Chung WK, Park J, Lee S, Kim MG, Oh J, et al. Feasibility study for a fully decentralized clinical trial in participants with functional constipation symptoms. Clin Transl Sci. 2023;16:2177–2188. doi: 10.1111/cts.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma NS. Patient centric approach for clinical trials: current trend and new opportunities. Perspect Clin Res. 2015;6:134–138. doi: 10.4103/2229-3485.159936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benbow JH, Rivera DR, Lund JL, Feldman JE, Kim ES. Increasing inclusiveness of patient-centric clinical evidence generation in oncology: real-world data and clinical trials. Am Soc Clin Oncol Educ Book. 2022;42:1–11. doi: 10.1200/EDBK_350574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional roles and responsibilities in DCTs (FDA vs. EMA)
Implementation strategies tailored to the decentralized elements (FDA vs. EMA)
Exception list by keyword
The list of drug-related DCT cases