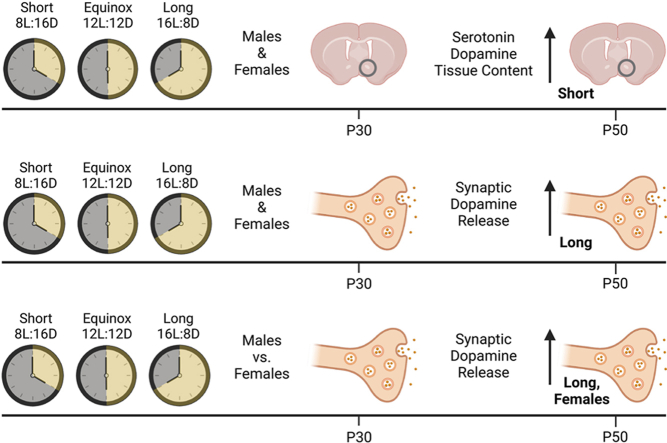
Abstract
Day length, or photoperiod, is a reliable environmental cue encoded by the brain's circadian clock that indicates changing seasons and induces seasonal biological processes. In humans, photoperiod, age, and sex have been linked to seasonality in neuropsychiatric disorders, as seen in Seasonal Affective Disorder, Major Depressive Disorder, and Bipolar Disorder. The nucleus accumbens is a key locus for the regulation of motivated behaviors and neuropsychiatric disorders. Using periadolescent and young adult male and female mice, here we assessed photoperiod's effect on serotonin and dopamine tissue content in the nucleus accumbens core, as well as on accumbal synaptic dopamine release and uptake. We found greater serotonin and dopamine tissue content in the nucleus accumbens from young adult mice raised in a Short winter-like photoperiod. In addition, dopamine release and clearance were greater in the nucleus accumbens from young adult mice raised in a Long summer-like photoperiod. Importantly, we found that photoperiod's effects on accumbal dopamine tissue content and release were sex-specific to young adult females. These findings support that in mice there are interactions across age, sex, and photoperiod that impact critical monoamine neuromodulators in the nucleus accumbens which may provide mechanistic insight into the age and sex dependencies in seasonality of neuropsychiatric disorders in humans.
Keywords: Photoperiod, Adolescence, Dopamine, Nucleus accumbens, Sex differences
Graphical abstract
Highlights
-
•
Short, winter-like photoperiod enhances serotonin and dopamine content in the NAc in adult mice.
-
•
Long, summer-like photoperiod enhances synaptic dopamine release in the NAc of adult mice.
-
•
Effects of photoperiod on dopamine tissue content and dopamine release are age and sex-specific to adult female mice.
1. Introduction
Photoperiodism is the response of organisms to seasonal changes in day length, or circadian photoperiod. Seasonality of neuropsychiatric disorders is a compelling example of human photoperiodism where photoperiod strongly influences physiology and behavior. There is converging evidence that photoperiod may modulate affective behaviors through its effects on the serotonin and dopamine systems (Wirz-Justice, 1974; Carlsson et al., 1980; Rosenthal et al., 1998; Neumeister et al., 2001; Praschak-Rieder et al., 2008; Willeit et al., 2008; Eisenberg et al., 2010; Kalbitzer et al., 2010; Otsuka et al., 2014; Goda et al., 2015; Green et al., 2015; McMahon et al., 2016; Tyrer et al., 2016). However, it is not well understood what interactions there may be between age, sex, and photoperiod impacting these neuromodulatory systems.
The nucleus accumbens (NAc) is a key node within the reward circuitry where serotonin and dopamine play important roles in mediating motivated behaviors (Floresco, 2015; Salgado and Kaplitt, 2015; Turner et al., 2018). Within the NAc, dopamine and serotonin are contained in and released from axon terminals originating in the ventral tegmental area (VTA) and dorsal raphe nucleus (DRN), respectively (Salgado and Kaplitt, 2015; Klawonn and Malenka, 2018; Turner et al., 2018). During adolescence, NAc dopamine tissue content has yet to reach mature levels and is still increasing (Reynolds and Flores, 2021). Released dopamine is rapidly taken back up by the dopamine transporter (DAT) (Giros et al., 1996; Jones et al., 1998) where it is repackaged for future release by the vesicular monoamine transporter (VMAT) (Erickson et al., 1992; Nolan et al., 2020). In adult rodents there are well-established sex differences in the mesolimbic dopamine system (Zachry et al., 2021; Jameson et al., 2023), where females show enhanced dopamine neuron activity, synaptic release, uptake by DAT, and enhanced VMAT activity compared to males (Walker et al., 2000; Dluzen and McDermott, 2008). However, the question remains as to whether the effect photoperiod has on the dopamine and serotonin systems differs by sex or age— two important factors that contribute to the risk of developing neuropsychiatric disorders.
Here, we describe the impact of age and sex on the effect of photoperiod on serotonin and dopamine in the NAc. Using periadolescent and young adult male and female mice raised in seasonally relevant photoperiods, our findings reveal interactions across age, photoperiod, and sex, that impact critical monoamine neuromodulators in the NAc.
2. Materials and methods
2.1. Animals and housing
Male and female C3Hf+/+ mice were used as these animals are melatonin-producing and lack the retinal degeneration alleles of the parent C3H strain (Ebihara et al., 1986; Goto et al., 1989; Tosini and Menaker, 1998; Contreras-Alcantara et al., 2010). Mice developed under Equinox (12 h of light and 12 h of darkness), Short winter-like (8 h of light and 16 h of darkness), or Long summer-like (16 h of light and 8 h of darkness) photoperiods. Light intensity for all photoperiod conditions was approximately 100 lux, and animals were continuously maintained in these photoperiod conditions from embryonic day 0 (E0) until they were used for experiments. Experimental animals consisted of two age groups: periadolescence (ranging from P30–P40) or young adulthood (ranging from P50–P90); three photoperiod groups: Equinox, Short, and Long photoperiod mice, and both sexes. All tissues were isolated within an hour of the mid-point of the light period on all light cycles. With lights off serving as zeitgeber time (ZT) 12, experiments were performed at ZT8 for Short, ZT6 for Equinox, and ZT4 for Long photoperiod animals. We treated lights off as zeitgeber time (ZT) 12 as our animal model is nocturnal, in which lights off is the relevant circadian cue. Work by Stowe et al. (2022); Ferris et al. (2014) found that diurnal rhythms of DA release peak at midday. Based on this work, and our own previous methods, we conducted all experiments at the midday time point. Short, Equinox, and Long photoperiods consisted of 8, 12, and 16 h of light respectively. Midday for each of these photoperiods would be 4, 6, and 8 h before lights off, respectively. Therefore, experiments occurred 4 h before lights off at ZT8 for Short, 6 h before lights off at ZT6 for Equinox, and 8 h before lights off at ZT4 for Long. Experiments were performed in accordance with the Vanderbilt University Institutional Animal Care and Use Committee and National Institutes of Health guidelines.
2.2. Fast-scan cyclic voltammetry
Ex vivo FSCV was used to assess dopamine release and clearance in the NAc core as previously described (Jameson et al., 2023) Mice were euthanized under isoflurane anesthesia. Using a Leica Vibratome, 250 μm-thick sagittal sections containing the NAc core were collected from whole brain tissue in oxygenated (95% O2; 5% CO2) artificial cerebrospinal fluid (aCSF) containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.2 MgCl2, 25 NaHCO3, 11 glucose, 0.4 L-ascorbic acid, and pH adjusted to 7.4. Slices were transferred to a chamber containing oxygenated aCSF. All experiments were performed using a Scientifica SliceScope Pro System in 32 °C aCSF with a flow rate of 2 mL/min. The carbon fiber microelectrode (100−200 μM length, 7 μM radius) and bipolar stimulating electrode were placed in close proximity in the NAc core. A single electrical pulse (750 μA, 4ms, monophasic) was applied to the tissue every 5 min to evoke dopamine release. We also evoked dopamine release using a train of 5 pulses at 5 Hz, 10 Hz, and 20 Hz (750 μA, 4ms, monophasic) every 5 min. Extracellular dopamine was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). Once peak evoked dopamine release reached a stable baseline (3 collections with <10% variability), peak amplitude (nA), and decay time (tau = time to fall to 33% of peak height; units in seconds). Tau values were derived from exponential fit curves based on the peak and post-peak baseline using the least squares constrained exponential fit algorithm (National Instruments) as previously described (Yorgason et al., 2011). Electrodes were replaced once every ∼7 days. For each electrode, we sampled across age, sex, and photoperiod groups to mitigate any potential impact of electrode background current variations.
2.3. Monoamine analysis
NAc-enriched tissue was collected from mouse brains (n = 10–14). The tissue was placed in 1.5 mL tubes and frozen in liquid nitrogen. Biogenic amine analysis was performed on the NAc tissue by the Vanderbilt Neurochemistry Core Laboratory. Briefly, samples were stored at −80 °C, then the tissue was homogenized with a tissue dismembrator, in 100–750 μl of 0.1M TCA. This contained 10−2 M sodium acetate, 10−4 M EDTA, 5 ng/mL isoproterenol (as internal standard) and 10.5 % methanol (pH 3.8). Samples were then spun in a microcentrifuge at 10000×g for 20 min with the supernatant being removed and stored at −80 °C. Supernatant was then thawed and spun for 20 min and the samples of the supernatant were analyzed for biogenic amines. These amines were determined by a specific HPLC assay using an Antec Decade II (oxidation: 0.4) (3 mm GC WE, HYREF) electrochemical detector operated at 33 °C. 20 μl samples of the supernatant were injected using a Water 2707 autosampler onto a Phenomenex Kintex (2.6u, 100A) C18 HPLC column (100 × 4.60 mm), biogenic amines were eluted with a mobile phase consisting of 89.5 % 0.1 M TCA, 10−2 M sodium acetate, 10−4 M EDTA and 10.5 % methanol (pH 3.8) and the solvent was then delivered at 0.6 mL/min using a Waters 515 HPLC pump. Using this HPLC solvent the following biogenic amines eluted were: DOPAC, dopamine, 5-HIAA, and serotonin. HPLC control and data acquisition were managed by Empower software. Methods were the same as those previously published (Siemann et al., 2019).
2.4. Statistical analysis
2.4.1. FSCV experiments
To assess the effects of sex, photoperiod, and age on synaptic dopamine dynamics, we employed a statistical analysis framework consistent with the study's objectives and the nature of the data. MANOVA is commonly believed to control Type I error rates when analyzing multiple dependent variables simultaneously (Smith et al., 2020; Warne, 2014). MANOVA and ANOVA answer distinct questions, with MANOVA focusing on overall group differences across multiple dependent variables and ANOVA specifically testing differences in means of individual variables. In our study, the primary interest lay in examining the differences between means based on sex, photoperiod, and age, making ANOVAs the more suitable choice given our sample sizes.
Statistical significance was determined by two-way ANOVA with an alpha value of 0.05. We used Tukey's or Holm-Sidak's multiple comparison tests for all post hoc analysis and standard error of the mean was used for all experiments. Demon voltammetry and analysis software (Yorgason et al., 2011) was used to analyze all FSCV data. Data were analyzed to determine peak height and tau as previously described (Yorgason et al., 2011; Everett et al., 2022; Jameson et al., 2023).
2.4.2. Monoamine analysis experiments
Prism 8 (Graphpad Software Inc., La Jolla, CA) was used for all statistical analyses. Statistical significance was determined by two-way ANOVA with an alpha value of 0.05 using the same statistical framework as aforementioned. We used Holm-Sidak's multiple comparison tests for all post hoc analysis and standard error of the mean was used for all experiments.
3. Results
3.1. Serotonin and dopamine content in the NAc is elevated by Short Winter-like photoperiod in an age-dependent manner
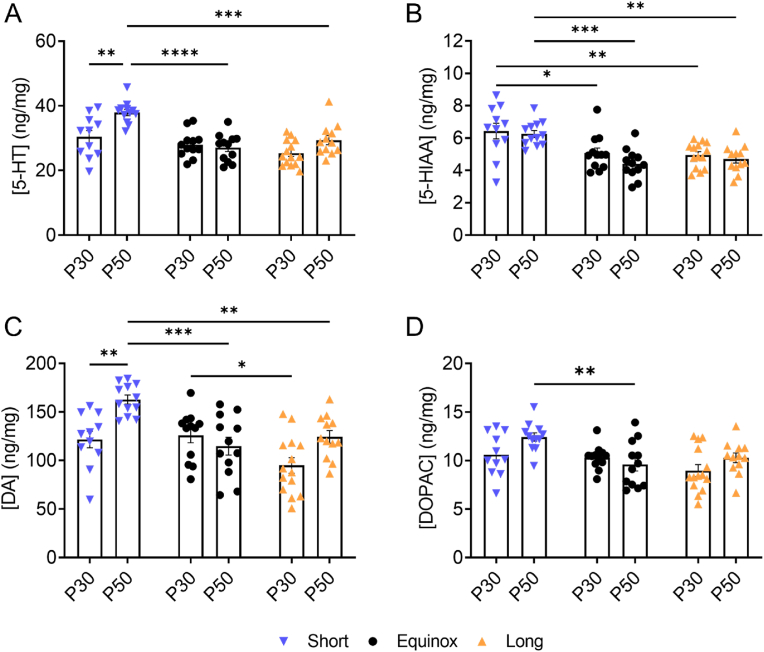
To test for age effects on photoperiod modulation of NAc monoamine content we evaluated serotonin and dopamine content along with their main metabolites, 5-hydroxyindoleacetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC), respectively (Cumming et al., 1992) in NAc punches isolated from periadoleascent (P30-40) and young adult (P50-90) male and female mice raised in different photoperiods (Short L:D 8:16; Equinox L:D 12:12; Long L:D 16:8). For 5-HT content, using a two-way ANOVA, we found a significant main effect of photoperiod (F (2, 67) = 17.15, p < 0.0001), a main effect of age (F (1, 67) = 11.03, p = 0.0015), and an interaction effect (F (2, 67) = 5.220, p = 0.0078) (Fig. 1A). Multiple comparison post hoc test revealed that 5-HT content was significantly elevated in NAc from young adults in Short vs. Long (p = 0.0002), and Short vs. Equinox (p < 0.0001) groups, as well as significantly increased in Short photoperiod in the young adult vs. periadolescent age groups (p = 0.0019). For 5-HIAA concentrations there was a significant main effect of photoperiod (F (2, 67) = 18.49, p < 0.0001) but no significant effect of age F(1, 67) = 2.341, p = 0.1307) (Fig. 1B). Post hoc tests showed increased NAc 5-HIAA content in Short vs Long (p = 0.0061), and in Short vs Equinox (p = 0.0189) within the periadolescence age group. Within the young adult age group, significant increases in 5-HIAA content were found between Short vs. Long (p = 0.0049), and between Short vs. Equinox (p = 0.0005) photoperiods. Dopamine content exhibited a significant main effect of photoperiod (F(2, 67) = 9.586, p = 0.0002), a main effect of age (F (1, 67) = 10.41, p = 0.0019), and an interaction effect (F (2, 67) = 6.453, p = 0.0027) (Fig. 1C). Significant increases in NAc dopamine content were observed in periadolescent mice in Equinox vs. Long (p = 0.0381) photoperiods. In young adults, significant increases in dopamine were found between Short vs. Long (p = 0.0078), and between Short vs. Equinox (p = 0.0004) photoperiods. We also found a significant increase in NAc dopamine content in the young adult vs. periadolescent age groups in the Short photoperiod animals (p = 0.0048). For DOPAC concentrations, a significant main effect of photoperiod (F (2, 67) = 6.611, p = 0.0024) and a non-significant effect of age (F (1, 67) = 3.198, p = 0.0783) were found (Fig. 1D). In young adults, a significant increase in NAc DOPAC was found between Short vs. Equinox (p = 0.0083) photoperiods. Together these results indicate that photoperiod modulates monoamine tissue content in the NAc in an age-dependent manner, in which Short photoperiod elevates NAc serotonin, 5-HIAA, dopamine, and DOPAC in the young adult age group.
Fig. 1.
Serotonin and Dopamine Content in the NAc is Elevated by Short Winter-like Photoperiod in an Age-Dependent Manner: Serotonin, 5-HIAA, dopamine, and DOPAC content in the NAc of Short, Equinox and Long periadolescent (P30) and young adult (P50) mice. A) For serotonin, there was a main effect of photoperiod (F (2, 67) = 17.15, p < 0.0001), age (F (1, 67) = 11.03, p = 0.0015), and an interaction effect (F (2, 67) = 5.220, p = 0.0078). B) For 5-HIAA concentrations, there was a main effect of photoperiod (F (2, 67) = 18.49, p < 0.0001). C) For dopamine content, there was a main effect of photoperiod (F(2, 67) = 9.586, p = 0.0002), age (F (1, 67) = 10.41, p = 0.0019), and an interaction effect (F (2, 67) = 6.453, p = 0.0027) D) For DOPAC concentrations, we found a main effect of photoperiod (F (2, 67) = 6.611, p = 0.0024). Data are represented as mean±SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Males: Short, P30 (N = 4), Short, P50 (N = 2); Equinox, P30 (N = 7), Equinox, P50 (N = 3); Long, P30 (N = 5), Long P50 (N = 5); Females: Short, P30 (N = 7), Short, P50 (N = 10); Equinox, P30 (N = 5), Equinox, P50 (N = 9); Long, P30 (N = 9), Long P50 (N = 7).
3.2. Synaptic dopamine release and clearance in the NAc Are Increased by Long Summer-like photoperiod in an age-dependent manner
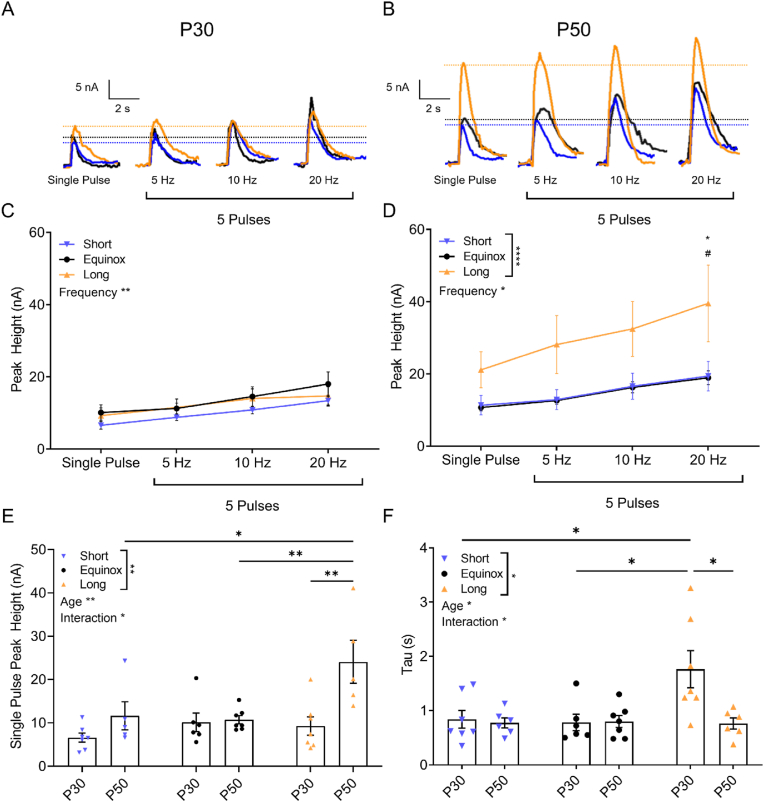
To further assess whether synaptic release and uptake of dopamine in the NAc may be differentially regulated by photoperiod we used Fast Scan Cyclic Voltammetry (FSCV). Electrically evoked synaptic dopamine release from VTA dopaminergic fibers was measured using FSCV in brain slices containing the NAc from Short, Equinox, and Long photoperiod periadolescent and young adult male and female mice. VTA dopamine neurons that project to the NAc have two functionally distinct firing modes: spontaneous, low frequency tonic firing, and high frequency phasic burst firing (Ljungberg et al., 1992; Schultz, 1998, 2007). To determine whether effects of photoperiod were dependent on firing mode, we stimulated dopamine release across a range of tonic and phasic frequencies (single pulses to 20Hz). In the periadolescent age group, two-way ANOVA revealed a significant main effect of frequency of stimulation (F(3, 68) = 5.981; p = 0.0011), and a trend-level main effect of photoperiod (F(2, 68) = 3.059, p = 0.0535) (Fig. 2A–C). Short, Equinox, and Long photoperiod young adult mice showed more striking differences in dopamine release exhibiting a significant main effect of photoperiod (F (2, 64) = 13.32; p < 0.0001), and frequency of stimulation (F (3, 64) = 3.172; p = 0.0301). There was a significant increase in peak dopamine release for the phasic 20 Hz stimulation in Long vs. Short photoperiod (p = 0.0111), as well as between Long vs. Equinox photoperiod (p = 0.0102) (Fig. 2B–D). In addition, in the single pulse condition there were also significant main effects of photoperiod (F (2, 31) = 5.374; p = 0.0099), and of age (F (1, 31) = 11.76; p = 0.0017), as well as a significant interaction effect (F (2, 31) = 4.453; p = 0.02). Post-hoc tests revealed significantly elevated dopamine release in young adult vs periadolescent age groups in the Long photoperiod condition (p = 0.0027), as well as significant increases within the young adult mice as follows: Long vs. Short (p = 0.0272), Long vs. Equinox (p = 0.0074) (Fig. 2E). Thus, while evoked dopamine release was increased at higher stimulation frequencies as expected, Long photoperiod induced increases in dopamine release at both 20Hz and in response to single pulses, suggesting photoperiod modulation occurs at both phasic and tonic frequencies. In addition, significant photoperiod effects were only observed in young adult mice, not in periadolescents.
Fig. 2.
Synaptic Dopamine Release and Clearance in the NAc Are Increased by Long Summer-like Photoperiod in an Age-Dependent Manner: A) Representative current traces across tonic and phasic frequencies in the NAc core of periadolescent (P30) or B) young adult (P50) mice raised in Short, Equinox, or Long photoperiods. C) Evoked NAc core dopamine release across tonic and phasic frequencies in the periadolescent age group showed a main effect of frequency of stimulation (F(3, 68) = 5.981; p = 0.0011). D) In the young adult group, there was a significant main effect of frequency of stimulation (F (3, 64) = 3.172; p = 0.0301) and photoperiod (F (2, 64) = 13.32; p < 0.0001) on evoked NAc dopamine release. E) NAc dopamine release evoked from the single pulse condition showed a main effect of photoperiod (F (2, 31) = 5.374; p = 0.0099), age (F (1, 31) = 11.76; p = 0.0017), as well as an interaction effect (F (2, 31) = 4.453; p = 0.02). F) Tau, the time it takes for the dopamine signal to decay to 1/3 of its peak value, was used to assess dopamine clearance. There was a main effect of photoperiod (F(2,33) = 3.915; p = 0.0298), age (F(1,33) = 5.0; p = 0.0322) and an interaction effect (F(2,33) = 4.369; p = 0.0207). Data are represented as mean±SEM. * = Short vs Long, # = Long vs. Equinox. *p < 0.05, **p < 0.01, ****p < 0.0001. Males: Short, P30 (N = 4), Short, P50 (N = 3); Equinox, P30 (N = 3), Equinox, P50 (N = 3); Long, P30 (N = 3), Long P50 (N = 3). Females: Short, P30 (N = 3), Short, P50 (N = 3), Equinox, P30 (N = 3), Equinox, P50 (N = 4), Long, P30 (N = 4), Long, P50 (N = 3).
We also assessed whether there were age-dependent effects of photoperiod on the rate of dopamine clearance in the NAc by measuring tau, the time it takes for the dopamine release signal to decay to 1/3 of it's peak value. Indeed, there were significant main effects of photoperiod (F(2,33) = 3.915; p = 0.0298), age (F(1,33) = 5.0; p = 0.0322), and a significant interaction effect (F(2,33) = 4.369; p = 0.0207). There were significant increases in tau (decreases in the rate of clearance) across photoperiods in the periadolescent age group as follows: Long vs. Short (p = 0.0128); Long vs. Equinox (p = 0.0115) with tau being greater in Long photoperiod in each case. We also found a significant increase in tau in the Long photoperiod periadolescent animals vs young adults (p = 0.0115) (Fig. 2F). Together, these results indicate that photoperiod impacts synaptic dopamine release and clearance in an age-dependent manner, where Long photoperiod enhances release in the young adult age group, and decreases dopamine clearance in the periadolescent group.
3.3. Photoperiod effects on NAc Dopamine Tissue Content and Release Are Sex-dependent
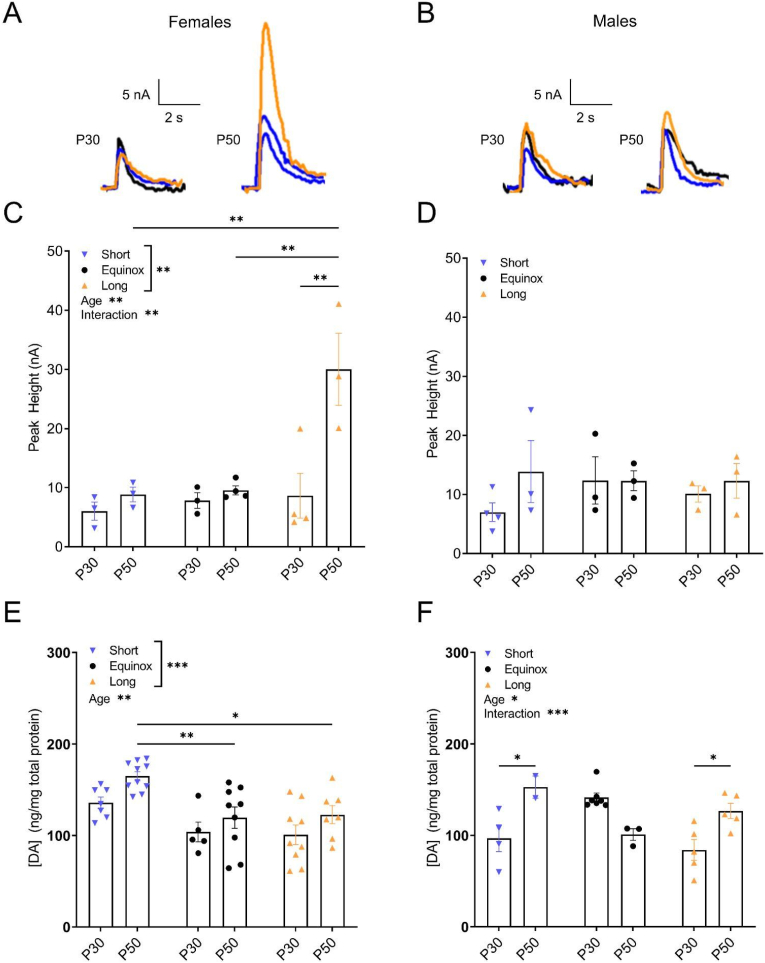
We have previously shown that photoperiod has a sex-specific effect on synaptic dopamine release in the NAc of young adult mice (Jameson et al., 2023). Therefore, to test for sex-specific effects here we separated male and female NAc DA release data. This uncovered striking sex-specific effects of photoperiod that were age-dependent. NAc dopamine release in females showed significant main effects of photoperiod (F (2, 14) = 9.144; p = 0.0029), and of age (F (1, 14) = 11.75; p = 0.0041), as well as a significant interaction effect (F (2, 14) = 6.596; p = 0.0096). Post-hoc tests revealed significant increases in dopamine release in the young adult vs. periadolescent age group in Long photoperiod females (p = 0.0026). There were also significant increased in dopamine release in the young adult females between Long vs. Short photoperiods (p = 0.0041) as well as between Long vs. Equinox photoperiods (p = 0.0034) (Fig. 3A–C). In contrast, when we assessed NAc dopamine release in the males, we did not find any significant main effects of photoperiod (F(2, 13) = 0.2062, p = 0.8162) or age (F(1, 13) = 1.485, p = 0.245) (Fig. 3B–D). Taken together these results independently reproduce our previous finding that NAc dopamine release is increased by Long photoperiod in female mice, and in addition demonstrate that this effect is specific to young adult females.
Fig. 3.
Photoperiod Effects on NAc Dopamine Tissue Content and Release Are Sex-Dependent: A) Representative current traces evoked from a single pulse in the NAc core from female and B) male periadolescent and young adult mice raised in Short, Equinox, or Long photoperiod. C) NAc core dopamine release in the females showed a main effect of photoperiod (F (2, 14) = 9.144; p = 0.0029), age (F (1, 14) = 11.75; p = 0.0041), and an interaction effect (F (2, 14) = 6.596; p = 0.0096). D) NAc core dopamine release in the males did not show significant differences by age or photoperiod. E) NAc dopamine tissue content in females showed a main effect of photoperiod (F(2,41) = 11.21; p = 0.0001) and age (F(1,41) = 7.885; p = 0.0076). F) NAc dopamine tissue content in males showed a main effect of age (F(1,20) = 5.174; p = 0.034) as well as an interaction effect (F(2,20) = 13.19; p = 0.0002). Data are shown as mean±SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Females: Short, P30 (N = 3), Short, P50 (N = 3); Equinox, P30 (N = 3), Equinox, P50 (N = 4); Long, P30 (N = 4), Long P50 (N = 3). Males: Short, P30 (N = 4), Short, P50 (N = 3); Equinox, P30 (N = 3), Equinox, P50 (N = 4); Long, P30 (N = 3), Long P50 (N = 3).
Due to these sex-specific effects of developmental photoperiod on NAc dopamine release, we further assessed whether the age-dependent photoperiod effects on dopamine tissue content in Fig. 1 could also be sex-specific by analyzing those data post-hoc by sex. When we analyzed post hoc male and female NAc DA content separately, we found that the female mice showed a significant main effect of photoperiod (F(2,41) = 11.21; p = 0.0001), with DA content in Short photoperiod elevated, and of age (F(1,41) = 7.885; p = 0.0076) with DA content in young adults elevated. There were significant increases female young adults as follows: Short vs Equinox (p = 0.007), Short vs. Long (p = 0.0278) (Fig. 3E). While we did not find a significant main effect of photoperiod on NAc DA content in the male mice (F(2, 20) = 2.193, p = 1376), we did find a significant main effect of age (F(1,20) = 5.174; p = 0.034), with young adults being greater, and a significant interaction effect (F(2,20) = 13.19; p = 0.0002). Additionally, post hoc tests revealed significant decreases in NAc DA content between the periadolescent vs. young adult age group within Short (p = 0.0461) and Long (p = 0.036) photoperiod males, as well as increases within the periadolescent condition as follows: Equinox vs. Short (p = 0.0261), Equinox vs. Long (p = 0.0013) (Fig. 3F). While we must be cautious in interpreting this post-hoc analysis because of limited numbers in some sex x age x photoperiod groups, these results do suggest that photoperiod has sex-dependent effects on dopamine tissue content that may parallel the sex-dependent photoperiod effects on release found here and in Jameson et al., (2023). Dopamine release is enhanced in Long photoperiod females while dopamine tissue content is enhanced in Short photoperiod females. Whereas in males, we found no main effects of photoperiod, but an interaction between age and photoperiod. Males raised in Short or Long photoperiod had increased dopamine tissue content in the young adults, while the Equinox males did not show any difference in dopamine content across age.
4. Discussion
Here we investigated interactions of photoperiod, age, and sex and their influence on neuromodulatory systems in the NAc by assessing serotonin and dopamine tissue content and evoked dopamine release and uptake within the NAc of periadolescent and young adult male and female mice raised in seasonally relevant photoperiods. We found that both maturational age from periadolescent to young adult, and sex impacted the photoperiodic regulation of these key neuromodulators in the NAc. Taken together, our results demonstrate that photoperiod differentially regulates NAc serotonin and dopamine in males and females in an age-dependent manner.
Photoperiod had age-dependent effects on serotonin tissue content in the NAc. Long photoperiod sequentially programs elevated serotonin neuron activity and serotonin tissue content in the DRN through development, and concomitantly Long photoperiod reduces depression-like and anxiety like behaviors in melatonin competent mice (Green et al., 2015; Siemann et al., 2019, 2020). With evidence that Long photoperiod enhances serotonergic content both in the DRN and midbrain (Green et al., 2015; Siemann et al., 2019, 2020), we were intrigued to find that serotonin and 5-HIAA tissue content in the Short photoperiod animals was elevated in the NAc. Our results reveal that the effects of photoperiod may reflect differential regulation of serotonin content in the DRN and regulation of serotonin content and signaling at terminals within the NAc.
In addition to serotonin content, we found a concomitant increase in NAc dopamine tissue content in young adult mice raised under Short photoperiod. Previous work from our lab showed that mice raised under Short photoperiod exhibited a greater depressive-like phenotype than mice raised in Long photoperiod (Green et al., 2015). Studies have reported elevated NAc serotonin and dopamine tissue content in a rat model of depression, and chronic treatment with antidepressants was able to normalize serotonin and dopamine levels (Zangen et al., 1997, 1999, 2001; Yadid et al., 2000). Considering these results, our findings that NAc serotonin and dopamine content is enhanced by Short photoperiod suggest their potential involvement in underlying mechanisms of photoperiod-driven changes in depressive-like behaviors (Green et al., 2015).
We found here, and in previous work from our group (Jameson et al., 2023), that synaptic NAc dopamine release is enhanced in female mice raised under Long summer-like photoperiod. In the present study, we replicated these findings in young adult mice, and also found that these sex-specific effects of photoperiod were age-dependent. Sex differences can emerge at several critical periods of development, including genetic influence from sex chromosomes during gestation, pre-pubertal sex hormone action during development, sex hormone action during the peripubertal period, and sex hormone action in adulthood (Becker et al., 2005; McCarthy and Arnold, 2011; Becker and Chartoff, 2019). It is important to note that the peripubertal period in rodents is between P30–P40 (Becker, 2009), which is the same age range at which we collected data from the periadolescent animals. Peripubertal female sex hormones have a direct impact on the sexual differentiation of the mesolimbic dopamine system that is separate from the pre-pubertal sexual differentiation of these systems which occurs earlier during development (Becker, 2009). Because we did not observe marked differences across photoperiod in our periadolescent age group, it is plausible that our female sex-specific photoperiod effects are age dependent due to the involvement of peripubertal female sex hormones or due to mature female sex hormone fluctuations through the estrous cycle, though we did not assess this in the current study. Future studies are necessary to explore the interaction of photoperiod and biological sex to determine how these factors may work in concert to modulate rhythms in monoaminergic systems, whether it occurs through sex hormones, sex chromosomes, sexual differentiation or developmental trajectory, among other possibilities (Arnold and Chen, 2009).
It is crucial to exercise caution when extrapolating age-dependent conclusions from our results, given the close proximity of these age groups. While our study revealed distinct age-dependent effects between 'periadolescent' and 'young adult' groups, the limited 10-day separation between them necessitates caution in generalizing age-specific conclusions. It is also crucial to acknowledge the inherent variability within the periadolescent age group, as our sampling did not specifically assess individual animals for sex hormones. Consequently, the P30–P40 age range of the periadolescent age group likely encompasses animals at various stages of peripubescence, introducing potential variability in the data. While our findings demonstrate a clear distinction between the periadolescent and young adult groups, this limitation necessitates caution in generalizing age-specific conclusions. On the other hand, fact that females transition to responsiveness to photoperiod across this relatively narrow time window which correlates with reproductive maturity and the onset of estrus cycling is highly suggestive. Future studies incorporating sex hormone assessment and age stratification will be pivotal in elucidating the nuanced interplay between age and photoperiodic effects on synaptic NAc dopamine release.
Previous work from our group has demonstrated critical periods of prenatal and perinatal development that are sensitive to photoperiod. In these critical periods of development, photoperiod can have lasting impact on circadian clock gene expression, circadian behavior, monoamine gene expression, and monoamine signaling (Ciarleglio et al., 2011; Siemann et al., 2021; Siemann et al., 2020; Siemann et al., 2019). Recently, using the same mouse model, we have shown that photoperiod sequentially programs the serotonin system during prenatal and postnatal development (Siemann, et al., 2019). Exposure to Long photoperiod prenatally programs increased DRN serotonin neuron excitability into adolescence and adulthood, while continuing postnatal exposure to Long photoperiod enhanced serotonin content and was protective against depressive-like behaviors (Siemann, et al., 2019). In the present study, dams selected for breeding experimental animals were housed in the same conditions as the experimental offspring. This ensured that all stages of development, from prenatal to postnatal, occurred within a consistent photoperiodic environment. Therefore, it is likely that the photoperiod effects we see here are due to combined effects of prenatal and postnatal exposure to photoperiod, and future studies are necessary to determine the developmental mechanisms and critical periods involved.
The hormone melatonin is synthesized and released upon the onset of darkness by the pineal gland, and is a key photoperiodic signal. Melatonin has been shown to play a lasting role in development, as late gestational maternal-fetal melatonin signaling can influence gonadal development later in the offspring (Goldman, 2003; van Dalum et al., 2020; Siemann et al., 2019). Previously, our group found that melatonin signaling is necessary to observe photoperiod effects in DRN serotonin neurons and photoperiod-induced depressive-like behaviors (Green et al., 2015). In that study, melatonin receptor 1 (MT1) knockout mice raised in Short, Equinox, and Long photoperiods did not show differences in DRN serotonin neuron firing rate, serotonin content, or affective behavior (Green et al., 2015). Another group found that, similar to exposure to short photoperiod, exogenous melatonin signaling administered to mimic short photoperiod can alter hippocampal dendritic morphology, impair spatial learning and memory, and impair hippocampal long term potentiation (LTP) in mice (Walton et al., 2013). From these studies it is clear that melatonin plays a role in photoperiodic modulation of circuit function and behavior in mice. In the present study, melatonin may be necessary upstream of the NAc for photoperiod to modulate dopamine, but more studies are necessary to determine the role of melatonin signaling here.
It is important to consider distinctions between measures of dopamine tissue content and evoked dopamine release. Dopamine tissue content, as measured by HPLC, captures both extracellular and intracellular dopamine to give a total dopamine concentration. In contrast, FSCV measures synaptic dopamine release and clearance on a millisecond time-scale. Studies have shown that dopamine synthesis rate can change in the opposite direction as dopamine tissue content (Gainetdinov et al., 1998), dopamine metabolism does not necessarily correlate with extracellular dopamine (Sharp et al., 1986), and that dopamine release can be inversely correlated to extracellular dopamine (Ferris et al., 2014). Therefore, while both techniques assess dopamine concentration, changes in these measures reflect distinct phenomena that may be differentially regulated by photoperiod. Future studies assessing levels of key enzymes within the dopamine synthesis pathway such as tyrosine hydroxylase (TH), MAOB, and COMT could give more insight to whether synthesis or degradation is impacted by photoperiod independently from synaptic release.
While our study has yielded valuable insights, it is important to acknowledge the limitations imposed by the small sample sizes utilized in some analyses. Given the constraints imposed by the sample size, our study was underpowered for conducting multiple regression analysis, which is the preferred statistical test for examining the relationships between our variables of interest (Warne, 2014). This prompted us to opt for multiple 2-way ANOVAs with post-hoc comparisons to address our research questions. However, we also performed and exploratory multiple regression analysis (Supplemental Figure 1). Notably, the results obtained from the multiple regression analysis were consistent with and supportive of the findings derived from our primary analytical approach using two-way ANOVAs. While acknowledging the inherent limitations of our study design, these findings underscore the robustness and reliability of our main findings.
It is also important to consider the limitations of this study in the context of potential future experiments. One limitation is that we have sampled monoamine content, release and uptake in the NAc at only one time point – the mid-point of each light cycle. Dopamine release is known to be driven in the NAc in a diurnal rhythm, (Ferris et al., 2014) and therefore it is not clear from our current results if the differences in content and release we observe are due to changes in the baseline levels, or the amplitude or timing of any diurnal rhythms that may change with photoperiod. Additional studies using 24-h experiments for content and release conducted with time points samples around the clock will be necessary to clarify the temporal aspects of the differences we have shown here.
5. Conclusion
In summary, we have elucidated novel interactions between photoperiod, age, and sex that influence serotonin and dopamine in the NAc and show that they are regulated differently in male and female mice by photoperiod across periadolescence and young adulthood. This study provides insight for future research examining potential mechanisms of sex-dependence and seasonality in neuropsychiatric disorders.
CRediT authorship contribution statement
Alexis N. Jameson: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Justin K. Siemann: Visualization, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Carrie A. Grueter: Methodology, Data curation, Conceptualization. BradA. Grueter: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Conceptualization. Douglas G. McMahon: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This research was supported by National Institutes of Health Grants NIH R01MH108562 to DGM and NIH R01MH130537 to DGM and BAG, JS was supported by NIMH T32 MH018921, ANJ was supported by NIMH T32 MH064913. The Vanderbilt Neurochemistry Core is supported by NIH U54 HD083211.
Handling Editor: Mark R. Opp
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbscr.2024.100103.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Arnold A.P., Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B. Sexual differentiation of motivation: a novel mechanism? Horm. Behav. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Arnold A.P., Berkley K.J., Blaustein J.D., Eckel L.A., Hampson E., Herman J.P., Marts S., Sadee W., Steiner M., Taylor J., Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019;44:166–183. doi: 10.1038/s41386-018-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A., Svennerholm L., Winblad B. Seasonal and circadian monoamine variations in human brains examined post mortem. Acta Psychiatr. Scand. 1980;61:75–85. doi: 10.1111/acps.1980.61.s280.75. [DOI] [PubMed] [Google Scholar]
- Contreras-Alcantara S., Baba K., Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity. 2010;18:1861–1863. doi: 10.1038/oby.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio C.M., Axley J.C., Strauss B.R., Gamble K.L., McMahon D.G. Perinatal photoperiod imprints the circadian clock. Nat. Neurosci. 2011;14(1):25–27. doi: 10.1038/nn.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P., Brown E., Damsma T., Fibiger H., Cumming P. Formation and clearance of interstitial metabolites of dopamine and serotonin in the rat striatum: an in vivo microdialysis study. J. Neurochem. 1992;59:1905–1914. doi: 10.1111/j.1471-4159.1992.tb11026.x. [DOI] [PubMed] [Google Scholar]
- Dluzen D.E., McDermott J.L. Sex differences in dopamine- and vesicular monoamine-transporter functions: implications for methamphetamine use and neurotoxicity. Ann. N. Y. Acad. Sci. 2008;1139:140–150. doi: 10.1196/annals.1432.010. [DOI] [PubMed] [Google Scholar]
- Ebihara S., Marks T., Hudson D.J., Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science (1979) 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.P., Kohn P.D., Baller E.B., Bronstein J.A., Masdeu J.C., Berman K.F. Seasonal effects on human striatal presynaptic dopamine synthesis. J. Neurosci. 2010;30:14691–14694. doi: 10.1523/JNEUROSCI.1953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.D., Eiden L.E., Hoffman B.J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc. Natl. Acad. Sci. USA. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. https://www.pnas.org Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A.C., Graul B.E., Ronström J.W., Robinson J.K., Watts D.B., España R.A., Siciliano C.A., Yorgason J.T. Effectiveness and relationship between biased and unbiased measures of dopamine release and clearance. ACS Chem. Neurosci. 2022 doi: 10.1021/acschemneuro.2c00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M.J., España R.A., Locke J.L., Konstantopoulos J.K., Rose J.H., Chen R., Jones S.R. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2751–2759. doi: 10.1073/pnas.1407935111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S.B. The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015;66:25–32. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R.R., Jones S.R., Fumagalli F., Wightman R.M., Caron M.G. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res. Rev. 1998;26:148–153. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Giros B., Jaber M., Jones S.R., Wightman R.M., Caron M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goda R., Otsuka T., Iwamoto A., Kawai M., Shibata S., Furuse M., Yasuo S. Serotonin levels in the dorsal raphe nuclei of both chipmunks and mice are enhanced by long photoperiod, but brain dopamine level response to photoperiod is species-specific. Neurosci. Lett. 2015;593:95–100. doi: 10.1016/j.neulet.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Goldman B.D. Pattern of melatonin secretion mediates transfer of photoperiod information from mother to fetus in mammals. Sci. STKE. 2003;2003(192) doi: 10.1126/stke.2003.192.pe29. [DOI] [PubMed] [Google Scholar]
- Goto M., Oshima I., Tomita T., Ebihara S. Melatonin content of the pineal gland in different mouse strains. J. Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Green N.H., Jackson C.R., Iwamoto H., Tackenberg M.C., McMahon D.G. Photoperiod programs dorsal raphe serotonergic neurons and affective behaviors. Curr. Biol. 2015;25:1389–1394. doi: 10.1016/j.cub.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson A.N., Siemann J.K., Melchior J., Calipari E.S., McMahon D.G., Grueter B.A. Photoperiod impacts nucleus accumbens dopamine dynamics. eNeuro. 2023;10(2):1–11. doi: 10.1523/ENEURO.0361-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.R., Gainetdinov R.R., Mark Wightman R., Caron M.G. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer J., Erritzoe D., Holst K.K., Nielsen F.Å., Marner L., Lehel S., Arentzen T., Jernigan T.L., Knudsen G.M. Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. 2010. http://www.fil.ion Available at: [DOI] [PubMed]
- Klawonn A.M., Malenka R.C. Nucleus accumbens modulation in reward and aversion. Cold Spring Harbor Symp. Quant. Biol. 2018;83:119–129. doi: 10.1101/sqb.2018.83.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T., Apicella P., Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J. Neurophysiol. 1992;67 doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M., Arnold A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon B., Andersen S.B., Madsen M.K., Hjordt L.v., Hageman I., Dam H., Svarer C., da Cunha-Bang S., Baare W., Madsen J., Hasholt L., Holst K., Frokjaer V.G., Knudsen G.M. Seasonal difference in brain serotonin transporter binding predicts symptom severity in patients with seasonal affective disorder. Brain. 2016;139:1605–1614. doi: 10.1093/brain/aww043. [DOI] [PubMed] [Google Scholar]
- Neumeister A., Willeit M., Praschak-Rieder N., Asenbaum S., Stastny J., Hilger E., Pirker W., Konstantinidis A., Kasper S. Dopamine transporter availability in symptomatic depressed patients with seasonal affective disorder and healthy controls. Psychol. Med. 2001;31:1467–1473. doi: 10.1017/s003329170105434z. [DOI] [PubMed] [Google Scholar]
- Nolan S.O., Zachry J.E., Johnson A.R., Brady L.J., Siciliano C.A., Calipari E.S. Direct dopamine terminal regulation by local striatal microcircuitry. J. Neurochem. 2020;155:475–493. doi: 10.1111/jnc.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T., Kawai M., Togo Y., Goda R., Kawase T., Matsuo H., Iwamoto A., Nagasawa M., Furuse M., Yasuo S. Photoperiodic responses of depression-like behavior, the brain serotonergic system, and peripheral metabolism in laboratory mice. Psychoneuroendocrinology. 2014;40:37–47. doi: 10.1016/j.psyneuen.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N., Willeit M., Wilson A.A., Houle S., Meyer J.H. Seasonal variation in human brain serotonin transporter binding. Arch. Gen. Psychiatr. 2008;65:1072–1078. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- Reynolds L.M., Flores C. Mesocorticolimbic dopamine pathways across adolescence: diversity in development. Front. Neural Circ. 2021;15 doi: 10.3389/fncir.2021.735625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N.E., Barnett R.L., Hardin T.A., Turner E.H., Lam G.K., Ozaki N., Goldman D. Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol. Psychiatr. 1998;3:175–177. doi: 10.1038/sj.mp.4000360. [DOI] [PubMed] [Google Scholar]
- Salgado S., Kaplitt M.G. The nucleus accumbens: a comprehensive review. Stereotact. Funct. Neurosurg. 2015;93:75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Adv. Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sharp T., Zetterstrom T., Ungerstedt U. An in vivo study of dopamine release and metabolism in rat brain regions. J. Neurochem. 1986;47:113–122. doi: 10.1111/j.1471-4159.1986.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Siemann J.K., Green N.H., Reddy N., McMahon D.G. Sequential photoperiodic programing of serotonin neurons, signaling and behaviors during prenatal and postnatal development. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann J.K., Williams P., Malik T.N., Jackson C.R., Green N.H., Emeson R.B., Levitt P., McMahon D.G. Photoperiodic effects on monoamine signaling and gene expression throughout development in the serotonin and dopamine systems. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann J.K., Grueter B.A., McMahon D.G. Rhythms, reward, and blues: consequences of circadian photoperiod on affective and reward circuit function. Neuroscience. 2021;457:220–234. doi: 10.1016/j.neuroscience.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.N., Lamb K.N., Henson R.K. Making meaning out of MANOVA: the need for multivariate post hoc testing in gifted education research. Gift. Child. Q. 2020;64(1):41–55. [Google Scholar]
- Stowe T.A., Pitts E.G., Leach A.C., Iacino M.C., Niere F., Graul B., Raab-Graham K.F., Yorgason J.T., Ferris M.J. Diurnal rhythms in cholinergic modulation of rapid dopamine signals and associative learning in the striatum. Cell Rep. 2022;39(1):110633. doi: 10.1016/j.celrep.2022.110633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G., Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Turner B.D., Kashima D.T., Manz K.M., Grueter C.A., Grueter B.A. Synaptic plasticity in the nucleus accumbens: lessons learned from experience. ACS Chem. Neurosci. 2018;9:2114–2126. doi: 10.1021/acschemneuro.7b00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer A.E., Levitan R.D., Houle S., Wilson A.A., Nobrega J.N., Meyer J.H. Increased seasonal variation in serotonin transporter binding in seasonal affective disorder. Neuropsychopharmacology. 2016;41:2447–2454. doi: 10.1038/npp.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dalum J., Melum V.J., Wood S.H., Hazlerigg D.G. Maternal photoperiodic programming: melatonin and seasonal synchronization before birth. Front. Endocrinol. 2020;10:901. doi: 10.3389/fendo.2019.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Q.D., Rooney M.B., Wightman R.M., Kuhn C.M. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Walton J.C., Chen Z., Travers J.B., Nelson R.J. Exogenous melatonin reproduces the effects of short day lengths on hippocampal function in male white-footed mice, Peromyscus leucopus. Neuroscience. 2013;248:403–413. doi: 10.1016/j.neuroscience.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne R. A primer on multivariate analysis of variance (MANOVA) for behavioral scientists. Practical Assess. Res. Eval. 2014;19(1) [Google Scholar]
- Willeit M., Sitte H.H., Thierry N., Michalek K., Praschak-Rieder N., Zill P., Winkler D., Brannath W., Fischer M.B., Bondy B., Kasper S., Singer E.A. Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology. 2008;33:1503–1513. doi: 10.1038/sj.npp.1301560. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Possible circadian and seasonal rhythmicity in an in vitro model: monoamine uptake in rat brain slices. Experientia. 1974;30:1240–1241. doi: 10.1007/BF01945159. [DOI] [PubMed] [Google Scholar]
- Yadid G., Nakash R., Deri I., Tamar G., Kinor N., Gispan I., Zangen A. Elucidation of the neurobiology of depression: insights from a novel genetic animal model. Prog. Neurobiol. 2000;62:353–378. doi: 10.1016/s0301-0082(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Yorgason J.T., España R.A., Jones S.R. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachry J.E., Nolan S.O., Brady L.J., Kelly S.J., Siciliano C.A., Calipari E.S. Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology. 2021;46:491–499. doi: 10.1038/s41386-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A., Nakash R., Overstreet D.H., Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155:434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- Zangen A., Overstreet D.H., Yadid G. High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. J. Neurochem. 1997;69:2477–2483. doi: 10.1046/j.1471-4159.1997.69062477.x. [DOI] [PubMed] [Google Scholar]
- Zangen A., Overstreet D.H., Yadid G. Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Res. 1999;824:243–250. doi: 10.1016/s0006-8993(99)01214-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.