Abstract
Background
Fibroids and endometriosis are sex hormone-mediated and exhibit cancer-like behavior. Breast cancer may be more common in women who have had these conditions, but the literature is conflicting and does not always address factors like hysterectomy/oophorectomy status, race/ethnicity, menopause, and hormone receptor subtypes.
Methods
Data are from the Sister Study, a cohort of 50,884 U.S. women enrolled in 2003–2009 and followed through 2020. Cox proportional hazards models with time-varying exposures and covariates assessed the relationship of fibroids or endometriosis with breast cancer. Logistic regression examined the association with estrogen receptor (ER) status among cases.
Results
Fibroids (19,932 cases) were positively associated with breast cancer (fully adjusted hazard ratio [HR]: 1.07, 95% confidence interval [CI]: 1.01–1.14), notably among Black participants (HR 1.34, 95% CI: 1.07–1.69) and women who had a hysterectomy (HR: 1.18, 95% CI: 1.05–1.31). Endometriosis (3,970 cases) was not associated with breast cancer (HR: 0.99, 95% CI: 0.91–1.08). Among 4,419 breast cancer cases, fibroids were positively associated with ER+ subtypes (odds ratio [OR]: 1.34, 95% CI: 1.10–1.65), while endometriosis was negatively associated with ER+ subtypes (OR: 0.78; 95% CI: 0.61–1.01).
Conclusions
We observed a modest positive association between fibroids and breast cancer, particularly ER+ breast cancer. No relationship with endometriosis and breast cancer incidence was found.
Impact
Fibroids, even in those with a family history of breast cancer, might modify breast cancer risk stratification tools. Future studies should further assess this link and interrogate shared risk factors.
Introduction
Breast cancer is the most common cancer diagnosis and the second-most common cause of cancer mortality in women(1). The etiology is multifactorial with genetic, lifestyle, and environmental links (2) (3). Understanding relationships between benign and malignant conditions represents an opportunity to elucidate shared risk factors, identify novel syndromes, and refine screening recommendations. Fibroids and endometriosis are fitting benign conditions to explore given their cancer-like qualities and sex hormone mechanisms.
Fibroids are benign tumors of monoclonal origin(4,5). They exhibit chromosomal abnormalities common among other somatic growths like lipomas, hamartomas, endometrial polyps, and salivary adenomas (6) as well as meningiomas (7) and are part of a rare syndrome of hereditary leiomyomatosis and renal cell carcinoma (8). Fibroid tissue upregulates aromatase and sex hormone receptors(9). They shrink with menopause and hormone modifying fibroid treatment (10) and may be prevented with depot medroxyprogesterone acetate (11),(12).
Endometriosis is also monoclonal and exhibits behavior akin to metastasis(4). In patients with endometriosis, endometrial tissue that has migrated to a different site, as compared to endometrial tissue in situ, is more likely to express MYC, Cyclin D1, and GREB1, proteins implicated in breast cancer (13). Endometriosis tissue also exhibits altered expression of oncogenes and tumor suppressors (14), including WNT4 (stabilizes B-catenin and is associated with familial adenomatous polyposis syndrome) (15) and VEZT (associated with gastric cancer) (16). Furthermore, endometriosis demonstrates increased expression of sex hormone receptors (13) and aromatase (17).
A recent 2022 meta-analysis(18) found that among 16 cohort and case-control studies, women with endometriosis had an overall increased risk of breast cancer (risk ratio [RR] 1.08; 95% CI: 1.00–1.17). However, results from individual studies suggest that risk estimates may vary by age. For example, a Danish cohort of 45,790 participants found increased risk only in those over age 50(19), whereas a Finnish cohort of 49,993 participants found an increased risk among 20–40-year-olds(20). A Danish case-cohort study of 114,327 participants found decreased risk from early-onset endometriosis but increased risk from late-onset endometriosis(21).
Studies on fibroids and breast cancer tend to suggest a positive association. A few Taiwan-based studies demonstrated a positive association between fibroids and breast cancer(22,23), with one retrospective cohort of 107,357 participants reporting a HR of 1.31 (95%: 1.13–1.52) (24). Interestingly, however, they reported an inverse association between fibroids and mortality among breast cancer cases. A Korean retrospective cohort of 630,523 participants also reported a HR of 1.30 (1.20–1.41)(25). A Swedish cohort study demonstrated a positive association between fibroids and benign breast disease in premenopausal women (26). In the Black Women’s Health Study, there was no relationship with breast cancer overall, but a positive association between early-onset fibroids and pre-menopausal breast cancer was observed and a possible positive relationship with estrogen receptor positive (ER+) breast cancer (27). A Mendelian randomization study on women of European descent demonstrated a positive association with breast cancer, especially ER+ breast cancer (28). Among the Sister Study cohort, it was previously reported that breast cancer was positively associated with a history of hysterectomy (29), the most common indication for which is fibroids (30).
The aim of this study is to assess the association between a history of fibroids or endometriosis and 1. incident breast cancer and 2. breast cancer hormone receptor status. Since prior studies noted differences by age and menopausal status, we examined early-onset fibroids and endometriosis, and additionally stratified by menopause status. Since fibroids and endometriosis may prompt hysterectomy and/or oophorectomy, which can in turn affect breast cancer susceptibility, we further stratify those with fibroids by surgery. Moreover, those with fibroids and hysterectomy may represent more clinically significant cases. In the case of fibroids, which are particularly common and severe in African American women (31), it is important to further examine race/ethnicity. While previous studies span multiple countries and ethnicities, each one was itself ethnically homogenous. Describing inter-disease associations can help clarify shared etiology and may improve risk calculators used for screening recommendations.
Materials and Methods
Study Population
The Sister Study is a prospective cohort designed to understand risk factors associated with breast cancer and other chronic diseases. It enrolled 50,884 women ages 35–74 years, across all US states, including Puerto Rico, during the period 2003–2009 (32). None of the women had a prior history of breast cancer themselves, but, as part of the eligibility criteria, all women had a sister (half or full) who was diagnosed with breast cancer. At enrollment, participants reported detailed medical, family, and social history, and were followed-up annually for breast cancer diagnoses, with more detailed questionnaires administered approximately every 3 years. Data are complete through October 2020 (data release 10.1).
Participants with self-reported invasive breast cancer, ductal carcinoma in situ (DCIS), and lobular carcinoma in situ (LCIS) were considered cases. DCIS and LCIS were included as cases because they have similar risk factors with breast cancer. DCIS can develop into invasive cancer and LCIS is associated with invasive cancer at other sites (33,34). Whenever possible, pathology reports from the medical records were used to confirm diagnoses, including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status. Medical records were obtained for 82% of known cases, and self-reported data was used when the medical record was not available. Self-reported breast cancer characteristics among this cohort was previously shown to agree with medical records, with positive predictive values of 99.1% (ER+), 83.0% (ER-), 98.9% (PR+), 71.6% (PR-), 66.1% (HER2+), and 99.1% (HER2-)35.
All participants provided written informed consent. The Sister Study was approved by the Institutional Review Board of the National Institutes of Health and conducted in accordance with recognized ethical guidelines including the US Common Rule.
Statistical Approach
Of the 50,884 participants, we excluded the following: 4 participants who withdrew; 115 who reported breast cancer prior to completing enrollment; 30 who had unclear incident breast cancer status or timing; 231 who had a pre-baseline prophylactic bilateral mastectomy; 16 who reported having a fibroid (n=11) or endometriosis (n=5) before the age of 10; and 287 who did not contribute any follow-up information. This left 50,201 participants for analysis. We do not exclude women with a history of hysterectomy because the prevalence of pre-enrollment hysterectomy is likely be unequal in our exposed and unexposed groups and may introduce bias.
Missing covariate data at baseline were imputed using multiple imputation by chained equations with 30 imputation sets using the mice package in R(35). Although data for history of fibroids or endometriosis were nearly complete, there was moderate missingness for the age of onset of fibroids (8%) and endometriosis (7%) diagnosed prior to enrollment. Additionally, we imputed missing or “indeterminate” hormone receptor status for some cases: ER (17%), PR (19%), HER2 (31%).
Some covariables were time-dependent, changing with each survey (initial missingness <1% for each): body mass index (BMI), alcoholic drinks per week, lifetime smoking pack years, physical activity (recreational or work-related) hours per week, total years of hormone replacement therapy (HRT) as interaction with ever used (categorized as estrogen only or combined estrogen plus progestin), total years taking oral contraceptives (OCs), parity, age of first pregnancy, hysterectomy, bilateral oophorectomy. Non-time dependent covariables (<1% missing) included age of menarche, highest level of education attained, and race and ethnicity (Non-Hispanic White, Non-Hispanic Black / African American [referred to henceforth as Black], Hispanic, and Other). If follow-up questionnaire data were missing, data from the prior questionnaire were carried over.
Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (Cis) for the association between having a prior diagnosis of fibroids or endometriosis and incident breast cancer diagnosis. Time started at age at enrollment for the entire cohort. The diagnosis status of fibroids or endometriosis was a time-dependent binary variable that could be updated at the time of each follow-up questionnaire. In other words, a participant contributed to both unexposed and exposed person-time if they developed a fibroid or endometriosis during the study follow-up period. Since these pathologies can be treated with hysterectomy and/or oophorectomy, each analysis was followed by a separate analysis that subdivided those with fibroids based on surgical status: “bilateral oophorectomy” includes everyone with bilateral oophorectomy with or without hysterectomy but not counting those with partial/unilateral oophorectomy; “hysterectomy only” includes those with hysterectomy (partial or total) but not bilateral oophorectomy; “without surgery” indicates neither hysterectomy nor bilateral oophorectomy. Those with fibroids and partial/unilateral oophorectomy would either be categorized as “fibroids with hysterectomy only” or “fibroids without surgery”. Surgical status can also change during follow-up. Nearly all women (97%, 8797/9031) who had a bilateral oophorectomy also had a hysterectomy. The referent group is no fibroids, regardless of surgical status. Fibroids and endometriosis were analyzed separately.
We stratified analyses by race/ethnicity and menopausal status (combining natural and surgical). Heterogeneity was assessed with Wald tests of the interaction between fibroids/endometriosis and menopause as well as fibroids and race. Some participants may have had hysterectomy before they could have been diagnosed with fibroids or endometriosis. These were simply included in the analysis as people who never developed those conditions. However, we also evaluated early-onset fibroids and endometriosis, defined as diagnosis before age 35. Further, because age 35 was the minimum age of enrollment and a time point experienced by all participants, it also serves as a useful cut-point for examining early-onset diagnosis, specifically.
We also assessed the association of fibroids and endometriosis with breast cancer subtypes among cases using logistic regression. Here we report odds ratios (ORs) and 95% Cis for the association between a diagnosis of fibroids and endometriosis at any time and risk of having a breast cancer that is ER+ versus ER-, PR+ versus PR-, HER2+ versus HER2-, triple negative (ER-, PR- and HER2-) versus not.
For each analysis, age-adjusted only, partially adjusted, and fully adjusted models were used. The partially adjusted analysis included the following: alcoholic drinks per week, pack-years of smoking, BMI, exercise hours per week, race/ethnicity, education status, parity, age at first pregnancy, months breastfeeding, and age at menarche. The fully adjusted analysis additionally included variables related to endogenous or exogenous hormones that may mediate the association: oophorectomy status, years taking OCs, and HRT use. We report fully adjusted HRs and ORs in the text unless otherwise specified.
Analyses were performed in R and R studio version 2022.2.3 (available at http://www.R-project.org/ and https://rstudio.com/products/rstudio/download), using the survival (available at https://cran.r-project.org/web/packages/survival/index.html) and survminer (available at https://cran.r-project.org/web/packages/survminer/index.html) packages and graphics made with the ggplot2 (available at https://cran.r-project.org/web/packages/ggplot2/index.html) and gtsummary (available at https://cran.r-project.org/web/packages/gtsummary/index.html) packages.
Data Availability Statement:
Data used in this manuscript are available as described on the Sister Study website (The Sister Study: Collaborations and Data Requests, nih.gov) or by request via the Sister Study tracking and review system (www.sisterstudystars.org; registration required). Computing code can be requested from the corresponding author.
Results
Participants were followed for a median of 11.6 years (range 0.1 to 15.3). Of the 50,201 eligible participants, 19,932 (40%) were diagnosed with fibroids and 8,951 (18%) were diagnosed with endometriosis by the end of follow-up (Table 1), while 4,419 participants developed breast cancer by the end of follow up. For fibroids, 14,719 (81%) were diagnosed pre-baseline and 4,230 (21%) were diagnosed before the age of 35. For endometriosis, 7,119 (86%) were diagnosed pre-baseline and 3,970 (44%) were diagnosed before the age of 35. The mean age of diagnosis for fibroids was 43.4 years old and endometriosis was 38.0 years old (Supplemental Figure 1).
Table 1.
Baseline (2003–2009) characteristics of Sister Study participants (n=50,201) according to their fibroid and endometriosis status at the end of follow-up (up to September 2020)
| Fibroid and Endometriosis Status at End of Follow-up | ||||||
|---|---|---|---|---|---|---|
| Baseline Characteristics3 | All Participants | Neither diagnosis | Fibroids | Fibroids diagnosed < 35 years | Endometriosis | Endometriosis diagnosed < 35 years |
| N = 50,201 | N = 26,547 (52.8%) | N = 19,9321 (39.7%) | N = 4,2301 (8.4%) | N = 8,9511 (17.8%) | N = 3,9701 (7.9%) | |
| Age at Baseline | 56 (9) | 55 (9) | 56 (9) | 56 (9) | 56 (9) | 54 (8) |
| Race | ||||||
| Non-Hispanic White | 42,038 (84%) | 22,996 (87%) | 15,773 (79%) | 2,891 (68%) | 7,547 (84%) | 3,316 (84%) |
| Non-Hispanic Black/ African American | 4,521 (9.0%) | 1,465 (5.5%) | 2,886 (14%) | 1,050 (25%) | 741 (8.3%) | 375 (9.4%) |
| Hispanic | 2,315 (4.6%) | 1,366 (5.1%) | 779 (3.9%) | 171 (4.0%) | 381 (4.3%) | 152 (3.8%) |
| Other | 1,327 (2.6%) | 720 (2.7%) | 494 (2.5%) | 118 (2.8%) | 282 (3.2%) | 127 (3.2%) |
| Education | ||||||
| High School | 7,683 (15%) | 4,284 (16%) | 2,781 (14%) | 617 (15%) | 1,345 (15%) | 553 (14%) |
| Some College | 16,930 (34%) | 8,606 (32%) | 6,956 (35%) | 1,559 (37%) | 3,314 (37%) | 1,538 (39%) |
| Undergraduate Degree | 13,556 (27%) | 7,418 (28%) | 5,208 (26%) | 1,035 (24%) | 2,223 (25%) | 998 (25%) |
| Graduate Degree | 12,032 (24%) | 6,239 (24%) | 4,987 (25%) | 1,019 (24%) | 2,069 (23%) | 881 (22%) |
| Body mass index (BMI) at Baseline (kg/m2) | ||||||
| <18.5 | 519 (1.0%) | 320 (1.2%) | 171 (0.9%) | 25 (0.6%) | 73 (0.8%) | 32 (0.8%) |
| 18.5–25 | 19,801 (39%) | 11,170 (42%) | 7,135 (36%) | 1,340 (32%) | 3,300 (37%) | 1,548 (39%) |
| 25–30 | 15,971 (32%) | 8,334 (31%) | 6,439 (32%) | 1,396 (33%) | 2,910 (33%) | 1,261 (32%) |
| >30 | 13,910 (28%) | 6,723 (25%) | 6,187 (31%) | 1,469 (35%) | 2,668 (30%) | 1,129 (28%) |
| Physical Activity Including Work | ||||||
| <10 hours/week | 18,901 (38%) | 9,907 (37%) | 7,620 (38%) | 1,610 (38%) | 3,285 (37%) | 1,471 (37%) |
| 10–20 hours/week | 20,738 (41%) | 11,083 (42%) | 8,126 (41%) | 1,652 (39%) | 3,655 (41%) | 1,616 (41%) |
| 20–30 hours/week | 8,403 (17%) | 4,406 (17%) | 3,345 (17%) | 779 (18%) | 1,583 (18%) | 691 (17%) |
| >30 hours/week | 2,159 (4.3%) | 1,151 (4.3%) | 841 (4.2%) | 189 (4.5%) | 428 (4.8%) | 192 (4.8%) |
| Alcohol | ||||||
| Non-Drinking | 9,538 (19%) | 4,939 (19%) | 3,885 (19%) | 965 (23%) | 1,804 (20%) | 815 (21%) |
| <7 drinks/week | 33,874 (67%) | 17,831 (67%) | 13,509 (68%) | 2,807 (66%) | 6,019 (67%) | 2,688 (68%) |
| 7+ drinks/week | 6,789 (14%) | 3,777 (14%) | 2,538 (13%) | 458 (11%) | 1,128 (13%) | 467 (12%) |
| Smoking (pack-years) | ||||||
| Never | 28,338 (56%) | 14,910 (56%) | 11,417 (57%) | 2,383 (56%) | 4,905 (55%) | 2,170 (55%) |
| <15 pack-years | 13,687 (27%) | 7,173 (27%) | 5,478 (27%) | 1,106 (26%) | 2,502 (28%) | 1,087 (27%) |
| 15–30 pack-years | 4,890 (9.7%) | 2,635 (9.9%) | 1,863 (9.3%) | 430 (10%) | 927 (10%) | 426 (11%) |
| >30 pack-years | 3,286 (6.5%) | 1,829 (6.9%) | 1,174 (5.9%) | 311 (7.4%) | 617 (6.9%) | 287 (7.2%) |
| Parity | ||||||
| No children | 9,079 (18%) | 4,374 (16%) | 3,828 (19%) | 811 (19%) | 2,180 (24%) | 1,018 (26%) |
| 1 child | 7,261 (14%) | 3,539 (13%) | 3,059 (15%) | 791 (19%) | 1,547 (17%) | 780 (20%) |
| 2 children | 18,450 (37%) | 9,882 (37%) | 7,242 (36%) | 1,483 (35%) | 3,107 (35%) | 1,388 (35%) |
| 3+ children | 15,411 (31%) | 8,752 (33%) | 5,803 (29%) | 1,145 (27%) | 2,117 (24%) | 784 (20%) |
| Age at First Pregnancy | ||||||
| Never Pregnant | 7,762 (15%) | 3,941 (15%) | 3,059 (15%) | 637 (15%) | 1,762 (20%) | 851 (21%) |
| <20 years old | 9,536 (19%) | 4,629 (17%) | 4,217 (21%) | 1,064 (25%) | 1,751 (20%) | 741 (19%) |
| 20–24 years old | 17,466 (35%) | 9,117 (34%) | 7,187 (36%) | 1,470 (35%) | 2,950 (33%) | 1,213 (31%) |
| 25–29 years old | 9,349 (19%) | 5,300 (20%) | 3,425 (17%) | 590 (14%) | 1,433 (16%) | 610 (15%) |
| 30+ years old | 6,088 (12%) | 3,560 (13%) | 2,044 (10%) | 469 (11%) | 1,055 (12%) | 555 (14%) |
| Breastfeeding | ||||||
| Never | 21,596 (43%) | 10,756 (41%) | 9,026 (45%) | 2,021 (48%) | 4,416 (49%) | 1,930 (49%) |
| <1 year | 16,407 (33%) | 8,556 (32%) | 6,628 (33%) | 1,393 (33%) | 2,927 (33%) | 1,323 (33%) |
| 1–2 years | 6,503 (13%) | 3,768 (14%) | 2,343 (12%) | 436 (10%) | 929 (10%) | 415 (10%) |
| >2 years | 5,695 (11%) | 3,467 (13%) | 1,935 (9.7%) | 380 (9.0%) | 679 (7.6%) | 302 (7.6%) |
| Age of Menarche | ||||||
| <12 years old | 10,254 (20%) | 4,847 (18%) | 4,606 (23%) | 1,123 (27%) | 2,093 (23%) | 965 (24%) |
| 12–14 years old | 28,194 (56%) | 15,027 (57%) | 11,109 (56%) | 2,304 (54%) | 4,874 (54%) | 2,124 (54%) |
| >14 years old | 11,753 (23%) | 6,673 (25%) | 4,217 (21%) | 803 (19%) | 1,984 (22%) | 881 (22%) |
| Years taking oral contraceptives | ||||||
| Never | 8,015 (16%) | 4,399 (17%) | 3,113 (16%) | 648 (15%) | 1,190 (13%) | 465 (12%) |
| 0–2 years | 7,948 (16%) | 3,916 (15%) | 3,415 (17%) | 763 (18%) | 1,559 (17%) | 696 (18%) |
| 2–10 years | 21,556 (43%) | 11,291 (43%) | 8,604 (43%) | 1,901 (45%) | 3,959 (44%) | 1,882 (47%) |
| 10+ years | 12,682 (25%) | 6,941 (26%) | 4,800 (24%) | 918 (22%) | 2,243 (25%) | 927 (23%) |
| Hormone Replacement Therapy | ||||||
| None | 28,056 (56%) | 16,404 (62%) | 10,044 (50%) | 2,038 (48%) | 3,832 (43%) | 1,728 (44%) |
| Estrogen and Progestin | 12,388 (25%) | 7,016 (26%) | 4,442 (22%) | 833 (20%) | 1,982 (22%) | 863 (22%) |
| Estrogen Only | 9,757 (19%) | 3,127 (12%) | 5,446 (27%) | 1,359 (32%) | 3,137 (35%) | 1,379 (35%) |
| Surgery2 | ||||||
| No Surgery | 34,241 (68%) | 21,940 (83%) | 10,482 (53%) | 1,765 (42%) | 3,716 (42%) | 1,685 (42%) |
| Hysterectomy Only | 6,929 (14%) | 2,374 (8.9%) | 3,913 (20%) | 1,259 (30%) | 1,763 (20%) | 865 (22%) |
| Bilateral Oophorectomy Only | 234 (0.5%) | 141 (0.5%) | 78 (0.4%) | 13 (0.3%) | 36 (0.4%) | 10 (0.3%) |
| Both | 8,797 (18%) | 2,092 (7.9%) | 5,459 (27%) | 1,193 (28%) | 3,436 (38%) | 2,092 (7.9%) |
Those subjects who have both fibroids and endometriosis contribute to the statistics of both the fibroids and endometriosis columns.
“Hysterectomy Only” indicates hysterectomy but not bilateral oophorectomy; “bilateral oophorectomy” indicates bilateral oophorectomy with or without hysterectomy; “no surgery” indicates neither hysterectomy nor bilateral oophorectomy. Partial/unilateral oophorectomies would either be classified under “no surgery” or “hysterectomy only”.
Characteristics are based on status at baseline, while fibroids and endometriosis refer to status at end of follow-up
Mean (SD) or Count (percentage)
Data represented here is the average of all 30 imputed data sets
Baseline characteristics sometimes differed between those with fibroids, endometriosis, and no pathology (Table 1). Nulliparity was more frequently reported by those participants with fibroids (19%, 3,828/19,932) or endometriosis (24%, 2,180/8,951) than participants with neither condition (16%, 4,374/26,547). Participants with fibroids or endometriosis more frequently reported hysterectomies and/or bilateral oophorectomies. For those without either diagnosis, 9.8% (6,929/26,547) had only hysterectomies and 8.4% (9,031/26,547) had bilateral oophorectomies. For those with fibroids, 20% (3,913/19,932) and 28% (5,537/19,932) had the procedures, respectively, and for endometriosis, 20% (1,763/8,951) and 39% (3,472/8,951). Participants with fibroids and endometriosis reported more use of HRT. Those with fibroids or endometriosis more often reported a BMI consistent with obesity. No large differences by race were apparent for endometriosis, but fibroids were more common in Black participants (64%, 2,886/4,521) than non-Black participants (37%, 17,046/45,680) in this cohort.
After imputation, among those with both fibroids and hysterectomy, 89% (9,921/11,118) of fibroids were reported as diagnosed prior to hysterectomy. For endometriosis, 88% (5,176/5,878) were reported prior to hysterectomy.
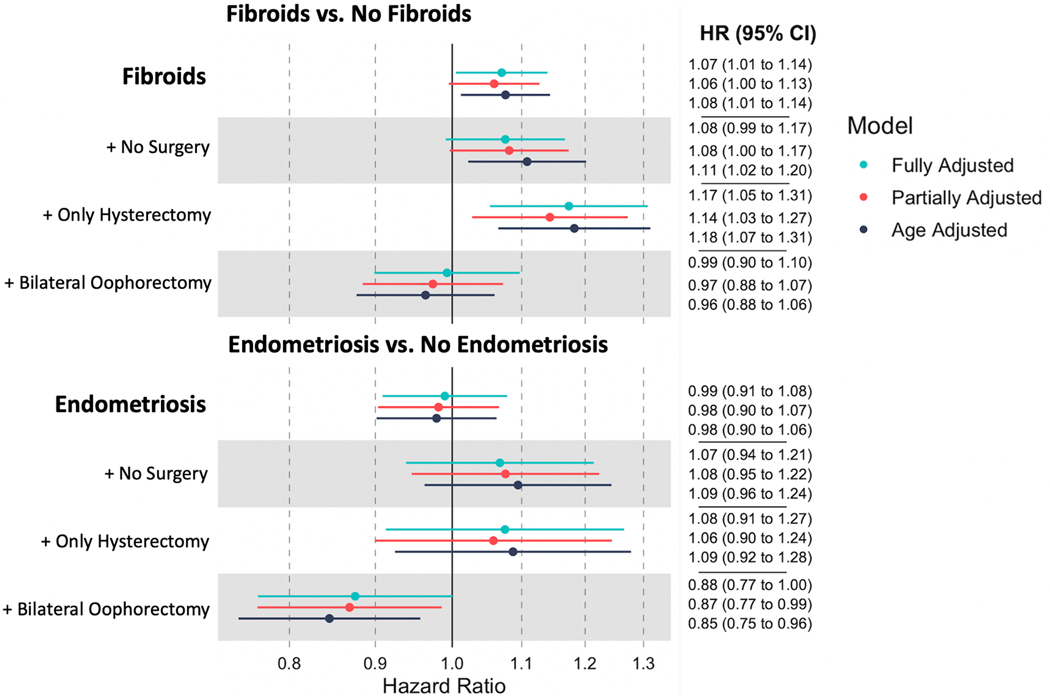
Fibroids were associated with a higher rate of incident breast cancer (fully adjusted HR: 1.07, 95% CI: 1.01–1.14) (Figure 1). For endometriosis, there was no evidence of an association with breast cancer (HR: 0.99, 95% CI: 0.91–1.08). Both models met the proportional hazards assumption (Schoenfeld tests p>0.05).
Figure 1:
Association of fibroids and endometriosis with breast cancer with and without hysterectomy and oophorectomy
Results from Cox proportional hazards model assessing the relationship between history of fibroids or endometriosis and incident breast cancer. Further analysis includes fibroids plus surgical history, either bilateral oophorectomy (regardless of hysterectomy status) and hysterectomy without oophorectomy. The referent group is no fibroids (or no endometriosis), regardless of surgical history. Age adjusted, partially adjusted, and fully adjusted models are included
In analyses where women with fibroids are separated by their hysterectomy and oophorectomy status, the referent group is no diagnosed fibroids, with or without gynecological surgery (Figure 1). The positive association between fibroids and breast cancer was most evident among participants who also had a hysterectomy only (HR: 1.17, 95% CI: 1.05–1.31). In contrast, fibroids were not associated with increased breast cancer rates among participants who underwent bilateral oophorectomy regardless of hysterectomy status (HR: 0.99, 95% CI: 0.90–1.10). Endometriosis was not associated with breast cancer among participants with a hysterectomy or neither gynecological surgery, but endometriosis with bilateral oophorectomy was associated with lower breast cancer incidence (HR: 0.88, 95% CI: 0.77–1.00).
Since fibroids are more common and often more severe among Black participants, we stratified by race and ethnicity, separating non-Hispanic Black women from everyone else (“non-Black”) (Table 2). Among Black participants, the hazard ratio between fibroids and breast cancer was higher (HR 1.34, 95% CI: 1.07–1.69) than that observed among non-Black participants (HR 1.07, 95% CI: 1.00–1.15; p-for-heterogeneity=0.08).
Table 2.
Association of fibroids with breast cancer, stratified by race/ethnicity
| Age Adjusted | Partially Adjusted | Fully Adjusted | |||
|---|---|---|---|---|---|
| Person-Years | Breast Cancer Events | HR (95% CI)1 | HR (95% CI)1 | HR (95% CI)1 | |
| non-Black women (includes non-Hispanic White women, Hispanic women, and other non-Black women) | |||||
| Fibroids | |||||
| No Fibroids | 29,501 | 2,609 | Ref | Ref | Ref |
| Fibroids | 15,018 | 1,439 | 1.05 (0.99 to 1.12) | 1.04 (0.98 to 1.11) | 1.07 (1.00 to 1.15) |
| Fibroids +/− Surgery | |||||
| No Fibroids | 29,501 | 2,609 | Ref | Ref | Ref |
| Fibroids Without Surgery | 6,861 | 658 | 1.09 (1.00 to 1.19) | 1.07 (0.98 to 1.16) | 1.06 (0.97 to 1.16) |
| Fibroids With Hysterectomy Only | 3,080 | 333 | 1.16 (1.04 to 1.31) | 1.13 (1.01 to 1.27) | 1.17 (1.04 to 1.31) |
| Fibroids With Bilateral Oophorectomy | 5,078 | 448 | 0.94 (0.85 to 1.04) | 0.95 (0.86 to 1.05) | 0.97 (0.87 to 1.08) |
| Non-Hispanic Black / African American women | |||||
| Fibroids | |||||
| No Fibroids | 1,561 | 121 | Ref | Ref | Ref |
| Fibroids | 2,420 | 250 | 1.30 (1.04 to 1.62) | 1.31 (1.05 to 1.64) | 1.34 (1.07 to 1.69) |
| Fibroids +/− Surgery | |||||
| No Fibroids | 1,561 | 121 | Ref | Ref | Ref |
| Fibroids Without Surgery | 1,037 | 103 | 1.31 (1.01 to 1.71) | 1.32 (1.01 to 1.73) | 1.34 (1.02 to 1.75) |
| Fibroids With Hysterectomy Only | 708 | 77 | 1.35 (1.01 to 1.80) | 1.34 (1.00 to 1.80) | 1.37 (1.02 to 1.85) |
| Fibroids With Bilateral Oophorectomy | 675 | 70 | 1.23 (0.91 to 1.66) | 1.26 (0.92 to 1.71) | 1.24 (0.90 to 1.71) |
| Wald Test for Heterogeneity of association between fibroids and breast cancer by race/ethnicity | |||||
| p-value | 0.12 | 0.10 | 0.08 | ||
HR = Hazard Ratio, CI = Confidence Interval
This association between fibroids and breast cancer was similar for pre- and post-menopausal person-time (p-for-heterogeneity = 0.51) (Table 3). The association between endometriosis and breast cancer continued to be null in models stratified by menopause status, also without significant heterogeneity (p-value = 0.38) (Figure 1, Table 3).
Table 3.
Association of fibroids or endometriosis with breast cancer, stratified by menopause-time
| Person-Years | Breast Cancer Events | Age Adjusted HR (95% CI) | Partially Adjusted HR (95% CI) | Fully Adjusted HR (95% CI) | |
|---|---|---|---|---|---|
| Pre-Menopausal Person-Time | |||||
| Fibroids | |||||
| No Fibroids | 11,309 | 890 | Ref | Ref | Ref |
| Fibroids | 5,242 | 464 | 1.07 (0.96 to 1.20) | 1.07 (0.95 to 1.20) | 1.09 (0.97 to 1.23) |
| Fibroids +/− Surgery | |||||
| No Fibroids | 11,309 | 890 | Ref | Ref | Ref |
| Fibroids Without Hysterectomy | 3,328 | 297 | 1.09 (0.95 to 1.24) | 1.08 (0.95 to 1.24) | 1.09 (0.95 to 1.24) |
| Fibroids With Hysterectomy | 1,914 | 167 | 1.04 (0.88 to 1.23) | 1.04 (0.87 to 1.23) | 1.07 (0.90 to 1.28) |
| Endometriosis | |||||
| No Endometriosis | 14,469 | 1,170 | Ref | Ref | Ref |
| Endometriosis | 2,082 | 184 | 1.08 (0.92 to 1.26) | 1.07 (0.91 to 1.25) | 1.09 (0.93 to 1.28) |
| Endometriosis +/− Surgery | |||||
| No Endometriosis | 14,469 | 1,170 | Ref | Ref | Ref |
| Endometriosis Without Hysterectomy | 1,238 | 110 | 1.10 (0.91 to 1.34) | 1.09 (0.90 to 1.33) | 1.09 (0.89 to 1.32) |
| Endometriosis With Hysterectomy | 845 | 74 | 1.04 (0.83 to 1.32) | 1.03 (0.82 to 1.31) | 1.08 (0.85 to 1.37) |
| Post-Menopausal Person-Time | |||||
| Fibroids | |||||
| No Fibroids | 19,753 | 1,840 | Ref | Ref | Ref |
| Fibroids | 12,196 | 1,225 | 1.08 (1.00 to 1.16) | 1.06 (0.98 to 1.14) | 1.09 (1.01 to 1.18) |
| Fibroids +/− Surgery | |||||
| No Fibroids | 19,753 | 1,840 | Ref | Ref | Ref |
| Fibroids Without Surgery | 4,597 | 464 | 1.10 (0.99 to 1.21) | 1.07 (0.97 to 1.19) | 1.07 (0.96 to 1.18) |
| Fibroids With Hysterectomy Only | 2,246 | 273 | 1.22 (1.07 to 1.38) | 1.19 (1.05 to 1.36) | 1.24 (1.08 to 1.41) |
| Fibroids With Bilateral Oophorectomy | 5,352 | 488 | 1.00 (0.91 to 1.11) | 0.98 (0.88 to 1.08) | 0.99 (0.89 to 1.10) |
| Endometriosis | |||||
| No Endometriosis | 26,536 | 2,580 | Ref | Ref | Ref |
| Endometriosis | 5,413 | 485 | 0.97 (0.88 to 1.06) | 0.95 (0.86 to 1.05) | 0.98 (0.88 to 1.09) |
| Endometriosis +/− Surgery | |||||
| No Endometriosis | 26,536 | 2,580 | Ref | Ref | Ref |
| Endometriosis Without Surgery | 1,430 | 142 | 1.07 (0.91 to 1.27) | 1.05 (0.89 to 1.25) | 1.04 (0.88 to 1.23) |
| Endometriosis With Hysterectomy Only | 843 | 92 | 1.06 (0.86 to 1.31) | 1.05 (0.86 to 1.30) | 1.08 (0.87 to 1.33) |
| Endometriosis With Bilateral Oophorectomy | 3,140 | 251 | 0.88 (0.78 to 1.01) | 0.87 (0.76 to 0.99) | 0.87 (0.76 to 1.00) |
| Heterogeneity testing with Wald test (p-value) for association between fibroids or endometriosis and breast cancer by menopausal status | |||||
| Fibroids | 0.91 | 0.83 | 0.51 | ||
| Endometriosis | 0.23 | 0.23 | 0.38 | ||
When examining fibroids and endometriosis diagnosed before the age of 35, most associations were near null, even when stratifying by surgical status and menopausal status (Supplemental Table 1–2). There was a possible positive association between fibroids with hysterectomy and breast cancer among post-menopausal participants (HR=1.20, 95% CI: 0.96–1.50).
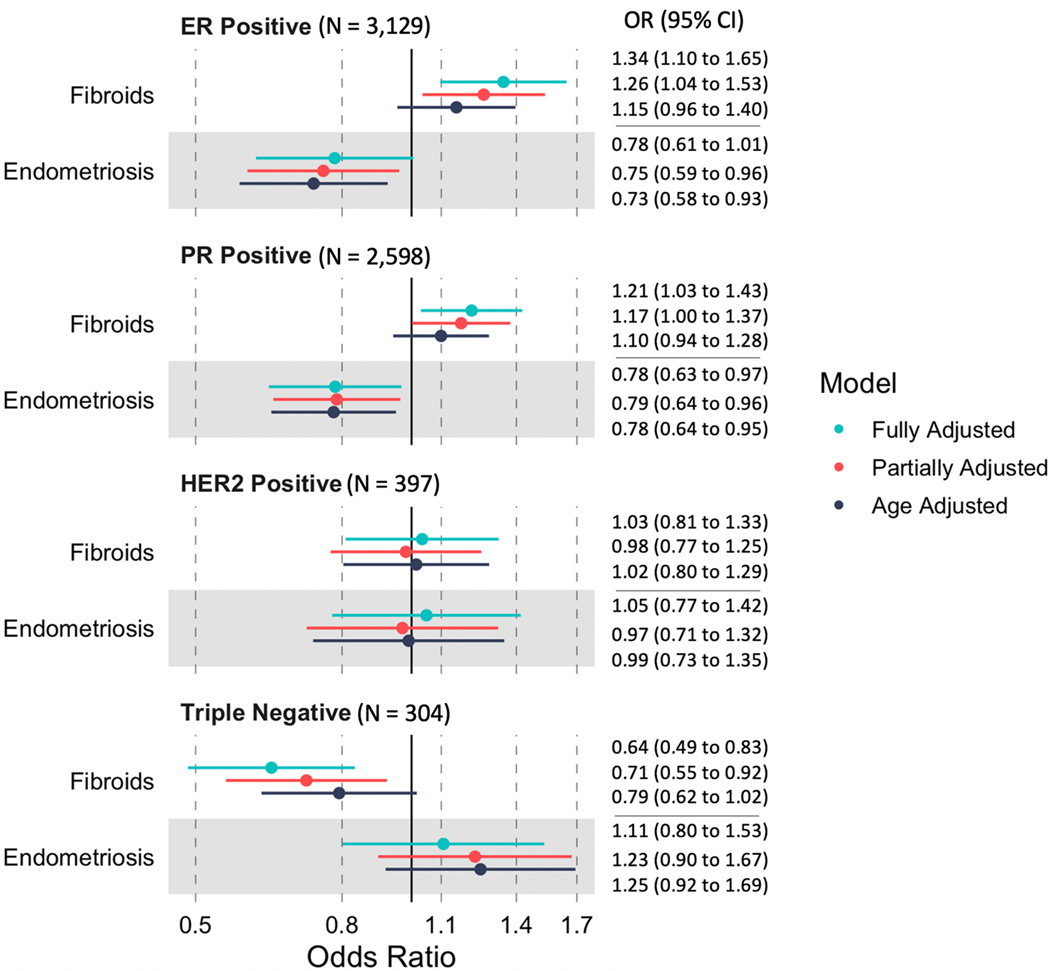
Among breast cancer cases, those with fibroids were more likely to have ER+ (versus ER-) disease (OR: 1.34, 95% CI: 1.10–1.65), whereas those with endometriosis were less likely to be ER+ (OR=0.78; 95% CI: 0.61–1.01) (Figure 2). Fibroids were inversely associated with triple negative breast cancer (versus non-triple negative; OR=0.64; 95% CI: 0.49–0.83), whereas endometriosis was non-significantly associated with triple negative breast cancer (OR=1.11; 95% CI: 0.80–1.53) (Figure 2).
Figure 2:
Association of fibroids and endometriosis with hormone receptor subtypes among 4,419 breast cancer cases
Results from logistic regression model among breast cancer cases assessing the relationship between history of fibroids or endometriosis and breast cancer subtype (ER+ versus ER-, PR+ versus PR-, HER2+ versus HER2- and triple-negative vs. non-triple-negative). The referent group is no fibroids (or no endometriosis), regardless of surgical history. Age adjusted, partially adjusted, and fully adjusted models are included
These associations potentially differed with regards to race/ethnicity (Supplemental Figure 2; Supplemental Tables 3-7). For example, among Black participants, fibroids were positively associated with HER2+ breast cancer (fully adjusted OR 1.80; 95% CI 0.80–4.04), but not associated with ER+ breast cancer (OR 1.04; 95% CI 0.55–1.97).
Discussion
This study investigated the association between previously diagnosed fibroids or endometriosis and incident breast cancer in the Sister Study, a cohort of 50,884 U.S. participants. Emphasis was placed on the intersection of these uterine pathologies with surgeries often used as treatment (hysterectomy and oophorectomy), race/ethnicity, menopausal status, and age of diagnosis. Finally, we examined the association of these pathologies with ER, PR, HER2, and triple negative subtypes of breast cancer.
History of fibroids was positively associated with incident breast cancer, with a HR of 1.07 of developing breast cancer. While this is statistically significant, it is a clinically modest effect size. As outlined in the introduction, most studies suggest a positive relationship with fibroids and breast cancer, but some are conflicting. Among those that report hazard ratios, a Taiwanese cohort with 107,357 participants reported a HR of 1.31 (95%: 1.13–1.52)(24), and South Korean cohort with 630,523 participants reported an HR of 1.30 (1.20–1.41). In this cohort, we showed that the hazard ratio was larger among Black women 1.34 (1.07 to 1.69), compared to non-Black women, and among women who had a hysterectomy without bilateral oophorectomy 1.17 (95% CI: 1.07–1.31). Contrary to our finding, the Black Women’s Health Study reported no association between fibroids and breast cancer, but a positive association between early-onset fibroids and pre-menopausal breast cancer(27). Fibroids here were positively associated with ER+ breast cancer subtypes and negatively associated with triple negative breast cancer.
It is not likely that fibroids are directly causing breast cancer. More plausibly, other factors are driving both simultaneously, likely those involving estrogen exposure. High serum estrogen levels are documented for both diseases, and several estrogenic risk factors are common between the two diseases, including obesity, alcohol use, early menarche, and nulliparity (2,9),(36). The multivariable models reported here account for these risk factors, suggesting that other environmental or genetic risks may drive the link between fibroids and breast cancer. For instance, di-(2-ethylhexyl)-phthalate has been associated with both fibroids and breast cancer (37), suggesting that sex hormone disrupting chemicals could contribute to both diseases. Our findings that fibroids are associated with a higher odds ratio of ER+ and PR+ subtypes, and oophorectomy removes the association between fibroids and breast cancer, further support similar estrogenic etiology. Because Sister Study participants all have a full- or half-sister with a history of breast cancer, our results suggest the positive association between history of fibroids and breast cancer is also present in in those with a known family history of breast cancer.
Mutagenic compounds may also be implicated, since 50% of fibroids have chromosomal abnormalities (9). Mediator complex subunit 12 (MED12) represents an interesting link. Inactivation of MED12 modulates CDK8 and upregulates TGF-B, and 50–80% of fibroids have point mutations in MED12 (9,38). MED12 mutations have also been associated with fibroadenoma, phyllodes tumors, more aggressive prostate and ovarian cancer, and chronic lymphocytic leukemia (38–40).
Among those with fibroids, those with only hysterectomy had a marginally elevated hazard ratio for breast cancer incidence. Hysterectomy is a historically common treatment of fibroids, with 75% of patients with fibroids electing to treat with hysterectomy, despite increasing availability of other treatment modalities (30). Fibroids treated with hysterectomy may represent a more severe subset of fibroids that are larger, more numerous, or more symptomatic. The association was also higher among Black women, who experience a higher prevalence of fibroids on average as well as more burdensome fibroids in terms of number and size(41). The marginally increased incidence rates among Black women and women who had hysterectomies potentially points to a dose-dependent relationship between fibroid severity and breast cancer. Studies that use ultrasound to precisely measure fibroid size would be best suited to corroborate this.
In addition to fibroids, Black women are also disproportionately affected by breast cancer (41). As a group, they have lower incidence but higher mortality from breast cancer, with a discrepancy thought to be largely explained by the fact that Black women are more likely to have early-onset, aggressive (high-grade), and difficult to treat (triple negative) forms of breast cancer (42). However, even within subtypes, Black women experience higher mortality (43). Differing from the rest of the cohort, Black women with both fibroids and breast cancer were not more likely to have ER+ or PR+ subtypes. This suggests that non-sex-hormonal exposures may be more relevant disease mechanisms for this subgroup. Possible candidates include factors strongly influenced by structural racism, such as diet, housing, stress, and exposure to pollution and other toxins (44).
Additionally, vitamin D deficiency is associated with both fibroids and breast cancer (45,46), via two potential mechanisms: its anti-estrogen/progesterone properties and its ability to promote differentiation and apoptosis (46,47). Among the Sister Study cohort, it was previously reported that serum vitamin D levels as well as self-reported supplementation were associated with a lower breast cancer incidence, (48) with a weakly negative association observed among Black women (49).
There was a near null but trending positive association between fibroids diagnosed before age 35 and breast cancer. While early-onset fibroids does not represent a strong risk, this does not invalidate the overall association, since 79% of fibroids in our cohort were diagnosed over the age of 35.
Endometriosis was not associated with breast cancer in this cohort, even when stratifying by race/ethnicity, menopause status, or separately considering early-onset endometriosis. This is conflicting with a recent meta-analysis demonstrating a positive association.(18) However, among cases, endometriosis was more strongly associated with ER- and PR- subtypes (relative to ER+/PR+). This may be due to endometriosis-induced ovarian damage. Twenty eight percent of ovarian endometriosis was bilateral in one case series (50), and women with endometriosis have lower circulating levels of estradiol and progesterone (51).
For both fibroids and endometriosis, bilateral oophorectomy was associated with a reduced hazard of breast cancer. For fibroids, the increased risk was largely ameliorated. These results seem to represent the protective effect of oophorectomy on breast cancer risk, which is consistent with previous findings from this cohort(29).
A strength of this study was the inclusion of many potential confounders in the regression models and the ability to stratify by factors that might help explain the true mechanisms behind the observed associations. Participants in this study must have full- or half-sisters with a history of breast cancer. This unique study sample may mean that our results are not fully generalizable to the general US population or other demographic groups. However, several previous studies that did not enrich for breast cancer family history have also demonstrated a positive association. Beyond the potential lack of generalizability, another limitation of our study is that history of fibroids was self-reported, which omits asymptomatic fibroids that can only be detected on imaging. Similar concerns are present for self-reported endometriosis, which is typically only diagnosed surgically.
The relationship between fibroids and breast cancer may reflect common risk factors. The higher odds of ER+ subtypes indicates that some of these shared risk factors involve sex hormone mechanisms. This includes reproductive factors such as parity and exogenous hormone use, but also suggests other factors like vitamin D or endocrine-disrupting exposures. Fibroids diagnoses may prove to be a useful variable to include in risk calculations that guide breast cancer screening recommendations. Large prospective cohort studies should replicate these findings, and a meta-analysis of existing studies should be performed to better characterize the association, with careful consideration of surgical status, race/ethnicity, and other factors. Further studies that use imaging to detect asymptomatic fibroids and measure fibroid severity may help quantify this risk more precisely.
Supplementary Material
Acknowledgements
Funding:
This work was funded by the Intramural Research Program at the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005 to DPS). JZ is supported by the NIH Medical Research Scholars Program.
Footnotes
Conflict of Interest:
None of the authors (Zeldin, Sandler, Ogunsina, or O’Brien) have conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nature Reviews Disease Primers 2019;5(1):66 doi 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 3.Winters S, Martin C, Murphy D, Shokar N. Chapter one—breast cancer epidemiology, prevention, and screening. Progress in Molecular Biology and Translational Science; Lakshmanaswamy, R, Ed:1–32. [DOI] [PubMed] [Google Scholar]
- 4.Nilbert M, Pejovic T, Mandahl N, Iosif S, Willen H, Mitelman F. Monoclonal origin of endometriotic cysts. International Journal of Gynecological Cancer 1995;5(1):61–3. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocrine reviews 2022;43(4):678–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medikare V, Kandukuri LR, Ananthapur V, Deenadayal M, Nallari P. The genetic bases of uterine fibroids; a review. Journal of reproduction & infertility 2011;12(3):181. [PMC free article] [PubMed] [Google Scholar]
- 7.Yen Y-S, Sun L-M, Lin C-L, Chang S-N, Sung F-C, Kao C-H. Higher risk for meningioma in women with uterine myoma: a nationwide population-based retrospective cohort study. Journal of Neurosurgery 2014;120(3):655–61. [DOI] [PubMed] [Google Scholar]
- 8.Menko FH, Maher ER, Schmidt LS, Middelton LA, Aittomäki K, Tomlinson I, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Familial cancer 2014;13(4):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and Risk Factors of Uterine Fibroids. Best Practice & Research Clinical Obstetrics & Gynaecology 2018;46:3–11 doi 10.1016/j.bpobgyn.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Sankaran S, Manyonda IT. Medical management of fibroids. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22(4):655–76. [DOI] [PubMed] [Google Scholar]
- 11.Harmon Q, Baird D. Use of depot medroxyprogesterone acetate and prevalent leiomyoma in young African American women. Human reproduction 2015;30(6):1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmon QE, Patchel SA, Zhao S, Umbach DM, Cooper TE, Baird DD. Depot medroxyprogesterone acetate use and the development and progression of uterine leiomyoma. Obstetrics and gynecology 2022;139(5):797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertility and sterility 2012;98(5):1200–8. [DOI] [PubMed] [Google Scholar]
- 14.Laudanski P, Szamatowicz J, Kowalczuk O, Kuźmicki M, Grabowicz M, Chyczewski L. Expression of selected tumor suppressor and oncogenes in endometrium of women with endometriosis. Human reproduction 2009;24(8):1880–90. [DOI] [PubMed] [Google Scholar]
- 15.Yedid N, Kalma Y, Malcov M, Amit A, Kariv R, Caspi M, et al. The effect of a germline mutation in the APC gene on β-catenin in human embryonic stem cells. BMC cancer 2016;16(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008;149(3):1190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, Peng H, Huang X, Qi X. The association between endometriosis and risk of endometrial cancer and breast cancer: a meta-analysis. BMC Womens Health 2022;22(1):455 doi 10.1186/s12905-022-02028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogensen JB, Kjaer SK, Mellemkjaer L, Jensen A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol Oncol 2016;143(1):87–92 doi 10.1016/j.ygyno.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 20.Saavalainen L, Lassus H, But A, Tiitinen A, Härkki P, Gissler M, et al. A cohort study of 49 933 women with surgically verified endometriosis: Increased incidence of breast cancer below the age of 40. Acta Obstetricia Et Gynecologica Scandinavica 2019;98(9):1113–9. [DOI] [PubMed] [Google Scholar]
- 21.Bertelsen L, Mellemkjær L, Frederiksen K, Kjær SK, Brinton LA, Sakoda LC, et al. Risk for breast cancer among women with endometriosis. International journal of cancer 2007;120(6):1372–5. [DOI] [PubMed] [Google Scholar]
- 22.Chuang SC, Wu GJ, Lu YS, Lin CH, Hsiung CA. Associations between Medical Conditions and Breast Cancer Risk in Asians: A Nationwide Population-Based Study in Taiwan. PLoS One 2015;10(11):e0143410 doi 10.1371/journal.pone.0143410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng J, Chen Y, Chiang H, Lin C. Increased risk of breast cancer in women with uterine myoma: a nationwide, population-based, case-control study. 28, 3, May 2017. DOI: https://doiorg/103802/jgo 2017:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen T-C, Hsia T-C, Hsiao C-L, Lin C-L, Yang C-Y, Soh K-S, et al. Patients with uterine leiomyoma exhibit a high incidence but low mortality rate for breast cancer. Oncotarget 2017;8(20):33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuk JS, Yang SW, Yoon SH, Kim MH, Seo YS, Lee Y, et al. Association between breast diseases and symptomatic uterine fibroids by using South Korean National Health Insurance database. Sci Rep 2023;13(1):16772 doi 10.1038/s41598-023-43443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindegård B. Breast cancer among women from Gothenburg with regard to age, mortality and coexisting benign breast disease or leiomyoma uteri. Oncology 1990;47(5):369–75. [DOI] [PubMed] [Google Scholar]
- 27.Wise LA, Radin RG, Rosenberg L, Adams-Campbell L, Palmer JR. History of uterine leiomyomata and incidence of breast cancer. Cancer Causes & Control 2015;26(10):1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Xiao C, Han Z, Zhang L, Zhao X, Hao Y, et al. Investigating the shared genetic architecture of uterine leiomyoma and breast cancer: A genome-wide cross-trait analysis. The American Journal of Human Genetics 2022;109(7):1272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovett SM, Sandler DP, O’Brien KM. Hysterectomy, bilateral oophorectomy, and breast cancer risk in a racially diverse prospective cohort study. JNCI: Journal of the National Cancer Institute 2023. doi 10.1093/jnci/djad038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043 doi 10.1038/nrdp.2016.43. [DOI] [PubMed] [Google Scholar]
- 31.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. American journal of obstetrics and gynecology 2014;210(3):194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, et al. The Sister Study cohort: baseline methods and participant characteristics. Environmental health perspectives 2017;125(12):127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves GK, Pirie K, Green J, Bull D, Beral V, Million Women Study C. Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer 2012;131(4):930–7 doi 10.1002/ijc.26460. [DOI] [PubMed] [Google Scholar]
- 34.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010;102(3):170–8 doi 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 35.Van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software 2011;45:1–67. [Google Scholar]
- 36.Park YMM, White AJ, Nichols HB, O’Brien KM, Weinberg CR, Sandler DP. The association between metabolic health, obesity phenotype and the risk of breast cancer. International journal of cancer 2017;140(12):2657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Z, Zhao F, Chen K, Xu J, Li P, Xia D, et al. Association between urinary phthalate metabolites and risk of breast cancer and uterine leiomyoma. Reproductive Toxicology 2017;74:134–42 doi 10.1016/j.reprotox.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez CG, Akula S, Burleson M. The role of mediator subunit 12 in tumorigenesis and cancer therapeutics (Review). Oncol Lett 2022;23(3):74 doi 10.3892/ol.2022.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piscuoglio S, Murray M, Fusco N, Marchiò C, Loo FL, Martelotto LG, et al. MED12 somatic mutations in fibroadenomas and phyllodes tumours of the breast. Histopathology 2015;67(5):719–29 doi 10.1111/his.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim WK, Ong CK, Tan J, Thike AA, Ng CC, Rajasegaran V, et al. Exome sequencing identifies highly recurrent MED12 somatic mutations in breast fibroadenoma. Nat Genet 2014;46(8):877–80 doi 10.1038/ng.3037. [DOI] [PubMed] [Google Scholar]
- 41.Moorman PG, Leppert P, Myers ER, Wang F. Comparison of characteristics of fibroids in African American and white women undergoing premenopausal hysterectomy. Fertility and Sterility 2013;99(3):768–76.e1 doi 10.1016/j.fertnstert.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman LA. Breast cancer in African-American women. 2005. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien KM, Cole SR, Tse C-K, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical Cancer Research 2010;16(24):6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams DR, Collins C. Racial Residential Segregation: A Fundamental Cause of Racial Disparities in Health. Public Health Reports 2001;116(5):404–16 doi 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de La Puente-Yagüe M, Cuadrado-Cenzual MA, Ciudad-Cabañas MJ, Hernández-Cabria M, Collado-Yurrita L. Vitamin D: And its role in breast cancer. The Kaohsiung Journal of Medical Sciences 2018;34(8):423–7 doi 10.1016/j.kjms.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Ciebiera M, Włodarczyk M, Ciebiera M, Zaręba K, Łukaszuk K, Jakiel G. Vitamin D and Uterine Fibroids—Review of the Literature and Novel Concepts. International Journal of Molecular Sciences 2018;19(7):2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holick MF. Vitamin D Deficiency. New England Journal of Medicine 2007;357(3):266–81 doi 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien KM, Sandler DP, Taylor JA, Weinberg CR. Serum vitamin D and risk of breast cancer within five years. Environmental health perspectives 2017;125(7):077004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien KM, Harmon QE, Jackson CL, Diaz-Santana MV, Taylor JA, Weinberg CR, et al. Vitamin D concentrations and breast cancer incidence among Black/African American and non-Black Hispanic/Latina women. Cancer 2022;128(13):2463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vercellini P, Aimi G, De Giorgi O, Maddalena S, Carinelli S, Crosignani PG. Is cystic ovarian endometriosis an asymmetric disease? BJOG: An International Journal of Obstetrics & Gynaecology 1998;105(9):1018–21. [DOI] [PubMed] [Google Scholar]
- 51.Cahill D, Hull M. Pituitary–ovarian dysfunction and endometriosis. Human reproduction update 2000;6(1):56–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this manuscript are available as described on the Sister Study website (The Sister Study: Collaborations and Data Requests, nih.gov) or by request via the Sister Study tracking and review system (www.sisterstudystars.org; registration required). Computing code can be requested from the corresponding author.