Abstract
Feline panleukopenia virus (FPLV) was shown to induce apoptosis to feline lymphoid cells and to reduce the expression of interleukin-2 receptor α on the cells. FPLV-induced apoptosis might be a key element in the pathophysiology of atrophy of lymphoid tissues associated with feline panleukopenia caused by FPLV.
Feline panleukopenia virus (FPLV) and canine parvovirus (CPV) are classified as host range variants of feline parvovirus (FPV). FPLV causes acute depression, gastroenteric symptoms such as diarrhea and vomiting, and lymphopenia, with a high mortality rate among nonimmune kittens (26). In vivo infectivity studies demonstrated that FPLV targets particularly lymphoid tissues and rapidly dividing cells such as those of the thymus, bone marrow, spleen, mesenteric and other lymph nodes, and the intestinal epithelium (27, 32, 35). Recently, we isolated FPVs from the peripheral blood mononuclear cells (PBMCs) of cats and wild felids, suggesting that FPV is lymphotropic in naturally infected animals (13, 16).
Apoptosis, or programmed cell death, is a physiological process important for normal cellular turnover and is characterized by pronounced morphological changes and internucleosomal DNA degradation (36). Studies have shown that it can be triggered by several viruses, and there is mounting evidence that induction of apoptosis contributes directly to the pathogenesis of a number of viruses, such as feline leukemia virus subgroup C (30), feline immunodeficiency virus (FIV) (24), influenza A and B viruses (8), measles virus (4), and, most significantly, human immunodeficiency virus type 1 (HIV-1) (7). In FPLV-infected cats, the decrease leukocyte counts is marked and lymphocytes disappear from the circulation, lymph nodes, bone marrow, and thymus (11, 26). It is probable that polymorphonuclear leukocyte stem cells are also destroyed (11, 26). On the other hand, in CPV-infected dogs, acute myocarditis and hemorrhagic enteritis are generally observed, while lymphopenia is not so regularly seen as it is in FPLV-infected cats (26). Although many of the clinical manifestations of parvovirus infection are thought to be caused by the lytic properties of the virus, there are limited reports regarding the mechanism of FPLV-induced lymphopenia. Furthermore, the reason why CPV does not regularly cause severe lymphopenia in dogs remains obscure. The purpose of the present study was to clarify the effects of FPLV and CPV infections on feline and canine lymphoid cells.
Feline PBMCs from a specific-pathogen-free cat and canine PBMCs from conventional dogs were purified by centrifugation over Ficoll-Paque (Pharmacia Biotech, Tokyo, Japan) and stimulated with 10 μg of concanavalin A (ConA) per ml for 3 days, as described previously (17). The ConA-stimulated feline and canine PBMCs and a feline T-lymphoblastoid cell line, MYA-1, were maintained in RPMI 1640 growth medium supplemented with 10% fetal calf serum (FCS), antibiotics, 50 mM 2-mercaptoethanol, 2 μg of Polybrene per ml, and 100 units of recombinant human interleukin-2 (IL-2) per ml (18). Feline and canine T-lymphoblastoid cell lines, FL74 and CL-1, respectively, were cultured in growth medium without recombinant human IL-2 (22, 31). Crandell feline kidney (CRFK) cells (3) were grown in Dulbecco’s modified Eagle’s medium supplemented with 8% FCS. The TU1 strain of FPLV (14) and the Cp49 strain of CPV (2) were used in this study. TU1 and Cp49 were classified as FPLV and CPV type 2, respectively, by using monoclonal antibodies (MAbs) (20). To prepare stock viruses, CRFK cells were inoculated with TU1 or Cp49. The infected cells were passaged twice at intervals of 5 days. After the second passage, the cultures were incubated further for 4 days and then frozen and thawed once, followed by centrifugation. The resulting supernatants were passed through 0.20-μm-pore-size filters and stored at −80°C as stock viruses. The viruses were titrated on CRFK cells, and the virus antigens were detected by an indirect immunofluorescence assay with a MAb described below. To examine the susceptibility of the feline and canine cells to FPLV and CPV, the cells were inoculated with TU1 or Cp49 at a multiplicity of infection (MOI) of 0.2. After adsorption for 2 h at 37°C, the cells were washed three times with phosphate-buffered saline (PBS) and suspended at a concentration of 2 × 105 cells per ml in growth medium.
For detection of FPLV or CPV antigen, anti-FPLV VP2 MAb 2D9, which reacted with both FPLV and CPV, was used (21). To detect feline IL-2 receptor α (IL-2Rα) on the infected cells, we used MAb 9F23 (23). MAbs f43 and vpg15, which reacted with feline CD5 and CD9, respectively, were used for control antibodies (1, 10). The indirect immunofluorescence assay was performed for detection of FPLV or CPV antigens. FPLV- or CPV-inoculated cells were washed twice in PBS and fixed on glass slides with acetone. After incubation with MAb 2D9 for 30 min at 37°C, the cells were washed with PBS. Then, the cells were incubated with goat anti-mouse immunoglobulin G conjugated with fluorescein isothiocyanate for 30 min at 37°C and washed with PBS. The stained cells were observed under a UV microscope. Flow cytometric analysis was performed to analyze the antigen-positive rates in the inoculated cells. Cells were washed once in cold sorter buffer (PBS containing 3% FCS and 0.1% NaN3) and incubated with MAbs 2D9, 9F27, f43, and vpg15 for 30 min on ice. After a wash with the sorter buffer, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G for 30 min on ice and washed again with the sorter buffer. The cells were then analyzed by FACScan (Becton Dickinson, Tokyo, Japan).
A DNA fragmentation assay was performed to determine whether DNA fragmentation occurred in the FPLV- or CPV-inoculated cells. The FPLV- or CPV-inoculated cells (107) were harvested and washed three times with PBS. The cells were resuspended in 0.1 M Tris-HCl (pH 9.0) containing 1% sodium dodecyl sulfate, 0.1 M NaCl, 1 mM EDTA and 100 μg of proteinase K per ml and incubated at 50°C for 1 h. DNA was extracted and purified with phenol, phenol-chloroform, and ether and precipitated with ethanol. Three micrograms of the extracted DNA was subjected to electrophoresis in a 1.7% agarose gel. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assays were carried out according to the manufacturer’s protocol (In Situ Cell Death Detection Kit [fluorescein]; Boehringer, Mannheim, Germany). Briefly, the FPLV- or CPV-infected cells were washed three times with PBS, fixed with PBS containing 4% paraformaldehyde for 30 min at room temperature, washed with PBS, permeabilized in 0.1% sodium citrate containing 0.1% Triton X-100 for 2 min on ice, and then treated with TUNEL reaction mixture. After the final washes with PBS, the cells were photographed under a UV microscope or analyzed by FACScan (Becton Dickinson).
The morphologies of FPLV-infected feline PBMCs were examined by electron microscopy. Feline PBMCs inoculated with strain TU1 at an MOI of 0.2 were maintained for 2 days, and then the cells were collected and fixed in 2.5% glutaraldehide in PBS. The cells were postfixed in 1% osmium tetroxide, embedded in Epok 812 (Ohken Co. Ltd., Tokyo, Japan), and processed for transmission electron microscopy.
Susceptibility of feline lymphoid cells to FPLV.
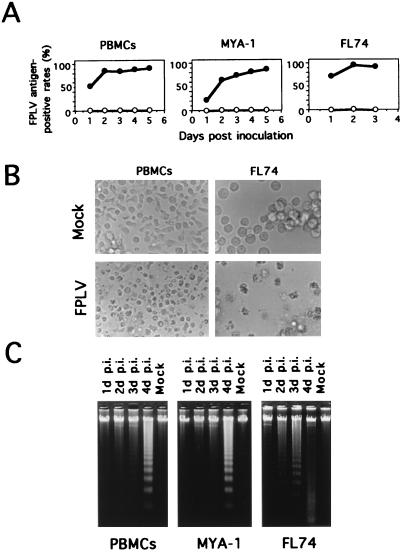
FPLV-specific antigens in the inoculated cells were sequentially analyzed by flow cytometric analysis by using anti-VP2 MAb. As shown in Fig. 1A, FPLV antigen was detected in the inoculated cells at 1 day postinoculation (p.i.). The antigen-positive rates increased rapidly and reached more than 80, 60 and 80% at 2 days p.i. in the infected PBMCs, MYA-1 cells, and FL74 cells, respectively. The FPLV-infected PBMCs showed cytopathic effects (CPEs) such as cell rounding and nuclear disintegration from 3 days p.i. (Fig. 1B), and most of the cells died within 8 days. The infected MYA-1 cells showed similar CPEs at 3 days p.i. (data not shown), and most of the cells died within 6 days. In FL74 cells, remarkable CPEs appeared from 1 day p.i. (Fig. 1B), and most of the cells were killed in 3 days. As shown in Fig. 1C, the DNA from FPLV-infected feline PBMCs demonstrated characteristic 180- to 200-bp nucleosomal ladders. The ladders were seen in samples of cellular DNA obtained at 2 days p.i. and were clearly visible at 4 days p.i. In the FPLV-infected MYA-1 and FL74 cells, the oligonucleosome-length ladders were clearly visible at 4 and 2 days p.i., respectively (Fig. 1C). Although an apparent discrepancy was observed between the low MOI with FPLV and the high FPLV antigen-positive rate in the infected lymphoid cells (Fig. 1A), this might have been due to the fact that virus titers were not determined in lymphoid cells but in CRFK cells, which are less sensitive to FPV (12).
FIG. 1.
Susceptibilities of feline cells to FPLV. (A) Growth curves of strain TU1 in feline lymphoid cells. Feline cells were inoculated with TU1 and collected at the indicated times. FPLV antigen-positive rates in the cells were measured with an anti-FPLV VP2 MAb. Symbols: ○, mock-inoculated cells; •, TU1-infected cells. (B) CPEs observed in FPLV-inoculated feline PBMCs and FL74 cells. FPLV-inoculated or mock-inoculated feline PBMCs and FL74 cells were harvested at 2 days and 1 day p.i., respectively. (C) Electrophoresis of total cellular DNA. Cellular DNA was extracted from cultures of inoculated or mock-inoculated PBMCs and MYA-1 and FL74 cells. Lanes 1, FPLV-inoculated cells at 1 day p.i.; lanes 2, 2 days p.i.; lanes 3, 3 days p.i.; lanes 4, 4 days p.i.; Mock, mock-inoculated cells at 4 days p.i. The extracted DNAs were analyzed by 1.7% agarose gel electrophoresis.
Morphologies of FPLV-infected lymphocytes.
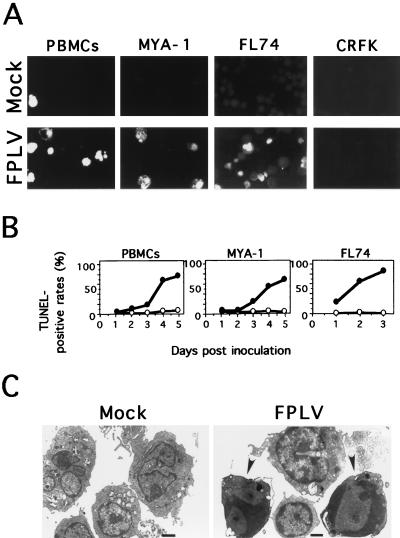
To reveal the proportion of cells undergoing apoptosis among FPLV-infected cells, TUNEL assays, which identify cells containing DNA strand breaks, were performed on the FPLV-inoculated feline PBMCs and MYA-1, FL74, and CRFK cells. As shown in Fig. 2A, the FPLV-inoculated feline PBMCs and MYA-1 cells at 2 days p.i. showed many brightly stained cells compared with mock-inoculated cells. In the inoculated FL74 cells, brightly stained cells appeared as early as 1 day p.i. (Fig. 2A). We could not rule out the possibility that the TUNEL assay would detect viral DNA. However, no brightly stained cells were observed among the infected CRFK cells at 8 days p.i., in which the FPLV antigen-positive rate reached more than 50%, suggesting that detection of viral DNA by the TUNEL assay might be negligible. Flow cytometric analysis revealed that the percentage of TUNEL-positive cells increased gradually after FPLV infection (Fig. 2B). Electron microscopic analysis revealed that the FPLV-inoculated PBMCs showed chromatin condensing along the inner aspect of the nuclear membrane (Fig. 2C).
FIG. 2.
Morphologies of FPLV-infected lymphocytes. (A) Detection of DNA strand breaks in FPLV-inoculated cells by the TUNEL assay. FPLV-inoculated or mock-inoculated PBMCs and MYA-1, FL74, and CRFK cells were harvested at 2, 2, 1, and 8 days p.i., respectively (B) Flow cytometric analysis of TUNEL-positive rates in the inoculated cells. The inoculated cells were collected at the indicated times and analyzed by FACScan. Symbols: ○, mock-inoculated cells; •, TU1-inoculated cells. (C) Electron microscopic analysis of infected PBMCs. FPLV-inoculated or mock-inoculated PBMCs were harvested at 2 days p.i. Arrowheads indicate cells showing chromatin condensation along the inner aspect of the nuclear membrane. Bars, 1 μm.
Effects of FPLV infection on feline lymphoid cells.
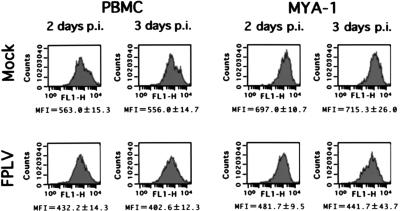
To examine the effects of FPLV infection on the cell surface antigens of infected feline cells, flow cytometric analyses with anti-feline CD5, CD9, and IL-2Rα MAbs were performed using FPLV-inoculated feline PBMCs and MYA-1 cells. As shown in Fig. 3, the mean fluorescence intensities of IL-2Rα of the inoculated cells decreased as early as 2 days p.i. compared with those of mock-inoculated cells. However, the IL-2Rα-positive rates were almost the same between FPLV-inoculated and mock-inoculated cells. On the other hand, when anti-feline CD5 and CD9 MAbs were used for flow cytometric analysis, no significant differences in these cell surface antigens were observed even 3 days after FPLV inoculation.
FIG. 3.
Effects of FPLV infection on feline IL-2Rα of inoculated feline lymphoid cells. Flow cytometric analysis was performed with FPLV-inoculated feline PBMCs and MYA-1 cells at 2 or 3 days p.i. Three independent experiments were performed, and the averages and standard deviations of mean fluorescence intensities (MFI) are presented. The results of flow cytometric analyses are representative of one of the three independent experiments.
Susceptibility of feline and canine lymphoid cells to CPV.
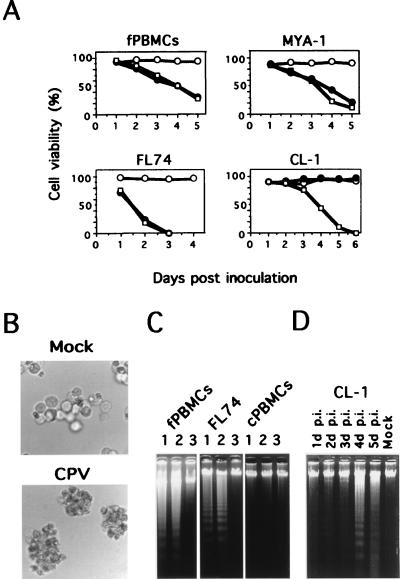
To examine the infectivity of CPV for feline and canine lymphoid cells, feline and canine PBMCs and MYA-1, FL74, and CL-1 cells were inoculated with strain TU1 or strain Cp49 at an MOI of 0.2. The cytotoxic activities of the two strains were sequentially examined by trypan blue dye exclusion. As shown in Fig. 4A, the effects of CPV infection on the viabilities of the feline cells were similar to those observed in FPLV infection. The CPEs observed in the CPV-inoculated feline cells were almost the same as in FPLV infection. On the other hand, the viability of CL-1 cells was reduced by CPV inoculation but was not affected by FPLV inoculation (Fig. 4A). The CPEs observed in the CPV-inoculated CL-1 cells are shown in Fig. 4B. Although no FPLV antigen was detected in the FPLV-inoculated CL-1 cells at 2 weeks p.i., CPV antigen was observed in the CPV-inoculated CL-1 cells at 4 days p.i. In contrast, the viability of the FPLV- or CPV-inoculated canine PBMCs was more than 85%, and no FPLV or CPV antigen was detected in the cells at 2 weeks after FPLV or CPV inoculation. These results indicate that TU1 can infect neither canine PBMCs nor CL-1 cells and that Cp49 can infect CL-1 cells but not canine PBMCs. Next, we examined by DNA fragmentation assays whether CPV infection induced apoptosis of the infected cells. As shown in Fig. 4C and D, the DNAs isolated from CPV-inoculated feline and CL-1 cells showed characteristic nucleosomal ladders, as observed in FPLV-inoculated feline cells, while the DNAs from both FPLV- and CPV-inoculated canine PBMCs did not show the characteristic ladders.
FIG. 4.
Susceptibilities of feline and canine cells to CPV. (A) Cell viabilities of FPLV- or CPV-inoculated feline and canine lymphoid cells. Feline and canine cells were inoculated with strain TU1 or strain Cp49 and collected at the indicated times. Symbols: ○, mock-inoculated cells; •, TU1-inoculated cells; □, Cp49-inoculated cells. (B) CPEs observed in CPV-inoculated CL-1 cells. CPV-inoculated or mock-inoculated CL-1 cells were harvested at 5 days p.i. (C) Electrophoresis of total cellular DNA. Cellular DNA was extracted from cultures of FPLV-inoculated (lanes 1), CPV-inoculated (lanes 2), or mock-inoculated (lanes 3) feline PBMCs (fPBMCs), FL74 cells, and canine PBMCs (cPBMCs). The inoculated fPBMCs, FL74 cells and cPBMCs were harvested at 4, 2, and 4 days p.i., respectively. The extracted DNAs were analyzed by 1.7% agarose gel electrophoresis. (D) Electrophoresis of total cellular DNA of the CPV-inoculated CL-1 cells. Cellular DNA was extracted from cultures of inoculated or mock-inoculated CL-1 cells. Lane 1, CPV-inoculated cells at 1 day p.i.; lane 2, 2 days p.i.; lane 3, 3 days p.i.; lane 4, 4 days p.i.; lane 5, 5 days p.i.; Mock, mock-inoculated cells at 4 days p.i. The extracted DNAs were analyzed by 1.7% agarose gel electrophoresis.
In the present study, we demonstrated that FPLV- or CPV-induced CPEs in the infected T-lymphoid cells were associated with apoptosis. As derangements of apoptosis contribute to the pathogenesis of several diseases (4, 7, 8, 24, 30), it might be possible that the induction of apoptosis in FPLV infection is a key element in the pathophysiology of atrophy of lymphoid tissues associated with feline panleukopenia caused by FPLV.
Induction of apoptosis has been well studied in HIV-1 systems (9, 28). A recent study of HIV-1 indicated that not only infected but also uninfected CD4+ T cells die in HIV-1-infected patients (9). As shown in Fig. 1A, FPLV antigen was detected in the infected feline cells at 1 day p.i., and the FPLV antigen-positive rates increased rapidly. However, the TUNEL-positive cell rates in FPLV-infected feline cells increased rather slowly and the ladders of DNA from FPLV-infected feline cells were clearly visible only at 2 to 4 days p.i. (Fig. 1C and 2B). These results suggest that FPLV-induced programmed cell death might result from direct infection of lymphocytes. Therefore, it is suggested that activation of an endogenous cell suicide program in FPLV-infected cells might serve as a host defense mechanism against viral proliferation. HIV-1-infected patients have also been reported to show both impaired expression rates of IL-2 receptor and a reduced proliferative response to IL-2 (29, 34). FIV infection was reported to suppress the mean fluorescence intensity of IL-2Rα expression (25), although suppressive effects on the expression of IL-2 receptor were smaller than those observed in HIV-1 infection. As shown in Fig. 3, almost the same results as those reported for FIV infection were observed in FPLV-infected lymphocytes. Therefore, it is possible that FPLV-induced apoptosis in the infected lymphocytes was partly due to a defect in the IL-2 signal transduction pathway.
Although FPLV and CPV have greater than 98% sequence identity (15, 32, 33), the host ranges of these viruses are complicated in vivo (6, 7, 19, 20, 32, 33). CPV does not regularly cause profound lymphopenia in dogs (26), while FPLV generally causes severe leukopenia in cats (11, 26). In the present study, FPLV was shown to grow efficiently in feline PBMCs but CPV was unable to infect canine PBMCs. These in vitro data seem to explain the phenomena observed in in vivo studies (26). However, Truyen and Parrish (32) reported that CPV strain CPV-d (CPV type 2) was able to grow efficiently in canine PBMCs. These conflicting data may be due to variabilities among CPV and FPLV isolates (20). The definite reason for the mild lymphopenia caused by CPV infection in dogs requires further studies using many FPLV and CPV isolates.
Recently, we isolated FPV strains from the PBMCs of domestic cats and wild felids with high levels of virus-neutralizing antibodies (13, 16). These observations indicate that FPV is more lymphotropic than we had considered and can persistently infect lymphocytes in vivo even in the presence of high levels of virus-neutralizing antibodies. In the present study, we report the apoptosis of lymphocytes induced by FPV infection. Thus, further studies using lymphoid cells will be necessary to assess the mechanisms of induction of apoptosis and persistent infection by FPV in cats.
Acknowledgments
We thank H. Tsujimoto (University of Tokyo, Tokyo, Japan) for providing anti-feline IL-2Rα MAb and CL-1 cells. We thank B. J. Willett (Glasgow University, Glasgow, Scotland) for providing anti-feline CD9 MAb.
This study was partly supported by grants from the Ministry of Education, Science, Sports and Culture and from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Ackley C D, Cooper M D. Characterization of a feline T-cell-specific monoclonal antibody reactive with a CD5-like molecule. Am J Vet Res. 1992;53:466–471. [PubMed] [Google Scholar]
- 2.Azetaka M, Hirasawa T, Konishi S, Ogata M. Studies on canine parvovirus isolation, experimental infection and serologic survey. Jpn J Vet Sci. 1981;43:243–255. doi: 10.1292/jvms1939.43.243. [DOI] [PubMed] [Google Scholar]
- 3.Crandell R A, Fabricant C G, Nelson-Rees W A. Development, characterization and viral susceptibility of a feline (Felis catus) renal cell line (CRFK) In Vitro (Rockville) 1973;9:176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- 4.Esolen L M, Park S W, Hardwick J M, Griffin D E. Apoptosis as a cause of death in measles virus-infected cells. J Virol. 1995;69:3955–3958. doi: 10.1128/jvi.69.6.3955-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto H, Hirano T, Uchida E, Watanabe K, Shinagawa M, Ichijo S, Shimizu K. Comparative studies of physicochemical and biological properties between canine parvovirus and feline panleukopenia virus. Jpn J Vet Sci. 1984;46:519–526. doi: 10.1292/jvms1939.46.519. [DOI] [PubMed] [Google Scholar]
- 6.Goto H, Hosokawa S, Ichijo S, Shimizu K, Morohoshi Y, Nakano K. Experimental infection of feline panleukopenia virus in specific pathogen-free cats. Jpn J Vet Sci. 1983;45:109–112. doi: 10.1292/jvms1939.45.109. [DOI] [PubMed] [Google Scholar]
- 7.Gougeon M L, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw V S, Olsen C W, Sissoko N D, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;331:78–81. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 10.Hosie M J, Willett B J, Dunsford T H, Jarrett O, Neil J C. A monoclonal antibody which blocks infection with feline immunodeficiency virus identifies a possible non-CD4 receptor. J Virol. 1993;67:1667–1671. doi: 10.1128/jvi.67.3.1667-1671.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu L, Esposito J J, Scott F W. Raccoon poxvirus feline panleukopenia virus VP2 recombinant protects cats against FPV challenge. Virology. 1996;218:248–252. doi: 10.1006/viro.1996.0186. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda, Y., T. Miyazawa, K. Kurosawa, R. Naito, S. Hatama, C. Kai, and T. Mikami. New quantitative methods for detection of feline parvovirus (FPV) and virus neutralizing antibody against FPV using a feline T lymphoid cell line. J. Vet. Med. Sci., in press. [DOI] [PubMed]
- 13.Ikeda, Y. Submitted for publication.
- 14.Konishi S, Mochizuki M, Ogata M. Studies on feline panleukopenia. 1. Isolation and properties of virus strains. Jpn J Vet Sci. 1975;37:439–449. doi: 10.1292/jvms1939.37.5_247. [DOI] [PubMed] [Google Scholar]
- 15.Martyn J C, Davidson B E, Studdert M J. Nucleotide sequence of feline panleukopenia virus: comparison with canine parvovirus identifies host-specific differences. J Gen Virol. 1990;71:2747–2753. doi: 10.1099/0022-1317-71-11-2747. [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa, T. Submitted for publication.
- 17.Miyazawa T, Furuya T, Itagaki S, Tohya Y, Nakano K, Takahashi E, Mikami T. Preliminary comparisons of the biological properties of two strains of feline immunodeficiency virus (FIV) isolated in Japan with FIV Petaluma strain isolated in the United States. Arch Virol. 1989;108:59–68. doi: 10.1007/BF01313743. [DOI] [PubMed] [Google Scholar]
- 18.Miyazawa T, Furuya T, Itagaki S, Tohya Y, Takahashi E, Mikami T. Establishment of a feline T-lymphoblastoid cell line highly sensitive for replication of feline immunodeficiency virus. Arch Virol. 1989;108:131–135. doi: 10.1007/BF01313750. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki M, Horiuchi M, Hiragi H, San Gabriel M C, Yasuda N, Uno T. Isolation of canine parvovirus from a cat manifesting clinical signs of feline panleukopenia. J Clin Microbiol. 1996;34:2101–2105. doi: 10.1128/jcm.34.9.2101-2105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mochizuki M, Harasawa R, Hakatani H. Antigenic and genomic variabilities among recently prevalent parvovirus of canine and feline origin in Japan. Vet Microbiol. 1993;38:1–10. doi: 10.1016/0378-1135(93)90070-n. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuki M, Konishi S, Ajiki M, Akaboshi T. Comparison of feline parvovirus subspecific strains using monoclonal antibodies against a feline panleukopenia virus. Jpn J Vet Sci. 1989;51:264–272. doi: 10.1292/jvms1939.51.264. [DOI] [PubMed] [Google Scholar]
- 22.Momoi Y, Okai Y, Watari T, Goitsuka R, Tsujimoto H, Hasegawa A. Establishment and characterization of a canine T-lymphoblastoid cell line derived from malignant lymphoma. Vet Immunol Immunopathol. 1997;59:11–20. doi: 10.1016/s0165-2427(97)00053-6. [DOI] [PubMed] [Google Scholar]
- 23.Ohno K, Goitsuka R, Kitamura K, Hasegawa A, Tokunaga T, Honda M. Production of a monoclonal antibody that defines the α-subunit of the feline IL-2 receptor. Hybridoma. 1992;11:595–605. doi: 10.1089/hyb.1992.11.595. [DOI] [PubMed] [Google Scholar]
- 24.Ohno K, Nakano T, Matsumoto Y, Watari T, Goitsuka R, Nakayama H, Tsujimoto H, Hasegawa A. Apoptosis induced by tumor necrosis factor in cells chronically infected with feline immunodeficiency virus. J Virol. 1993;67:2429–2433. doi: 10.1128/jvi.67.5.2429-2433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohno K, Okamoto Y, Miyazawa T, Mikami T, Watari T, Goitsuka R, Tsujimoto H, Hasegawa A. Induction of apoptosis in a T lymphoblastoid cell line infected with feline immunodeficiency virus. Arch Virol. 1994;135:153–158. doi: 10.1007/BF01309772. [DOI] [PubMed] [Google Scholar]
- 26.Parrish C R. Parvoviruses: cats, dogs and mink. In: Webster R G, Granoff A, editors. Encyclopedia of virology. London, England: Academic Press; 1994. pp. 1061–1067. [Google Scholar]
- 27.Parrish C R. Molecular epidemiology of parvoviruses. Semin Virol. 1995;6:415–418. [Google Scholar]
- 28.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 29.Prince H E, Kermani-Arab V, Fahey J L. Depressed interleukin 2 receptor expression in acquired immune deficiency and lymphoadenopathy syndromes. J Immunol. 1984;133:1313–1317. [PubMed] [Google Scholar]
- 30.Rojko J L, Fulton R M, Rezanka L J, Williams L L, Copelan E, Cheney C M, Reichel G S, Neil J C, Mathes L E, Fisher T G, Cloyd M W. Lymphocytotoxic strains of feline leukemia virus induced apoptosis in feline T4-thymic lymphoma cells. J Clin Investig. 1992;66:418–426. [PubMed] [Google Scholar]
- 31.Theilen G H, Kawakami T G, Rush J D, Munn R J. Replication of the cat leukemia virus in cell suspension cultures. Nature. 1969;222:589–590. doi: 10.1038/222589b0. [DOI] [PubMed] [Google Scholar]
- 32.Truyen U, Parrish C R. Canine and feline host ranges of canine parvovirus and feline panleukopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. J Virol. 1992;66:5399–5408. doi: 10.1128/jvi.66.9.5399-5408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truyen U, Gruenberg A, Chang S-F, Obermaier B, Veijalainen P, Parrish C R. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelstein A, Kingsley L A, Klein R S, Lyter D W, Evans T L, Rinaldo C R, Weaver L D, Machen L L, Schadle R C. Defective T-cell colony formation and IL-2 receptor expression at all stages of HIV infection. Clin Exp Immunol. 1988;71:417–422. [PMC free article] [PubMed] [Google Scholar]
- 35.Wosu L O. Feline panleucopenia—in vivo infectivity studies. Vet Microbiol. 1988;16:137–143. doi: 10.1016/0378-1135(88)90038-7. [DOI] [PubMed] [Google Scholar]
- 36.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]