Abstract
Objective
In the small intestine, the products of digestion of dietary triacylglycerol (TAG), fatty acids (FA) and monoacylglycerol, are taken up by absorptive cells, enterocytes, for systemic energy delivery. These digestion products can also bind receptors on endocrine cells to stimulate the release of hormones capable of influencing systemic energy metabolism. The initial phase of intestinal FA absorption involves the acylation of FAs to acyl-CoA by the acyl-CoA long chain synthetase (ACSL) enzymes. ACSL5 is abundantly expressed in the small intestinal epithelium where it is the major ACSL isoform, contributing approximately 80% of total ACSL activity. In mice with whole body deficiency of ACSL5, the rate of dietary fat absorption is reduced and energy expenditure is increased. However, the mechanisms by which intestinal ACSL5 contributes to intestinal FA metabolism, enteroendocrine signaling, and regulation of energy expenditure remain undefined. Here, we test the hypothesis that intestinal ACSL5 regulates energy metabolism by influencing dietary fat absorption and enteroendocrine signaling.
Methods
To explore the role of intestinal ACSL5 in energy balance and intestinal dietary fat absorption, a novel mouse model of intestine specific ACSL5 deficiency (ACSL5IKO) was generated by breeding ACSL5 floxed (ACSL5loxP/loxP) to mice harboring the tamoxifen inducible, villin-Cre recombinase. ACSL5IKO and control, ACSL5loxP/loxP mice were fed chow (low in fat) or a 60% high fat diet (HFD), and metabolic phenotyping was performed including, body weight, body composition, insulin and glucose tolerance tests, energy expenditure, physical activity, and food intake studies. Pair-feeding studies were performed to determine the role of food intake in regulating development of obesity. Studies of dietary fat absorption, fecal lipid excretion, intestinal mucosal FA content, and circulating levels of glucagon like peptide 1 (GLP-1) and peptide YY (PYY) in response to a TAG challenge were performed. Treatment with a GLP-1 receptor antagonist was performed to determine the contribution of GLP-1 to acute regulation of food intake.
Results
We found that ACSL5IKO mice experienced rapid and sustained protection from body weight and fat mass accumulation during HFD feeding. While intestine specific deficiency of ACSL5 delayed gastric emptying and reduced dietary fat secretion, it did not result in increased excretion of dietary lipid in feces. Energy expenditure and physical activity were not increased in ACSL5IKO mice. Mice deficient in intestinal ACSL5 display significantly reduced energy intake during HFD, but not chow feeding. When HFD intake of control mice was matched to ACSL5IKO during pair-feeding studies, no differences in body weight or fat mass gain were observed between groups. Postprandial GLP-1 and PYY were significantly elevated in ACSL5IKO mice secondary to increased FA content in the distal small intestine. Blockade of GLP-1 signaling by administration of a long-acting GLP-1 receptor antagonist partially restored HFD intake of ACSL5IKO.
Conclusions
These data indicate that intestinal ACSL5 serves as a critical regulator of energy balance, protecting mice from diet-induced obesity exclusively by increasing satiety and reducing food intake during HFD feeding. The reduction in food intake observed in ACSL5IKO mice is driven, in part, by increased postprandial GLP-1 and PYY secretion. These effects are only observed during HFD feeding, suggesting that altered processing of dietary fat following intestinal ACSL5 ablation contributes to GLP-1 and PYY mediated increases in satiety.
Keywords: Intestine, Acyl-CoA long chain synthetase 5, Glucagon like peptide-1, Food intake, Dietary fat absorption
Highlights
-
•
Mice with intestine-specific deficiency of Acyl-CoA Synthetase 5 (ACSL5IKO) are protected against diet-induced obesity.
-
•
Reduced energy intake is responsible for the reduced body weight and adiposity observed in HFD fed ACSL5IKO mice.
-
•
Postprandial GLP-1 and PYY secretion is significantly elevated in ACSL5iKO mice.
-
•
GLP-1 receptor antagonism partially restores HFD energy intake in ACSL5iKO mice.
1. Introduction
In addition to the intestine's role in the absorption of dietary nutrients, the intestine also participates in the regulation of energy homeostasis. This regulation occurs, in part, through endocrine signaling networks capable of sensing dietary nutrients and their digestion products. Dietary fat is the most energy dense nutrient. After consumption, dietary fat in the form of triacylglycerol (TAG), is digested in the lumen of the small intestine to monoacylglycerol (MAG) and fatty acids (FAs). These digestive products are then taken up by absorptive cells of the small intestine, enterocytes, where they are further metabolized into TAG and incorporated into chylomicrons for systemic delivery [1,2]. Although the bulk of FAs and MAG are efficiently absorbed, FAs and MAG in the lumen of the small intestine can also bind receptors on enteroendocrine cells to promote secretion of hormones that regulate physiological metabolism (e.g. food intake, gut transit time, insulin secretion). The success of bariatric surgery [3] and drugs targeting enteroendocrine signaling (e.g. glucagon like peptide 1 (GLP-1) receptor agonists) [4,5] in improving adiposity and metabolic health highlights the importance of dietary fat digestion, absorption, and signaling in regulating energy homeostasis.
The initial step of dietary fat absorption occurs within enterocytes, where coenzyme A (CoA) is enzymatically added to FAs to form acyl-CoAs. The formation of acyl-CoAs effectively traps FAs inside enterocytes (referred to as vectorial acylation) and promotes further metabolism of FAs. This step is catalyzed by one of five long-chain acyl-coenzyme A synthetases (ACSLs) [6,7]. ACSL5 is the major small intestinal ACSL isoform and is expressed primarily in enterocytes, with lower levels of expression in liver, white preadipocytes, and brown adipose tissue [8,9]. Previously, we found that mice with whole body ACSL5 deficiency (ACSL5KO) have reduced adiposity, improved insulin sensitivity, and increased energy expenditure when fed a low fat, chow diet [10]. The increase in energy expenditure was associated with increased hepatic secretion of fibroblast growth factor 21 (FGF21) and increased expression of uncoupling protein 1 (UCP1) in white adipose. In addition to increased energy expenditure, ACSL5KO mice also showed reduced rates of dietary fat absorption, suggesting an essential role for intestinal ACSL5 in metabolism of dietary FAs. However, whether deficiency of ACSL5 in the intestine alters dietary fat metabolism to impact enteroendocrine signaling and whole-body energy metabolism is unclear.
To test the hypothesis that intestinal ACSL5 regulates energy metabolism by influencing dietary fat absorption and enteroendocrine signaling, we generated mice with intestine specific deficiency of ACSL5 (ACSL5IKO). We found that high fat diet (HFD) fed ACSL5IKO mice are resistant to the development of diet induced obesity and associated metabolic complications due, primarily, to reduced food intake. Although gastric emptying was delayed and the rate of dietary fat absorption was slowed, quantitative absorption of dietary fat was not reduced in HFD fed ACSL5IKO mice. In response to HFD, ACSL5IKO mice had increased circulating levels of the enteroendocrine hormones, GLP-1 and PYY. Blockade of GLP-1 signaling with a GLP-1 receptor antagonist partially restored food intake in HFD fed ACSL5 IKO mice. These results highlight the important role of intestinal ACSL5 in regulating body weight, adiposity, and obesity-associated metabolic complications through its influence on food intake via potentiated postprandial secretion of GLP-1 and PYY.
2. Materials & methods
2.1. Generation of mice with intestine specific loss of ACSL5
Mice harboring loxP sequences flanking exons 16 and 17 of the ACSL5 gene (ACSL5loxP/loxP) were generated on a C57BL/6 J background as previously described [10]. To generate a mouse line in which ACSL5 is ablated specifically in the intestine, ACSL5loxP/loxP mice were mated with C57BL6/J mice carrying an intestinal epithelial specific, tamoxifen (TAM) inducible Cre transgene, villin-Cre-ERT2, to yield heterozygous ACSL5wildtype/loxP-vilCRE-ERT2 mice [11]. Heterozygotes carrying the inducible villin-Cre transgene were subsequently mated to ACSL5loxP/loxP mice to generate ACSL5loxP/loxP homozygotes carrying inducible villin-Cre (ACSL5IKO). At 12 weeks of age, both ACSL5loxP/loxP and ACSL5IKO mice were treated with 100 μl of TAM (Sigma) in sunflower oil (Sigma) via oral gavage once per day for three days. During initial experiments TAM was administered at 2.0 mg/day. In later experiments, we found that both 0.5 mg/day (“low-dose”) and 2.0 mg/day (“high-dose”) TAM were equally effective at reducing ACSL5 gene expression for up to 12 weeks (data not shown). As such, low-dose TAM was used for subsequent experiments. Use of high-dose TAM is specified in the figure legends where appropriate. Following TAM treatment, mice were allowed 10 days to recover from possible acute, negative side effects of TAM administration [12]. All experiments were performed subsequently using this TAM regiment. With respect to selection of experimental controls, we did not observe any independent effects of TAM treatment, dose, or the presence of the Cre transgene on ACSL5 gene expression or body composition using the stated experimental protocol (data not shown). Thus, all experiments described herein were performed with TAM treated ACSl5loxP/loxP mice lacking villin-Cre-ERT2 serving as controls. ACSL5 and Cre genotypes of mice were determined by PCR.
2.2. Mouse care and use
All experiments were conducted at the Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging in accordance with guidelines and regulations approved by the Institutional Animal Care and Use Committee of Tufts University. ACSL5loxP/loxP and ACSL5IKO littermates were individually housed at 10 weeks of age. At 12 weeks of age, TAM treatment was administered, and mice were randomized into experimental groups. For diet studies mice were either fed chow (Teklad, 2016S) or a 60% high fat diet (HFD, Research Diets D12492). Prior to euthanasia food was removed at the start of the light cycle. Mice were euthanized in the fed state, defined as 2 h after the start of the light cycle, or fasted state, defined as 6 h after the start of the light cycle. Fasting time is indicated in figure legends. Mice were euthanized under isoflurane anesthesia by exsanguination and subsequent cervical dislocation. Blood was collected in EDTA coated tubes, centrifuged at 2,000×g for 20 min at 4 °C and the plasma fraction was collected and stored at −80 °C. Tissues were collected, and flash frozen in liquid nitrogen. For mucosal scrapings, the intestine was removed from base of stomach to ileocecal junction, flushed in ice cold phosphate buffered saline (PBS), cut into five equal length sections, and mucosa was scraped using a glass slide and flash frozen in liquid nitrogen. The most proximal section, section 1, was designated as duodenum, sections 2 & 3 were pooled and designated as jejunum, and the most distal sections, sections 4 & 5, were pooled and designated as ileum. Prior to analysis all tissues were pulverized under liquid nitrogen.
2.3. Tissue protein levels
Pulverized intestinal tissue (75–100 mg) was homogenized in 500 μl of ice cold RIPA Buffer (50 mM Tris–HCl, pH 7.4; 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitors (Thermo Scientific) with ten strokes of a Dounce homogenizer. Samples were incubated on ice for 30 min and spun at 16,000×g for 10 min at 4 °C. The infranatant was collected and passed through a 26G needle for 30 strokes. Protein concentrations of homogenate were determined by BCA Assay (Thermo Scientific). Homogenate was added to 2x Laemmle sample buffer (BioRad) and denatured by boiling for 5 min at 95 °C. Protein homogenate (25 μg) was loaded onto a 7.5% SDS-Page gel (Biorad) and proteins were separated by SDS-PAGE at 100 V for 90 min. Protein was transferred to a 45 μm nitrocellulose membrane for 1 h at 350 mA at 4 °C, blocked in 5% milk at room temperature, and incubated with primary antibody overnight. Following primary antibody incubation, membranes were washed in PBS with 0.1% Tween-20, incubated with secondary antibody for 1 h at room temperature and washed again. Blots were subsequently treated with ECL Reagent (Thermo Scientific) and proteins were visualized using autoradiography.
2.4. Tissue relative mRNA levels
Pulverized liver and intestine (30–50 mg) and adipose tissue (250 mg) were mechanically homogenized in TRIzol (Invitrogen) and total RNA was extracted with RNeasy Mini columns (Qiagen) per the manufacturer's instructions. RNA concentration and purity of extracts were determined using a Nanodrop 100 spectrophotometer. cDNA was generated from 2 μg RNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative real-time PCR (qPCR) was performed using SYBR Green (Applied Biosystems) on an Applied Biosystems 7300 Real-Time PCR system. Relative expression was quantified using the 2−ΔΔCt method [13].
2.5. Metabolic phenotyping & tissue collection
The body composition of mice was determined by magnetic resonance imaging (EchoMRI) within 4 h of the start of the light cycle. Energy expenditure was determined using indirect calorimetry (TSE Systems). Briefly, mice were placed in metabolic chambers and allowed to acclimate for 48 h prior to data collection. Gas exchange was then recorded for a minimum of 72 h. Hourly averages were determined by pooling daily data. To control for differences in body composition average daily energy expenditure was analyzed by linear regression using lean body mass as a predictor [14].
Glucose and insulin tolerance tests were performed as previously described [13]. Briefly, for glucose tolerance tests mice were fed HFD for six weeks and fasted for 6 h prior to intraperitoneal administration of 1 mg/kg glucose in sterile saline. Blood glucose was measured from tail vein nicks via glucometer (OneTouch Ultra). For insulin tolerance tests, mice fed HFD for eight weeks were fasted for 4 h and injected intraperitoneally with 1 IU/kg insulin in sterile saline. Glucose from tail vein blood was measured via glucometer.
Fasting plasma metabolites were measured from cardiac plasma collected at time of sacrifice after a 6 h fast, except for blood glucose which was measured from tail vein blood via glucometer at time of sacrifice. Plasma insulin was measured via ELISA (Ultra-Sensitive Mouse Insulin ELISA, Crystal Chem). Blood lipoproteins were measured on a clinical chemistry analyzer (AU480 Clinical Chemistry Analyzer, Beckman Coulter, Inc.) as specified in the manufacturer's procedural documentation. HDL was measured by a two-phase reaction with colorimetric endpoint detection as specified in the Olympus AU480 Procedural Insert (HDL-Cholesterol OSR6187 and OSR6287). The Friedewald formula was used to estimate VLDL and LDL cholesterol when TAG levels were less than 400 mg/dL.
Hepatic lipids were measured as previously described using a modified Folch extraction [13]. Non-esterified fatty acids (NEFA) from intestinal mucosa of mice fed a HFD for two weeks and euthanized in the fed state were extracted and analyzed using a colorimetric kit following kit instructions (Free Fatty Acid Quantitation Kit, Sigma)
2.6. Postprandial lipemia and intestinal TAG secretion rate
For postprandial lipemia experiments, male mice (16-week-old) fed a chow diet were fasted for 6 h and 100 μl of blood from a tail vein puncture was collected in a K2-EDTA coated tube to serve as a baseline for serum TAG. Mice were then oral gavaged with 200 μl olive oil and blood was collected at one, two, and four hours post gavage. For TAG secretion assays, the experimental protocol above was repeated in the presence of tyloxapol. For tyloxapol treatment, after baseline collection, 18-week-old, male mice fed chow diet received an intraperitoneal injection of 500 mg/kg of tyloxapol and were oral gavaged 30 min later with 200 μl olive oil. Following experiments blood was spun at 1,500g for 10 min and serum TAG was determined using a colorimetric assay (Wako Diagnostics).
2.7. Rate of gastric emptying
Male mice (18-20-week-old) fed a chow diet were fasted for 6 h before receiving 1 μCi of carboxy-14C labeled triolein suspended in olive oil. Four hours following gavage mice were euthanized via cervical dislocation and stomach was removed. The stomach was opened, solid contents were removed, and both contents and stomach lumen were washed in 0.5 mM sodium taurocholate for 30 s by gently mixing in a 2 ml Eppendorf tube. Stomach tissue was subsequently discarded. Wash solutions from stomach was mixed with 5 ml of scintillation buffer and equilibrated overnight at 4 °C before counts were measured via scintillation counter.
2.8. Fecal lipid excretion
Male mice (16-week-old) fed HFD for four weeks were individually housed in wire bottom cages for five days. Mice were acclimated to wire bottom housing for two days. After two days of acclimation, food and feces were weighed each morning for three consecutive days. Feces were freeze dried and subsequently pulverized using a mortar & pestle on dry ice. Lipid from 200 to 300 mg of dry feces was extracted using a modified Folch Extraction [15]. Lipid extracts were dried under nitrogen gas and gross lipid weight was measured on a microgram balance. Lipid content (%w/w) of feces was determined by dividing weight of dried lipid extract by weight of feces used for extraction. Daily fecal lipid output was calculated by multiplying the lipid content of the feces by the average mass of feces output per day. This was then normalized to average daily food intake and presented as average daily fecal lipid excretion per average daily food intake.
2.9. Food intake & pair-feeding
Chow food intake was recorded from 14-week-old, male mice maintained in normal housing conditions. HFD food intake was recorded in 18-week-old male mice following four weeks of diet feeding. Daily intake was averaged over 72 h.
For fasting/refeeding studies, 18-week-old, male mice fed a chow diet were fasted overnight for 16 h with free access to water. Following the fast, chow diet was re-introduced, and food intake was recorded every 2 h for 12 h and again at 24 h. Mice were fed chow until bodyweight returned to pre-experiment levels (approximately 5–7 days). Ten days following chow fasting/refeed, mice were fasted overnight for 16 h and refed a 60% HFD. Food intake was recorded as indicated above.
For pair-feeding studies, 16-week-old, chow fed male mice were randomized into bodyweight matched groups and acclimated to twice daily handling for one week. Following acclimation, on day one, ACSL5loxP/loxP and ACSL5IKO mice were started on ad-libitum HFD feeding and food intake was recorded every 12 h. Starting on day three, the 12 h average food intake of ACSL5IKO ad-libitum mice was administered to all ACSL5loxP/loxP pair-fed mice every 12 h (at the start and end of the light cycle). After one week of acclimation to restricted food intake, pair-fed mice were transitioned to once daily feeding. At this time the average daily intake of ACSL5IKO ad-libitum fed mice was given to all pair-fed mice at the start of the dark cycle. During pair-feeding experiments body weight was recorded every 72 h at the start of the light cycle. Body composition was recorded every two weeks within the first 4 h of the start of the light cycle.
2.10. Postprandial GLP-1 and PYY
Male mice (14-week-old) were fasted overnight and either treated with 200 μl olive oil delivered via oral gavage or refed with 60% HFD. For studies in HFD mice, experiments were performed in male mice (20-week-old) fed a HFD for six weeks. For both gavage and refeeding experiments, blood samples were collected 1 h prior to the start of the experiment (after overnight fast) and again 2 h following gavage or refeeding. Blood was collected via submandibular vein puncture, stored in in K2-EDTA tubes, and mixed immediately following collection with 10 μl DPP4 Inhibitor (Millipore). Active GLP-1 was measured via immunoassay (Meso Scale Discovery). PYY was analyzed by ELISA (Crystal Chem).
2.11. GLP-1 antagonism
Male mice (9-month-old) were intraperitoneally injected with 2000 μmol/kg of a long acting GLP-1 agonist dissolved in saline at 8 am [16]. At 4 pm food was removed, and mice were fasted overnight. At 8 am the following morning, mice were treated again with the GLP-1 agonist. At 10 am mice were refed a pre-weighed amount of HFD and 24-hour cumulative food intake was recorded.
2.12. Food preference
Individually housed, male mice (16-week-old) fed a chow diet were fed an equal weight of pellets of 60% HFD (Research Diets, D12492) and sucrose-matched 10% low fat diet (LFD, Research Diets, D12450J) at the start of the dark cycle. Pellets were deposited directly into the cage food hopper. Food was weighed every 12 h, at the start and end of the light cycle, for 48 h. Energy intake was calculated from food weight consumed based on energy density (kcal/g) of each diet.
2.13. Statistical analysis
Results are expressed as means ± standard error of the mean. For 2 × 2 factorial study designs, data were analyzed by two-way analysis of variance (ANOVA) followed by Tukey's HSD post-hoc testing. For time series (repeated measures) experiments, differences in outcomes were analyzed by fitting a linear mixed model to the indicated outcome, using genotype as a between subjects factor, time as a within subjects factor, and individual animal as a random factor. When a significant interaction between within and between subjects factors was observed, differences between estimated marginal means of groups was assessed by Student's t-tests, adjusting for multiple comparisons using the Bonferroni method. All data analysis was conducted using using R (versions 4.2.3.) and RStudio (2023.03.0 + 386) using the following packages: tidyverse (v2.0.0), emmeans (v1.8.5), nlme_(v3.1-162). Values of p 0.05 were considered significant.
3. Results
3.1. Intestine specific deficiency of ACSL5 protects against diet-induced obesity & insulin resistance
To determine the role of intestinal ACSL5 in dietary fat metabolism and regulation of energy homeostasis we generated mice with intestine specific deficiency of ACSL5 by crossing ACSL5 floxed (ACSL5loxP/loxP) mice to mice carrying the tamoxifen inducible villin-Cre-ERT2 transgene (ACSL5IKO) [10,11]. For all experiments, TAM was administered to both ACSL5loxP/loxP (control) and ACSL5IKO at 12 weeks of age. Following TAM administration, mice were fed either a low fat, chow diet or a 60% HFD for 12 weeks. ACSL5 protein deficiency was confirmed in jejunal extracts of ACSL5IKO mice by western blotting (Figure 1A). Acsl5 mRNA expression was reduced in duodenum, jejunum, and ileum but not in liver, confirming recombination was limited to the intestine (Figure 1B). No increase in mRNA expression of other ACSL isoforms or other genes expressing proteins with acyl-CoA synthetase activity (e.g. fatty acid transport protein 4 (FATP4)) was observed in ACSL5IKO mice (Figure 1C).
Figure 1.
Intestine Specific ACSL5-deficiency in ACSL5IKOMice. (A) Representative western blots of ACSL5 protein in jejunal mucosal scrapings of chow fed ACLS5loxP/loxP and ACSLIKO mice 12 weeks post-TAM treatment (2.0 mg/day). (B) Relative mRNA levels of ACSL5 in tissues of ACLS5loxP/loxP and ACSL5IKO mice fed 60% HFD for 12 weeks, n = 14 mice/group. (C) Relative mRNA levels of ACSL isoforms in jejunal mucosal scrapings of ACLS5loxP/loxP and ACSL5IKO mice fed chow for 12 weeks, n = 12–14 mice/group. For all experiments mice were euthanized following a 6 h fast. Data are presented as means ± SEM. ∗ = p ≤ 0.05 via student's t-test.
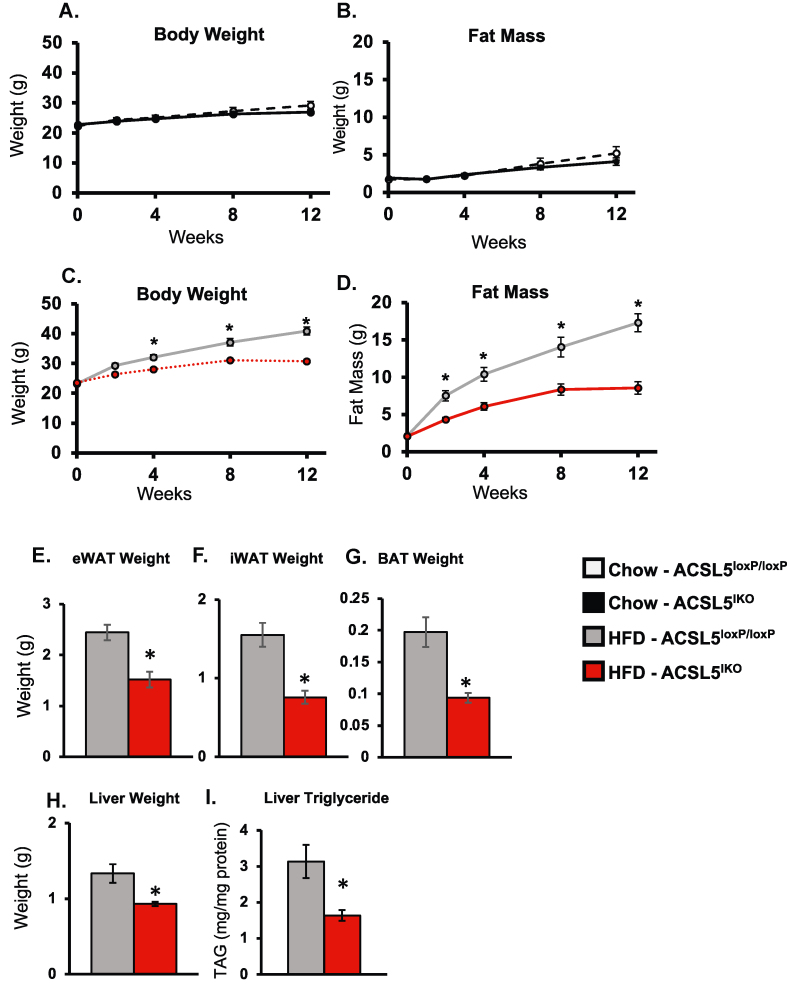
To investigate if intestinal ACSL5 regulates body composition and energy metabolism, we fed ACSL5IKO and ACSL5loxP/loxP mice either a chow or a 60% HFD for up to 12 weeks. During chow feeding, no differences in body composition, adipose depot weights, or liver weights were observed (Figure 2A-B). In contrast to chow feeding, body weight and fat mass were significantly reduced in ACSL5IKO within one week of HFD feeding (Figure S1a–b). Consistent with reduced adiposity, weights of adipose depots were significantly reduced in ACSL5IKO mice after two weeks of HFD feeding (Figure S1c–e). Liver weight and liver TAG content also trended lower in ACSL5IKO compared to ACSL5loxP/loxP mice at this timepoint (Figure S1f–g). After 12 weeks of HFD feeding, ACSL5IKO mice continued to demonstrate significant protection from body weight gain, along with reduced fat mass and lower adipose depot weights (Figure 2C–G). In addition, liver weight and liver TAG content were significantly lower in ACSL5IKO compared to ACSL5loxP/loxP mice after 12 weeks of HFD (Figure 2H–I). In summary, ACSL5IKO demonstrated immediate and persistent protection from DIO and hepatic steatosis when fed HFD.
Figure 2.
Protection from 60% High Fat Diet (HFD)-induced Obesity in ACSL5IKOMice. (A–D) Body weight and fat mass of chow (A,B) or 60% HFD (C,D) fed mice over 12 weeks beginning two weeks following high-dose tamoxifen treatment (2.0 mg/d for three days). (E–H) Tissue weights of epididymal white adipose tissue (eWAT) (E), inguinal white adipose tissue (iWAT) (F), brown adipose tissue (BAT) (G) and liver (H) after 12 weeks of HFD feeding, n = 12–14 mice/group. (I) Hepatic TAG after 12 weeks of HFD feeding, n = 7–9 mice/group. Data are presented as means ± SEM. ∗ = p ≤ 0.05 via student's t-test.
We next investigated if intestinal ACSL5 regulates glucose-insulin homeostasis in mice fed HFD. After 12 weeks of HFD feeding, fasting glucose was significantly reduced and serum insulin levels trended lower in ACSL5IKO mice compared to controls (Figure 3A–B). An insulin tolerance test revealed significantly reduced glucose excursions in HFD fed ACSL5IKO compared to ACSL5loxP/loxP mice as determined by glucose area under the curve (AUC) (Figure 3C). The reduction in glucose following insulin injection remained significant when controlling for differences in initial glucose values (Figure 3D). Blood glucose excursions were also significantly reduced in HFD fed ACSL5IKO mice following an intraperitoneal glucose tolerance test (Figure 3E–F).
Figure 3.
Improved Glucose Tolerance and Insulin Sensitivity in 60% HFD Fed ACSL5IKO. (A) Fasting glucose and (B) insulin in mice following 12 and 18 weeks of 60% HFD feeding, respectively. n = 12–14 mice/group. (C-D) Blood glucose levels following intraperitoneal (IP) injection of 1 Unit/mL insulin in mice fed 60% HFD for 4 weeks presented as (C) absolute glucose and (D) change in glucose from baseline. (E–F) Blood glucose levels following IP injection of 1 g/kg glucose in mice fed 60% HFD for 6 weeks presented as (E) absolute glucose and (F) change in glucose from baseline. Area under the curve (AUC) is presented alongside the corresponding plot. n = 5–9 mice/group/timepoint. ∗ = p ≤ 0.05. For time series data each timepoint was analyzed separately via student's t-test.
To determine if intestinal ACSL5 deficiency influences blood lipids, we determined fasting blood lipid concentrations in HFD fed ACSL5IKO and ACSL5loxP/loxP male mice. Total plasma cholesterol, low density lipoprotein (LDL) cholesterol and very low-density lipoprotein (VLDL) cholesterol concentrations were significantly reduced in ACSL5IKO mice compared to ACSL5loxP/loxP controls (Table 1). In addition, high-density lipoprotein (HDL) cholesterol concentration was significantly elevated in ACSL5IKO mice (Table 1). Although not statistically significant, fasting blood TAG concentration tended to be reduced in HFD fed ACSL5IKO mice compared to ACSL5loxP/loxP mice (p = 0.08) (Table 1).
Table 1.
Improved Serum Lipid Profile in ACSL5IKOMice. Six hour fasting serum lipids in ACLS5loxP/loxP and ACSL5IKO fed 60% HFD for 12 weeks. n = 10–12 mice/group. Data are presented as means ± SEM. ∗ = p ≤ 0.05 - ACSL5loxP/loxP.
| Measure | Floxed |
ACSL5IKO |
p-value |
|---|---|---|---|
| mean ± s.e.m | mean ± s.e.m | ||
| Triglyceride (mg/dL) | 50.4 ± 3.05 | 42.9 ± 5.84 | 0.08 |
| Cholesterol (mg/dL) | 188 ± 9.2 | 153 ± 7.3 | <0.01 |
| HDL-C (mg/dL) | 82.3 ± 1.2 | 93.4 ± 3.1 | <0.01 |
| LDL-C (mg/dL) | 100.8 ± 7.7 | 56.9 ± 6.2 | <0.01 |
| VLDL-C (mg/dL) | 10.2 ± 0.6 | 8.5 ± 0.4 | <0.05 |
| Glucose (mg/dL) | 172 ± 4 | 155 ± 5 | <0.05 |
3.2. ACSL5IKO mice have a blunted postprandial triglyceridemia, a reduced rate of gastric emptying, but no change in quantitative dietary fat absorption
Because addition of CoA to FAs is critical for uptake and cellular trafficking in enterocytes, we investigated the impact of ACSL5 deficiency in intestinal epithelial cells on morphological aspects of the intestine and dietary fat absorption when fed HFD. We found no differences in intestinal length nor morphology but found a significant increase in the height of the jejunal villi in ACSL5IKO mice (Figure 4A–C). To investigate the kinetics of dietary fat absorption, we challenged chow fed mice with a 200 μl oral bolus of olive oil. We found postprandial triglyceridemia was significantly reduced in ACSL5IKO compared to ACSL5loxP/loxP mice (Figure 4D). To rule out a contribution from increased in TAG clearance to this response, we blocked TAG clearance using an inhibitor of lipoprotein lipase, tyloxapol and repeated the experiment. With TAG clearance blocked, we again observed a significant reduction in the rate of postprandial TAG accumulation in circulation (Figure 4E). In total, these results are consistent with a reduced rate of dietary fat absorption in ACSL5IKO mice. The reduced rate of dietary fat absorption was not associated with reduced expression of genes known to contribute to the rate of dietary fat absorption, such as MGAT2 and DGAT1 (Figure 4F).
Figure 4.
Delayed Gastric Emptying but No Reduction in Gross Lipid Absorption in 60% HFD Fed ACSL5IKOMice. (A) Intestine length in ACLS5loxP/loxP and ACSL5IKO mice fed 60% HFD for 12 weeks, n = 11–13 mice/group. (B) Representative jejunal cross sections from mice fed 60% HFD for 4 weeks. (C) Average villus height, from base to tip, of jejunal villi. Data represent average height collected from 10 villi per mouse. n = 3 mice/group ∗ = p ≤ 0.05 via student's t-test. (D–E) Serum TAG following 200 μl olive oil gavage in the absence (D) or presence (E) of tyloxapol treatment in chow fed ACLS5loxP/loxP and ACSL5IKOmice, n = 5–7 mice/group. ∗ = p ≤ 0.05 via student's t-test at individual time points (F) Relative mRNA levels of lipid metabolism genes in jejunum of chow and 60% HFD fed (2 weeks) ACLS5loxP/loxP and ACSL5IKOmice n = 10–12 mice/group. Data are presented as means ± SEM. ∗ = p ≤ 0.05 vs Chow - ACSL5loxP/loxP, @ = p ≤ 0.05 vs. HFD - ACSL5loxP/loxP analyzed via two-ANOVA and Tukey's HSD post-hoc testing. (G) Gastric lipid content and (H) intestinal mucosal lipid content, as measured by scintillation counts 2 h following a gavage of 1 μCi carboxy-14C-triolein in chow fed mice. ∗ = p ≤ 0.05 via student's t-test. (I) Average daily fecal lipid excretion normalized to food intake (as measured by mg lipid per mg feces ∗ average mg feces excreted per day/average mass food consumed per day) in ACLS5loxP/loxP and ACSL5IKO mice fed 60% HFD for 4 weeks, n = 5–6 mice/genotype. ∗ = p ≤ 0.05 via student's t-test. Data are presented as means ± SEM.
One possible explanation for the delayed rate of dietary fat absorption is a reduced rate of gastric emptying. To investigate this possibility, mice were oral gavaged with olive oil containing racemic 14C-triolein and radioactivity of gastric contents and intestinal mucosa was quantified. Four hours following gavage, we observed a significant 200% increase in levels of radioactivity in the stomach of ACSL5IKO mice compared to ACSL5loxP/loxP mice (Figure 4G). In addition, radioactivity in intestinal mucosal scrapings was 50% lower in ACSL5IKO compared to ACSL5loxP/loxP mice at this time point (Figure 4H). In total, these observations are consistent with a reduced a rate of gastric emptying in ACSL5IKO mice.
To determine if the reduced rate of dietary fat absorption could also be due to reduced quantitative, total dietary fat absorption, we quantified food intake and fecal lipid output during HFD feeding. Our studies revealed that daily fecal lipid excretion normalized to food intake was similar between ACSL5IKO and ACSL5loxP/loxP mice fed HFD (Figure 4I). In summary our studies indicate intestinal ACSL5 deficiency reduces the rate of dietary fat absorption potentially through reduced rates of gastric emptying but does not impact total dietary fat absorption.
3.3. Protection from DIO in ACSL5IKO mice is not due to increased metabolic rate
To determine if increased metabolic rate contributes to protection from DIO in ACSL5IKO mice, we investigated energy expenditure of ACSL5loxP/loxP and ACSL5IKO mice fed chow or HFD. When fed chow, ACSL5IKO mice had similar energy expenditure to ACSL5loxP/loxP mice (Figure S2a–b). After one week of HFD feeding, energy expenditure of ACSL5IKO mice was slightly, but significantly lower than ACSL5loxP/loxP controls in the absence of any changes in physical activity (Figure S2c–e). However, after 12 weeks of HFD feeding no differences in metabolic rate were observed between ACSL5loxP/loxP and ACSL5IKO mice (Figure 5A–B). In our previous publication, increased energy expenditure in whole body ACSL5 KO mice was associated with increased hepatic secretion of FGF21 [10]. In contrast to ACSL5 KO mice, circulating FGF21 and hepatic expression of FGF21 were reduced in ACSL5IKO mice fed HFD (Figure S3a–b). In summary, protection from DIO in ACSL5IKO mice is not due to increased metabolic rate.
Figure 5.
Similar Energy Expenditure in ACSL5loxP/loxPand ACSL5IKOMice Fed 60% HFD for 12 weeks. (A–B) Representative energy expenditure (A) and average daily energy expenditure regressed by lean body mass (B) in mice fed 60% HFD for 12 weeks. Data were averaged over 72 h n = 5–6 mice/group. Data presented as mean ± SEM ∗ = p ≤ 0.05. n = 5–6 mice/group. p values calculated from linear regression model: EE = m (Lean Mass) + Genotype.
3.4. Protection from DIO in ACSL5IKO mice is due to reduced energy intake
To determine if intestinal ACSL5 deficiency resulted in reduced energy intake, we monitored energy intake for 30 days in chow and HFD fed mice. When fed chow, no differences in average daily energy intake were observed (Figure 6A). However, when fed the HFD, ACSL5IKO mice had significantly lower average daily energy intake compared to ACSL5loxP/loxP mice (Figure 6A). Similarly, 30 day cumulative energy intake of HFD was significantly reduced in ACSL5IKO mice compared ACSL5loxP/loxP controls (Figure 6B).
Figure 6.
Reduced Energy Intake in 60% HFD Fed ACSL5IKOMice. (A) Average daily energy intake of ACLS5loxP/loxP and ACSL5IKO mice fed chow or 60% HFD for 4 weeks, n = 5–6 mice/group, data represent a three day average ± SEM. (B) Cumulative energy intake in ACLS5loxP/loxP and ACSL5IKO mice fed 60% HFD for 30 days, n = 5–6 mice/group/timepoint. (C) Body weight gain over time in ACLS5loxP/loxP, ACSL5IKO and pair-fed ACLS5loxP/loxP mice in response to 60% HFD feeding. Pair-fed mice each received the daily average intake of the ACSL5IKOad-libitum fed group. ACSL5loxP/loxP group was included as a control, n = 10–12 mice/group. Each timepoint during time series experiments was analyzed separately via one-way ANOVA. ∗ = p ≤ 0.05. (D) Body weight and (E) fat mass at baseline and after four weeks of 60% HFD pair-feeding, n = 10–12 mice/group. ∗ = p ≤ 0.05 via student's t-test vs ACSL5loxP/loxP. (F–I) Average periodic (2 h) and cumulative energy intake of six-week-old mice fed chow, fasted 16 h and refed chow (F–G) or 60% HFD (H–I), n = 5–7 mice/group. Data presented as mean ± SEM ∗ = p ≤ 0.05 via student's t-test between groups at each timepoint. Data are presented as means ± SEM.
To determine if reduced energy intake was sufficient to explain the reduced body weight and fat mass observed in HFD fed ACSL5IKO mice, we pair-fed ACSL5loxP/loxP mice to ad-libitum fed ACSL5IKO mice and monitored body composition. When ACSL5loxP/loxP mice received the same amount of HFD consumed by ACSL5IKO mice, they gained significantly less body weight and fat mass than ACSL5loxP/loxP ad-libitum fed controls (Figure 6C–E). Importantly, there were no differences in body weight or fat mass gain between pair-fed ACSL5loxP/loxP and ACSL5IKO mice during 4 weeks of pair-feeding. These results provide strong evidence that reduced intake of the HFD is the primary mechanism by which ACSL5IKO mice are protected from DIO.
To determine whether intestinal deficiency of ACSL5 influences satiety, as measured by food intake following a period of food deprivation, we challenged mice with a fast-refeeding protocol. When overnight fasted mice were refed with chow diet, ACSL5IKO mice had a small, but significant 25% reduction in energy intake compared to ACSL5loxP/loxP mice in the 2 h period immediately following reintroduction of a chow diet (Figure 6F). However, within 4 h following refeeding, cumulative chow diet energy intake between ACSL5loxP/loxP and ACSL5IKO was not different (Figure 6H). No differences in chow diet intake were observed for the remainder of the study and 24 h cumulative energy intake was similar between chow refed ACSL5IKO and ACSL5loxP/loxP mice (Figure 6G). In contrast to chow refeeding, within 2 h of HFD refeeding energy intake was reduced by 60% in ACSL5IKO relative to controls (Figure 6H). Energy intake of ACSL5IKO mice remained lower throughout HFD refeeding, resulting in a 50% reduction in 24-hour cumulative energy intake compared to ACSL5loxP/loxP mice (Figure 6I). The results from these fasting-refeeding experiments are suggestive of increased satiety in ACSL5IKO mice during HFD, but not chow, feeding.
3.5. HFD fed ACSL5IKO mice have increased circulating GLP-1 concentrations
The enteroendocrine horomone, glucagon like peptide 1 (GLP-1), has been to shown to slow gastric emptying, reduce rates of dietary fat absorption, and increase satiety – all physiological phenomena observed in ACSL5IKO mice [[17], [18], [19], [20]]. Thus, we next explored whether intestinal ACSL5 influences circulating GLP-1 levels. We hypothesized that postprandial, circulating GLP-1 levels may be sufficiently elevated in ACSL5IKO mice to reduce HFD intake [21,22]. To determine if intestinal deficiency of ACSL5 alters the secretion of GLP-1 in response to dietary fat, chow fed mice were fasted overnight and then challenged with an oral bolus of olive oil [19]. Two hours post olive oil gavage, circulating active GLP-1 levels were over 75% higher in ACSL5IKO compared to ACSL5loxP/loxP mice (Figure 7A). A similar increase in circulating active GLP-1 was observed in ACSL5IKO refed with HFD after an overnight fast (Figure 7B). Chronic ingestion of a HFD has been suggested to reduce GLP-1 secretion [23,24]. To determine if increases in postprandial GLP-1 are maintained in ACSL5IKO mice following chronic exposure to HFD, mice fed a HFD for 6 weeks were fasted overnight and then gavaged with 300 μl of olive oil. Three hours following oil gavage, plasma active GLP-1 was elevated over 150% in HFD-fed ACSL5IKO mice compared to controls (Figure 7C).
Figure 7.
Intestinal Loss of ACSL5 Reduced Food Intake, in Part, by Elevated GLP-1 Levels in ACSL5IKOMice. (A–B) Plasma active GLP-1 in chow fed ACLS5loxP/loxP and ACSL5IKO mice, fasted for 16 h and oral gavaged with 200 μl olive oil (A) or refed 60% HFD (B), n = 5–6 mice/group/timepoint. Data are presented as means ± SEM. ∗ = p ≤ 0.05 via student's t-test. (C) Plasma active GLP-1 in ACLS5loxP/loxP and ACSL5IKO mice, fed 60% HFD for six weeks, fasted for 16 h, and oral gavaged with 300 μl olive oil. Blood was collected pre-gavage and 3 h post-gavage “Post”. n = 4 mice/group/timepoint. ∗ = p ≤ 0.05 via student's t-test. (D) 24 h energy intake in 9-month old chow fed mice following a 16 h overnight fast and refed 60% HFD. Mice were treated with 2000 nmol/kg GLP1r antagonist 26 h and again 2 h before introduction of diet. n = 5–7 mice/group. Data are presented as means ± SEM. ∗ = p ≤ 0.05 via two-way ANOVA and Tukey's HSD. (E) Plasma peptide YY (PYY) levels in chow fed ACLS5loxP/loxP and ACSL5IKO mice, fasted for 16 h and refed 60% HFD, n = 12–13 mice/group/timepoint. Data are presented as means ± SEM. ∗ = p ≤ 0.05 via student's t-test. Data are presented as means ± SEM. ∗ = p ≤ 0.05 via student's t-test. (F) Non-esterified fatty acid (NEFA) content of ileal mucosal scrapings from ACLS5loxP/loxP and ACSL5IKO mice fed 60% HFD for two weeks and euthanized in the fed state. (G-I) 16-week-old male mice fed a chow diet were presented with pre-weighed pellets of both 60% high fat diet (HFD) and sucrose-matched 10% low fat diet (LFD) at the start of the dark cycle. After 48 h the pellets were weighed and total energy intake (G), relative energy intake from HFD (H) and LFD (I) was calculated based on the gram amount of each diet consumed. n = 6–7/group. ∗ = p ≤ 0.05 via student's t-tests. Data are presented as means ± SEM.
To determine whether the elevated circulating GLP-1 levels observed in ACSL5IKO mice were sufficient to reduce intake of the HFD, we treated mice with a long acting GLP-1 receptor antagonist (GLP1Ri) [16]. Using the fast-refeeding paradigm, we treated ACSL5loxP/loxP and ACSL5IKO mice with 2 μmol/kg GLPRi 26 h before and 2 h before re-introduction of HFD. We found that pretreatment with GLPRi did not affect 24 h cumulative energy intake of ACSL5loxP/loxP mice (Figure 7D). In contrast, GLP1 receptor antagonism increased the food intake of ACSL5IKO mice by ∼85% compared to untreated ACSL5IKO mice. In summary these studies demonstrate that during both chow and HFD feeding, intestinal ACSL5 deficiency increases postprandial active GLP-1 levels in circulation and that the increased circulating GLP-1 contributes to reduced HFD intake.
As acute GLP-1 inhibition only partially restored food intake, we hypothesized that deficiency of intestinal ACSL5 may potentiate the postprandial release of additional enteroendocrine hormones that could contribute to increased satiety. To test this hypothesis, we measured circulating levels of peptide YY (PYY) in response to HFD refeeding and olive oil gavage, respectively. We observed a significant increase in postprandial PYY in ACSL5IKO mice compared to controls (Figure 7E).
One possible explanation for the increased levels of circulating GLP-1 and PYY is that reduced levels of ACSL5 may result in decreased “trapping” of dietary FA within enterocytes. This could result in increased levels of non-esterified free fatty acids (NEFA) in the intestine which can bind to receptors on enteroendocrine cells and increase GLP-1 and PYY secretion [19,[25], [26], [27]]. In support of this hypothesis, we observed increased NEFA levels in the intestinal mucosa of ACSL5IKO fed high fat diet for 2 weeks (Figure 7F).
In mouse studies using semaglutide, a long acting GLP-1 receptor agonist, and Tirzepatide, the dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) agonist, GLP-1 agonism was found to alter food preference, reducing intake of HFD and increasing intake of low fat diet (LFD) [28,29]. To determine if increased GLP-1 was associated with altered food preference in ACSL5IKO mice, we provided ACSL5loxP/loxP and ACSL5IKO mice with an equal amount of pre-weighed HFD and a sucrose matched LFD to determine the relative amount of each food type consumed. When presented with a choice of LFD or HFD, ACSL5IKO mice consumed fewer total calories than ACSL5loxP/loxP controls, consistent with increased satiety (Figure 7G). When the relative amount of HFD and LFD was compared, we found that that the ACSL5loxP/loxP mice almost exclusively ingested HFD, with HFD comprising 98% of their total energy intake and LFD making up 2% of total energy consumption (Figure 7H–I). In contrast, ACSL5IKO mice consumed significantly less HFD and more LFD as a percent of total calorie consumption compared to ACSL5loxP/loxP controls. These data suggest a reduced preference for HFD in ACSL5IKO mice, which is consistent with the established actions of GLP-1 on food preference.
4. Discussion
We previously reported that mice with whole body ACSL5 deficiency have lower body weight, fat mass, and blood TAG along with increased metabolic rates and improved insulin sensitivity [10]. In addition, we found reduced rates of dietary fat absorption, indicating a role for ACSL5 in dietary fat metabolism. Because ACSL5 is abundantly expressed in the intestinal mucosa, and altered intestinal FA metabolism is associated changes in enteroendocrine signaling and energy homeostasis, we hypothesized that intestinal ACSL5 regulates energy metabolism by influencing dietary fat absorption and enteroendocrine signaling. In the present study, we test this hypothesis demonstrating that intestine-specific ablation of ACSL5 protects against HFD-induced obesity, hepatic steatosis, and insulin resistance. We found that the resistance to HFD-induced obesity was a result of reduced energy intake due, in part, to increased postprandial GLP-1 and PYY secretion. In sum, these results highlight the importance of intracellular dietary FA metabolism in intestinal enterocytes in regulation of systemic energy balance and co-morbidities associated with obesity.
ACSL5 is highly expressed in enterocytes along the entire length of the small intestine where the role of FA activation is well established. ACSL5 accounts for >80% of ACSL activity in the small intestine [10]. The residual acyl-CoA synthetase activity may come from other ACSL family members or other proteins with acyl-CoA synthetase activity, such as fatty acid transport protein 4 (FATP4). In ACSL5IKO mouse jejunum we did not find upregulation of other ACSL or FATP4 mRNA levels. Despite ACSL5's major contribution to small intestinal ACLS activity, the absorption of dietary fat was not quantitatively reduced in ACSL5IKO mice. This result suggests the small intestine has the capacity to maintain highly efficient energy absorption in the presence of significantly reduced ACSL activity. Interestingly, we observed increased villus height suggestive of increased absorptive surface area which may contribute to energy absorption. The mechanisms by which loss of intestinal ACSL5 influences villus height are unclear and will require additional studies to elucidate.
Our results demonstrate the importance of intestinal ACSL5 in the regulation of food intake and systemic energy balance. Significantly ACSL5IKO mice fed HFD had reduced food intake that accounted for their reduced weight gain as shown in pair-feeding studies. We hypothesized that the reduced food intake in ACSL5IKO mice may result from altered enterocyte dietary FA metabolism. Specifically, we hypothesized that intestinal ACSL5 deficiency would reduce acyl-CoA generation within intestinal enterocytes thereby increasing the availability of FAs to activate enteroendocrine cells and increase secretion of the satiety hormone, GLP-1. In support of this hypothesis, we observed that NEFA levels in intestinal mucosa were increased in HFD fed ACSL5IKO mice. In addition, we found that ACSL5IKO mice had elevated GLP-1 and PYY levels in response to a dietary fat challenge. Furthermore, the reduction in food intake in ACSL5IKO mice was only observed following HFD feeding, suggesting that a digestion product enriched during high fat diet feeding, such as FAs resulting from TAG digestion, contributes to the increased satiety we observed in this model.
Interestingly, while pharmacologic analogues of GLP-1 have been demonstrated to significantly and robustly decrease food intake and reduce adiposity in mice and humans [4,5], physiologically normal levels of postprandial GLP-1 do not influence food intake [30]. However, elevating circulating levels of GLP-1 by exogenous administration in rodents and humans was found to increase satiety and reduce body weight, suggesting that circulating levels of GLP-1 need to reach a sufficient concentration or threshold to alter satiety [20,[31], [32], [33]]. In these studies, we show that inhibition of the glucagon-like peptide-1 receptor partially restores food intake in ACSL5IKO mice, arguing that intestinal loss of ACSL5 increases endogenous postprandial GLP-1 levels sufficiently to contribute to increased satiety.
Our observations that GLP-1 receptor antagonism only partially restored food intake in high fat fed ACSL5IKO mice suggest that, in addition to GLP-1, other intestinal hormones and/or lipid metabolites may contribute to the satiety observed in these mice. In this study, we observed a significant increase in PYY 2hr following HFD refeeding in ACSL5IKO. PYY is produced in enteroendocine L-cells and released following ingestion of a meal [27,34]. While all dietary macronutrients have been shown to increase PYY release, meals rich in fat or protein produce greater postprandial PYY secretion than meals rich in carbohydrate. In addition, direct infusion of the small intestine with fatty acids has been shown to potentiate PYY release, establishing PYY as a FA sensitive enteroendocrine factor. Like GLP-1, exogenously administered PYY reduces energy intake, slows gastric emptying, increases feelings of fullness, and reduces body weight [34,35]. In total, these data suggest that the increased postprandial PYY in ACSL5IKO mice may contribute to several aspects of the phenotype, including delayed gastric emptying, reduced food intake, and protection from DIO. In addition, the link between FA and increased PYY further supports our hypothesis that increased availability of free fatty acids in the small intestine resulting from the absence of intestinal ACSL5 underlies the potentiated postprandial eneteroendocrine response we observe in these mice. Importantly, while we have limited our investigation to GLP-1 and PYY in this study, it is likely that additional fatty acid responsive incretins may also be elevated in the postprandial state and contribute to the increased satiety observed in ACSL5IKO mice.
The resistance to DIO and insulin resistance as well as the reduced postprandial triglyceridemia observed in ACSL5IKO mice are like phenotypes observed in other mouse models with deficiencies in enzymes required for TAG synthesis, Dgat1 and Mgat2. However, the mechanisms by which deficiency of these enzymes influences systemic energy balance are distinct from what is observed in ACSL5IKO mice. Unlike ACSL5IKO mice, Dgat1KO and Mgat2IKO mice expend more energy and have lower respiratory exchange ratios, indicative of higher oxidation of FAs, compared to control mice [36,37]. In addition, ACSL5IKO mice differ from Dgat1KO and Mgat2IKO mice in food intake behavior. ACSL5IKO mice have persistent reductions in food intake that account for their resistance to weight and adipose tissue gain when fed HFD. Mgat2IKO have brief, transient reductions in food intake during HFD feeding, and then eat similarly to control mice [37,38] while Dgat1KO mice eat more than control mice [36]. Despite these differences in food intake behavior, all three mouse models have increases in GLP-1 in response to dietary fat [37,39]. It remains unclear why deficiencies in these three enzymes that mediate sequential steps in the TAG synthesis have differing effects on food intake. One explanation may be related to acyl-CoA formation as both Mgat2 and Dgat1 deficiency do not prevent generation of acyl-CoAs while ACSL5 deficiency robustly blocks the generation of acyl-CoA within the intestine.
The role of the intestine in regulating obesity and metabolism in humans is highlighted by the clinical success of surgical procedures such as gastric bypass surgery and the remarkable success of GLP-1 analogues in regulation of obesity and associated metabolic disorders [4,5,22,40]. While our data indicate that intestinal ACSL5 influences intestinal regulation of satiety in mice, it is unclear whether these observations translate to humans. Like mice, in humans ACSL5 is robustly expressed in the small intestine [41]. In one consanguineous family, six individuals were identified to have a mutation in their intestinal ACSL5 which resulted in a loss of function of ACSL5's catalytic activity [41]. During only the neonatal period, the newborn had diarrhea and vomiting which required total parental nutrition or a formula that provided medium chain TAG. After the neonatal period the children were able to develop on a normal diet without issue, indicating that in humans ACSL5 deficiency may influence health early after birth without lasting effects into adulthood. Importantly, any effects of ACSL5 loss on intestinal development in early life were mitigated in our studies by initiating tamoxifen induced recombination at 12 weeks of age when the intestine is fully developed.
In summary, we demonstrated that mice with intestinal ACSL5 deficiency were significantly protected against the development of HFD-induced obesity and associated metabolic complications. We found that the reduced body weight and fat mass observed in HFD fed ACSL5IKO was a result of reduced food intake due, in part, to increased endogenous GLP-1 levels. Importantly, loss of ACSL5 did not impair quantitative dietary fat absorption.
4.1. Conclusion
In total, these studies highlight the importance of intestinal ACSL5 in the regulation of food intake and whole-body energy homeostasis. Our data indicate that these effects are likely the consequence of alterations in dietary fat metabolism resulting from enterocyte deficiency of ACSL5 which subsequently influences enteroendocrine signaling. Importantly, these results suggest intestinal ACSL5 may serve as a therapeutic target for obesity.
4.2. Limitations
One limitation of this study is that a direct link between chronic elevations in GLP-1 and protection from HFD-induced obesity in ACSL5IKO mice was not confirmed. Herein, we demonstrate that acute treatment with a GLP-1 receptor antagonist partially restores 24hr high fat diet intake of overnight fasted ACSL5IKO, suggesting that GLP-1 contributes to satiety acutely. However, this result does not provide insight into whether increased postprandial GLP-1 contributes chronically to reductions in food intake and, by extension, protection from high-fat diet in mice deficient in intestinal ACSL5. A second limitation of these studies is the exclusive use of male mice, limiting our understanding of how deficiency in intestinal ACSL5 affects energy metabolism of female animals. Interestingly, in humans GLP-1 receptor agonist therapies produce greater weight loss in women than in men [42]. As the sexual dimorphism in GLP-1Ra therapies appears related to drug exposure, it is unclear how targeting intestinal ACSL5 and regulation of endogenous enteroendocrine hormones may differentially affect male and female metabolism.
CRediT authorship contribution statement
John D. Griffin: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ying Zhu: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Andrew Reeves: Writing – review & editing, Investigation. Kimberly K. Buhman: Writing – review & editing, Methodology, Investigation, Data curation, Conceptualization. Andrew S. Greenberg: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:John D. Griffin was an employee of Pfizer when this manuscript was prepared.
Acknowledgements
This work was supported by USDA NIFA2022-67018-37186 (ASG), USDA Agricultural Service Cooperative Agreement 58-8050-9-004, NIH/NIDDK P30DK2022-6708-37186 (ASG), NIH/NIDDK098606. ASG also receives support from the Robert C and Veronica Atkins Foundation. KKB receives support from the McKinley Educational Initiative. We thank Dr.Brian Finan for generation of GLP-1 inhibitor. The authors would like to thank Dr. David Tybor (Tufts University) for his assistance with statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2024.101918.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.Iqbal J., Hussain M.M. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296(6):E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao C., Stahel P., Carreiro A.L., Buhman K.K., Lewis G.F. Recent advances in triacylglycerol mobilization by the gut. Trends Endocrinol Metab. 2018;29(3):151–163. doi: 10.1016/j.tem.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Courcoulas A.P., Christian N.J., Belle S.H., Berk P.D., Flum D.R., Garcia L., et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;(384):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 5.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 6.Mashek D.G., Li L.O., Coleman R.A. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2(4):465–476. doi: 10.2217/17460875.2.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis J.M., Frahm J.L., Li L.O., Coleman R.A. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21(3):212–217. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber A.L., Biton M., Rogel N., Herbst R.H., Shekhar K., Smillie C., et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551(7680):333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikawa E., Iijima H., Suzuki T., Sasano H., Sato H., Kamataki A., et al. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J Biochem. 1998;124(3):679–685. doi: 10.1093/oxfordjournals.jbchem.a022165. [DOI] [PubMed] [Google Scholar]
- 10.Bowman T.A., O'Keeffe K.R., D'Aquila T., Yan Q.W., Griffin J.D., Killion E.A., et al. Acyl CoA synthetase 5 (ACSL5) ablation in mice increases energy expenditure and insulin sensitivity and delays fat absorption. Mol Metabol. 2016;5(3):210–220. doi: 10.1016/j.molmet.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.el Marjou F., Janssen K.P., Chang B.H., Li M., Hindie V., Chan L., et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 12.Ye R., Wang Q.A., Tao C., Vishvanath L., Shao M., McDonald J.G., et al. Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metabol. 2015;4(11):771–778. doi: 10.1016/j.molmet.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin J.D., Bejarano E., Wang X.D., Greenberg A.S. Integrated action of autophagy and adipose tissue triglyceride lipase ameliorates diet-induced hepatic steatosis in liver-specific PLIN2 knockout mice. Cells. 2021;10(5) doi: 10.3390/cells10051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschop M.H., Speakman J.R., Arch J.R., Auwerx J., Bruning J.C., Chan L., et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida A., Lee H.J., Cheng J.X., Buhman K.K. Imaging cytoplasmic lipid droplets in enterocytes and assessing dietary fat absorption. Methods Cell Biol. 2013;116:151–166. doi: 10.1016/B978-0-12-408051-5.00014-0. [DOI] [PubMed] [Google Scholar]
- 16.Patterson J.T., Ottaway N., Gelfanov V.M., Smiley D.L., Perez-Tilve D., Pfluger P.T., et al. A novel human-based receptor antagonist of sustained action reveals body weight control by endogenous GLP-1. ACS Chem Biol. 2011;6(2):135–145. doi: 10.1021/cb1002015. [DOI] [PubMed] [Google Scholar]
- 17.Nahmias A., Stahel P., Tian L., Xiao C., Lewis G.F. GLP-1 (Glucagon-Like peptide-1) is physiologically relevant for chylomicron secretion beyond its known pharmacological role. Arterioscler Thromb Vasc Biol. 2021;41(6):1893–1900. doi: 10.1161/ATVBAHA.121.316311. [DOI] [PubMed] [Google Scholar]
- 18.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabol. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Muller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R., et al. Glucagon-like peptide 1 (GLP-1) Mol Metabol. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutzwiller J.P., Goke B., Drewe J., Hildebrand P., Ketterer S., Handschin D., et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44(1):81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers A.P., Sorrell J.E., Haller A., Roelofs K., Hutch C.R., Kim K.S., et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metabol. 2017;25(4):927–934 e923. doi: 10.1016/j.cmet.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drucker D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metabol. 2022;57 doi: 10.1016/j.molmet.2021.101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duca F.A., Swartz T.D., Sakar Y., Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes. 2013;37(3):375–381. doi: 10.1038/ijo.2012.45. [DOI] [PubMed] [Google Scholar]
- 24.Richards P., Pais R., Habib A.M., Brighton C.A., Yeo G.S., Reimann F., et al. High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides. 2016;77:21–27. doi: 10.1016/j.peptides.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu V.B., Gribble F.M., Reimann F. Free fatty acid receptors in enteroendocrine cells. Endocrinology. 2018;159(7):2826–2835. doi: 10.1210/en.2018-00261. [DOI] [PubMed] [Google Scholar]
- 26.Feltrin K.L., Patterson M., Ghatei M.A., Bloom S.R., Meyer J.H., Horowitz M., et al. Effect of fatty acid chain length on suppression of ghrelin and stimulation of PYY, GLP-2 and PP secretion in healthy men. Peptides. 2006;27(7):1638–1643. doi: 10.1016/j.peptides.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Kozimor A., Chang H., Cooper J.A. Effects of dietary fatty acid composition from a high fat meal on satiety. Appetite. 2013;69:39–45. doi: 10.1016/j.appet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Geisler C.E., Antonellis M.P., Trumbauer W., Martin J.A., Coskun T., Samms R.J., et al. Tirzepatide suppresses palatable food intake by selectively reducing preference for fat in rodents. Diabetes Obes Metabol. 2023;25(1):56–67. doi: 10.1111/dom.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Ronne J., Alanentalo T., Baquero A.F., et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6) doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y., Koehler J.A., Baggio L.L., Powers A.C., Sandoval D.A., Drucker D.J. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metabol. 2019;30(5):976–986 e973. doi: 10.1016/j.cmet.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neary N.M., Small C.J., Druce M.R., Park A.J., Ellis S.M., Semjonous N.M., et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146(12):5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 32.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutzwiller J.P., Drewe J., Goke B., Schmidt H., Rohrer B., Lareida J., et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276(5):R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 34.Cooper J.A. Factors affecting circulating levels of peptide YY in humans: a comprehensive review. Nutr Res Rev. 2014;27(1):186–197. doi: 10.1017/S0954422414000109. [DOI] [PubMed] [Google Scholar]
- 35.Moran T.H., Smedh U., Kinzig K.P., Scott K.A., Knipp S., Ladenheim E.E. Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R384–R388. doi: 10.1152/ajpregu.00535.2004. [DOI] [PubMed] [Google Scholar]
- 36.Smith S.J., Cases S., Jensen D.R., Chen H.C., Sande E., Tow B., et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25(1):87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 37.Nelson D.W., Gao Y., Yen M.I., Yen C.L. Intestine-specific deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem. 2014;289(25):17338–17349. doi: 10.1074/jbc.M114.555961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banh T., Nelson D.W., Gao Y., Huang T.N., Yen M.I., Yen C.L. Adult-onset deficiency of acyl CoA:monoacylglycerol acyltransferase 2 protects mice from diet-induced obesity and glucose intolerance. J Lipid Res. 2015;56(2):379–389. doi: 10.1194/jlr.M055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ables G.P., Yang K.J., Vogel S., Hernandez-Ono A., Yu S., Yuen J.J., et al. Intestinal DGAT1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J Lipid Res. 2012;53(11):2364–2379. doi: 10.1194/jlr.M029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puzziferri N., Roshek T.B., 3rd, Mayo H.G., Gallagher R., Belle S.H., Livingston E.H. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Thihli K., Afting C., Al-Hashmi N., Mohammed M., Sliwinski S., Al Shibli N., et al. Deficiency of acyl-CoA synthetase 5 is associated with a severe and treatable failure to thrive of neonatal onset. Clin Genet. 2021;99(3):376–383. doi: 10.1111/cge.13883. [DOI] [PubMed] [Google Scholar]
- 42.Rentzeperi E., Pegiou S., Koufakis T., Grammatiki M., Kotsa K. Sex differences in response to treatment with glucagon-like peptide 1 receptor agonists: opportunities for a tailored approach to diabetes and obesity Care. J Personalized Med. 2022;12(3) doi: 10.3390/jpm12030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.