Abstract
Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) is a revolutionary tool for the diagnosis and staging of mediastinal disorders. Nevertheless, its diagnostic capability is reduced in certain disorders such as lymphoproliferative diseases. EBUS‐guided transbronchial mediastinal cryobiopsy (EBUS‐TBMC) is a novel technique that can provide larger samples with preserved tissue architecture, with an acceptable safety profile. In this case report, we present a middle‐aged gentleman with a huge anterior mediastinal mass and bilateral mediastinal and hilar lymphadenopathy. He underwent EBUS‐TBNA with rapid on‐site evaluation (ROSE) followed by EBUS‐TBMC, all under general anaesthesia. Histopathological analysis showed discordance between EBUS‐TBNA and EBUS‐TBMC in which only TBMC samples provided adequate tissue to attain a diagnosis of primary mediastinal large B‐cell lymphoma. This case report reinforced the diagnostic role of EBUS‐TBMC in the diagnosis of lymphoproliferative diseases.
Keywords: bronchoscopy, endobronchial ultrasound, lymphoma, mediastinum
Endobronchial ultrasound‐guided transbronchial mediastinal cryobiopsy (EBUS‐TBMC) is a novel technique that can provide larger samples with preserved tissue architecture, with an acceptable safety profile. We present a gentleman with anterior mediastinal mass who underwent EBUS‐transbronchial needle aspirate (TBNA) followed by EBUS‐TBMC. TBMC samples provided adequate tissue to attain a diagnosis of primary mediastinal large B‐cell lymphoma.
INTRODUCTION
Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) is a well‐established tool for the diagnosis and staging of mediastinal disorders. Nevertheless, the utility of EBUS‐TBNA in lymphoproliferative disorders remains restricted due to low diagnostic sensitivity. 1 Ongoing efforts to obtain larger mediastinal samples with preserved tissue architecture led to the idea of performing EBUS‐guided transbronchial mediastinal cryobiopsy (EBUS‐TBMC). Recent studies have confirmed the safety and feasibility of EBUS‐TBMC, highlighting its superiority over EBUS‐TBNA in certain pathologies including lymphoproliferative diseases. 2 Herein, we present a middle‐aged gentleman that underwent EBUS‐TBNA with rapid on‐site evaluation (ROSE) followed by EBUS‐TBMC for a huge anterior mediastinal mass. TBMC samples provided adequate tissue to attain a final diagnosis of primary mediastinal large B‐cell lymphoma.
CASE REPORT
A 44‐year‐old gentleman, non‐smoker, with no prior medical illness presented with 2 weeks of worsening shortness of breath, orthopnoea, and paroxysmal nocturnal dyspnoea. He reported no constitutional symptoms or fever. Physical examination on arrival revealed bilateral lower zone lung crepitations with reduced air entry over the right lower zone. Cardiovascular examination was unremarkable but his liver was palpable at two finger breaths below the costal margin. There was bilateral pitting oedema up till mid‐shin but no lymph nodes were palpable. Chest radiograph showed a right sided pleural effusion while echocardiogram demonstrated a global pericardial effusion with a widest fluid margin of 1.8 cm, leading to right ventricle collapse. Urgent diagnostic and therapeutic thoracentesis were performed, which drained cloudy milky fluid. The fluid was exudative in nature, and was biochemically confirmed to be a chylothorax. Other pleural fluid investigations including bacterial cultures, acid‐fast bacilli, cytology, and fungal cultures were all unremarkable. Concurrently, pericardial fluid obtained from emergency pericardiocentesis revealed multiple lymphocytes but without malignant cells. Computed tomography (CT) of the thorax showed a right pleural effusion with a huge anterior mediastinal mass measuring up to 11.6 cm at its widest margin with invasion into adjacent vascular structures including the brachiocephalic, subclavian, and internal jugular veins together with bilateral mediastinal and hilar lymphadenopathies (Figure 1).
FIGURE 1.
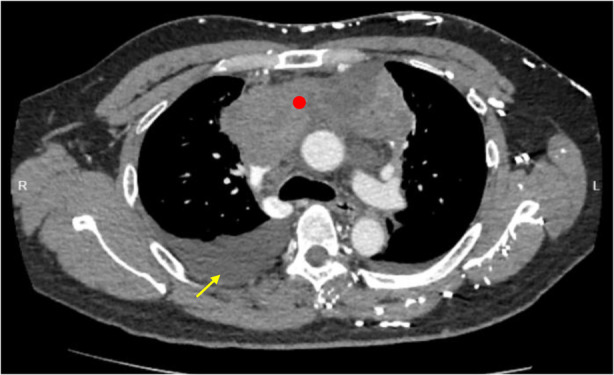
Computed tomography of the thorax showing right pleural effusion (yellow arrow) and a huge anterior mediastinal mass (red dot) with invasion into adjacent vascular structures including the brachiocephalic, subclavian, and internal jugular veins together as well as bilateral mediastinal and hilar lymphadenopathies.
Under general anaesthesia, and via laryngeal mask airway, he underwent EBUS‐TBNA of the enlarged mediastinal mass at right lower paratracheal (4R) region, which measured more than 30 mm on EBUS (Pentax EB‐1970UK Linear‐Array Ultrasound Bronchoscope, Japan). The lesion showed ill‐defined margins with areas of central hypodensities. A total of four passes were made using a 22 Gauge EBUS needle (EchoTip ProCore Needle, Cook Medical, USA) with ROSE showed only lymphoid cells. A decision was then made by the bronchoscopist to proceed with EBUS‐TBMC as lymphoproliferative disease was likely in this case. A 1.1 mm cryoprobe (ERBE, Medizintechnik, Germany) inserted through the working channel of EBUS scope was advanced directly into the mediastinal lesion via a tract that was already created by EBUS‐TBNA attempts earlier (Figure 2A,B). A total of three samples were obtained, with activation times ranging from 3 to 7 s. The procedure was uneventful.
FIGURE 2.
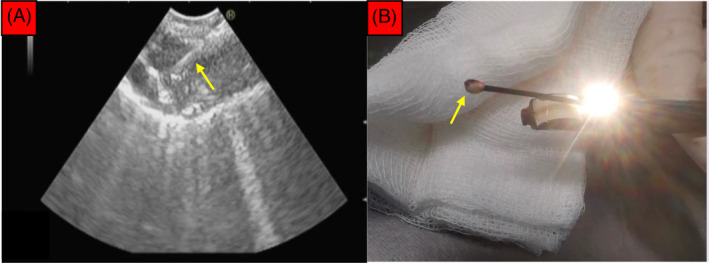
(A) The position of the cryoprobe (yellow arrow) within the mediastinal lesion was confirmed under EBUS before activation for a duration of 3–7 s. (B) The frozen tissue specimen (yellow arrow) can be seen at the tip of the cryoprobe immediately after the EBUS scope was removed en bloc along with the cryoprobe.
Smears and cell block from EBUS‐TBNA showed only mixed inflammatory cells which composed of lymphocytes, plasma cells and occasional neutrophils (Figure 3A) while samples obtained from EBUS‐TBMC demonstrated tumour tissue containing a sheet of atypical lymphoid cells (Figure 3B). These cells were medium in size and displayed irregular hyperchromatic nuclei, inconspicuous nucleoli, and scanty cytoplasm with frequent mitosis and apoptotic bodies. Immunohistochemistry was positive for CD20(diffuse), BCL6(>30%), MUM1(>30%), C‐Myc(>40%), and BCL2(>50%) and negative for CKAE1/AE3, CD3, and CD10. The Ki‐67 proliferative index was >80%. The overall morphological and immunohistochemical features were diagnostic for high‐grade B‐cell lymphoma favouring primary mediastinal large B‐cell lymphoma (Figure 4A,D). He was subsequently referred to haematology team where chemotherapy (R‐EPOCH regime: rituximab, etoposide, prednisolone, oncovin, cyclophosphamide and hydroxydaunorubicin) was commenced. Repeated CT thorax 6 weeks post treatment showed resolution of pleural effusion together with reduction in size of his anterior mediastinal mass (Figure 5).
FIGURE 3.
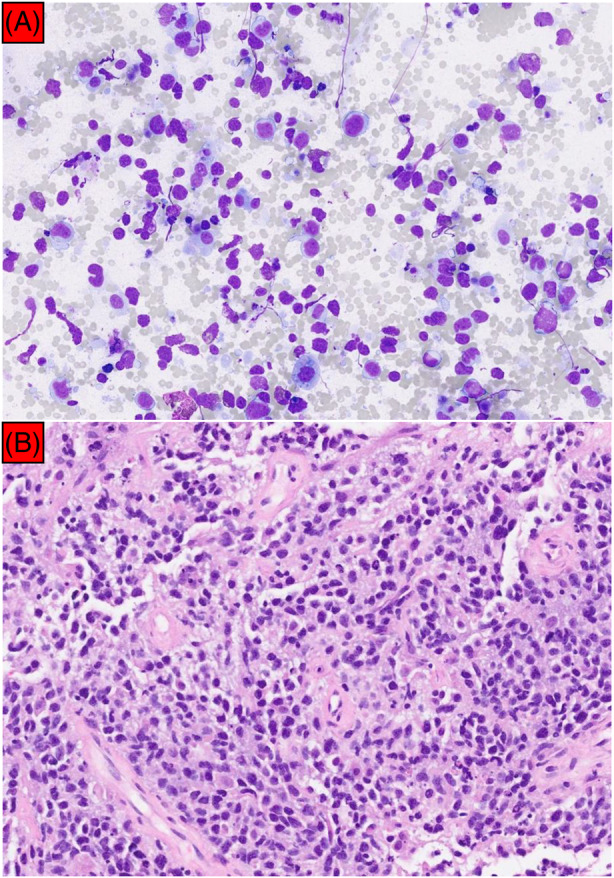
(A) Cell block from EBUS‐TBNA showed polymorphous lymphocytes with a background of mixed inflammatory cells. No atypical lymphocytes or malignant cells were seen (200× magnification). (B) In comparison, samples obtained from EBUS‐TBMC demonstrated tumour tissue containing atypical lymphoid cells, consistent with primary mediastinal large B‐cell lymphoma (200× magnification).
FIGURE 4.
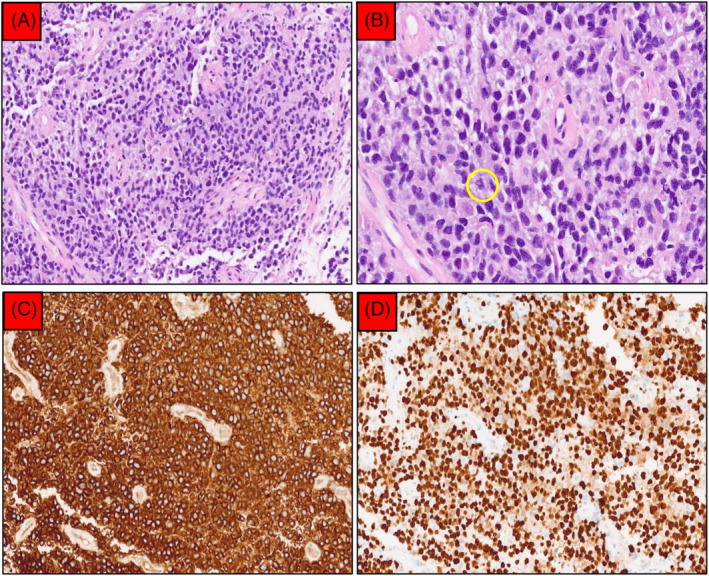
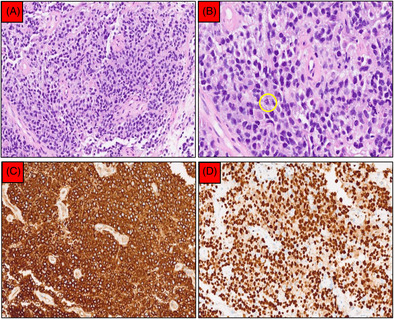
EBUS‐TBMC confirmed a diagnosis of primary mediastinal large B‐cell lymphoma. (A) Sheets of medium‐sized atypical lymphoid cells separated by fibrosis (200× magnification). (B) Further magnification (400×) showed that the nuclei were round with inconspicuous nucleoli and scanty cytoplasm. Mitosis (yellow circle) and apoptotic bodies were frequent seen. (C) CD20 was diffusely positive (200× magnification). (D) Ki‐67 proliferative index was approximately 80% (200× magnification).
FIGURE 5.
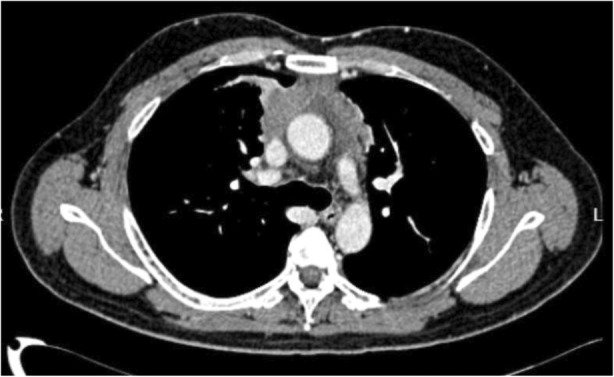
Surveillance computed tomography thorax 6 weeks post chemotherapy showing resolution of pleural effusion together with a size reduction of the anterior mediastinal mass.
DISCUSSION
Whilst diagnostic yields are high at beyond 90% for lung malignancies, 3 the utility of EBUS‐TBNA in lymphoma remains restricted due to low diagnostic sensitivity at only 65%. 1 This is unsurprising as cytology or cell block samples provided by TBNA may be insufficient for pathologies with complex morphologies and/or requiring further immunohistochemical or molecular analyses. The key advantage of EBUS‐TBMC lies in its ability to obtain larger mediastinal samples with preserved tissue architecture. 2 , 4 EBUS‐TBMC was first described in literature back in 2020, 5 followed by emergence of multiple case reports and case series from various centres worldwide. 6 , 7 , 8 , 9 A randomized controlled trial conducted in 2021 which enrolled 197 patients demonstrated that EBUS‐TBMC has a better diagnostic yield compared to EBUS‐TBNA (91.8% vs 79.9%). 10 The superiority of EBUS‐TBMC is particularly noticeable in benign mediastinal disorders (80.9% vs. 53.2%) and in rare tumours (91.7% vs. 25%). 10 These study outcomes were subsequently reiterated in another follow‐up randomized trial which involved 271 patients. 11 In this trial, the authors reported that incorporating TBMC to standard TBNA sampling can significantly increase the overall diagnostic yield for mediastinal lesions (93% vs. 81%, risk ratio [RR] 1·15 [95% CI 1·04–1·26]; p = 0·0039). 11 In our case, a final diagnosis of B‐cell lymphoma was only possible using samples obtained from TBMC.
Existing literature reiterates EBUS‐TBMC as a safe procedure, with reported complications rates such as self‐limiting pneumothorax and pneumomediastinum at between 0.5% and 1.0%. 2 , 11 Our patient did not experience any adverse events directly related to TBMC. Nevertheless, some fundamental technical aspects of EBUS‐TBMC remain unanswered, ranging from the optimal cryoprobe freezing time to the number of cryo‐passes and its impacts of specimen size. 2 , 4 We performed a total of 3 cryo‐passes for our patient, with a gradually increasing freezing time from 3 to 5 s and finally 7 s. The selected freezing times pays homage to recommendations from diagnosing interstitial lung disease using transbronchial cryobiopsy (ILD‐TBLC). 12 Unlike in EBUS‐TBMC, various technical aspects of ILD‐TBLC, from freezing time, number of passes and their correlation with specimen size and quality have been extensively studied in ILD‐TBLC. 13 Moreover, different methods have been described to create a tract for cryoprobe insertion during EBUS‐TBMC. 9 We managed to insert the cryoprobe through a tract that was created by repeated TBNA attempts in our case. Other tools such as high‐frequency needle knife 10 , 11 and Nd:YAG laser 14 have been used for tract creation as well.
In conclusion, existing literature suggest EBUS‐TBMC as an effective and safe technique to acquire sufficient diagnostic tissue samples for mediastinal diseases when conventional EBUS‐TBNA is not conclusive. Future studies focusing on patient selection and technical aspects of EBUS‐TBMC such as optimal passes, freezing times and cryoprobe tract creation methods are needed to further solidify its position in the diagnosis and staging of mediastinal pathologies.
AUTHOR CONTRIBUTIONS
N‐C Huan contributed to the design and implementation of the case report. N‐C Huan, WL Lee, MSL Tsen and LE Nyanti wrote the manuscript. N‐C Huan, HM Ramarmuty and D Yunus carried out the procedures mentioned. HM Ramarmuty supervised the project. All authors discussed and contributed to the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Huan N‐C, Lee WL, Ramarmuty HY, Nyanti LE, Tsen MSL, Yunus D. The utility of endobronchial ultrasound‐guided transbronchial mediastinal cryobiopsy (EBUS‐TBMC) for the diagnosis of mediastinal lymphoma. Respirology Case Reports. 2024;12(4):e01342. 10.1002/rcr2.1342
Nai‐Chien Huan and Wei Lun Lee are joint first authors.
Associate Editor: David Lam
Contributor Information
Nai‐Chien Huan, Email: naichien_1@yahoo.com.
Wei Lun Lee, Email: calvinlee1106@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Erer OF, Erol S, Anar C, Aydoğdu Z, Özkan SA. Diagnostic yield of EBUS‐TBNA for lymphoma and review of the literature. Endosc Ultrasound. 2017;6(5):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramarmuty HY, Oki M. Endobronchial ultrasound‐guided transbronchial mediastinal cryobiopsy: a narrative review. Mediastinum. 2024;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torre M, Reda M, Musso V, Danuzzo F, Mohamed S, Conforti S. Diagnostic accuracy of endobronchial ultrasound‐transbronchial needle aspiration (EBUS‐TBNA) for mediastinal lymph node staging of lung cancer. Mediastinum. 2021;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poletti V, Petrarulo S, Piciucchi S, Dubini A, De Grauw AJ, Sultani F, et al. EBUS‐guided cryobiopsy in the diagnosis of thoracic disorders. Pulmonology. 2024;23:00223‐4. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Fu WL, Huang ZS, Guo JR, Li Q, Herth FJF, et al. Primary mediastinal seminoma achieved by Transbronchial mediastinal cryobiopsy. Respiration. 2020;99(5):426–430. [DOI] [PubMed] [Google Scholar]
- 6. Gonuguntla HK, Shah M, Gupta N, Agrawal S, Poletti V, Nacheli GC. Endobronchial ultrasound‐guided transbronchial cryo‐nodal biopsy: a novel approach for mediastinal lymph node sampling. Respirol Case Rep. 2021;9(8):e00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Genova C, Tagliabue E, Mora M, Aloè T, Dono M, Salvi S, et al. Potential application of cryobiopsy for histo‐molecular characterization of mediastinal lymph nodes in patients with thoracic malignancies: a case presentation series and implications for future developments. BMC Pulm Med. 2022;22(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ariza‐Prota MA, Pérez‐Pallarés J, Fernández‐Fernández A, López‐González F, Cascón JA, García‐Alfonso L, et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lymph nodes: a case series: how to do it. Arch Bronconeumol. 2022;58(10):718–721. [DOI] [PubMed] [Google Scholar]
- 9. Ramarmuty HY, Huan NC, Nyanti LE, Khoo TS, Renganathan T, Manoh AZ, et al. Early experience of endobronchial ultrasound‐guided transbronchial nodal cryobiopsy: a case series from Sabah, Malaysia. Ther Adv Respir Dis. 2024;18:17534666241231122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Guo JR, Huang ZS, Fu WL, Wu XL, Wu N, et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: a randomised trial. Eur Respir J. 2021;58(6):2100055. [DOI] [PubMed] [Google Scholar]
- 11. Fan Y, Zhang AM, Wu XL, Huang ZS, Kontogianni K, Sun K, et al. Transbronchial needle aspiration combined with cryobiopsy in the diagnosis of mediastinal diseases: a multicentre, open‐label, randomised trial. Lancet Respir Med. 2023;11(3):256–264. [DOI] [PubMed] [Google Scholar]
- 12. Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S, et al. Transbronchial Cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the Cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95(3):188–200. [DOI] [PubMed] [Google Scholar]
- 13. Kho SS, Nyanti LE, Chai CS, Tie ST. Exploring the optimal freeze time and passes of the ultrathin cryoprobe in transbronchial cryobiopsy of peripheral pulmonary lesions. ERJ Open Res. 2024;10(1):506–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gershman E, Amram Ikan A, Pertzov B, Rosengarten D, Kramer MR. Mediastinal “deep freeze”‐transcarinal lymph node cryobiopsy. Thorac Cancer. 2022;13(11):1592–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


