Abstract
Introduction
Managing cognitive function in care homes is a significant challenge. Individuals in care have a variety of scores across standard clinical assessments, such as the Mini-Mental Status Exam (MMSE), and many of them have scores that fall within the range associated with dementia. A recent methodological advance, brain vital sign monitoring through auditory event-related potentials, provides an objective and sensitive physiological measurement to track abnormalities, differences, or changes in cognitive function. Taking advantage of point-of-care accessibility, the current study evaluated the methodological feasibility, the assessment of whether a particular research method can be successfully implemented, of quantitatively measuring cognition of care home residents using brain vital signs. Secondarily, the current study examined the relationship between brain vital signs, specifically the cognitive processing associated N400 component, and MMSE scores in care home residents.
Materials and methods
Brain vital signs used the established N100 (auditory sensation), P300 (basic attention), and N400 (cognitive processing) event-related potential (ERP) components. A total of 52 residents were enrolled, with all participants evaluated using the MMSE. Participants were assigned into homogeneous groups based on their MMSE scores, and were categorized into low (n = 14), medium (n = 17), and high (n = 13) MMSE groups. Both brain vital sign measures and underlying ERP waveforms were examined. Statistical analyses used partial least squares correlation (PLS) analyses in which both MMSE and age were included as factors, as well as jackknife approaches, to test for significant brain vital sign changes.
Results
The current study successfully measured and analyzed standardized, quantifiable brain vital signs in a care home setting. ERP waveform data showed specific N400 changes between MMSE groups as a function of MMSE score. PLS analyses confirmed significant MMSE-related and age-related differences in the N400 amplitude (p < 0.05, corrected). Similarly, the jackknife approach emphasized the N400 latency difference between the low and high MMSE groups.
Discussion and conclusion
It was possible to acquire brain vital signs measures in care home residents. Additionally, the current study evaluated brain vital signs relative to MMSE in this group. The comparison revealed significant decreasing in N400 response amplitude (cognitive processing) as a function of both MMSE score and age, as well as a slowing of N400 latency. The findings indicate that objective neurophysiological measures of impairment are detectable in care home residents across the span of MMSE scores. Direct comparison to MMSE- and age-related variables represents a critical initial step ahead of future studies that will investigate relative improvements in sensitivity, validity, reliability and related advantages of brain vital sign monitoring.
Keywords: Electroencephalography (EEG), Event-related potentials (ERPs), Cognitive evoked potentials (EPs), Dementia, Alzheimer's disease
1. Introduction
1.1. Background
According to the Alzheimer's Association, somewhere between one-half and two-thirds of all residents in assisted living and nursing homes have some form of dementia, including Alzheimer's disease [1]. In a longitudinal study of 690 care home residents, the reported median mortality is 2.2 years (range: 1.9–2.4 years) with the severity of dementia associated with higher mortality rates [2]. Cognitive impairment can be among the most significant challenges to providing person-centred care for residents. The odds of residents having moderate to severe cognitive impairment are 2.76 times greater for those in long-versus short-term care [3]. This situation directly impacts critical care and quality of life issues, such as the quality use of medicines and the well-documented over-prescription of antipsychotic medications in long-term care [4,5].
Accurate clinical decision-making around cognitive impairment relies on time-consuming and costly baseline investigations (e.g., a neuropsychological test battery). Accordingly, in-depth cognitive evaluation is rarely, if ever, conducted in care home environments. These challenges prevent any utilization of cognitive evaluation for day-to-day management, especially for care home residents. Screening for global cognitive performance using the Mini-Mental Status Exam (MMSE), and tests like it, represent the default option. However, this screening is subjective and error-prone, with a reported misdiagnosis rate as high as 43% [6], and it shows a floor effect in more advanced stages of dementia [7]. Accordingly, there is a priority need for a sensitive, objective, physiological, and practically accessible measure of cognitive function in clinical settings, such as care home environments.
Cognitive evoked potentials (EPs), also known as event-related potentials (ERPs), are derived from electroencephalography (EEG) and provide an objective, physiological measure of cognitive function [6]. With more than 160,000 published ERP studies in the National Library of Medicine's PubMed dating back to 1960s, there is well-established literature characterizing the relationship between ERPs and cognitive processing. Recent hardware (e.g., portable EEG) and software (e.g., signal processing and artificial intelligence) advances have enabled ERP technology to move beyond restricted research laboratory settings to be deployed clinically [8,9]. Consequently, it has been possible to utilize ERPs within a brain vital sign framework for monitoring cognitive function, similar to other vital sign monitoring capabilities [10].
The brain vital signs framework translated well-established ERPs into a portable, accessible, rapid, and standardized normative evaluation for cognitive function [10]. It was possible to compress hours of non-standardized, controlled laboratory testing into a quick (∼5 min), deployable, and user-friendly acquisition within the brain vital signs framework. Extracted as “vital signs,” the procedure has facilitated the possibility of objective cognitive evaluation across various clinical settings [11]. As such, three auditory ERP responses are used to create a “brain vital sign” set: the N100 for auditory sensation [12], the P300 for basic attention, which may comprise one or both of its subcomponents (P300a and P300b) [13], and the N400 [14] for cognitive processing. The N100, P300, and N400 components are key elements of our auditory ERP study. The N100 is elicited by a short series of standard (75 dB, 80%) and unexpected louder deviant tones (100 dB, 20%). This auditory response is essential for assessing auditory sensation within the brain vital signs framework. Following the N100, the P300 component, is produced by the same tone series. The P300 reflects basic attention mechanisms in the brain.
Subsequently, the N400 component is elicited using an integrated auditory tone and prime word pair sequence. The short tone sequence primes the listener for paired spoken word pairs, which are either congruent prime pairs (e.g., bread-butter, 50%) or incongruent prime pairs (e.g., Romeo-table, 50%). The incongruent pairs are particularly effective in disrupting semantic expectations and eliciting the N400, a critical component for cognitive processing. Our research team, authors of reference [10], further explored the N400 through a magnetoencephalography (MEG) study. This study supported the neuroanatomical correlates of the N400, highlighting its pivotal role in the brain vital signs framework. We observed that this response shows maximal amplitudes at midline central or parietal sites, and it is observed from 200 to 600 ms post-stimulus across various modalities. Finally, to comprehensively assess brain function, response latencies and amplitudes for all three ERPs (N100, P300, and N400) are normalized. This normalization process allows us to derive six standardized scores, providing a robust measure of cognitive health and function.
Previous studies have supported the use of brain vital signs in clinical applications through regulatory-approved medical devices throughout Canada and the USA; across a wide array of brain health conditions [[15], [16], [17], [18]]. The initial study evaluated healthy brain function in both young and older healthy individuals, including cross-validating brain vital signs with high-density functional brain mapping [10,11]. In the foundational study [10], brain vital signs were reliably detected in healthy adults at the individual level across the lifespan. Importantly, brain vital signs’ neurophysiological measures of cognitive function were sensitive to small but significant age-related latency delays in older adults, specifically in the P300. In contrast, the standard cognitive screening MMSE results detected no difference between younger and older healthy adults. Specifically, from the brain vital signs framework, the N400 ERP component has been connected to being a dependent variable for examining almost every aspect of language processing and, thus, higher-order cognitive processing [19]. As dementia and pathological aging are known to affect cognitive and syntactic processing [20,21], the cognitively associated N400 should and has prior literature connecting its modulation to both healthy and pathological aging [22,23].
1.2. Objectives and hypothesis
The primary objective of this study was to assess the methodological feasibility of quantitatively measuring cognitive function in care home residents using the brain vital signs model described above. The methodological feasibility in the current study refers to the assessment of whether the brain vital signs framework can be used to elicit the N100, P300, and N400 ERP components for analysis to provide meaningful results of neurophysiology in care home residents. Secondarily, we aimed to employ these measures to distinguish varying degrees of cognitive impairment (using the Alzheimer's Association scale) among the residents. The primary hypothesis predicted that brain vital signs components will be elicited in care home residents and will prove to be an appropriate approach leading to meaningful results. Secondarily, the N400, sensitive to cognitive processing, would vary as a function of individual MMSE score and age.
2. Materials and Methods
2.1. Participants
Fifty-two (N = 52) residents in Laurel Place (Fraser Health, Surrey, BC, Canada) consented to enroll in an EEG cognitive evaluation study (Fraser Health Authority provided research ethics approval [FHREB 2015-092] for the study; informed consent was received from each participant or a designated signing authority where required before enrolment). Thirty-two participants were receiving specific care for dementia. Five residents passed away between consent and data collection, consistent with annual mortality rate estimates of about 32% [24]. Three participant scans were removed due to EEG noise (described below under ERP signal processing) for a final adjusted N = 44.
The grouping strategy was based on MMSE groups defined by the Alzheimer's Association Scale rather than traditional diagnostic cutoffs. This strategy was used to enable comparable sample sizes. Specifically, participants were categorized into the low MMSE group (0–12; n = 15), the medium MMSE group (13–24; n = 17), and the high MMSE group (25–30; n = 13) with homogeneity in age distribution. Table 1 provides an overview of the descriptive statistics for the three MMSE groups. A one-way ANOVA was used to evaluate ages between groups (Table 1).
Table 1.
Sample characteristic.
| All | Low MMSE (0-12) | Medium MMSE (13–24) | High MMSE (25–30) | |
|---|---|---|---|---|
| N | 44 | 14 | 17 | 13 |
| Age (range)a | 85.10 ± 9.50 (59–102) | 88.40 ± 8.60 (73–102) | 86.20 ± 8.80 (65–101) | 80 ± 9.00 (59–94) |
| Sex: female/male | 29/16 | 11/4 | 10/7 | 8/5 |
| MMSE (range) | 17.10 ± 9.00 | 6.53 ± 3.60 | 18.20 ± 3.20 | 27.80 ± 1.90 |
One-way ANOVA: No significant difference (p ≥ 0.050) related to difference in age between Low and High MMSE that was also not significant in the alternative group breakdown.
2.2. Data collection
EEG data were recorded from three midline scalp electrodes (Fz, Cz, and Pz; Ag/AgCl electrodes) using a portable 8-channel g. Nautilus system (Gtec Medical Engineering: bandpass: dc-250Hz, 500Hz sampling, 3-axis head motion accelerometers) and a portable computer. Ground (forehead) and reference (earlobe) along with electro-oculogram (EOG: left the supra-orbital ridge and outer canthus) were recorded using disposable electrodes. Skin-electrode impedances were maintained below 30 kΩ impedance at each active electrode site, a value that is low enough to not influence the recorded measurement. Following signal amplification, conditioning, and digitization, data were transmitted via Bluetooth to the computer using a custom USB-TTL converter subsystem to mark stimulus events. Brain vital sign recordings were repeated for each participant (see ERP processing below) [10].
Participants were instructed to sit motionlessly and pay attention to the auditory stimuli while maintaining visual fixation on a cross located 2 m away. The 5-min stimulus sequence comprised auditory stimuli combining interlaced tones and spoken word pairs to enable full optimization of near-maximum trials per minute (e.g., 5s/stimulus cycle x 60 cycles = 5 min). A number of prior methodological studies developed and validated the compressed sequence used in the study [11,15]. Tone stimuli consisting of standard (75 dB, 92%) and deviant (100 dB, 8%) conditions elicited the N100 and P300 responses. In contrast, spoken words consisting of congruent (e.g., Romeo-Juliet, 50%) and incongruent (e.g., Romeo-Coffee, 50%) prime word pairs elicited the N400. For more methodological detail, see Ghosh Hajra et al., 2016 [10].
2.3. ERP signal processing
Python was used for offline data processing. A fourth-order Butterworth filter (0.3–20Hz) and a notch filter (60Hz) were applied to the raw EEG data. EOG artifacts were corrected with adaptive filtering. Established methods, namely, segmentation (−100–900 ms), baseline correction (−100 to 0 ms), and conditional averaging (tones: standard vs deviant; and word pairs: congruent vs incongruent), were used to derive the ERP waveforms. Signal-to-noise (SNR) optimizations were carried out using a 30 μV peak-to-peak threshold to eliminate participant epochs compromised by noise, where three participants had all epochs dropped.
2.4. Statistical analysis
Significant brain vital sign differences as a function of MMSE score and age were analyzed across the N100, P300, and N400 using time intervals for each response. Each time interval was divided into three equal windows. The N100 (180–255 ms [window 1: 180–205 ms, window 2: 205–230 ms, window 3: 230–255 ms]), P300 (400–550 ms [window 1: 400–450 ms, window 2: 450–500 ms, window 3: 500–550 ms]), and N400 (500–800 ms [window 1: 500–600 ms, window 2: 600–700 ms, window 3: 700–800 ms]) response windows were then identified using the grand average ERP waveforms.
Peak amplitudes and latencies for each MMSE group were extracted from the grand average ERP waveforms, as shown in Fig. 1 and Table 2. For the N100, amplitude measures were taken at the Fz electrode site, targeting the highest negative peaks within the 180–255 ms range. The P300 amplitude for each group was identified at the Cz electrode site, representing the highest positive peak within the 400–550 ms range. Finally, the N400 amplitude measures were taken at the Pz electrode site, targeting the highest negative peak within the 500–800 ms window. The corresponding latencies to these peak amplitudes were retrieved. These site selections are based on the typical maximal response locations for each component [10,25]. The individual participant amplitude values from these ranges and electrode sites were then used in the partial least square correlation (PLS) analysis to examine the relationship between brain vital signs and cognitive function.
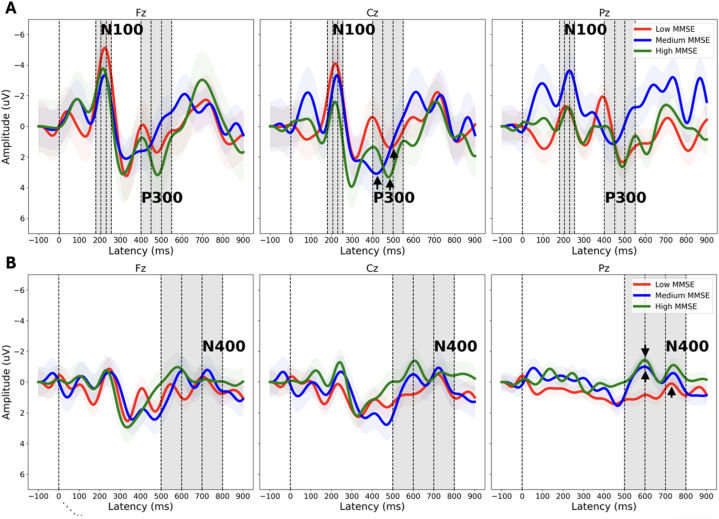
Fig. 1.
Brain vital sign ERP waveforms: grand average waveforms from tones (A) and word pairs (B) at each of the three center electrodes (Fz, Cz, Pz), showing N100, P300, and N400 changes for the different levels of cognitive impairment. Note the peak amplitude reductions and latency delays across the N100, P300 (peaks at Cz denoted with arrow), and N400 (peaks at Pz denoted with arrow). While this is relatively consistent across all three MMSE groups, there is also a clear drop in the N400 (Pz) for the Low MMSE group, which was quantitatively evaluated in the PLS analyses Table 3 and 4). Negative is up on y-axis.
Table 2.
Grand average amplitude and latencies.
| Groups | Measures | N100 | P300 | N400 |
|---|---|---|---|---|
| Low MMSE (0–12) | Amplitude | −5.12 | 1.40 | 0.10 |
| Latency (ms) | 223.94 | 485.50 | 729.00 | |
| Medium MMSE (13–24) | Amplitude | −3.33 | 3.1 | −0.9 |
| Latency (ms) | 221.93 | 417.10 | 598.00 | |
| High MMSE (25–30) | Amplitude | −3.78 | 3.30 | −1.5 |
| Latency (ms) | 213.90 | 477.50 | 598.00 |
Statistical analysis was performed using the PLS [26]. PLS for neuroimaging was written and carried out in MATLAB (MATLAB Version: 9.7.0 (R2019b)) [27]. This toolbox provided the PLS methods to analyze data from multiple responses collected on the same observation. PLS is a statistical technique used to identify relationships between multiple sets of variables and is based on maximizing the covariance between two sets of variables while minimizing the variance of one of the sets. PLS can be divided into two types: contrast-task-based PLS and behavioural PLS [26]. In the current study, behavioural PLS was used to identify significant ERP changes as a function of MMSE score and age. Both contrast task and behavioural PLS can handle high-dimensional data and are robust to noise, outliers and multicollinearity. To address the multiple comparisons issue, which could lead to false positives, the Holm-Sidak correction technique was applied to ensure robust identification of relationships and rigorous control of statistical errors [26,[28], [29], [30]].
Additionally, the jackknife approach [[31], [32], [33]] was used to directly examine N400 latency. Briefly, for each MMSE group, waveform grand averages are created across all but one of the n individual data sets in a subsample, with each data set being left out once. The N400 latency data were then extracted from each of the n leave-one-out grand averages. The N400 component was identified as the largest negative going peak after 400 ms in the n leave-one-out grand averages. Given that the data can be resampled as a function of the number of averages within the grand average (typically n – 1 multiplied by the number of individual averages), any latency estimate is much less influenced by noise. The decreased variance between the leave-one-out derived N400 latencies is corrected with appropriately adjusted statistical tests. In this case, an independent t-test was used to examine the N400 latency differences between the group extremes (low vs. high MMSE groups) as well as the medium vs. high groups. The resulting t-statistic was then divided by n – 1 as a correction [34]. A one-way analysis of variance (ANOVA) was also carried out with the jackknife scores to examine the trend across all three MMSE groups and their N400 latencies. The resulting F-value was corrected by dividing it by (n - 2)2 [34].
3. Results
Brain vital sign data were successfully obtained in 44 of 52 (85%) care home residents. On a group level, this data showed a successful elicitation of the N100, P300, and N400 ERP components (Fig. 1) to analyze in groups of care home residents. The N400 brain vital sign component showed variation across the three MMSE groups. Fig. 1 shows the grand average ERP waveform responses for the brain vital sign results. Examination of MMSE group differences across the N100, P300, and N400 responses demonstrated reduced N400 amplitude for the low MMSE group relative to the other two MMSE groups. In conjunction with Fig. 1, Table 2 provides a more comprehensive representation, quantitatively showcasing the reduction in amplitude. The ERP results indicated a specific N400 sensitivity to clinical factors associated with cognitive impairment.
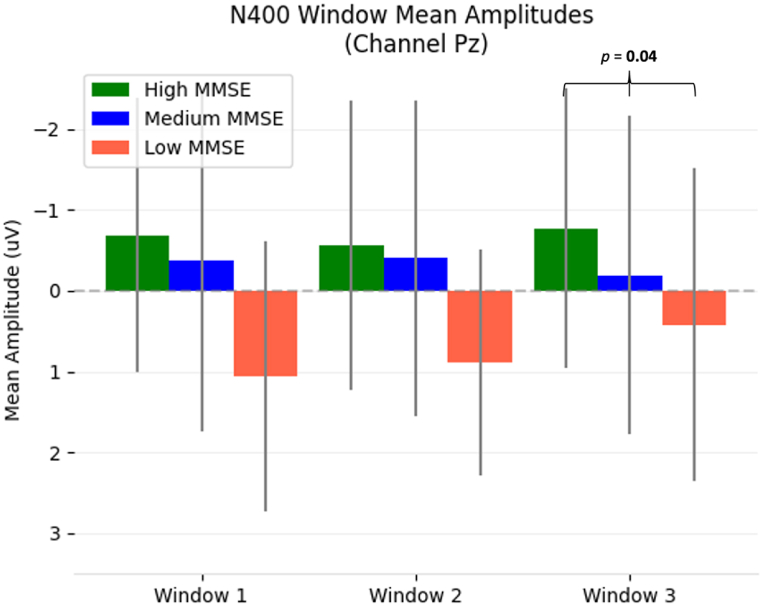
To substantiate these observations, Table 3 (MMSE) and Table 4 (Age) showed the behaviour PLS analysis across all three ERP response intervals and time windows for each response. The PLS analysis was not bound by MMSE groups, instead, the analysis treated MMSE and Age as continuous variables. Results are quantitatively reported as p-values (corrected for multiple comparisons). Examination of the MMSE results (Table 3) showed a significant relationship between the N400 amplitude differences and MMSE scores at Pz in time window 3 (p=0.04). This was also visualized in Fig. 2. Examination of the Age results (Table 4) showed significant relationships between the N400 amplitude differences and Age for Fz (window 1, p = 0.03) and Pz (window 3, p = 0.04). Additionally, PLS was carried out with MMSE as a behavioural variable and age as a covariate, which did not provide significant results.
Table 3.
Behaviour (MMSE) PLSC Analysis (p-values: *p < 0.05, corrected).
|
Window |
N100 |
P300 |
N400 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Fz | Cz | Pz | Fz | Cz | Pz | Fz | Cz | Pz | |
| 1 | 0.31 | 0.20 | 0.53 | 0.32 | 0.67 | 1.00 | 0.83 | 0.60 | 0.54 |
| 2 | 0.22 | 0.52 | 0.47 | 0.51 | 0.27 | 0.35 | 0.79 | 0.38 | 0.90 |
| 3 | 0.56 | 0.94 | 0.31 | 0.47 | 0.64 | 0.97 | 0.28 | 0.27 | 0.04* |
Table 4.
Behaviour (Age) PLSC Analysis (p-values: *p < 0.05, corrected).
|
Window |
N100 |
P300 |
N400 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Fz | Cz | Pz | Fz | Cz | Pz | Fz | Cz | Pz | |
| 1 | 0.91 | 0.31 | 0.31 | 0.68 | 0.36 | 0.86 | 0.83 | 0.65 | 0.52 |
| 2 | 0.11 | 0.28 | 0.28 | 0.76 | 0.37 | 0.58 | 0.03* | 0.43 | 0.85 |
| 3 | 0.20 | 0.16 | 0.32 | 0.09 | 0.45 | 0.50 | 0.33 | 0.95 | 0.04* |
Fig. 2.
Bar plots with standard deviation lines of the N400 mean amplitude for all three windows of each MMSE sub-group. Significant result windows from PLS analysis of MMSE scores and N400 mean amplitudes are annotated with the resulting corrected p-value. Similar to waveform axes, negative is up.
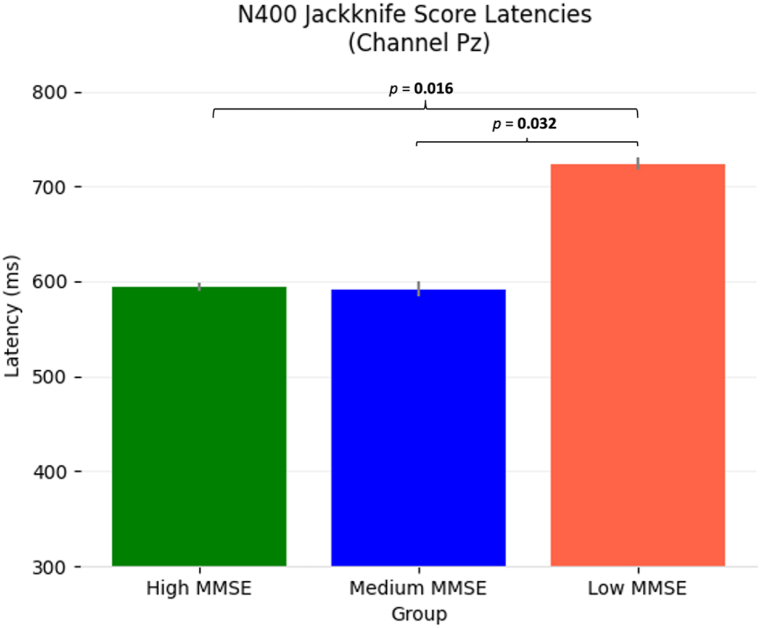
Furthermore, the jackknifing method (mean scores shown in Table 5) also showed a difference between the high and low MMSE groups. The mean N400 jackknife latency scores are outlined in Table 5 and visualized in Fig. 3. An independent t-test showed the high MMSE group to have a significantly faster N400 than the low MMSE group [t(25) = −2.29, p = 0.016]. Similarly, the medium MMSE group had a significantly faster N400 than the low MMSE group [t(25) = −1.93, p = 0.032]. The one-way ANOVA between all three MMSE groups did not reach significance [F(2,25) = 0.99, p = 0.16].
Table 5.
Jackknifing N400 latency scores.
| Group | Mean N400 Latency Score (±SD) | Significance of difference to low MMSE group (p-value) |
|---|---|---|
| Low MMSE (0–12) | 724 ± 6.6 ms | – |
| Medium MMSE (13–24) | 592 ± 7.9 ms | 0.032 |
| High MMSE (25–30) | 594 ± 4.4 ms | 0.016 |
Fig. 3.
Bar plots with standard deviation lines of the N400 jackknife scores for each MMSE sub-group. Note the small standard deviation due to the jackknifing approach. Statistics were adjusted to correct for the reduced variance levels that resulted from the jackknife procedure. Significant differences are annotated with resulting p-values.
4. Discussion
This study tested the methodological feasibility of measuring brain vital signs in care home residents. Furthermore, the current study evaluated the relationship between brain vital signs, specifically the N400, and MMSE scores. Examination of the ERP waveforms (Fig. 1) revealed a variation of peak latencies and amplitudes, with the specific sensitivity of the N400 to the low MMSE group. While there were also individual variations in the P300 latency, they were not large enough to show a significant group difference. Behavioural PLS analysis confirmed the significant differences in the N400 time interval as a function of both MMSE scores (Table 3) and age (Table 4). Overall, the results supported the longstanding identification of ERPs for clinical application for the evaluation of cognitive impairment, ranging from mild cognitive impairment to dementia, including Alzheimer's disease [[35], [36]], with the translation into brain vital signs enabling practical deployment to care home evaluation.
As the N400 is centrally linked to cognitive processing, specifically in the semantic processing of incongruent word stimuli, its significance is evident across various contexts [14]. The N400 has been strongly associated with a central-parietal distribution, aligning with the significant differences observed at the Pz electrode site. Such findings are highly consistent with prior literature that has linked the N400 to semantic integration mechanisms, which evolve throughout the human lifespan [22,37]. For instance, significant age-related variations in the N400 ERP component suggest an evolving neural processing mechanism for semantic integration across different ages [22,37]. Joyal et al. conducted a systematic review of N400 differences in healthy aging and pathological conditions like Alzheimer's disease [22]. This review highlighted electrophysiological variations between elderly and young adults, potentially indicating functional reorganization or compensatory mechanisms within the semantic cognition network [22]. They noted not only reduced semantic association effects in elderly adults compared to younger ones but also further reductions in Alzheimer's disease patients [22]. This suggests potential underlying differences in semantic access, the richness of semantic representations, and cognitive processes, particularly in pathological aging [22]. Building upon this, our findings reveal a nuanced relationship between ERP components, especially the N400, and cognitive impairment as quantified by the MMSE scores.
The sensitivity of the N400 to semantic incongruities mirrors the markers of cognitive decline in the MMSE, offering a unique lens through which to view semantic processing deficits in dementia. Notably, variations in N400 amplitude and latency shift with MMSE scores, suggesting a potential electrophysiological signature of the severity of cognitive impairment. These observations underscore the N400's critical role as a biomarker in cognitive assessments, bridging the gap between traditional screening tools like the MMSE and more direct measures of cognitive processing. Furthermore, the differential responses in the N400 among participants with varying MMSE scores illustrate the complex interplay between cognitive decline and neural processing. This insight implies that the N400, used in conjunction with MMSE scores, can serve as a comprehensive assessment tool. It captures the subtle nuances of cognitive impairment in care home residents, enhancing our understanding of cognitive decline and facilitating the development of targeted interventions, ultimately improving the quality of care in these settings.
While many studies also link ERPs such as the P300 to cognitive impairment at different stages of dementia [36], increasing studies have specifically examined the N400 as a biomarker of synaptic dysfunction in cognitive impairment [[38], [39], [46]]. A recent review proposed that the N400 changes in MCI and AD result from “inhibitory hypofunction” related to semantic network disinhibition [40]. The authors speculate that factors such as cholinergic dysfunction, Aβ load, and somatostatin-positive cell loss may lead to a failure to differential semantic representations and the retrieval of lexical semantic links, which results in a dampening of the N400 activity. While the underlying factors relating to N400 variation in aging and pathological conditions remain a mystery, the current findings highlight the feasibility and the need for more sensitive objective measures of cognitive impairment for management rather than relying on subjective cognitive screening with measures like the MMSE.
As observed in N400 amplitude and latency, exploratory examination of other brain vital sign components evidently shows that the high MMSE group trended toward faster N100 latencies and stronger P300 amplitudes compared to the medium and low MMSE groups. However, these trends did not reach statistical significance. This may be due to a number of factors, including small sample size, measurement error, and confounding variables such as age, attention/fatigue, medication usage, and educational background. The current study did not require active behavioral responses from participants, which is often challenging in cognitively impaired individuals. Despite the paradigm's expected elicitation of internal neural processes, attention variability was anticipated during the 5-min sequence. Monitoring for attention is an important aspect during cognitive testing. It involves continuous observation of participants for signs of disengagement or fatigue, complemented by immediate post-data collection quality checks. This ensures the reliability of the data being collected. However, one of the unique advantages of this test is that it is less dependent on the participant's active attention. As the test relies on a signal-averaged response, a total lack of attention or disengagement to the stimuli would result in no detectable responses. Differences in attention levels are effectively accounted for during the data processing stage, allowing for accurate assessments even in cases of varying attention spans. Additionally, this study took an exploratory step by including the N100 component, which is part of the three brain vital sign components, alongside P300 and N400. The inclusion of the N100 adds a layer of comprehensiveness. It is essential to recognize that the N100 may offer supplementary insights into the neurological underpinnings of cognitive impairments (e.g., differentiating between central auditory processing deficits versus cognitive impairments). It is also important to consider the potential influence of medications and special treatments on neural signals, as these interventions, commonly prescribed to individuals with dementia, may modulate neural activity and impact the ERP components. Future research should document and control medication usage to dissect its potential confounding effects. This emphasizes the need for a more holistic approach in further research to understand the roles of N100, P300, and N400 within the context of cognitive impairment and dementia.
The current proof-of-concept study showed the feasibility of measuring brain vital signs and the significance of using them as an objective and sensitive measure for evaluating cognitive impairment in care home residents. The decline of the N400 with low MMSE highlights the necessity for sensitive and accessible measures of cognitive function in care home environments. With further expected developments, using brain vital signs to evaluate cognitive impairment in care home residents provides new avenues for monitoring the effectiveness of various interventions, such as medications, environmental and interior design modifications, therapies, and activities, at the individual level.
5. Limitations
Brain vital sign monitoring in the care home environment represents an emerging and promising capability to overcome cognitive evaluation challenges. However, more work is needed to achieve practical accessibility in this challenging application. Specifically, it is important to highlight that the current results were obtained at the group level, and future studies are required to confirm that individual-level results are possible for the care home population. Additionally, as part of our pilot study focusing on the feasibility of using the brain vital signs framework in a care home setting, future research should incorporate a healthy control group of older individuals. This will enable a more thorough understanding of electrophysiological measures across the spectrum of cognitive function, from normal aging to severe impairment. Such a longitudinal study is vital for comparing healthy aging with cognitive decline, especially considering the variability in attention and engagement among individual residents which influences the results.
Further signal quality improvements, such as novel denoising approaches for low-density ERP studies [41] and dynamic signal combinations [42], may further improve assessments and have since been implemented to enable individual-level evaluation in challenging care home environments. However, even with the current noisier dataset, jackknifing methods included in the current study have been used as a more accurate method of determining latencies [43] when single-subject ERPs are considered too noisy. Furthermore, improved age-specific norms should be integrated with the brain vital signs to further offer an age-related reference comparison to better characterize the differences between individuals in care, matched individuals in independent living, and those in a normal control group.
The groupings used provided a quantitative grouping of MMSE scores, as opposed to grouping by diagnosis/treatment of dementia (n = 32). It should be noted that other groupings using different clinical-based MMSE scales were evaluated based on different clinical subdivisions (i.e., 30 for no dementia, 26–29 for questionable dementia, 21–25 for mild dementia, 11–20 for moderate dementia, and 0–10 for severe dementia) [44]. This group breakdown also showed comparable results even though the sample sizes were less balanced relative to the current group breakdown. With these future directions, the clinical value of brain vital signs lies in routine monitoring of cognitive functioning/impairment, predictive discrimination, and comparison with a normative control group. In this respect, brain vital sign data combined with existing clinical evaluations, like the MMSE, should be examined with artificial intelligence/machine learning algorithms and potentially integrated with innovative emerging measures such as the frailty index [45].
Data availability statement
The dataset collected and analyzed during this study are not publicly available due to the sensitive nature of the information, which includes personal and health-related data of care home residents, as well as ethical considerations and privacy agreements with the participants and their care facilities. However, these data are available from the corresponding author upon reasonable request, subject to adherence to the ethical guidelines and privacy policies governing this research.
Ethics approval and consent to participate
This study was conducted in strict accordance with the ethical standards of the research community. Formal approval was granted by the Fraser Health Human Research Ethics Board under the reference number FHREB2015-092. All participants provided written informed consent in line with the guidelines set by the aforementioned board, ensuring the integrity and ethical conduct of the research. Conflict of Interest: Several authors are associated with HealthTech Connex Inc., which may qualify them to financially benefit from the commercialization of a NeuroCatch® Platform capable of measuring brain vital signs.
Funding
This study received research-funding support from the Canadian Frailty Network (CFNCAT2017-25). Additional funding for trainee support was from the Surrey Hospitals Foundation (FHAG2017-001). F. Knoefel acknowledges funding for the University of Ottawa Brain and Mind – Bruyère Research Institute Chair in Primary Health Care Dementia Research.
CRediT authorship contribution statement
Joshua Ighalo: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Formal analysis, Data curation. Eric D. Kirby: Writing – review & editing, Data curation, Formal analysis, Methodology, Software, Validation, Visualization. Xiaowei Song: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Shaun D. Fickling: Writing – review & editing, Supervision, Methodology, Investigation, Data curation. Gabriela Pawlowski: Investigation, Data curation. Sujoy Ghosh Hajra: Writing – review & editing, Investigation, Data curation, Conceptualization. Careesa C. Liu: Writing – review & editing, Methodology, Conceptualization. Carlo Menon: Writing – review & editing, Validation, Supervision, Conceptualization. Sudhin A. Shah: Writing – review & editing, Visualization, Validation. Frank Knoefel: Writing – review & editing, Validation, Supervision, Funding acquisition. Ryan C.N. D'Arcy: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Ryan D'Arcy reports financial support was provided by Canadian Frailty Network. Ryan D'Arcy reports financial support and administrative support were provided by Surrey HospitalsFoundation. Shaun Fickling reports a relationship with HealthTech Connex Inc that includes: employment. Several authors are associated with HealthTech Connex Inc., which may qualify them to financially benefit from the commercialization of a NeuroCatch® Platform capable of measuring brain vital signs.
Acknowledgements
The authors sincerely acknowledge the administrative and medical/nursing staffs at the Laurel Place long-term care home for assistance with participant recruitment and data collection. The authors acknowledge T. Arvan for her contributions in data collection, as well as C. Stunden, H. Low, B Chinda and Drs. D. Samoil, ED Zhang, and R. Kelly, for critical discussions and help with the project development, and the Department of Evaluation and Research Services of Fraser Health and Surrey Memorial Hospital for research administrative assistance. We would like to thank the volunteers for participating in the research. We acknowledge the staff at Laurel Place Nursing home for their support in the data collection.
References
- 1.Gaugler J.E., Yu F., Davila H.W., Shippee T. Alzheimer's disease and nursing homes. Health Aff. 2014 Apr;33(4):650–657. doi: 10.1377/hlthaff.2013.1268. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5767317/ [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vossius C., Bergh S., Selbæk G., Benth J.Š., Myhre J., Aakhus E., et al. Mortality in nursing home residents stratified according to subtype of dementia: a longitudinal study over three years. BMC Geriatr. 2022 Apr 5;22(1):282. doi: 10.1186/s12877-022-02994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansbach W.E., Mace R.A., Clark K.M., Firth I.M. Meaningful activity for long-term care residents with dementia: a comparison of activities and raters. Gerontol. 2016 Feb 16;57(3) doi: 10.1093/geront/gnv694. [DOI] [PubMed] [Google Scholar]
- 4.Disalvo D., Luckett T., Bennett A., Davidson P.M., Agar M. Multidisciplinary perspectives on medication-related decision-making for people with advanced dementia living in long-term care: a critical incident analysis. Eur. J. Clin. Pharmacol. 2020 Jan;14 doi: 10.1007/s00228-019-02820-z. [DOI] [PubMed] [Google Scholar]
- 5.Harrison F., Cations M., Jessop T., Shell A., Brodaty H. Inappropriate prescribing of antipsychotic medications in long term-care residents: the halt project. Innovation in Aging. 2017 Jun 30;1(suppl_1) 968–8. [Google Scholar]
- 6.Gawryluk J.R., D'Arcy R.C., Connolly J.F., Weaver D.F. Improving the clinical assessment of consciousness with advances in electrophysiological and neuroimaging techniques. BMC Neurol. 2010 Jan 29;10:11. doi: 10.1186/1471-2377-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galasko D., Gould R.O., Abramson I., Salmon D.P. Measuring cognitive change in a cohort of patients with Alzheimer's disease. Stat. Med. 2000 Jun 15;19(11–12):1421–1432. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1421::aid-sim434>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Connolly J.F., D'Arcy R.C.N. Innovations in neuropsychological assessment using event-related brain potentials. Int. J. Psychophysiol. 2000 Jul;37(1):31–47. doi: 10.1016/s0167-8760(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 9.Fleck-Prediger C.M., Ghosh Hajra S., Liu C.C., Gray D.S., Weaver D.F., Gopinath S., et al. Point-of-care brain injury evaluation of conscious awareness: wide scale deployment of portable HCS EEG evaluation. Neuroscience of Consciousness. 2018 Jan 1;2018(1) doi: 10.1093/nc/niy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajra S.G., Chang Liu C., Song X., Fickling S., Pawlowski G., D'Arcy R.C.N. P4-203: developing brain vital signs: initial assessments across the adult lifespan. Alzheimer's Dementia. 2016 Jul;12 P1102–2. [Google Scholar]
- 11.D'Arcy R.C.N., Ghosh Hajra S., Liu C., Sculthorpe L.D., Weaver D.F. Towards brain first-aid: a diagnostic device for conscious awareness. IEEE (Inst. Electr. Electron. Eng.) Trans. Biomed. Eng. 2011 Mar;58(3):750–754. doi: 10.1109/TBME.2010.2090880. [DOI] [PubMed] [Google Scholar]
- 12.Davis P.A. Effects of acoustic stimuli on the waking human brain. J. Neurophysiol. 1939 Nov 1;2(6):494–499. [Google Scholar]
- 13.Sutton S., Tueting P., Zubin J., John E.R. Information delivery and the sensory evoked potential. Science. 1967 Mar 17;155(3768):1436–1439. doi: 10.1126/science.155.3768.1436. [DOI] [PubMed] [Google Scholar]
- 14.Kutas M., Hillyard S.A. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984 Jan;307(5947):161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh Hajra S., Liu C.C., Song X., Fickling S., Liu L.E., Pawlowski G., et al. Developing brain vital signs: initial framework for monitoring brain function changes over time. Front. Neurosci. 2016 May;12:10. doi: 10.3389/fnins.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fickling S.D., Poel D.N., Dorman J.C., D’Arcy R.C., Munce T.A., Subconcussive changes in youth football players: objective evidence using brain vital signs and instrumented accelerometers, Brain Communications 4 (2) (2021 Dec 15) fcab286. [DOI] [PMC free article] [PubMed]
- 17.Kirby E.D., Jones C.B., Fickling S.D., Pawlowski G., Brodie S.M., Boyd L.A., et al. Real world evidence of improved attention and cognition during physical therapy paired with neuromodulation: a brain vital signs study. Front. Hum. Neurosci. 2023;17 doi: 10.3389/fnhum.2023.1209480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fickling S.D., Greene T., Greene G., Frehlick Z., Campbell N., Etheridge T., et al. Brain vital signs detect cognitive improvements during combined physical therapy and neuromodulation in rehabilitation from severe traumatic brain injury: a case report. Front. Hum. Neurosci. 2020;14 doi: 10.3389/fnhum.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutas M., Federmeier K.D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu. Rev. Psychol. 2011 Jan 10;62(1):621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedden T., Gabrieli J.D.E. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience [Internet] 2004 Feb;5(2):87–96. doi: 10.1038/nrn1323. https://www.nature.com/articles/nrn1323 Available from: [DOI] [PubMed] [Google Scholar]
- 21.Hoffman P. An individual differences approach to semantic cognition: divergent effects of age on representation, retrieval and selection. Sci. Rep. 2018 May 25;8(1) doi: 10.1038/s41598-018-26569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyal M., Groleau C., Bouchard C., Wilson M.A., Fecteau S. Semantic processing in healthy aging and Alzheimer's disease: a systematic review of the N400 differences. Brain Sci. 2020 Oct 23;10(11):770. doi: 10.3390/brainsci10110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Z., Hou X., Yang Y. Reduced syntactic processing efficiency in older adults during sentence comprehension. Front. Psychol. 2018 Mar 1;9 doi: 10.3389/fpsyg.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alzheimer's Association. 2020 Alzheimer’s Disease Facts and Figures Alzheimer’s & Dementia [Internet] 2020 Mar;16(3):391–460. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.12068 Available from: [Google Scholar]
- 25.Pawlowski G.M., Ghosh-Hajra S., Fickling S.D., Liu C.C., Song X., Robinovitch S., et al. Brain vital signs: expanding from the auditory to visual modality. Front. Neurosci. 2019 Jan;18:12. doi: 10.3389/fnins.2018.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011 May;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 27.MATLAB Version: 9.13.0 (R2022b) The MathWorks Inc.; Natick, Massachusetts: 2022. [Google Scholar]
- 28.Bailey K., West R., Mullaney K.M. Neural correlates of processing negative and sexually arousing pictures. Martinez L.M., editor. PLoS One. 2012 Sep 20;7(9) doi: 10.1371/journal.pone.0045522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee T.W., Younger W.-Y. Yu, Wu H.C., Chen T.J. Do resting brain dynamics predict oddball evoked-potential? BMC Neurosci. 2011 Nov 24;12(1) doi: 10.1186/1471-2202-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holm S. Board of the foundation of the scandinavian journal of statistics A simple sequentially rejective multiple test procedure A simple sequentially rejective multiple test procedure. Source. Scand. J. Stat. 1979;6(2):65–70. [Google Scholar]
- 31.Miller J., Patterson T., Ulrich R. Jackknifebased method for measuring LRP onset latency differences. Psychophysiology. 1998;35(1):99–115. doi: 10.1111/14698986.3510099. [cited 2023 Jul 20] [DOI] [PubMed] [Google Scholar]
- 32.Ulrich R., Miller J. Using the jackknifebased scoring method for measuring LRP onset effects in factorial designs. Psychophysiology. 2001;38(5):816–827. doi: 10.1111/14698986.3850816. [cited 2023 Jul 20] [DOI] [PubMed] [Google Scholar]
- 33.Smulders Fren T.Y. Simplifying jackknifing of ERPs and getting more out of it: retrieving estimates of participants' latencies. Psychophysiology. 2010;47(2):387–392. doi: 10.1111/j.14698986.2009.00934.x. [cited 2023 Jul 20] [DOI] [PubMed] [Google Scholar]
- 34.Luck S.J. Mit Press; Cambridge, Mass: 2014. An Introduction to the Event-Related Potential Technique. [Google Scholar]
- 35.Olichney J.M., Hillert D.G. Clinical applications of cognitive event-related potentials in Alzheimer's disease. Phys. Med. Rehabil. Clin. 2004 Feb;15(1):205–233. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 36.Parra M.A., Ascencio L.L., Urquina H.F., Manes F., Ibáñez A.M. P300 and neuropsychological assessment in mild cognitive impairment and alzheimer dementia. Front. Neurol. 2012 Dec 5;3:172. doi: 10.3389/fneur.2012.00172. https://www.frontiersin.org/articles/10.3389/fneur.2012.00172/full [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto T., Katayama J., Koyama T. ERPs, semantic processing and age. Int. J. Psychophysiol. 1998 Jun;29(1):43–51. doi: 10.1016/s0167-8760(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh Hajra S., Liu C.C., Song X., Fickling S.D., Cheung T.P.L., D'Arcy R.C.N. Multimodal characterization of the semantic N400 response within a rapid evaluation brain vital sign framework. J. Transl. Med. 2018 Jun 4;16(1) doi: 10.1186/s12967-018-1527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh Hajra S., Liu C.C., Song X., Fickling S.D., Cheung T.P.L., D'Arcy R.C.N. Accessing knowledge of the “here and now”: a new technique for capturing electromagnetic markers of orientation processing. J. Neural. Eng. 2018 Dec 3;16(1) doi: 10.1088/1741-2552/aae91e. [DOI] [PubMed] [Google Scholar]
- 40.Paitel E.R., Samii M.R., Nielson K.A. A systematic review of cognitive event-related potentials in mild cognitive impairment and Alzheimer's disease. Behav. Brain Res. 2021 Jan;396 doi: 10.1016/j.bbr.2020.112904. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh Hajra S., Gopinath S., Liu C., Pawlowski G., Fickling S., Song X., et al. Electronics and Mechatronics Conference (IEMTRONICS) IEEE International; 2020. Enabling event-related potential assessments using low-density electrode arrays: a new technique for denoising individual channel EEG data. IOT. [Google Scholar]
- 42.Hajra S.G., Liu C.C., Fickling S.D., Pawlowski G.M., Song X., D'Arcy R.C.N. Event related potential signal capture can Be enhanced through dynamic SNR-weighted channel pooling. Sensors. 2021 Oct 31;21(21):7258. doi: 10.3390/s21217258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller J., Ulrich R., Schwarz W. Why jackknifing yields good latency estimates. Psychophysiology. 2009;46(2):300–312. doi: 10.1111/j.14698986.2008.00761.x. [cited 2023 Jul 20] [DOI] [PubMed] [Google Scholar]
- 44.Perneczky R., Wagenpfeil S., Komossa K., Grimmer T., Diehl J., Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatr. 2006 Feb;14(2):139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 45.Song X., MacKnight C., Latta R., Mitnitski A.B., Rockwood K. Frailty and survival of rural and urban seniors: results from the Canadian Study of Health and Aging. Aging Clin. Exp. Res. 2007 Apr;19(2):145–153. doi: 10.1007/BF03324681. [DOI] [PubMed] [Google Scholar]
- 46.Olichney J.M., Yang J.C., Taylor J., Kutas M., Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer's disease, J. Alzheimer's Dis. 26 (3) (2011 Jan) 215–228. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset collected and analyzed during this study are not publicly available due to the sensitive nature of the information, which includes personal and health-related data of care home residents, as well as ethical considerations and privacy agreements with the participants and their care facilities. However, these data are available from the corresponding author upon reasonable request, subject to adherence to the ethical guidelines and privacy policies governing this research.