Abstract
Intralesional therapies are used for recalcitrant warts, but no Food and Drug Administration–approved treatment exists nor is there consensus regarding the most efficacious therapy. Therefore, this systematic review aims to summarize efficacy and adverse events reported in 62 randomized controlled trials (RCTs) of intralesional therapies for cutaneous warts. The most studied intralesional therapies included measles, mumps, rubella (MMR) vaccine (n = 24 studies), purified protein derivative (PPD) (n = 19 studies), vitamin D3 (n = 15 studies), and Candida antigen (n = 14 studies). Most studies included adult and pediatric patients or adults alone, with only 4 studies on pediatric patients alone. MMR vaccine was the most studied treatment (n = 853 patients). MMR had a complete response rate of 27–90%. The next most common treatment, PPD, had a complete response rate of 45–87%. Other treatments included Candida antigen and vitamin D3, with complete response rates of 25–84% and 40–96%, respectively. The most frequent side effects were injection-site reactions and flu-like symptoms. This systematic review represents a useful summary of intralesional therapy RCTs for clinician reference. This study also highlights the lack of large multi-institutional RCTs, despite many patients being treated for this widespread problem.
Keywords: Cutaneous warts, Intralesional therapy, Systematic review, Verruca, Wart
Introduction
Warts are verrucous, exophytic lesions caused by human papillomaviruses (HPVs) that slip through disrupted epithelial barriers to infect basal keratinocytes. Different wart subtypes, including common warts (verruca vulgaris), flat warts (verruca plana), filiform warts (verruca filiformis), and genital warts (condyloma acuminatum), can result from HPV types 1, 2, 4, 27, 57, and 63 (James et al, 2011). The infected keratinocytes excessively and abnormally proliferate within the epidermal layer to create a thickened, warty papule on the skin. Warts most commonly occur at sites of regular trauma, where the virus can slip through even minimally damaged epithelial barriers to infect the underlying basal stem cells (Al Aboud and Nigam, 2023). Considered one of the most common complaints of routine dermatological practice, warts affect approximately 10% of the population. Cutaneous warts often occur in children, with incidence rates peaking in White teenagers (ranging from 12 to 18 years) (Al Aboud and Nigam, 2023; Hay et al, 2014). Although warts are largely a benign condition, their undesirable appearance and potentially painful placement make them a cause for concern and frustration for patients. In addition, these flesh-colored papules can fissure, be uncomfortable or tender, and bleed. Owing to the recurring nature of warts, they may be difficult to remove, which can be disheartening for both patients and providers (Al Aboud and Nigam, 2023; Hay et al, 2014; Muršić et al, 2020).
There is a range of treatment options available; aggressive approaches may risk normal tissue destruction, and less aggressive therapies may leave the patient with a higher chance of recurrence. In addition, treatment options depend on the lesion type, size, and frequency of recurrence (Hay et al, 2014; James et al, 2011; Muršić et al, 2020). Removal may be done through destructive therapies, immunotherapies, application of antiproliferative agents, and/or antiviral therapies. Of these therapy options, common warts are most often treated with liquid nitrogen cryotherapy, the application of keratolytic agents under occlusion, or electrocauterization. Other traditional treatment methods include surgery with curettage, pulse dye lasers, vitamin derivatives, immune response–modifying treatments, or cytostatic treatments (such as 5-fluorouracil [5-FU]). Despite a variety of treatment options, the effectiveness of treatment methods remains unclear, and some treatment methods may only inhibit further growth (Mattoo and Bhatia, 2018; Muršić et al, 2020). In addition, several of those treatments are inconvenient for the patient because they may require frequent medical visits and prescriptions. The lack of definitive, convenient, and cost-effective therapies for common warts has led to patients attempting home remedies that use occlusion alone (ie, duct tape therapy), which are less effective (Loo and Tang, 2014).
Intralesional therapies are an emerging and effective approach for treating recalcitrant warts, particularly those situated in challenging regions such as palmoplantar and periungual areas, which often exhibit resistance to conventional treatments (Mattoo and Bhatia, 2018). Several intralesional therapies for warts have emerged, including vitamin D3; tuberculin purified protein derivative (PPD); Candida antigen (Candida); the measles, mumps, rubella (MMR) vaccine; as well as various pharmaceutical mixtures, including immunotherapeutic drugs, digoxin–furosemide combinations, and antivirals such as acyclovir (Aldahan et al, 2016; Fields et al, 2020; Ju et al, 2022). Unlike various other treatment options, immunotherapeutic approaches upregulate the immune system to recognize and destroy the lesions at both the target site and distant locations. This approach has proven to be an inexpensive and effective method, particularly for individuals with multiple recalcitrant warts (Aldahan et al, 2016). In comparison with conventional therapeutic methods, intralesional immunotherapy has demonstrated shorter treatment times, increased efficacy, a lower incidence of side effects, and reduced recurrence rates (Ju et al, 2022; Martin et al, 2022). However, no Food and Drug Administration–approved treatment exists, nor is there consensus regarding the most efficacious intralesional therapy. Thus, a systematic review of intralesional therapies is reported in this paper to achieve a better understanding of the intralesional treatment modalities currently available to patients and to understand the efficacy and safety of intralesional therapies for treating nongenital warts.
Methods
Data sources and search strategies
We searched MEDLINE, EMBASE, The Cochrane Library, CINAHL, and ClinicalTrials.gov from database origin up to May 17, 2022 for articles regarding intralesional therapy for cutaneous warts. A librarian peer review of the EMBASE search strategy was performed, and keywords included locations such as hand OR facial OR periungual but not genital, verruca vulgaris OR wart OR verruca, and intra lesional OR intralesional. Searches were limited to articles available in English. No age group limits were applied. Only ClinicalTrials.gov trials that had been completed through January 2023 were included in the analysis.
Study selection and data collection
To ensure the highest quality of evidence, this study only included randomized controlled trials (RCTs). Exclusion criteria were articles about animal models, non-HPV–related verruca, noncutaneous warts (anogenital warts, oral warts), and article types other than RCTs. In addition, only RCTs that reported outcomes by treatment type and number of patients could be included. For example, trials that reported response by number of warts that responded per patient were excluded because these response rates could not be compared with rates reported as number/percentage of patients treated. Complete response was chosen as the primary outcome because it is clinically meaningful and the most standardized across studies. In contrast, partial responses were variably defined and less comparable across studies.
Two separate reviewers (SAM and ELM) reviewed articles for inclusion or exclusion, with a third person (MJW) settling disagreement on study inclusion. For data collection, 2 separate reviewers (2 of ELM, SAM, KTN, TAH, DW, or RLB) abstracted data, and a third reviewer (SAM) combined these results and settled any discrepancies through a third review of the article.
Data collected included wart type, treatment type, concentration, volume, dose, frequency, duration, and number of warts treated per person; number of patients treated and number lost to follow-up; age group of patients defined as adult (age ≥18 years), pediatric, or both; number and percentage of complete response, partial response, and no response; adverse effects; and previous treatments. If a treatment concentration was not given, the standard commercially available dose was assumed. Concentrations are reported in milligrams per milliliter (mg/ml) or tuberculin units (ie, TU). Vitamin D concentrations were often listed in international units (ie, IU) and then converted using 1 IU of vitamin D to equal 0.025 μg.
Assessing level of evidence
The Oxford Centre for Evidence-Based Medicine (CEBM) 2009 grading criteria were utilized to evaluate the quality of each study (Oxford Centre for Evidence-Based Medicine, 2009). Additional data collected and reviewed included blinding (double blinded, single blinded, not blinded, or not reported), confidence interval (CI), and percentage lost to follow-up. Per CEBM criteria, RCTs are either level 1B (individual RCT with narrow CI) or 2B (low-quality RCT) (eg, <80% follow-up rate). Studies with no blinding or with blinding not reported are level 2B. A total of 20 studies were 1B, and 42 studies were 2B. Per CEBM criteria, grade A recommendations are consistent with level 1 studies, and grade B are consistent with level 2 or 3 studies. Because most studies were 2B, the grade of recommendation for this review is grade B (Table 1).
Table 1.
Assessment of Evidence
| Level of Evidence | Number of Studies |
|---|---|
| 1B | 20 |
| 2B | 42 |
Abbreviation: CEBM, Centre for Evidence-Based Medicine.
The Oxford CEBM 2009 was used to determine the level of evidence of each study and overall grade of recommendation. On the basis of a majority of studies presenting level 2 evidence, the overall grade of recommendation is grade B.
Analysis
A descriptive analysis was performed to characterize each study. Quantitative analysis reported the number of times each treatment was used across studies, the number of times each control was used, the ranges for treatment regimens, the number of studies in each age group, the complete response rate range, and the frequency of adverse effects. The analysis was performed using SAS/STAT software (version 9.4, © 2016) of the SAS System for Windows.
Results
Search results
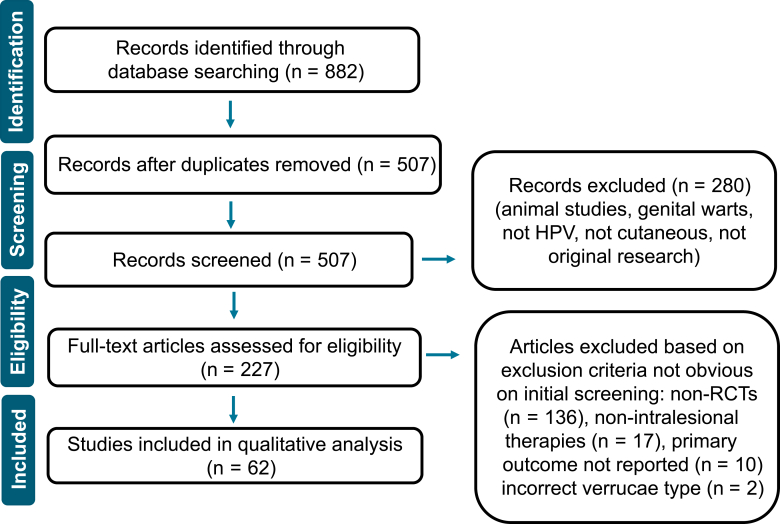
A total of 882 studies were identified, and 507 remained after deduplication. After screening respective abstracts and titles, an additional 280 records were excluded. A total of 227 full-text articles were reviewed, and of these, 62 were chosen for inclusion in the final analysis (Figure 1). These RCTs ranged in date from 1974 to 2022 and originated from 12 different countries.
Figure 1.
PRISMA Diagram. PRISMA diagram defines our final studies included. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
Description of included studies
A total of 4789 patients were treated across studies, and 21 unique intralesional solitary or combination therapies were identified. Seven different controls were used across studies, with intralesional normal saline (n = 21) and cryotherapy (n = 13) being the most common (Table 2). Notably, 19% (12 of 62) of studies described previous therapies undergone by their patients. The most common statement was that patients had been unsuccessfully treated with at least 1 therapy, which often varied among patients, before entering the trial. Wart types most commonly included verruca vulgaris, followed by verruca plantaris and verruca palmaris, and least commonly included verruca plana and periungual warts. Only 4 therapies were trialed in greater than 10 RCTs: these 4 most common therapies were MMR (n = 24), PPD (n = 19), vitamin D (n = 15), and Candida (n = 14) (Table 3) (Abd-Elazeim et al, 2014; Abd El-Magiud et al, 2020; Agrawal et al, 2018; Ahmed et al, 2020; Amin et al, 2021; Amirnia et al, 2016; Awad et al, 2023; Awal and Kaur, 2018; Baaniya et al, 2021; Bhalala et al, 2021; Eldahshan et al, 2022; ElGhareeb, 2019; El Sayed et al, 2020; Elsayed Ghaly et al, 2021; Fathy et al, 2019; Fawzy et al, 2020; Hodeib et al, 2021; Ibrahim et al, 2022, 2020; Jain and Marfatia, 2021; Jartarkar et al, 2021; Kareem et al, 2019; Kaur et al, 2021; Khozeimeh et al, 2017; Khurshid and Pal, 2009; Mehta et al, 2021; Milante et al, 2019; Mohammed et al, 2021; Mohta et al, 2022, 2021; Nassar et al, 2022; Nofal et al, 2022a, 2022b, 2022c, 2021a, 2021b, 2020b; Nofal and Nofal, 2010; Rageh et al, 2021; Rajegowda et al, 2020; Rezai et al, 2019; Rutnin et al, 2023; Shaldoum et al, 2020; Wan Ahmad Kammal et al, 2021; Zamanian et al, 2014; Zainab et al, 2021). The most common therapy, MMR, was reported in 807 patients across studies. All 4 common therapies had significantly variable treatment regimens, making it difficult to compare across studies. Another 18 therapies were trialed in less than 10 studies and are reported in Table 4 (Adalatkhah et al, 2007; Barkat et al, 2018; Choi et al, 2002; Dhakar et al, 2016; Dhar et al, 2009; Ebrahim et al, 2021; Eldahshan et al, 2022; ElGhareeb, 2019; Ghonemy et al, 2020; Hodeib et al, 2021; Horn et al, 2005; Ibrahim et al, 2022; Kaur et al, 2021; Mohta et al, 2022; Muhammad et al, 2019; Niimura, 1990; Nofal et al, 2022b, 2022c, 2020a, 2020b; Sarin and Dfwan, 1974; Soni et al, 2011; Yazdanfar et al, 2008).
Table 2.
Controls
| Type of Control | Number of Studies |
|---|---|
| Intralesional normal saline | 21 |
| Cryotherapy | 13 |
| Photodynamic therapy/pulsed dye laser | 2 |
| Intralesional Water | 1 |
| Electrosurgery | 1 |
| Microneedling | 1 |
| Intralesional Collomak1 | 1 |
Abbreviations: MMR, measles, mumps, rubella vaccine; PPD, purified protein derivative; RCT, randomized controlled trial.
All controls used in RCTs are shown. Some studies compared treatment groups without a control (eg. MMR vs PPD) and thus are not listed here.
Collomak: Active ingredients include salicylic acid, acetic acid, decussate sodium, castor oil, pyroxylin, ethanol, acetone, and isobutyl acetate, depending on pharmacy formulation.
Table 3.
Most Common Intralesional Therapies
| Therapy | Number of Studies Using Therapy | Number of Patients across Studies1 | Type of Warts (Number of Studies) | Dose Concentration and Amount | Dose Frequency and Number of Sessions2 | Complete Response Rate3 |
|---|---|---|---|---|---|---|
| MMR | 24 | 807 | vulgaris (13), palmaris (8), plantaris (13), periungual (7), plana (3), not specified (4) | 20–100 IU/ml, 0.1–0.5 ml | Every 2, 3, or 4 wk, 2–8 sessions | 27–90% |
| PPD | 19 | 631 | vulgaris (7), palmaris (5), plantaris (8), periungual (6), plana (2), not specified (3) | 2.5–100 TU/ml, 0.1–0.3 ml | Every 1, 2, or 4 wk, 3–12 sessions | 48–87% |
| Vitamin D3 | 15 | 423 | vulgaris (7), palmaris (5), plantaris (11), periungual (4), plana (2), not specified (1) | 2.5–15 mg/ml, 0.1–0.6 ml | Every 2, 3, or 4 wk, 2–8 sessions | 40–96% |
| Candida | 14 | 508 | vulgaris (7), palmaris (2), plantaris (9), periungual (2), plana (3), not specified (0) | 0.1–10 mg/ml, 0.1–0.3 ml | Every 2, 3, or 4 wk, 3–6 sessions | 25–84% |
Abbreviations: MMR, measles, mumps, rubella vaccine; PPD, purified protein derivative; RCT, randomized controlled trial.
All therapies are described in greater than 10 RCTs.
With complete follow-up (excludes those lost to follow-up).
Sessions are reported as treatment until complete response or maximum number of sessions.
Reported as a range from lowest to highest complete response rate.
Table 4.
Less Common Intralesional Therapies
| Treatment | Number of Studies | Total Number of Patients |
|---|---|---|
| Bleomycin | 6 | 234 |
| MIP vaccine (Mw) | 3 | 114 |
| 5-FU | 3 | 84 |
| Zinc sulfate | 2 | 73 |
| Human serum albumin and lactose | 1 | 64 |
| IFN beta | 1 | 64 |
| IFN alfa-2b | 2 | 56 |
| Mumps, Candida, or trichophyton antigens | 1 | 54 |
| PPD + cryotherapy | 2 | 50 |
| Mumps /trichophyton/candida antigens + IFN alfa-2b | 1 | 41 |
| Alternating PPD and Candida | 1 | 40 |
| BCG-PSN vaccine | 1 | 40 |
| Intralesional Candida with oral isotretinoin | 1 | 36 |
| Vitamin A | 1 | 24 |
| HPV vaccine | 1 | 22 |
| IFN alfa-2b + pulse-dyed laser | 1 | 13 |
| Hydrocortisone acetate | 1 | 6 |
Abbreviations: 5-FU, 5 fluorouracil; BCG-PSN, Bacillus Calmette–Guerin polysaccharide nucleic acid; Candida, Candida antigen; HPV, human papillomavirus; MIP, Mycobacterium indicus pranii; Mw, Mycobacterium; PPD, purified protein derivative; IFN, interferons; RCT, randomized controlled trial.
MIP is formerly known as Mw. All of these therapies are described in less than 10 RCTs.
Treatment response
Among the 4 most common therapies, vitamin D3 had the highest reported complete response rate (96%) in a study of 23 adults treated with injection of 0.2 ml of 15 mg/ml vitamin D every 2 weeks for up to 4 sessions (Zainab et al, 2021). The next highest response rate was 90% complete response after treatment with MMR in a study of 19 adults treated with 0.3 ml every 2 weeks for up to 5 sessions (Rutnin et al, 2023). For PPD, the highest response rate was 87% reported in a study of 15 adults treated with 1 ml of 2.5 TU every 2 weeks for up to 6 sessions (Wan Ahmad Kammal et al, 2021). Finally, for Candida, the highest response rate was 84% in a study of 50 adults treated with 0.3 ml of 10 mg/ml every 2 weeks for up to 5 sessions (Nofal et al, 2022a) (Table 3).
Other therapies with greater than 80% response rate include bleomycin in 4 different studies (Adalatkhah et al, 2007; Dhar et al, 2009; Hodeib et al, 2021; Soni et al, 2011), bleomycin with cryotherapy (Muhammad et al, 2019), Candida in 2 additional studies (Nofal et al, 2021a; Rageh et al, 2021), PPD with cryotherapy (Ibrahim et al, 2022), HPV vaccine (Nofal et al, 2020a), IFN alfa-2b with pulsed dye laser (Choi et al, 2002), Mycobacterium indicus pranii (MIP) vaccine (Kaur et al, 2021), MMR in 6 additional studies (Baaniya et al, 2021; Mohammed et al, 2021; Mohta et al, 2021; Nofal and Nofal, 2010; Shaldoum et al, 2020), PPD in 1 additional study (Rutnin et al, 2023), and vitamin D3 in 3 additional studies (Amin et al, 2021; Ibrahim et al, 2020; Nofal et al, 2021a).
Safety profiles
Of the 62 RCTs, almost all (n = 60) reported adverse events (AEs). Among the 4 most common therapies, the most frequent AE was injection-site reaction, reported in 90–93% of studies depending on the therapy. Flu-like symptoms were the next most common AE, reported in 35–79% of studies, with the highest rate occurring after Candida. Other less common AEs included pigment changes, bullae/vesicles, desquamation, infection, hematoma, necrosis, and nail dystrophy (Table 5).
Table 5.
Adverse Effects
| Therapy | Injection-Site Reaction | Flu-like Symptoms | Dyspigmentation | Bullae/Vesicles | Desquamation |
|---|---|---|---|---|---|
| MMR (n = 24) | 92% (N = 22) | 46% (N = 11) | 4% (N = 1) | — | — |
| PPD (n = 19) | 90% (N = 18) | 35% (N = 7) | 25% (N = 5) | 20% (N = 4) | 5% (N = 1) |
| Vitamin D3 (n = 15) | 93% (N = 14) | — | 20% (N = 3) | 7% (N = 1) | — |
| Candida (n = 14) | 93% (N = 13) | 79% (N = 11) | 7% (N = 1) | 14% (N = 2) | 7% (N = 1) |
Abbreviations: MMR, measles, mumps, rubella vaccine; PPD, purified protein derivative.
n = number of studies including each respective therapy that reported any adverse effects. N = number of studies reporting that specific adverse effect. The number of studies reporting specific adverse effects for each of the most common therapies is shown.
Discussion
This systematic review summarizes 62 RCTs regarding intralesional verruca therapies. Previous systematic reviews and meta-analyses have looked at complete response rates (both local and distant), recurrence rates, and adverse effects. Salman et al (2019) performed a network meta-analysis of only RCTs, including 17 RCTs and 10 intralesional therapies, and found that PPD (OR = 39.56, 95% CI = 7.13–219.42), MMR (OR = 17.46, 95% CI = 5.02–60.74), and IFN-b (OR = 15.55, 95% CI = 2.37–101.85) had the highest complete response rate at the primary site compared with placebo. Martin et al (2022) reported on intralesional therapies for plantar warts among 26 studies and 6 therapies, discussing the median cure rates for vitamin D3 (80%), bleomycin (74%), 5-FU (59%), Candida (66%), zinc sulfate (70%), and PPD (67%). Ju et al (2022) reported on intralesional immunotherapies and found a pooled 60.6% (95% CI = 54.8–66.5%) local complete response rate across MMR, Candida, vitamin D, PPD, IFN-a, MIP vaccine, bacillus Calmette–Guerin (BCG) vaccine, and 8 other therapies. Unique to their study is a reported pooled recurrence rate of 2.0% (95% CI = 1.1–2.9%). In addition, they found AEs in 51 of 54 studies, with pain and flu-like symptoms being the most common, similar to this study’s findings (Ju et al, 2022). Despite previous reviews on this topic, this study includes information on more RCTs (many published after the most recent systematic review) and more treatments, including less common therapies such as bleomycin, 5-FU, and HPV vaccine. This study found response rates similar to those of previous studies but also highlighted the breadth of studied therapies and lack of RCT evidence.
MMR was the most commonly trialed therapy, and 6 studies of different regimens showed MMR to have a significantly higher complete response rate or complete response plus partial response rate than cryotherapy or normal saline (Abd El-Magiud et al, 2020; Agrawal et al, 2018; Awal and Kaur, 2018; Nofal and Nofal, 2010; Rezai et al, 2019; Zamanian et al, 2014). The highest rate of complete response, 90%, was achieved using 0.3 ml every 2 weeks for up to 5 sessions (Rutnin et al, 2023). When compared with other therapies, 0.1 ml of MMR every 2 weeks for up to 5 sessions was shown to have a significantly higher complete response than Candida but no difference compared with BCG vaccine (P = .03, P > .5) (Eldahshan et al, 2022). However, 2 separate RCTs found the same dose, frequency, and number of treatments of MMR to be not significantly more efficacious than Candida or PPD (P > .05 and P = .853) (Fawzy et al, 2020; Nofal et al, 2021a). Again, when MMR was compared with PPD, the same dose and frequency of MMR with only up to 4 sessions was found to be equally efficacious compared with PPD (P = .917) (Bhalala et al, 2021). A different dose of MMR, 0.3 ml every 2 weeks for up to 5 sessions, was found to have a higher complete response and lower adverse effects than PPD, but there was no statistical significance between the groups (P = .135) (Rutnin et al, 2023). One RCT comparing MMR dosed as 0.3 ml every 3 weeks for up to 6 sessions compared with vitamin D found that both are well-tolerated, effective, and cost-effective, with no statistically significant difference between the 2 groups (P = .889) (Shaldoum et al, 2020).
PPD was the second most common therapy. PPD across many studies was dosed on the basis of a widely accepted protocol that requires a previous intradermal injection of 0.1 ml of PPD and measurement of the reaction in millimeters (mm) 48–72 hours later. Intralesional PPD for treatment of verruca is later injected at a range of 0.1–0.3 ml — 0.3 ml if the PPD test reaction was 5–9 mm, 0.2 ml if the reaction was 10–15 mm, and 0.1 ml if the reaction was >15 mm. Assume this regimen in the discussion unless otherwise noted. PPD at various regimens has 4 studies showing its superiority to cryotherapy or normal saline (Abd-Elazeim et al, 2014; Amirnia et al, 2016; Ibrahim et al, 2022; Wan Ahmad Kammal et al, 2021). The highest rate of complete response, 87%, was achieved using 1 ml of 2.5 TU injected into the largest wart every 2 weeks for up to 6 sessions (Wan Ahmad Kammal et al, 2021). As mentioned previously, 0.1 ml of PPD every 2 weeks for up to 5 sessions was shown to have a complete response rate similar to MMR and Candida in an RCT comparing all 3 therapies. One unique RCT trialed 0.1 ml PPD every 2 weeks for up to 6 sessions or 0.1 ml of 1/1000 solution of Candida every 2 weeks for up to 6 sessions compared with a regimen alternating the 2 therapies and found that the difference in therapeutic response (complete response and partial response) was statistically significant when alternating PPD and Candida versus either agent alone (P < .0001) (Nofal et al, 2020b). In another interesting RCT, PPD was trialed every 2 weeks up to 12 sessions in patients with multiple verruca as a single injection into the largest lesion versus injection into every verruca, and multiple injections were shown to have a significantly higher rate of complete clearance (Milante et al, 2019). PPD every 2 weeks for up to 3 sessions was shown to have a lower complete response than 0.2 ml vitamin D3 (300,000 IU, 7.5 mg/ml) monthly for up to 3 sessions (50 vs 70%) and required more sessions to see a complete response. No analysis compared the 2 therapies’ response rates with each other; rather, the reference group was normal saline (Elsayed Ghaly et al, 2021). No other RCT looked at just PPD and vitamin D, but an RCT comparing 0.1 ml of PPD every 2 weeks for up to 4 sessions with vitamin D3 and with MMR found no statistical difference in response rates (P = .676) (Jain and Marfatia, 2021).
Vitamin D3 was the third most common therapy, and various regimens were shown in 3 studies to be superior to Collomak, normal saline, and cryotherapy (Ibrahim et al, 2020; Kareem et al, 2019; Zainab et al, 2021). The highest rate of complete response, 96%, was achieved using 0.2 ml of 15 mg/ml suspension every 2 weeks for up to 4 sessions (Zainab et al, 2021). However, 1 RCT showed 0.2–0.4 ml of 0.5 mg/ml vitamin D3 every 3 weeks for up to 3 sessions to be not significantly superior to cryotherapy (Amin et al, 2021). One RCT looked at vitamin D3 and photodynamic therapy versus Collomak, and although it did not directly compare vitamin D3 with photodynamic therapy, it found that vitamin D3 required half the number of treatments as photodynamic therapy (Ibrahim et al, 2020). Vitamin D3 at 0.6 ml of 2.5 mg/ml every 3 weeks for up to 3 weeks had a higher complete response rate than Candida (P = .021) (Fathy et al, 2019).
Candida has been discussed earlier in comparison with MMR, PPD, and vitamin D3. The highest rate of complete response, 84%, was achieved using 0.3 ml of 10 mg/ml suspension every 2 weeks for up to 5 sessions (Nofal et al, 2022a). Candida in various regimens had a statistically significantly higher complete response rate than cryotherapy (Khozeimeh et al, 2017) and normal saline (Khurshid and Pal, 2009; Nassar et al, 2022). Nofal et al (2022c) studied a new regimen combining Candida and oral isotretinoin versus either alone and found significantly higher response in the Candida group (56%) than in oral isotretinoin (44%) or the combination therapy group (39%) (P = .003).
Less common therapies of particular interest are bleomycin, 5-FU, and HPV vaccine because of their historic use in dermatology or emerging interest. The MIP vaccine was trialed in 3 RCTs (Dhakar et al, 2016; Kaur et al, 2021; Mohta et al, 2022), but this therapy is not available in the United States. Although bleomycin was mentioned in multiple studies in the search results, many were excluded owing to lack of randomization, leaving only 6 RCTs reporting bleomycin efficacy (Adalatkhah et al, 2007; Barkat et al, 2018; Dhar et al, 2009; Muhammad et al, 2019, 2019; Soni et al, 2011). Bleomycin is shown in these RCTs to be superior to cryotherapy and normal saline. Although 5-FU is an interesting option owing to its widespread availability and provider experience with the medication, only 3 studies used 5-FU (Ghonemy et al, 2020; Hodeib et al, 2021; Yazdanfar et al, 2008). 5-FU given as 4 ml of 50 mg/ml solution mixed with 1 ml of 20 mg/ml lidocaine and 0.0125 mg/ml epinephrine every week for up to 4 sessions was superior to saline control (P < .05) (Yazdanfar et al, 2008). Notably, all 3 studies including 5-FU reported injection-site pain as a major adverse effect, explaining why it was combined with lidocaine in 1 study (Yazdanfar et al, 2008). Hodeib et al (2021) compared bleomycin, 5-FU, and Candida and found bleomycin to have a higher complete response rate than Candida (P = .001) and 5-FU (P = .009). The only RCT regarding HPV vaccine compared HPV given intralesionally as 0.1–0.3 ml until blanching every 2 weeks for up to 6 sessions with a 3 dose series of 0.5 ml intramuscular HPV and found no significant difference in response (P = .287) (Nofal et al, 2020a).
Combination therapies, including intralesional therapy, are emerging options, and 4 RCTs describing combination therapies were identified in this study. Intralesional Candida combined with oral isotretinoin had a 39% complete response rate compared with 44% in oral isotretinoin alone and 56% with Candida. Candida alone was statistically superior to the other therapies (Nofal et al, 2022c). Two studies trialed intralesional PPD in the largest wart compared with cryotherapy for all warts plus intralesional PPD in the largest wart and found no significant difference in response rates. However, both found a shortened time to clearance in the combination group (Awad et al, 2023; Ibrahim et al, 2022). A combination of IFN alfa-2b with pulsed dye laser had a statistically significant higher clearance rate than either therapy alone (Choi et al, 2002). Finally, adding IFN alfa-2b to intralesional Mumps, Candida, or Trichophyton antigens did not improve response compared with injection of antigens alone (Horn et al, 2005).
Intralesional immunotherapeutic agents work by introducing an antigen to promote T-cell–mediated immunity nonspecifically against HPV, resulting in clearance of HPV at local and distant sites (Awad et al, 2023; Ju et al, 2022). More specifically, these therapies result in (i) delayed hypersensitivity reactions against HPV, (ii) trauma induced by injection, and (iii) proliferation of peripheral mononuclear cells that then promote T helper 1 (Th1) cell cytokine responses (Abd-Elazeim et al, 2014). Some more specific mechanisms of commonly studied immunotherapies are defined. For example, MMR stimulates the Th1 response to increase IL-2, IL-4, IL-5, and TNF-α (Mohta et al, 2021). HPV vaccine may promote clearance of warts in part owing to overlap between the vaccine targets and the HPV strain type(s) causing the warts. In addition, evidence suggests that the HPV vaccine promotes immunity to a capsid antigen common among most HPV strains (Nofal et al, 2020a). PPD is nonspecifically immunogenic, similar to MMR, and is also known to increase Th1 signaling and cytokine levels (Abd-Elazeim et al, 2014). Candida’s mechanism of promoting wart clearance is less studied but likely related to immunogenicity similar to those of PPD and vaccines. Vitamin D is effective owing to its antiviral effects, such as activation of toll-like receptors and release of reactive oxygen species, and by regulation of epidermal proliferation and differentiation, such as promotion of keratinocyte apoptosis (Mohta et al, 2021; Shaldoum et al, 2020).
Although AEs were frequently reported, almost all were mild and transient. No RCTs recommended against future use of a therapy owing to side effects. A few RCTs utilized unique strategies to mitigate side effects, such as combining the intralesional injection with lidocaine and epinephrine or giving antipyretics after for symptomatic relief. The side effects seen were often related to injection-site reactions that can occur with any injectable therapy, including pain at the injection site, swelling, and redness. Notably, Dhar et al (2009) describe that pain was shorter in their bleomycin group than in their cryotherapy group, which tended to report pain for hours after treatment. Intralesional normal saline resulted in a complete response in some patients, which Agrawal et al (2018) propose can be explained by the trauma itself activating a strong, nonspecific immune response, including macrophages, T helper cells, neutrophils, and natural killer cells. The injection trauma and administration of fluid as well as the local immune response to the specific immunotherapy injected may also explain the formation of bullae/vesicles and desquamation. Hyperpigmentation and hypopigmentation are caused by local inflammatory reactions resulting in postinflammatory dyspigmentation. Dyspigmentation was more extreme in cryotherapy-treated patients than in intralesional bleomycin–treated patients in Dhar et al (2009)’s study. Flu-like illness, possibly the most adverse systemic effect, is possibly due to release of antigens into circulation and the resulting immune response (ElGhareeb, 2019).
Conclusion
Warts are very common, with various treatment strategies employed in the clinic, including destructive modalities, immunotherapy, and intralesional approaches such as those reviewed in this paper. Although over 500 articles pertaining to intralesional therapy of warts were identified, less than 10% were RCTs. Even the most common treatment studied, MMR, had less than 1000 patients across all trials. Response rates and treatment regimens of the most common therapies varied significantly. Importantly, although warts are most common in children, few studies focused on a pediatric population. This study identified the need for multi-institutional RCTs with standardized treatment regimens in children and adults.
Data availability statement
The dataset created during this systematic review and the search strategy used can be found at https://doi.org/10.17632/9766x3dcsf.1 hosted at Mendeley Data. Search this entire citation to access: Mullen et al (2023) “Systematic Review of Intralesional Therapies for Cutaneous Warts,” Mendeley Data, V1, https://doi.org/10.17632/9766x3dcsf.1.
ORCIDs
Sarah A. Mullen: http://orcid.org/0000-0002-7598-0985
Emma L. Myers: http://orcid.org/0000-0002-1257-0022
Rebecca L. Brenner: http://orcid.org/0009-0009-8379-0231
Kim T. Nguyen: http://orcid.org/0009-0001-8034-2910
Tara A. Harper: http://orcid.org/0009-0005-9886-6770
Darby Welsh: http://orcid.org/0009-0004-2635-9342
Storm Keffer: http://orcid.org/0009-0001-5920-9576
Jenna Mueller: http://orcid.org/0000-0001-5198-849
Melodi Javid Whitley: http://orcid.org/0000-0002-8163-6881
Conflict of interest
MJW and JM have filed a prepatent application for an intralesional wart therapy device. JM works with Calla Health Foundation from which she may benefit financially. The other authors declare no conflicts.
Acknowledgments
Author Contributions
Conceptualization: Methodology: MJW, SAM, RLB; Data Curation: SAM, ELM, KTN, TAH, DW, RLB; Formal Analysis: SK; Project Administration: SAM; Software: SK; Supervision: MJW, JM; Validation: SAM; Visualization: SAM, ELM, MJW; Writing – Original Draft Preparation: SAM, ELM, MJW; Writing – Review and Editing: SAM, ELM, KTN, MJW, JM
corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2024.100264
References
- Abd El-Magiud E.M., Abd El-Samea G.M., Gaber H.D. Intralesional injection of measles, mumps, and rubella vaccine versus cryotherapy in treatment of warts: a randomized controlled trial. Dermatol Ther. 2020;33 doi: 10.1111/dth.13257. [DOI] [PubMed] [Google Scholar]
- Abd-Elazeim F.M., Mohammed G.F.A., Fathy A., Mohamed R.W. Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts, treated by intralesional tuberculin antigen. J Dermatolog Treat. 2014;25:264–267. doi: 10.3109/09546634.2013.768760. [DOI] [PubMed] [Google Scholar]
- Adalatkhah H., Khalilollahi H., Amini N., Sadeghi-Bazargani H. Compared therapeutic efficacy between intralesional bleomycin and cryotherapy for common warts: a randomized clinical trial. Dermatol Online J. 2007;13:4. [PubMed] [Google Scholar]
- Agrawal C., Vyas K., Mittal A., Khare A.K., Gupta L.K. A Randomized double Blind Controlled Study Comparing the Efficacy of intralesional MMR Vaccine with Normal Saline in the Treatment of cutaneous Warts. Indian Dermatol Online J. 2018;9:389–393. doi: 10.4103/idoj.IDOJ_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Bhadbhade S.P., Noojibail B., Shetty S.M., Varghese A. Comparative study in efficacy and safety of intralesional injections of vitamin D3, measles rubella (MR) vaccine, and purified protein derivative (PPD) in the management of cutaneous warts. J Cutan Aesthet Surg. 2020;13:326–332. doi: 10.4103/JCAS.JCAS_39_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Aboud A.M., Nigam P.K. StatPearls Publishing; 2023. Wart. Treasure Island (FL) [PubMed] [Google Scholar]
- Aldahan A.S., Mlacker S., Shah V.V., Kamath P., Alsaidan M., Samarkandy S., et al. Efficacy of intralesional immunotherapy for the treatment of warts: a review of the literature. Dermatol Ther. 2016;29:197–207. doi: 10.1111/dth.12352. [DOI] [PubMed] [Google Scholar]
- Amin U., Fatima H., Tahir K., Iqbal S., Saleem A.A., Asghar A. Comparison of efficacy of liquid nitrogen and vitamin D3 in treatment of warts on hands and feet. J Pak Assoc Dermatol. 2021;31:184–190. [Google Scholar]
- Amirnia M., Khodaeiani E., Fouladi D.F., Masoudnia S. Intralesional immunotherapy with tuberculin purified protein derivative (PPD) in recalcitrant wart: A randomized, placebo-controlled, double-blind clinical trial including an extra group of candidates for cryotherapy. J Dermatolog Treat. 2016;27:173–178. doi: 10.3109/09546634.2015.1078871. [DOI] [PubMed] [Google Scholar]
- Awad S.M., Gomaa A.S., Hassan H.A., Tawfik Y.M. Efficacy of cryotherapy combined with intralesional purified protein derivative (PPD) versus intralesional PPD monotherapy in the treatment of multiple common warts. J Cutan Med Surg. 2023;27:117–125. doi: 10.1177/12034754231152224. [DOI] [PubMed] [Google Scholar]
- Awal G., Kaur S. Therapeutic outcome of intralesional immunotherapy in cutaneous warts using the mumps, measles, and rubella vaccine: a randomized, placebo-controlled trial. J Clin Aesthet Dermatol. 2018;11:15–20. [PMC free article] [PubMed] [Google Scholar]
- Baaniya D.B., Shah N., Marahatta S. 2021. A comparative study to assess efficacy of intralesional MMR (measles, mumps, rubella) vaccine and intralesional vitamin D3.https://classic.clinicaltrials.gov/ProvidedDocs/59/NCT04428359/Prot_SAP_ICF_000 (accessed February 29, 2024) [Google Scholar]
- Barkat M.T., Abdel-Aziz R.T.A., Mohamed M.S. Evaluation of intralesional injection of bleomycin in the treatment of plantar warts: clinical and dermoscopic evaluation. Int J Dermatol. 2018;57:1533–1537. doi: 10.1111/ijd.14092. [DOI] [PubMed] [Google Scholar]
- Bhalala K.B., Poojary S., Shah K.S. Comparative study of efficacy of intralesional purified protein derivative (PPD) versus intralesional measles, mumps, and rubella (MMR) vaccine in management of multiple viral warts. J Cutan Aesthet Surg. 2021;14:397–403. doi: 10.4103/JCAS.JCAS_166_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G.S., Park J.H., Kim Y.K., Choi G.S. Combination therapy with intralesional interferon α-2b and pulsed dye laser for the treatment of periungual warts. Ann Dermatol. 2002;14:82–87. [Google Scholar]
- Dhakar A.K., Dogra S., Vinay K., Sarangal R., Kanwar A.J., Singh M.P. Intralesional mycobacterium w vaccine versus cryotherapy in treatment of refractory extragenital warts: A randomized, open-label, comparative study. J Cutan Med Surg. 2016;20:123–129. doi: 10.1177/1203475415616962. [DOI] [PubMed] [Google Scholar]
- Dhar S.B., Rashid M.M., Islam A., Bhuiyan M. Intralesional bleomycin in the treatment of cutaneous warts: a randomized clinical trial comparing it with cryotherapy. Indian J Dermatol Venereol Leprol. 2009;75:262–267. doi: 10.4103/0378-6323.48428. [DOI] [PubMed] [Google Scholar]
- Ebrahim H.M., Asaad A.M., El desoky F., Morsi H.M. Bacillus Calmette-Guerin polysaccharide nucleic acid vs bacillus Calmette-Guerin vaccine in the treatment of warts: A comparative, double-blind, controlled study. Dermatol Ther. 2021;34 doi: 10.1111/dth.14549. [DOI] [PubMed] [Google Scholar]
- El Sayed M.H., Sayed F.S., Afify A.A. Intralesional zinc sulfate 2% vs intralesional vitamin D in plantar warts: a clinicodermoscopic study. Dermatol Ther. 2020;33 doi: 10.1111/dth.13308. [DOI] [PubMed] [Google Scholar]
- Eldahshan R.M., Ashry W.M.O., Elsaie M.L. Comparative study between intralesional injection of MMR, BCG, and candida albicans antigen in treatment of multiple recalcitrant warts. J Cosmet Dermatol. 2022;21:1120–1126. doi: 10.1111/jocd.14737. [DOI] [PubMed] [Google Scholar]
- ElGhareeb M.I. Comparative study of autoimplantation therapy and intralesional injection of MMR vaccine in warts treatment. Dermatol Ther. 2019;32 doi: 10.1111/dth.13135. [DOI] [PubMed] [Google Scholar]
- Elsayed Ghaly N., El-Ashmawy A.A., Abou Zeid M., E Shaker E.S. Efficacy and safety of intralesional injection of vitamin D3 versus tuberculin PPD in the treatment of plantar warts: A comparative controlled study. J Cosmet Dermatol. 2021;20:1231–1240. doi: 10.1111/jocd.13712. [DOI] [PubMed] [Google Scholar]
- Fathy G., Sharara M.A., Khafagy A.H. Intralesional vitamin D3 versus Candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts: A comparative case-control study. Dermatol Ther. 2019;32 doi: 10.1111/dth.12997. [DOI] [PubMed] [Google Scholar]
- Fawzy M.M., Nofal A., Alakad R. Intralesional antigen immunotherapy for the treatment of plane warts: a comparative study. Dermatol Ther. 2020;33 doi: 10.1111/dth.13807. [DOI] [PubMed] [Google Scholar]
- Fields J.R., Saikaly S.K., Schoch J.J. Intralesional immunotherapy for pediatric warts: a review. Pediatr Dermatol. 2020;37:265–271. doi: 10.1111/pde.14094. [DOI] [PubMed] [Google Scholar]
- Ghonemy S., Ibrahim Ali M., Ebrahim H.M. The efficacy of microneedling alone vs its combination with 5-fluorouracil solution vs 5-fluorouracil intralesional injection in the treatment of plantar warts. Dermatol Ther. 2020;33 doi: 10.1111/dth.14179. [DOI] [PubMed] [Google Scholar]
- Hay R.J., Johns N.E., Williams H.C., Bolliger I.W., Dellavalle R.P., Margolis D.J., et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- Hodeib A.A.E., Al-Sharkawy B.G., Hegab D.S., Talaat R.A.Z. A comparative study of intralesional injection of Candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J Dermatolog Treat. 2021;32:663–668. doi: 10.1080/09546634.2019.1688236. [DOI] [PubMed] [Google Scholar]
- Horn T.D., Johnson S.M., Helm R.M., Roberson P.K. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589–594. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- Ibrahim H., El Taieb M., Nada E., Kamal E., Hegazy E. Combined intralesional injection of tuberculin purified protein derivative plus cryotherapy versus each alone in the treatment of multiple common warts. Dermatol Ther. 2022;35 doi: 10.1111/dth.15350. [DOI] [PubMed] [Google Scholar]
- Ibrahim N.A., Abdel Fadeel D.A., Sadek A., Fadel M., Tawfik A. Intralesional vitamin D3 versus new topical photodynamic therapy in recalcitrant palmoplanter warts Randomized comparative controlled study. Photodiagnosis Photodyn Ther. 2020;32 doi: 10.1016/j.pdpdt.2020.101979. [DOI] [PubMed] [Google Scholar]
- Jain S., Marfatia Y.S. A comparative study of intralesional vitamin D3, measles mumps rubella vaccine, and tuberculin purified protein derivative in the treatment of recalcitrant warts: an approach to solve a therapeutic conundrum. J Clin Aesthet Dermatol. 2021;14:26–34. [PMC free article] [PubMed] [Google Scholar]
- James W.D., Elston D., Berger T. Elsevier Health Sciences; Amsterdam, The Netherlands: 2011. Andrew’s diseases of the skin e-book: clinical dermatology. [Google Scholar]
- Jartarkar S., Kadnur M., Mamatha P., Mishra S., Spoorthy B. A comparative study of therapeutic efficacy of intralesional measles, mumps, and rubella vaccine and intralesional vitamin D3 in the treatment of recurrent warts. J Dermatol Dermatol Surg. 2021;25:14–17. [Google Scholar]
- Ju H.J., Park H.R., Kim J.Y., Kim G.M., Bae J.M., Lee J.H. Intralesional immunotherapy for non-genital warts: a systematic review and meta-analysis. Indian J Dermatol Venereol Leprol. 2022;88:724–737. doi: 10.25259/IJDVL_1369_20. [DOI] [PubMed] [Google Scholar]
- Kareem I.M.A., Ibrahim I.M., Mohammed S.F.F., Ahmed A.A.B. Effectiveness of intralesional vitamin D 3 injection in the treatment of common warts: single-blinded placebo-controlled study. Dermatol Ther. 2019;32 doi: 10.1111/dth.12882. [DOI] [PubMed] [Google Scholar]
- Kaur A., Brar B.K., Kumar S., Brar S.K., Boparai A.S., Puri N. A randomized comparative study of MIP and MMR vaccine for the treatment of cutaneous warts. Indian J Dermatol. 2021;66:151–158. doi: 10.4103/ijd.IJD_700_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khozeimeh F., Jabbari Azad F., Mahboubi Oskouei Y., Jafari M., Tehranian S., Alizadehsani R., et al. Intralesional immunotherapy compared to cryotherapy in the treatment of warts. Int J Dermatol. 2017;56:474–478. doi: 10.1111/ijd.13535. [DOI] [PubMed] [Google Scholar]
- Khurshid K., Pal S.S. Role of Candida antigen in treatment of viral warts: A placebo-controlled study. J Pak Assoc Dermatol. 2009;19:146–150. [Google Scholar]
- Loo S.K.-F., Tang W.Y. Warts (non-genital) BMJ Clin Evid. 2014;2014:1710. [PMC free article] [PubMed] [Google Scholar]
- Martin A., Thatiparthi A., Nourmohammadi N., Nguyen C., Sung C., Atanaskova Mesinkovska N. Emerging intralesional treatments for plantar warts: a systematic review. J Drugs Dermatol. 2022;21:1322–1329. doi: 10.36849/JDD.6735. [DOI] [PubMed] [Google Scholar]
- Mattoo A., Bhatia M. Verruca vulgaris of the buccal mucosa: a case report. J Cancer Res Ther. 2018;14:454–456. doi: 10.4103/jcrt.JCRT_47_17. [DOI] [PubMed] [Google Scholar]
- Mehta K.S., Chandel M., Chauhan P.S., Mahajan V.K., Verma Y., Sharma H.K., et al. A study to evaluate the efficacy and safety of intradermal and intralesional purified protein derivative (PPD) for treatment of common warts in children. IP Indian J Clin Exp Dermatol. 2021;7:296–310. [Google Scholar]
- Milante R.R., Venida-Tablizo A., King-Ismael D. Efficacy and safety of single versus multiple intralesional immunotherapy with purified protein derivative (PPD) in the treatment of multiple verruca vulgaris. Int J Dermatol. 2019;58:1477–1482. doi: 10.1111/ijd.14652. [DOI] [PubMed] [Google Scholar]
- Mohammed Y.F., Ibrahim H.S., Elbarbary M.A., Elsaie M.L. Comparative study of intralesional tuberculin protein purified derivative (PPD) and intralesional measles, mumps, rubella (MMR) vaccine for multiple resistant warts. J Cosmet Dermatol. 2021;20:868–874. doi: 10.1111/jocd.13634. [DOI] [PubMed] [Google Scholar]
- Mohta A., Jain S.K., Mehta R.D., Arora A. Intralesional purified protein derivative of tuberculin versus intralesional mycobacterium W vaccine in treatment of recalcitrant extragenital warts: A randomized, single-blinded, comparative study. Indian J Dermatol. 2022;67:26–30. doi: 10.4103/ijd.ijd_521_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohta A., Kushwaha R.K., Agrawal A., Sharma M.K., Gautam U., Jain S.K. Evaluation of the efficacy of intralesional measles, mumps, and rubella vaccine with intralesional vitamin D3 as immunotherapies in the treatment of recalcitrant cutaneous warts in adult- a randomized placebo-controlled study. Indian Dermatol Online J. 2021;12:879–887. doi: 10.4103/idoj.IDOJ_573_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad J., Akhtar N., Ahmad M.K., Anwar M.I. Comparison of efficacy between intralesional bleomycin and cryotherapy in plantar warts. J Pak Assoc Dermatol. 2019;29:35–39. [Google Scholar]
- Muršić I., Včev A., Kotrulja L., Kuric I., Milavić T., Šustić N., et al. Treatment of verruca vulgaris in traditional medicine. Acta Clin Croat. 2020;59:745–750. doi: 10.20471/acc.2020.59.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar A., Nofal A., Bakr N.M., Essam R., Alakad R. Correlation of serum interleukin 17 and macrophage migration inhibitory factor levels with clinical response to intralesional Candida antigen and their potential use as predictors of clinical outcome in patients with multiple common warts. J Cosmet Dermatol. 2022;21:3970–3978. doi: 10.1111/jocd.14688. [DOI] [PubMed] [Google Scholar]
- Niimura M. Application of beta-interferon in virus-induced papillomas. J Invest Dermatol. 1990;95:149S–151S. doi: 10.1111/1523-1747.ep12875129. [DOI] [PubMed] [Google Scholar]
- Nofal A., Adel L., Fawzy M., Elkholy B.M. Intralesional immunotherapy for multiple recalcitrant plantar warts: candida antigen is superior to intralesional purified protein derivative. Dermatol Ther. 2022;35 doi: 10.1111/dth.15440. [DOI] [PubMed] [Google Scholar]
- Nofal A., Alakad R., Fouda I., Fawzy M.M. Intralesional antigen immunotherapy in the treatment of periungual warts. J Cutan Med Surg. 2021;25:286–292. doi: 10.1177/1203475420988859. [DOI] [PubMed] [Google Scholar]
- Nofal A., Albalat W., Ismail A., Khattab F.M. Immunotherapeutic modalities for the treatment of recalcitrant plantar warts: a comparative study. J Dermatolog Treat. 2022;33:922–927. doi: 10.1080/09546634.2020.1789540. [DOI] [PubMed] [Google Scholar]
- Nofal A., El-Arab R.E., Nasr M., Alakad R. Intralesional measles, mumps, and rubella vaccine versus intralesional candida antigen in the treatment of common and plantar warts. J Cutan Med Surg. 2021;25:377–383. doi: 10.1177/1203475421991130. [DOI] [PubMed] [Google Scholar]
- Nofal A., Khedr A., Fathy M. Combined oral isotretinoin and Candida antigen versus either agent alone in the treatment of plane warts. J Dermatolog Treat. 2022;33:342–347. doi: 10.1080/09546634.2020.1754325. [DOI] [PubMed] [Google Scholar]
- Nofal A., Marei A., Ibrahim A.-S.M., Nofal E., Nabil M. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94–100. doi: 10.1016/j.jaad.2019.07.070. [DOI] [PubMed] [Google Scholar]
- Nofal A., Nofal E. Intralesional immunotherapy of common warts: successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166–1170. doi: 10.1111/j.1468-3083.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- Nofal A., Yehia E., Khater E., Bessar H. Alternating intralesional purified protein derivative and Candida antigen versus either agent alone in the treatment of multiple common warts. J Am Acad Dermatol. 2020;83:208–210. doi: 10.1016/j.jaad.2020.01.054. [DOI] [PubMed] [Google Scholar]
- Oxford Centre for Evidence-Based Medicine . 2009. Levels of evidence.https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009; [Google Scholar]
- Rageh R.M., Hewedy E.-S.S., Hegab D.S. Intralesional injection of Candida albicans antigen versus measles, mumps, and rubella vaccine for treatment of plantar warts. Acta Dermatovenerol Alp Pannonica Adriat. 2021;30:1–5. [PubMed] [Google Scholar]
- Rajegowda H.M., Kalegowda D., Madegowda S.B., Palanayak J.K. Intralesional measles, mumps, and rubella vaccine versus cryotherapy in treatment of warts: a prospective study. J Dermatol Dermatol Surg. 2020;24:110–115. [Google Scholar]
- Rezai M.S., Ghasempouri H., Asqary Marzidareh O., Yazdani Cherati J., Rahmatpour Rokni G. Intralesional injection of the measles-mumps-rubella vaccine into resistant palmoplantar warts: A randomized controlled trial. Iran J Med Sci. 2019;44:10–17. [PMC free article] [PubMed] [Google Scholar]
- Rutnin S., Namasondhi A., Pomsoong C., Kositkuljorn C., Anuntrangsee T., Thadanipon K. Intralesional measles, mumps, rubella vaccine versus tuberculin purified protein derivative injections in the treatment of palmoplantar and periungual warts: a double-blind randomized controlled trial. Dermatology. 2023;239:109–115. doi: 10.1159/000526601. [DOI] [PubMed] [Google Scholar]
- Salman S., Ahmed M.S., Ibrahim A.M., Mattar O.M., El-Shirbiny H., Sarsik S., et al. Intralesional immunotherapy for the treatment of warts: A network meta-analysis. J Am Acad Dermatol. 2019;80:922–930.e4. doi: 10.1016/j.jaad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Sarin R.C., Dfwan S.P. Injection treatment of verrucae. Indian J of Dermatol, Venereol, and Leprology. 1974;40:60–62. [Google Scholar]
- Shaldoum D.R., Hassan G.F.R., El Maadawy E.H., El-Maghraby G.M. Comparative clinical study of the efficacy of intralesional MMR vaccine vs intralesional vitamin D injection in treatment of warts. J Cosmet Dermatol. 2020;19:2033–2040. doi: 10.1111/jocd.13272. [DOI] [PubMed] [Google Scholar]
- Soni P., Khandelwal K., Aara N., Ghiya B.C., Mehta R.D., Bumb R.A. Efficacy of intralesional bleomycin in palmo-plantar and periungual warts. J Cutan Aesthet Surg. 2011;4:188–191. doi: 10.4103/0974-2077.91250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Ahmad Kammal W.S.L., Jamil A., Md Nor N. Efficacy and safety of intralesional tuberculin purified protein derivative versus cryotherapy in the treatment of warts: an assessor-blinded, randomized controlled trial. Dermatol Ther. 2021;34 doi: 10.1111/dth.15080. [DOI] [PubMed] [Google Scholar]
- Yazdanfar A., Farshchian M., Fereydoonnejad M., Farshchian M. Treatment of common warts with an intralesional mixture of 5-fluorouracil, lidocaine, and epinephrine: a prospective placebo-controlled, double-blind randomized trial. Dermatol Surg. 2008;34:656–659. doi: 10.1111/j.1524-4725.2007.34123.x. [DOI] [PubMed] [Google Scholar]
- Zainab Z., Malik N.A., Malik S., Mashhood A., Obaid S., Aftab K., et al. Role of intralesional vitamin-D in viral Wartse. J Ayub Med Coll Abbottabad. 2021;33:598–601. [PubMed] [Google Scholar]
- Zamanian A., Mobasher P., Jazi G.A. Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomed Res. 2014;3:107. doi: 10.4103/2277-9175.129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset created during this systematic review and the search strategy used can be found at https://doi.org/10.17632/9766x3dcsf.1 hosted at Mendeley Data. Search this entire citation to access: Mullen et al (2023) “Systematic Review of Intralesional Therapies for Cutaneous Warts,” Mendeley Data, V1, https://doi.org/10.17632/9766x3dcsf.1.