Abstract
Background
The tumor microenvironment is an emerging biomarker of underlying genomic heterogeneity and response to immunotherapy-based treatment regimens in solid malignancies. How tumor mutational burden influences the density, distribution, and presence of a localized immune response in meningiomas is unknown.
Methods
Representative hematoxylin and eosin slides were reviewed at 40X to assess for the density of inflammatory cells. Lymphocytes and macrophages were quantified in the following ordinal manner: 0 = not present, 1 = 1–25 cells present, and 2 = greater than 26 cells present. Immune cell infiltrate grade was scored for both scattered and aggregated distributions. Next generation targeted sequencing was performed on all meningiomas included in this study.
Results
One hundred and forty-five meningiomas were evaluated in this study. Lymphocytes were observed in both scattered (95.9%) and aggregated (21.4%) distributions. A total of 115 (79.3%) meningiomas had 1–25 scattered lymphocytes, and 24 (16.6%) had > 25 scattered lymphocytes, and 6 (4.1%) had no scattered lymphocytes. Twenty (13.8%) meningiomas had 1–25 aggregated lymphocytes. Eleven (7.6%) had > 25 aggregated lymphocytes and 114 (78.6%) had no aggregated lymphocytes. Six (4.1%) meningiomas had 1–25 aggregated macrophages, 5 (3.4%) had > 25 aggregated macrophages, and 134 (92.4%) had no aggregated macrophages. Density of aggregated lymphocytes and aggregated macrophages were associated with higher tumor grade, P = 0.0071 and P = 0.0068, respectively. Scattered lymphocyte density was not associated with meningioma grade. The presence of scattered lymphocytes was associated with increased tumor mutational burden. Meningiomas that did not have scattered lymphocytes had a mean number of single mutations of 2.3 ± 2.9, compared with meningiomas that had scattered lymphocytes, 6.9 ± 20.3, P = 0.03. NF2 mutations were identified in 59 (40.7%) meningiomas and were associated with increased density of scattered lymphocytes. NF2 mutations were seen in 0 (0%) meningiomas that did not have scattered lymphocytes, 46 (40.0%) meningiomas that had 1–25 scattered lymphocytes, and 13 (54.2%) meningiomas that had > 25 scattered lymphocytes, P = 0.046.
Conclusions
Our findings suggest that distribution of immune cell infiltration in meningiomas is associated with tumor mutational burden. NF2 mutational status was associated with an increasing density of scattered lymphocytes. As the role of immunotherapy in meningiomas continues to be elucidated with clinical trials that are currently underway, these results may serve as a novel biomarker of tumor mutational burden in meningiomas.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02671-z) contains supplementary material, which is available to authorized users.
Keywords: Meningioma, Immunotherapy, Sequencing, Inflammation, Biomarker
Introduction
Meningiomas are extra-axial brain tumors with an estimated prevalence of approximately 2–7.5 in 100,000 [1–3]. While the majority of meningiomas are WHO grade I and are classically associated with a benign outcome, 5–7% of meningiomas are atypical (WHO grade II), and 1–3% are classified as anaplastic (WHO grade III). A typical and anaplastic meningioma grades are associated with worse clinical courses with reported 10-year survival rates of 79% and 34.5%, respectively [4]. However, while patients with Grade I meningiomas typically experience favorable oncological outcomes compared with higher grade tumors, Grade I meningiomas recur for a subset of patients, warranting further examination of histopathological features of low-grade meningiomas that may be associated with recurrence [5]. Higher-grade meningiomas may also be treated with adjuvant radiotherapy depending upon neuropathological features of the tumor such as brain invasion. Emerging insight into the underlying genomic heterogeneity of meningiomas is valuable to develop novel treatment paradigms for lesions that portend dismal clinical sequela.
Accumulating evidence suggest that the immune microenvironment mediates tumorigenesis and cancer progression [6–8]. For example, macrophage infiltration has been reported in all three grades of meningiomas with higher grade tumors expressing more numerous macrophages [9]. Interestingly, M1 macrophages have shown to exhibit anti-tumoral effects with low M1:M2 ratios reported in higher grade and recurrent meningiomas [10]. To further understand immune cell microenvironment in meningiomas, we quantified pathological evidence of macrophage and lymphocyte infiltration and examined the association between underlying mutational status and inflammatory response in meningiomas. Further understanding of how mutational influences immune response may augment our understanding of the pathogenesis of high-grade meningiomas, as well as help guide the emerging role of immunotherapy in meningiomas.
Methods
Study cohort
This study was reviewed and approved by the human subjects institutional review board and complied with HIPAA (Health Insurance Portability and Accountability Act of 1996) guidelines. Informed consent was waived. We reviewed the electronic health record for patients diagnosed with a meningioma who underwent neurosurgical resection at our institution between the years of 1995 and 2017. We identified 145 patients with available archival formalin-fixed, paraffin-embedded (FFPE) tissue. A board-certified neuropathologist reviewed histopathological diagnosis, grade, and purity of each case according to 2016 WHO guidelines [11].
Next-generation targeted sequencing
We performed next-generation targeted sequencing in a large series of 145 meningiomas. DNA was extracted from representative FFPE tissue using Maxwell FFPE Plus DNA Purification Kit (Promega, Madison, WI). DNA libraries were generated from tissue using the AmpliSeq Oncomine Comprehensive research panel versions 2.0 and 3.0 (ThermoFisher Scientific, Waltham, MA) as described previously [12]. Sequencing data analysis was performed using Torrent Suite (versions 5.6.0. and 5.8.0.) (ThermoFisher Scientific, Waltham, MA) and Ion Reporter (versions 5.2, 5.6, and 5.8) (ThermoFisher Scientific, Waltham, MA).
Histopathologic analysis of immune infiltration
WHO grade was determined by review of at least five board certified neuropathologist. A single board-certified neuropathologist with over 20 years of experience performed the quantification of immune cells. Representative hematoxylin and eosin (H&E) slides were reviewed at 40X for the density of inflammatory cells. Lymphocytes and macrophages were quantified using an ordinal scale: 0 = not present, 1 = 1–25 cells, and 2 = greater than 26 cells (Fig. 1). Immune cell infiltrate grade was assessed for both scattered and aggregated distributions (Fig. 2).
Fig. 1.
H&E at 40X demonstrating density of scattered (arrowheads) and aggregate (circled) lymphocytes in meningioma
Fig. 2.
Bar graphs of a scattered lymphocyte, b aggregated lymphocyte, and c aggregated macrophage distributions binned by not present, 1–25, > 25. Values above bars are data counts
Statistical analysis
Statistical analysis performed using JMP Pro 14.2 (SAS Institute Inc., Cary, NC). Fisher’s exact test used to detect statistical differences in categorical variables; t test used for continuous variables. P ≤ 0.05 used for statistical significance.
Results
Clinical characteristics of 145 meningiomas included in Table 1. Data on a sub-set of these tumors have been previously reported; however, none of these studies included data on immune characterization [13–16]. We were limited to the content of our targeted sequencing panel and were not able to assess known meningioma driver genes, such as TRAF, KLF4, and POLR2A [17, 18]. The genes included in this study are shown in Supplementary Table 1.
Table 1.
Clinical characteristics
| Clinical characteristics | |
|---|---|
| Age | |
| Median (IQR) | 60.7 (49.0, 70.9) |
| Range | 20.3–95.7 |
| Sex (female) | 95 (65.5) |
| WHO grade | |
| I | 43 (29.7%) |
| II | 93 (64.1%) |
| III | 9 (6.2%) |
| Occurrence | |
| Primary | 77 (53.1%) |
| Recurrent | 68 (46.9%) |
| Mitotic count | |
| Median (IQR) | 1 (0, 5) |
| Ki-67 | |
| Median (IQR) | 20 (10, 30) |
Data in parenthesis represent percentage in column, unless otherwise indicated
Lymphocytes were observed in both scattered (95.9%) and aggregated (21.4%) distributions, Fig. 1. A total of 115 (79.3%) meningiomas had 1–25 scattered lymphocytes, whereas 24 (16.6%) had > 25 scattered lymphocytes, Fig. 2a. A total of 20 (13.8%) meningiomas had 1–25 aggregated lymphocytes, whereas 11 (7.6%) had > 25 aggregated lymphocytes, Fig. 2b. There were 77 cases that were primary and 68 cases that were recurrent (Table 1). There were no significant differences in scattered lymphocytes, aggregated lymphocytes, or aggregated macrophages between primary and recurrent tumors (P > 0.05, for all).
No meningioma specimens had scattered macrophages. A total of 6 (4.1%) meningiomas had 1–25 aggregated macrophages, whereas 5 (3.4%) had > 25 aggregated macrophages, Fig. 2c. Density of aggregated lymphocytes (P = 0.0071, Fisher’s exact) and aggregated macrophages (P = 0.0068, Fisher’s Exact) were significantly associated with higher grade meningiomas, Table 2. Scattered lymphocyte density was not associated with meningioma grade. An example of meningioma specimens exhibiting high focal aggregation of lymphocytes and macrophages is shown in Fig. 3.
Table 2.
Immune infiltration by meningioma grade
| Grade I | Grade II | Grade III | P value* | |
|---|---|---|---|---|
| Scattered lymphocyte | 0.6 | |||
| Not present | 2 (33.3) | 4 (66.7) | 0 (0) | |
| 1–25 | 32 (27.8) | 74 (64.4) | 9 (7.8) | |
| > 25 | 9 (37.5) | 15 (62.5) | 0 (0) | |
| Aggregated lymphocyte | 0.0071 | |||
| Not present | 38 (33.3) | 73 (64.0) | 3 (2.6) | |
| 1–25 | 3 (15.0) | 14 (70.0) | 3 (15.0) | |
| > 25 | 2 (18.2) | 6 (54.6) | 3 (27.3) | |
| Aggregated macrophage | 0.0068 | |||
| Not present | 42 (31.3) | 86 (64.2) | 6 (4.5) | |
| 1–25 | 1 (16.7) | 5 (83.3) | 0 (0) | |
| > 25 | 0 (0) | 2 (40.0) | 3 (60.0) | |
Bold values indicate statistical significance (P < 0.05)
Data in parenthesis represent percentage in row
*P value within row, Fisher’s exact test
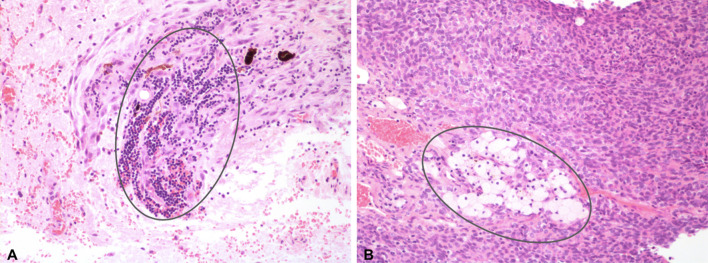
Fig. 3.
H&E at 40X demonstrating high aggregate lymphocyte (a) and macrophage (b) distributions (circled) seen in meningioma
The presence of scattered lymphocytes was associated with increased tumor mutational burden.
The mean number of single nucleotide variants was 6.7 ± 19.9 (range 0–192). Mean number of single nucleotide variants was 2.3 ± 2.9 for meningiomas that did not have scattered lymphocytes compared to 6.9 ± 20.3 for meningiomas that did have scattered lymphocytes (P = 0.03). Mean number of single nucleotide variants was 7.1 ± 20.7 for meningiomas that did not have aggregate macrophages present compared to 1.7 ± 1.2 for meningiomas that did have aggregate macrophages (P = 0.004).
A total of 59 (40.7) meningiomas harbored NF2 mutations. AKT1, SMO, and PIK3CA mutations were identified in 8 (5.6%), 4 (2.8%), and 4 (2.8%), respectively. NF2 mutation status was associated with increased density of scattered lymphocytes. NF2 mutations were seen in 0 (0%) meningiomas that did not have scattered lymphocytes, 46 (40.0%) meningiomas that had 1–25 scattered lymphocytes, and 13 (54.2%) meningiomas that had > 25 scattered lymphocytes (P = 0.046, Fisher’s exact). Among the 11 cases with aggregate macrophages, an NF2 mutation was seen in only 1 (9.1%) case (P = 0.03, Fisher’s exact). There was no significant association between scattered lymphocytes, aggregated lymphocytes, or aggregated macrophages and AKT1, SMO, and PIK3CA mutations status.
Discussion
Meningiomas are extra-axial tumors that reside outside of the blood–brain barrier. Systemic immune regulators can infiltrate these tumors [19]. While there has been considerable interest in studying immune-mediated processes to guide immunotherapies in other brain tumors such as gliomas [19–21], there is also a growing interest in assessing the immune system response to meningiomas and multiple immunotherapies trials are currently underway [19, 22]. Further understanding the complex association between immune infiltration and genomic mutations may help to increase our understanding of the complex role of inflammation in the tumor microenvironment as well as guide emerging immunotherapies for treating meningiomas.
Patterns of immune infiltrates in meningioma have been qualitatively described. They include a variety of cells including B and T lymphocytes, macrophages, plasma cells, and mast cells. Macrophages are found in all meningioma grades; however, they tend to have greater density in grades II and III lesions [9]. Grund et al. found that higher grade meningiomas with invasion of the pial–glial basement membrane exhibited greater degrees of microglial cells that expressed CD14 or CD163 [23]. These results support our finding of increased aggregated macrophages in higher grade meningiomas in the present study.
Similarly, CD4 + and CD8 + T lymphocytes are found in both low- and high-grade meningiomas, and tend to exhibit greater density in atypical and anaplastic meningiomas [9, 24]. Interestingly, increased presence of infiltrating CD8 + T cells has been shown to correlate with increased survival in meningiomas, as well as breast and lung cancer [25–27]. Higher PD-L1 + /CD68 − expression has been shown to be associated with reduced overall survival. Increased expression of CD68 −, PD-L1 + cells is correlated with meningioma grade [28]. Elevated PD-L1 expression in high-grade meningiomas has been found in numerous studies, suggesting that immune checkpoint inhibitors may be useful in treating aggressive high-grade meningiomas [29–32].
Several studies have evaluated how systemic serum markers of inflammation mediate immune response against meningiomas. Comtesse et al. found that patients with meningiomas exhibited an elevated serum antibody level to numerous antigens compared with healthy individuals, supporting that meningioma do in fact elicit a systemic immune response. CD69, a surface marker of natural killer cells and lymphocyte activation, may be elevated in the serum of patients with meningiomas [33].
We modeled our immune cell infiltration quantification after the study by Keren et al., which described the impact of tumor infiltrating immune cells in cold (no infiltration), mixed (scattered immune cells), and compartmentalized (aggregated immune cells) on tumor behavior and prognosis in breast cancer [34]. Keren et al. demonstrated that the compartmentalized immune cell phenotype was associated with increased survival compared with the mixed phenotype [34]. Linking underlying genomic status with tumor immune microenvironment features may further our understanding of the complex interplay between inflammation and tumor progression and aide in patient stratification and developing immunotherapies in the future. The results from this study suggest that potentially clinically relevant information is provided by studying the spatial arrangement of immune infiltration in the context of genomic heterogeneity in meningiomas.
Our results suggest that meningiomas that harbor more mutations generate increased levels of neoantigens that result in a more marked immune response. Meningiomas with scattered lymphocyte infiltration had threefold mutational burden compared with meningiomas without lymphocyte infiltration. Indeed, meningiomas with high tumor mutational burden may display higher susceptibility to immunotherapies compared with tumors with low mutational burden. Prior studies have shown that tumors with greater tumor mutational burden tend to have higher immunogenicity, which leads to a more robust repertoire of neoantigens and confers susceptibility to immunotherapies [35, 36]. There are a few studies that have examined the association between tumor mutational burden and immune response in meningiomas [37]. These findings may be useful in the selection of clinical trial candidates for the application and development of immunotherapeutic strategies in high-grade meningiomas, as seen in gliomas [20, 38].
While the role of cancer immunotherapy continues to expand in the treatment of advanced cancers such as melanoma [39], lung cancer [40], and glioma [20, 21, 38], the role of immunotherapy in the treatment of meningiomas must still be elucidated. Interestingly, a recent case report documented an incidental 24% decrease in meningioma volume in a patient receiving nivolumab for concomitant stage IV lung adenocarcinoma, providing preliminary evidence for the efficacy of a single-agent monoclonal antibody targeting PD-1 in treating meningiomas [41].
Clinical trials aimed at determining the efficacy nivolumab (NCT03173950), pembrolizumab (NCT03279692), avelumab (NCT03267836), and nivolumab with and without ipilimumab in combination with multifraction stereotactic radiosurgery (NCT03604978) are currently underway in patients with meningiomas. These studies, as well as clinical trials that utilize other checkpoint inhibitors against targets such as cytotoxic T lymphocyte-associated protein 4 (CTLA4), will be important for determining the role of immune checkpoint inhibitors in patients with high-grade meningiomas.
Limitations and future directions
The main limitation of the present study was the inability to quantify specific immune cell populations beyond the distinction between lymphocytes and macrophages that was permitted by H&E stains. Meningiomas can be invaded by a wide variety of immune cells and future studies are required to perform advanced immunohistochemical staining to quantify specific cell types to correlate with molecular findings. In particular, CD4, CD8, CD25, and FOXP3 are important immune cell markers that have been described in distinct meningioma phenotypes that should be investigated in the context of genomic alterations [24, 31, 42, 43]. Immune cells expressing immune checkpoint molecules such as PD-1, CTLA-4, and Tim-3 should be examined in the context of genomic alterations. Moreover, prior studies have provided evidence for the prognostic significance of activity of immune cells at the tumor border; such focused evaluation ought to occur in meningiomas [34]. Given the lack of differentiation of immune cell sub-phenotypes using immunohistochemistry, the present study serves to provide preliminary data with regard to the correlation of macrophage and lymphocyte patterns with tumor mutational status. Future work will include immunohistochemical interrogation of specific immune cell markers to further elucidate the role of immune cell infiltration in meningiomas.
Given the nature of the surgical resections, it was difficult to determine the location that each histological specimen originated from within the tumor. Future work should focus on methods by which to document the location of histological specimens within the tumor such as deeper aspects versus sections at the brain–tumor interface.
Conclusion
There are limited treatment options for patients with meningiomas in the recurrent setting [44, 45]. Immunotherapy is currently being explored in clinical trials as an adjuvant modality for treating aggressive meningiomas that recur after neurosurgical resection. However, there is an unmet need to sufficiently characterize the association between genomic heterogeneity and immune response in meningiomas. The present study revealed that both tumor mutational burden and NF2 mutation status are correlated with density of lymphocytic infiltration. These findings may serve as a novel biomarker to guide the emerging role of immunotherapy in high-grade meningiomas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
John Rutland was supported by the Rare diseases foundation and the BC Children’s Hospital Foundation.
Author contributions
JWR—manuscript drafting, data collection, critical revision, reviewed final manuscript, and submitted on behalf of the authors; CMG—manuscript drafting, statistical analysis, and reviewed final manuscript; JL—data collection, data analysis, and reviewed final manuscript; HA—data analysis and reviewed final manuscript; MP—data analysis and reviewed final manuscript; MU—data analysis and reviewed final manuscript; YK—data analysis and reviewed final manuscript; RBM—data analysis and reviewed final manuscript; JB—data analysis and reviewed final manuscript; MD—data analysis and reviewed final manuscript; RS—reviewed final manuscript and data analysis; RKS—reviewed final manuscript, study supervision, and critical revision; MF—data collection, reviewed final manuscript, study supervision, and critical revision.
Funding
This study was internally funded by the Icahn School of Medicine at Mount Sinai.
Compliance with ethical standards
Conflicts of interest
Dr. Joshua Bederson (a Significant Contributor in this study and Chair of the Department of Neurosurgery) owns equity in Surgical Theater, LLC [manufacturer of the Surgical Navigation Advanced Platform (SNAP) system that may be used for intraoperative image guidance in the study]. The remaining authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical approval
This study was by the Icahn School of Medicine at Mount Sinai Institutional Review Board, and we certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
John W. Rutland and Corey M. Gill contributed equally to this work.
References
- 1.Hasseleid BF, Meling TR, Ronning P, Scheie D, Helseth E. Surgery for convexity meningioma: simpson grade i resection as the goal: clinical article. J Neurosurg. 2012;117:999–1006. doi: 10.3171/2012.9.Jns12294. [DOI] [PubMed] [Google Scholar]
- 2.Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.Jns131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surov A, Gottschling S, Bolz J, Kornhuber M, Alfieri A, Holzhausen HJ, Abbas J, Kosling S. Distant metastases in meningioma: an underestimated problem. J Neurooncol. 2013;112:323–327. doi: 10.1007/s11060-013-1074-x. [DOI] [PubMed] [Google Scholar]
- 4.Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86:793–800. doi: 10.3171/jns.1997.86.5.0793. [DOI] [PubMed] [Google Scholar]
- 5.Nanda A, Vannemreddy P. Recurrence and outcome in skull base meningiomas: do they differ from other intracranial meningiomas. Skull Base Off J North Am Skull Base Soc. 2008;18:243–252. doi: 10.1055/s-2007-1016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, Kim J. Genetic variation in PPARGC1A may affect the role of diet-associated inflammation in colorectal carcinogenesis. Oncotarget. 2017;8:8550–8558. doi: 10.18632/oncotarget.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhou Z, Zhang X, et al. Inflammatory Molecule, PSGL-1, deficiency activates macrophages to promote colorectal cancer growth through NFkappaB signaling. Mol Cancer Res MCR. 2017;15:467–477. doi: 10.1158/1541-7786.Mcr-16-0309. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Xu X. Mutation-promoting molecular networks of uncontrolled inflammation. Tumour Biol J Int Soc Oncodev Biol Med. 2017 doi: 10.1177/1010428317701310. [DOI] [PubMed] [Google Scholar]
- 9.Rossi ML, Cruz Sanchez F, Hughes JT, Esiri MM, Coakham HB. Immunocytochemical study of the cellular immune response in meningiomas. J Clin Pathol. 1988;41:314–319. doi: 10.1136/jcp.41.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor DT, Huang J, Lama S, Albakr A, Van Marle G, Sutherland GR. Tumor-associated macrophage infiltration in meningioma. Neuro-Oncol Adv. 2019 doi: 10.1093/noajnl/vdz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 12.Pain M, Wang H, Lee E, Strahl M, Hamou W, Sebra R, Zhu J, Yong RL. Treatment-associated TP53 DNA-binding domain missense mutations in the pathogenesis of secondary gliosarcoma. Oncotarget. 2018;9:2603–2621. doi: 10.18632/oncotarget.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill CM, Loewenstern J, Rutland JW, et al. STK11 mutation status is associated with decreased survival in meningiomas. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2020 doi: 10.1007/s10072-020-04372-y. [DOI] [PubMed] [Google Scholar]
- 14.Gill CM, Loewenstern J, Rutland JW, et al. Recurrent IDH mutations in high-grade meningioma. Neuro-oncology. 2020 doi: 10.1093/neuonc/noaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill CM, Loewenstern J, Rutland JW, et al. In reply: retention of ATRX and DAXX expression in meningiomas. Neurosurgery. 2020;86:E244–E246. doi: 10.1093/neuros/nyz504. [DOI] [PubMed] [Google Scholar]
- 16.Loewenstern J, Rutland J, Gill C, et al. Comparative genomic analysis of driver mutations in matched primary and recurrent meningiomas. Oncotarget. 2019;10:3506–3517. doi: 10.18632/oncotarget.26941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark VE, Harmancı AS, Bai H, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48:1253–1259. doi: 10.1038/ng.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Bi WL, Dunn IF. Medical management of meningioma in the era of precision medicine. Neurosurg Focus. 2018;44:E3. doi: 10.3171/2018.1.Focus17754. [DOI] [PubMed] [Google Scholar]
- 20.Johnson LA, Sampson JH. Immunotherapy approaches for malignant glioma from 2007 to 2009. Curr Neurol Neurosci Rep. 2010;10:259–266. doi: 10.1007/s11910-010-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.Ccr-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brastianos PK, Galanis E, Butowski N, et al. Advances in multidisciplinary therapy for meningiomas. Neuro-oncology. 2019;21:i18–i31. doi: 10.1093/neuonc/noy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grund S, Schittenhelm J, Roser F, Tatagiba M, Mawrin C, Kim YJ, Bornemann A. The microglial/macrophagic response at the tumour-brain border of invasive meningiomas. Neuropathol Appl Neurobiol. 2009;35:82–88. doi: 10.1111/j.1365-2990.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 24.Becker I, Roggendorf W. Immunohistological investigation of mononuclear cell infiltrates in meningiomas. Acta Neuropathol. 1989;79:211–216. doi: 10.1007/BF00294381. [DOI] [PubMed] [Google Scholar]
- 25.Rapp C, Dettling S, Liu F, et al. Cytotoxic T cells and their activation status are independent prognostic markers in meningiomas. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:5260–5270. doi: 10.1158/1078-0432.Ccr-19-0389. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Lang R, Zhao J, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 27.Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han SJ, Reis G, Kohanbash G, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130:543–552. doi: 10.1007/s11060-016-2256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YD, Veliceasa D, Lamano JB, Lamano JB, Kaur G, Biyashev D, Horbinski CM, Kruser TJ, Bloch O. Systemic and local immunosuppression in patients with high-grade meningiomas. Cancer Immunol Immunother CII. 2019;68:999–1009. doi: 10.1007/s00262-019-02342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giles AJ, Hao S, Padget M, et al. Efficient ADCC killing of meningioma by avelumab and a high-affinity natural killer cell line, haNK. JCI Insight. 2019 doi: 10.1172/jci.insight.130688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Z, Abedalthagafi M, Aizer AA, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6:4704–4716. doi: 10.18632/oncotarget.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han SJ, Reis G, Kohanbash G, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neuro-Oncology. 2016;130:543–552. doi: 10.1007/s11060-016-2256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erkan EP, Ströbel T, Dorfer C, Sonntagbauer M, Weinhäusel A, Saydam N, Saydam O. Circulating tumor biomarkers in meningiomas reveal a signature of equilibrium between tumor growth and immune modulation. Front Oncol. 2019 doi: 10.3389/fonc.2019.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keren L, Bosse M, Marquez D, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. 2018;174:1373–87.e19. doi: 10.1016/j.cell.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.Mct-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunn IF, Du Z, Touat M, et al. Mismatch repair deficiency in high-grade meningioma: a rare but recurrent event associated with dramatic immune activation and clinical response to PD-1 blockade. JCO Precis Oncol. 2018 doi: 10.1200/po.18.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGranahan T, Therkelsen KE, Ahmad S, Nagpal S. Current state of immunotherapy for treatment of glioblastoma. Curr Treat Option Oncol. 2019;20:24. doi: 10.1007/s11864-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelerstein E, Berger A, Jonas-Kimchi T, et al. Regression of intracranial meningioma following treatment with nivolumab: case report and review of the literature. J Clin Neurosci Off J Neurosurg Soc Australas. 2017;37:51–53. doi: 10.1016/j.jocn.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Ding Y, Qiu L, Xu Q, Song L, Yang S, Yang T. Relationships between tumor microenvironment and clinicopathological parameters in meningioma. Int J Clin Exp Pathol. 2014;7:6973–6979. [PMC free article] [PubMed] [Google Scholar]
- 43.Fang L, Lowther DE, Meizlish ML, et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro-oncology. 2013;15:1479–1490. doi: 10.1093/neuonc/not110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, Riley K. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3. doi: 10.3171/foc/2008/24/5/e3. [DOI] [PubMed] [Google Scholar]
- 45.Simon M, Bostrom J, Koch P, Schramm J. Interinstitutional variance of postoperative radiotherapy and follow up for meningiomas in Germany: impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry. 2006;77:767–773. doi: 10.1136/jnnp.2005.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.