Abstract
Background
Tissue tumor mutation burden (tTMB) assessed by whole-exome sequencing (WES), which has been regarded as the gold standard method of tTMB measurement, can predict the clinical benefits of immune checkpoint inhibitors (ICIs). Multiple studies have investigated the feasibility of utilizing large panels to evaluate TMB but have obtained conflicting results. Furthermore, whether blood TMB (bTMB) can also be a predictive biomarker in NSCLC has not been determined.
Methods
Fifty-six advanced NSCLC patients treated with ICIs were enrolled, including an exploratory cohort (n = 42) and a small independent validation cohort (n = 14). Next-generation sequencing was performed on tumor and plasma samples collected prior to ICI treatment using a panel consisting of 520 cancer-related genes (OncoScreen) to evaluate tTMB/bTMB. WES was also performed on tumor samples to serve as references.
Results
A positive correlation between tTMB derived from WES and OncoScreen was observed. OncoScreen-derived tTMB showed a positive correlation with OncoScreen-derived bTMB. Patients with OncoScreen-derived tTMB 7 mutations/Mb (p = 0.003) or bTMB 11 mutations/Mb (p = 0.0029) had superior progression-free survival (PFS). In the small validation cohort, patients with OncoScreen-derived bTMB 11 mutations/Mb exhibited longer PFS (p = 0.192) with a nonsignificant difference. In all 42 patients who had available bTMB and PFS, patients with bTMB 11 mutations/Mb had significantly longer PFS (p = 0.011) than those with bTMB 11 mutations/Mb.
Conclusion
Our study confirmed the feasibility of using large panels to estimate TMB. We also demonstrated that bTMB can serve as a potential biomarker for predicting the efficacy of ICIs in NSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02943-2.
Keywords: Non-small cell lung cancer, Tumor mutation burden, Immune-checkpoint inhibitors, Capture-based targeted sequencing
Introduction
Immune checkpoint inhibitors (ICIs) targeting the programmed cell death-1 (PD-1)-programmed cell death ligand-1 (PD-L1) axis elicit remarkable clinical efficacy in both treatment-naïve and previously treated advanced or metastatic non-small cell lung cancer (NSCLC) patients [1–5]. The National Comprehensive Cancer Network (NCCN) guidelines currently recommend ICI in combination with chemotherapy as first-line treatment for advanced or metastatic NSCLC patients negative for actionable driver mutations regardless of their PD-L1 expression status. ICI monotherapy is a standard first-line treatment for PD-L1-expressing ( 1%) advanced or metastatic NSCLC patients, and is the preferred first-line therapy for patients with high PD-L1 expression ( 50%). Moreover, ICI monotherapy is preferred for second or successive lines of treatment for advanced or metastatic NSCLC patients regardless of their PD-L1 expression status [6]. However, as only a fraction of patients respond to ICIs, there is strong interest in deriving biomarkers that predict ICI response. Several biomarkers have been reported to predict the efficacy of ICIs across a spectrum of malignancies, including but not limited to the status of microsatellite instability (MSI)/DNA mismatch repair (MMR), PD-L1 expression and tumor mutation burden (TMB). The predictive value of the status of MSI/MMR or PD-L1 expression in NSCLC is debatable. MSI-high (MSI-H)/MMR deficiency (dMMR) is a rare event (0.6%) in NSCLC; therefore, MSI status is not routinely tested [7]. Although PD-L1 expression is a widely accepted biomarker for predicting ICI benefit in NSCLC, only 15–31% of patients with positive PD-L1 expression benefit from ICI monotherapy. On the other hand, 10–24% of patients with negative PD-L1 expression also respond to ICI monotherapy [8–10]. The poor reliability of PD-L1 expression can be attributed to the following: 1) PD-L1 expression can be transient, and intrapatient or even intratumor heterogeneity in PD-L1 expression is possible; 2) and there is a lack of uniformity in thresholds used for defining PD-L1 positivity [11, 12].
TMB, the number of non-synonymous somatic mutations, is primarily measured from a tissue biopsy sample using WES and has emerged as a potential biomarker for predicting the response to ICIs. Elevated TMB increases the odds of generating immunogenic neoantigens, thereby inducing a response to ICIs [13]. The association between tissue TMB (tTMB) and the efficacy of ICIs was first observed in melanoma and NSCLC and subsequently in a number of other solid tumors. An exploratory analysis from the CA209-038 trial showed an association between high tTMB and greater overall survival in ipilimumab-naïve melanoma patients treated with nivolumab [14]. KEYNOTE-001 demonstrated that high tTMB was also associated with improved objective response rate (ORR) and progression-free survival (PFS) in NSCLC patients receiving pembrolizumab [15]. Furthermore, a meta-analysis spanning 27 cancer types confirmed the positive association [16]. Based on KEYNOTE-158, the U.S. Food and Drug Administration (FDA) has approved pembrolizumab as monotherapy for the treatment of adult and pediatric patients with unresectable or metastatic TMB-high ( 10 mutations/megabase [Mb]) solid tumors, as determined by an FDA-approved test [17]. However, there is no consensus on how to measure TMB. Due to the high cost, requirement of a large amount of input DNA and extensive data management involved in WES, a large panel-based targeted sequencing approach for evaluating tTMB is currently being explored as an alternative. Studies have shown that TMB assessed using the FoundationOne assay targeting 315 genes, spanning 1.1 Mb of the coding region with a mean coverage depth of more than 500 × , is highly correlated with tTMB assessed by WES across a spectrum of tumor types [18]. tTMB measured by Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) targeting the coding region of 468 genes (~ 1.2 Mb) with a mean coverage depth of 744 × is consistent with WES-derived tTMB in NSCLC [19].
Obtaining tissue biopsy from advanced or metastatic NSCLC patients can be challenging with potential complications. There are conflicting findings regarding whether blood TMB (bTMB) assessed by panel-based targeted sequencing has the potential to predict prognosis in NSCLC patients treated with ICIs [20, 21]. Most studies concluded that a high bTMB predicts superior clinical benefit and PFS in patients treated with PD-1/PD-L1 inhibitors [20–23]. However, a recent study demonstrated that high bTMB predicted shorter PFS and overall survival (OS) in PD-1/PD-L1 inhibitor-treated NSCLC patients [24].
It remains debatable whether bTMB derived from panel-based targeted sequencing could be used for predicting the clinical response to ICIs in advanced NSCLC patients. In the present study, we investigated the potential of bTMB measured by a panel consisting of 520 cancer-related genes in predicting the responses to ICIs in advanced NSCLC patients.
Materials and methods
Patient selection
Between Jan 2017 and Jun 2019, advanced or metastatic NSCLC patients (stage IIIB-IV) who met the following inclusion criteria were prospectively enrolled: (1) patients were between the age of 18–80; (2) patients were treated with anti-PD-1/PD-L1 agent as monotherapy; (3) patients had at least 1 measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria; (4) patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; (5) patients had a life expectancy of 3 months or more. Both treatment-naïve and previously treated patients were enrolled. Patients who had previously received anti-PD-1/PD-L1 agents were excluded. Both tumor tissue and blood samples were collected from each patient prior to anti-PD-1/PD-L1 therapy. Eight patients lacked tumor tissue samples, and 4 patients had no plasma samples. Thirty patients had matched tumor tissue and plasma samples. This study was approved by the Ethics Committee of the First Affiliated Hospital of College of Medicine of Zhejiang University. Informed consent was obtained from each patient for the use of their peripheral blood and tumor tissue samples.
The clinical outcomes of patients treated with anti-PD-1/PD-L1 therapy were evaluated according to RECIST 1.1 criteria [25]. Progression-free survival (PFS) was defined as the interval between the initiation of the treatment and the time of progressive disease (PD) or the last follow-up. Objective response rate (ORR) was defined as the percentage of patients with complete response (CR) or partial response (PR). Durable clinical benefit (DCB) was defined as CR/PR/stable disease (SD) lasting more than 6 months. No durable benefit (NDB) was defined as PD within 6 months of beginning therapy.
Tissue DNA extraction, plasma isolation, ctDNA extraction
Tissue DNA was extracted with a QIAamp DNA formalin-fixed paraffin-embedded (FFPE) tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Ten milliliters of whole blood was collected and centrifuged at 2,000 g for 10 min at 4℃. The supernatant was transferred to a new centrifuge tube and centrifuged at 16,000 g for 10 min at 4℃. The supernatant (prepared plasma) was transferred to a new tube and stored at -80℃ for further analysis. Circulating tumor DNA (ctDNA) was extracted from plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The concentrations of tissue DNA and ctDNA were measured by Qubit 2.0 Fluorometer with a Qubit double-stranded DNA assay kit (Life Technologies, Carlsbad, CA, USA).
DNA library construction
DNA was fragmented by a Covaris M220 focused ultrasonicator (Covaris, Inc., Woburn, MA, USA) followed by end repair, phosphorylation, dA addition and adaptor ligation for library construction. Next, the DNA library was purified by using Agencourt AMPure beads (Beckman Coulter, Fullerton, CA, USA). Samples with at least 50 ng of ctDNA were used for library construction.
Capture-based targeted sequencing and whole-exome sequencing
Capture-based targeted sequencing was performed on both tumor tissue and plasma samples using a panel (OncoScreen, Burning Rock Biotech, Guangzhou, China) consisting of 520 cancer-related genes (including whole exons of 312 genes and selected exons, introns, or promoter regions of the remaining 208 genes), spanning 1.7 Mb of human genome. The library of tumor tissue DNA from 22 patients was also enriched with the whole-exome panel (Agilent SureSelect All Human Exon V5 (50 M), Agilent, Santa Clara, CA, USA) as a reference. Libraries were sequenced on an Illumina NextSeq 500 (Illumina, Inc., San Diego, CA, USA) with paired-end reads.
Sequencing data analysis
The raw sequencing data were preprocessed by using Trimmomatic 0.36 for trimming adaptors, low-quality reads and reads less than 50 base pairs. Preprocessed sequencing data were mapped to the human genome (hg19) by using Burrows-Wheeler Aligner 0.7.10 and Genome Analysis Toolkit 3.2 (Broad Institute, Cambridge, MA). Variant calling was performed by using VarScan and variants were annotated with ANNOVAR and SnpEff v3.6. The allele frequency (AF) of mutations was calculated. Mutations were then filtered against common single-nucleotide polymorphisms (SNPs) found in 1000 Genomes ExAC, dbSNP, ESP6500SI-V2, and ClinVar databases.
Tumor mutation burden
TMB count was defined as the number of somatic single-nucleotide variants (SNVs) and small insertions and deletions (InDels) (copy number variants and fusions were excluded) located at the coding region and its 20 bp upstream/downstream region after the removal of known and probably oncogenic driver events (including EGFR and ALK) and germline SNPs [26]. Tissue samples with maximum mutation AF (maxAF) 5% and plasma samples with maxAF 0.5% were screened for TMB calculation. TMB was calculated according to the following equation:
TMB = .
PD-L1 expression
FFPE tissue from NSCLC tumors was evaluated for PD-L1 expression by immunohistochemistry staining, reported as the percentage of tumor cells with membranous PD-L1 staining. Specimens were stained with antibodies, including DAKO 22C3 (Agilent, Santa Clara, CA, USA), DAKO 28–8 (Agilent, Santa Clara, CA, USA), and Ventana SP263 (Roche, Indianapolis, IN, USA). Membranous expression of PD-L1 on tumor cells was scored semiquantitatively; 1% membranous staining was considered positive, and 1% was considered to be negative.
Statistical analysis
The correlations among WES-derived tTMB, OncoScreen-derived tTMB and OncoScreen-derived bTMB were described by the Spearman correlation coefficient. Differences between two-groups were assessed by a two-tailed, unpaired t-test for continuous variables with normal distribution, by the Mann–Whitney U test for continuous variables with non-normal distribution or by Fisher’s exact test for discontinuous variables. Receiver operating characteristic (ROC) curves were generated to derive the best cutoffs. The corresponding area under the curve (AUC) of the ROC curves was calculated. The cutoff value of the ROC curve was calculated [27]. For progression-free survival (PFS) analyses, Kaplan–Meier curves were compared by using the log-rank test. Univariate analysis and multivariate analysis with the Cox proportional hazards model were applied. The p-value was corrected for the false discovery rate (FDR). p-values 0.05 were considered to be significant. All statistical analyses were performed in R 3.3.3.
Results
Patient characteristics
From Jan 2017 to Jun 2019, 42 patients diagnosed with advanced or metastatic NSCLC and treated with an anti-PD-1/PD-L1 agent as monotherapy were prospectively enrolled in our study. Both tumor tissue and peripheral blood samples prior to anti-PD-1/PD-L1 treatment were collected from each patient. The median age of the enrolled patients, including 7 females and 35 males, was 61.5 years (ranging from 44 to 80 years). Twenty-nine patients had a history of smoking, and 13 were nonsmokers. Fourteen patients were diagnosed with adenocarcinoma (AD), and 28 patients were diagnosed with squamous cell carcinoma (SCC). PD-L1 expression was assessed in 32 patients, and 16 showed positive expression ( 1% expression). Most patients (95.2%, 40/42) had received platinum-doublet chemotherapy as a first-line treatment. Forty patients were treated with PD-1 inhibitors (including 22 with nivolumab, 13 with pembrolizumab and 5 with IBI308), and 2 patients were treated with a PD-L1 inhibitor (durvalumab).
The clinical characteristics of the exploratory cohort are summarized in Table 1. A schematic design of the study is shown in Figure S1. WES was also performed on 22 tumor samples to serve as references. The mean coverage depth for WES, targeted sequencing for tissue samples and targeted sequencing for plasma samples were 261 , 1,274 and 7,233 , respectively.
Table 1.
Clinical characteristics of 42 NSCLC patients enrolled in exploratory cohort
| Characteristic | n (%) |
|---|---|
| Median age (range), years | 61.5 (44–80) |
| Gender | |
| Female | 7 (16.7%) |
| Male | 35 (83.3%) |
| Histological subtype | |
| Adenocarcinoma | 14 (33.3%) |
| Squamous cell carcinoma | 28 (66.7%) |
| Smoking status | |
| Nonsmokers | 13 (31.0%) |
| Smokers | 29 (69.0%) |
| Number of prior lines of treatment | |
| 0 | 2 (4.8%) |
| 1 | 37 (88.1%) |
| ≥ 2 | 3 (7.1%) |
| Stage | |
| IIIB | 1 (2.4%) |
| IIIC | 8 (19.0%) |
| IVA | 19 (45.2%) |
| IVB | 14 (33.4%) |
| Immunotherapy | |
| anti-PD-1 | 40 (95.2%) |
| anti-PD-L1 | 2 (4.8%) |
| PD-L1 expression | |
| Positive | 16 (38.1%) |
| Negative | 16 (38.1%) |
| Unknown | 10 (23.8%) |
NSCLC non-small cell lung cancer PD-1 programmed cell death-1 PD-L1 programmed cell death ligand-1
Somatic mutational profiling in tumor and plasma samples
Somatic mutational profiles obtained from 30 patients with matched tumor tissue and plasma samples were compared. Collectively, 436 genetic alterations spanning 200 genes were identified in tumor tissue samples, including 315 SNVs, 7 InDels and 114 CNVs (including copy number amplification and copy number deletion). The most commonly mutated genes were TP53, CDKN2A, KRAS, LRP1B and SPTA1, which were altered in 70.0% (n = 21), 43.3% (n = 13), 33.3% (n = 10), 33.3% (n = 10) and 30.0% (n = 9) of patients, respectively (Figure S2A). There were 9 patients who had no mutations detected in their plasma samples. Collectively, 261 genetic alterations spanning 135 genes were identified from plasma samples, including 240 SNVs, 4 InDels and 17 CNVs. The most commonly mutated genes were TP53, CDKN2A, LRP1B and SPTA1, altering in 56.7% (n = 17), 26.7% (n = 8), 23.3% (n = 7) and 23.3% (n = 7) of patients, respectively (Figure S2B). The number of mutations in tissue samples and matched blood samples in each of the thirty patients is shown in Figure S2C. Among those mutations, 74 mutations (14.5%) were plasma-specific, 249 mutations (48.8%) were tissue-specific, and 187 mutations (36.7%) were shared between the two (Figure S2D).
Correlations between WES-derived and OncoScreen-derived tTMB
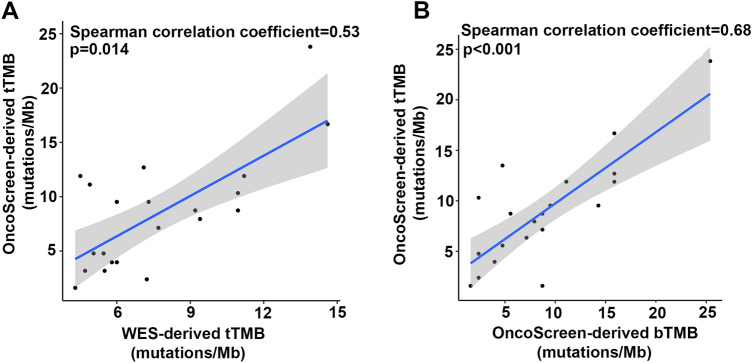
To determine whether TMB assessed by the OncoSreen could reliably reflect the TMB status, we compared TMB derived from WES and OncoScreen by performing WES and targeted sequencing on 22 tumor tissue samples. One tissue sample with maxAF 5% was excluded from further analyses. The average WES-derived tTMB and OncoScreen-derived tTMB were 7.70 mutations/Mb (ranging from 4.30 to 14.61) and 8.46 mutations/Mb (ranging from 1.59 to 23.81), respectively (Figure S3A-B). Our results showed that the 2 methods yielded comparable TMB (p = 0.94). In agreement with published data, our analysis revealed a positive correlation between the tTMB derived from both methods with a Spearman correlation coefficient of 0.53 and a p-value of 0.014 (Fig. 1a).
Fig. 1.
Correlation between tTMB and bTMB. a. Spearman correlation between WES-derived tTMB and OncoScreen-derived tTMB. b. Spearman correlation between tTMB and bTMB obtained by targeted sequencing
Correlation between OncoScreen-derived tTMB and bTMB
Next, we investigated whether OncoSreen-derived tTMB and bTMB were comparable. Twenty patients with qualified matched tumor and plasma samples were subjected to analysis. The average tTMB and bTMB were 8.73 mutations/Mb (ranging from 1.59 to 23.81) and 8.73 mutations/Mb (ranging from 1.59 to 25.40), respectively (Figure S4). TMB derived from tissue or plasma was comparable (p = 0.81). Furthermore, our analysis also showed a positive correlation between tTMB and bTMB, with a Spearman correlation coefficient of 0.68 and a p-value of less than 0.001 (Fig. 1b).
Association between PD-L1 expression and TMB
The correlation between PD-L1 expression and tTMB was investigated. Of the 32 patients with PD-L1 expression assessed, 26 patients also had tTMB results available. Of the 26 patients, 12 had positive PD-L1 expression ( 1%). We observed significantly higher tTMB in patients with positive PD-L1 expression (n = 12) than in those with negative expression (n = 14) (p = 0.02, Fig. 2a). However, of the patients in with both bTMB and PD-L1 expression were evaluated, bTMB was comparable in both PD-L1 expression-positive (n = 12) and PD-L1-negative (n = 10) patients (p = 0.62, Fig. 2b).
Fig. 2.
Difference of OncoScreen-derived TMB between patients with positive PD-L1 expression and negative expression. a Difference in tTMB between patients with positive PD-L1 expression and negative expression. b Difference in bTMB between patients with positive PD-L1 expression and negative expression
Targeted panel-derived TMB predicts clinical benefit
Next, we evaluated the associations between TMB (both tTMB and bTMB) and clinical outcomes, including PFS and DCB, in patients treated with PD-1/PD-L1 inhibitors. In this cohort, 11 (26.2%), 14 (33.3%) and 17 (40.5%) patients achieved PR, SD and PD as their best response, respectively, resulting in an ORR of 26.2% and a disease control rate (DCR) of 59.5%. DCB and NDB were observed in 15 and 22 patients, respectively. Our study demonstrated that patients with DCB had a trend of higher tTMB (p = 0.06, Fig. 3a) and higher bTMB (p = 0.03, Fig. 3b) than patients with NDB. Next, ROC analyses were performed to derive the optimal cutoffs for both tTMB and bTMB levels to distinguish patients with DCB from those with NDB (Fig. 3c, d). Of 29 patients with available tTMB and DCB/NDB, patients with tTMB 7 mutations/Mb (n = 16) showed a significantly higher DCB rate than patients with tTMB 7 mutations/Mb (n = 13) (62.5% versus. 26.1%, Fisher’s exact test p = 0.033, Figure S5A). Of 26 patients with available bTMB and DCB/NDB, no significantly higher DCB rate was observed in patients with bTMB 11 mutations/Mb (n = 7) and bTMB 11 mutations/Mb (n = 19) (57.1% versus. 21.0%, Fisher’s exact test p = 0.149, Figure S5B).
Fig. 3.
Correlations between OncoScreen-derived TMB and clinical benefit from PD-1/PD-L1 inhibitors. a The difference in tTMB between patients with DCB and NDB. b The difference in bTMB between patients with DCB and NDB. c ROC analysis for deriving the optimal cutoff for tTMB level. d ROC analysis for deriving the optimal cutoff for bTMB level
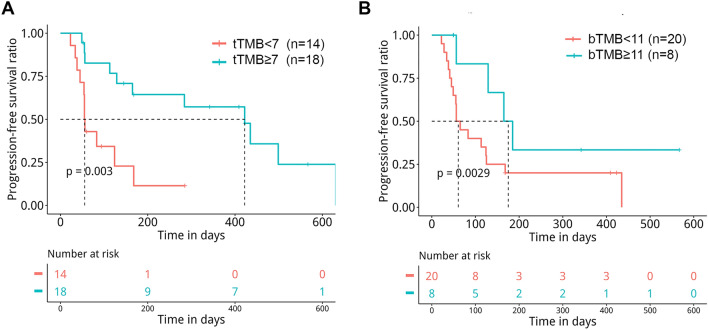
The median PFS for the cohort was 2.7 months (ranging from 0.7 to 20.8 months). Of 32 patients with available tTMB and PFS, patients with tTMB 7 mutations/Mb (n = 18) showed significantly longer PFS than patients with tTMB 7 mutations/Mb (n = 14) (13.9 months versus. 1.8 months, p = 0.003, Fig. 4a) after adjusting for stage. Of 28 patients with available bTMB and PFS, patients with bTMB 11 mutations/Mb (n = 8) also showed significantly longer PFS than those with bTMB 11 mutations/Mb (n = 20) (5.8 months versus. 2.0 months, p = 0.0029, Fig. 4b) after adjusting for stage. Next, independent factors associated with PFS were evaluated. In the univariable Cox proportional hazards regression model, tTMB (hazard ratio [HR] 0.24, 95% confidence interval [CI] 0.09–0.64, p = 0.004), smoking status (HR 0.42, 95% CI 0.18–0.97, p = 0.043) and histological subtype (HR 2.32, 95% CI 1.05–5.13, p = 0.037) were associated with PFS, and bTMB (HR 0.39, 95% CI 0.13–1.17, p = 0.092) and stage (HR 2.41, 95% CI 0.89–6.56, p = 0.085) showed a trend of association with PFS (Table 2). PD-L1 expression was not associated with PFS (HR 0.49, 95% CI 0.20–1.21, p = 0.120, Table 2). In addition, PFS between patients with positive PD-L1 expression (n = 16) and those with negative PD-L1 expression (n = 16) was comparable (9.3 months versus. 2.7 months, p = 0.108, Figure S6). In the multivariate Cox proportional hazards regression model that included tTMB, smoking status and histological subtype, tTMB remained significantly associated with PFS (HR 0.26, 95% CI 0.08–0.84, p = 0.025) (Table 2). In this cohort, 20 patients had both tTMB and bTMB assessed. Among these patients, 6 patients’ tTMB and bTMB were both above the cutoffs and 7 patients’ tTMB and bTMB were both below the cutoffs, resulting in a concordance of 65% (13/20) between tTMB and bTMB, which suggested consistency between tTMB and bTMB. Taken together, our data demonstrated that bTMB can potentially predict the efficacy of ICIs in NSCLC.
Fig. 4.
Progression-free survival by TMB status assessed by OncoScreen in our cohort. a Correlation of OncoScreen-derived tTMB with progression-free survival. b Correlation of OncoScreen-derived bTMB with progression-free survival
Table 2.
Univariable and multivariable analysis of risk factors of PFS
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender Male vs. female | 0.94 (0.32–2.76) | 0.915 | NA | NA |
| Age ≥ 65 versus. < 65 years | 0.77 (0.35–1.71) | 0.519 | NA | NA |
| Smoking status smoker versus. nonsmoker | 0.42 (0.18–0.97) | 0.043 | 1.29 (0.41–3.98) | 0.664 |
| Histological subtype AD versus. SCC | 2.32 (1.05–5.13) | 0.037 | 1.94 (0.68–5.56) | 0.219 |
| PD-L1 expression positive versus. negative | 0.49 (0.20–1.21) | 0.120 | NA | NA |
| tTMB ≥ 7 versus. < 7 | 0.24 (0.09–0.64) | 0.004 | 0.26 (0.08–0.84) | 0.025 |
| bTMB ≥ 11 versus. < 11 | 0.39 (0.13–1.17) | 0.092 | NA | NA |
| Stage IV versus. III | 2.41 (0.89–6.56) | 0.085 | NA | NA |
PFS progression-free survival HR hazard ratio CI confidence interval TMB tumor mutation burden tTMB tumor tissue TMB bTMB blood TMB AD adenocarcinoma SCC squamous cell carcinoma NA not available
Next, a small independent validation cohort (n = 14) was enrolled to preliminarily verify the association between bTMB and PFS. All patients in this cohort were males, and the median age was 67.5 years (ranging from 57 to 78 years). Thirteen patients had a history of smoking, and 1 was a nonsmoker. Thirteen patients were diagnosed with SCC, and 1 patient was diagnosed with AD. Thirteen patients were treated with PD-1 inhibitors (including 6 with IBI308, 3 with pembrolizumab, 2 with BGB-A317, 1 with nivolumab and 1 with AK105), and 1 patient was treated with the PD-L1 inhibitor KL-A167. As Figure S7 shows, patients with bTMB 11 mutations/Mb exhibited longer PFS (4.6 months versus. 1.6 months, p = 0.192) with a nonsignificant numerical difference compared with those with bTMB 11 mutations/Mb. We subsequently investigated the associations between bTMB and survival outcome in all 42 patients who had available bTMB and PFS, including 14 patients in the small validation cohort. As shown in Fig. 5, patients with bTMB 11 mutations/Mb had significantly longer PFS (6.1 months versus. 1.8 months, p = 0.011) than those with bTMB 11 mutations/Mb.
Fig. 5.

Progression-free survival by bTMB status in all 42 patients who had available bTMB and progression-free survival, including 14 patients enrolled in the small independent validation cohort
Discussion
Our findings suggest that the OncoScreen panel could be used for both tTMB and bTMB evaluation. We also validated bTMB as a potential biomarker to identify advanced NSCLC patients who are more likely to benefit from ICIs.
The current study demonstrated a positive correlation between tTMB obtained from the OncoScreen panel and WES, which is consistent with previous studies [18, 28]. Currently, commercial next-generation sequencing panels used for assessing TMB normally target the coding region of hundreds of genes, including FoundationOne assay (315 genes, 1.1 Mb), MSK-IMPACT (468 genes, 1.5 Mb), Oncomine Tumor Mutational Load Assay (409 genes, ~ 1.7 Mb), QIAseq TMB panel (468 genes, 1.2 Mb), TruSight Oncology 500 panel (500 genes, 1.95 Mb) and NEOplus RUO assay (more than 340 genes, 1.1 Mb). tTMB assessed by these panels demonstrates good concordance with whole-exome panel-derived tTMB [8, 18, 19, 28–32]. The size of the targeted genome region varies across the panels. The accuracy of panel-derived tTMB is compromised when the coding region size is below 1 Mb [18, 32]. Therefore, sufficient panel size (preferably more than 1 Mb) is needed for clinical application and is more critical than the exome coverage of the panel [32].
Our study demonstrated that less than 40% of identified variants were shared by both blood and tumor samples, and there was a concordance of 65% between tTMB and bTMB. The discordance between tTMB and bTMB might be attributed to a few factors, including intratumoral heterogeneity, low abundance of mutant ctDNA fragments, and different bioinformatic pipelines and cutoffs used for tTMB and bTMB. Mutations detected only in blood and not in tumor samples might be attributed to intratumoral heterogeneity in NSCLC, as localized biopsies obtained from different parts of the tumor harbor different mutations, resulting in differential mutational profiles between a single biopsy sample and ctDNA [33, 34]. Mutant ctDNA fragments with low abundance are masked by a large amount of background DNA, which may cause false-negative results in which some mutations are detected only in tumors and not in blood samples. In addition, different cutoffs used for tTMB and bTMB resulted in 3 patients with the same value of tTMB and bTMB were labeled as patients with high tTMB and low bTMB. Evidence suggests that the main source of variation between tTMB and bTMB originates from intratumoral heterogeneity, rather than analytic factors, including bioinformatic pipelines [20]. Although discordance exists between bTMB and tTMB, we found that OncoScreen-derived tTMB had a positive correlation with the same panel-derived bTMB, which was consistent with prior studies showing that tTMB and bTMB assessed by targeted panels were positively correlated (including FoundationOne CDx assay and 1021-gene panel) in NSCLC [20, 35].
No association between bTMB and PD-L1 expression was observed in our work, which is consistent with previous results [20, 21]. Several previous studies have revealed no association between PD-L1 expression level and tTMB in Western patients with NSCLC [19, 36, 37]; however, the association between PD-L1 expression level and tTMB in Chinese patients remains elusive. Our study showed that patients with positive PD-L1 expression had higher tTMB based on a small sample size. The correlation between PD-L1 expression and tTMB needs to be further investigated in a large cohort of Chinese patients with NSCLC.
Our findings suggested that higher tTMB assessed by targeted panel sequencing was associated with significantly longer PFS in NSCLC patients treated with PD-1/PD-L1 inhibitors, which is consistent with previous studies [19, 38, 39]. tTMB assessed by the FoundationOne panel predicts ORR, PFS and OS in NSCLC patients treated with atezolizumab as first-line, second-line or higher treatment [40]. tTMB assessed by the FoundationOne panel also predicts OS in NSCLC patients treated with nivolumab [39]. Targeted panel-derived bTMB as a predictive biomarker in predicting the clinical benefit from ICIs in NSCLC has attracted widespread attention [34]. In our work, we demonstrated that high bTMB ( 11 mutations/Mb) assessed by OncoScreen was associated with significantly longer PFS. This result is in agreement with previous studies showing higher bTMB assessed by targeted panels, including a 329 pan cancer-related gene panel (0.637 Mb) and FoundationOne CDx, predicted significantly longer PFS [20, 22]. In this study, multivariable analysis showed that bTMB had a trend of association with PFS, whereas tTMB was significantly associated with PFS. The above discrepancy might be due to the small sample size. A large prospective cohort is needed to explore the association between bTMB and PFS. Previous studies have demonstrated that PD-1/PD-L1 inhibitor-treated advanced NSCLC patients who achieved CR or PR had higher bTMB than those who achieved SD or PD [21]. In this study, we observed that patients with DCB harbored higher bTMB and a trend of higher tTMB than those with NDB.
There are several limitations to our study. First, the sample size for investigating bTMB as a predictor of response to ICIs was small which might weaken the statistical significance of our conclusion. Second, patients treated with different anti-PD-1 and anti-PD-L1 therapies were combined in our analyses, and further studies should explore each of the PD-1 and PD-L1 inhibitors separately in a large cohort.
In conclusion, our findings demonstrated the feasibility of TMB estimation using targeted panel. Patients with DCB had higher bTMB and a trend of higher tTMB. Our analyses also indicated that targeted panel-derived tTMB and bTMB were correlated with PFS in patients receiving PD-1/PD-L1 inhibitors. This study demonstrated that bTMB assessed by targeted panel has the potential to predict the clinical response to ICIs in advanced NSCLC patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- TMB
Tumor mutation burden
- WES
Whole-exome sequencing
- ICI
Immune checkpoint inhibitors
- NSCLC
Non-small cell lung cancer
- PFS
Progression-free survival
- DCB
Durable clinical benefit
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- MSI
Microsatellite instability
- MMR
DNA mismatch repair
- RECIST
Response Evaluation Criteria in Solid Tumors
- PD
Progressive disease
- ORR
Overall response rate
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- NDB
No durable benefit
- FFPE
Formalin fixed paraffin embedded
- ctDNA
Circulating tumor DNA
- AF
Allele frequency
- SNP
Single-nucleotide polymorphism
- SNV
Single-nucleotide variant
- InDels
Insertions and deletions
- ROC
Receiver operating characteristic curves
- AUC
Area under curve
- FDR
False discovery rate
- AD
Lung adenocarcinoma
- SCC
Lung squamous cell carcinoma
- CNV
Copy number variant
- ECOG PS
Eastern Cooperative Oncology Group performance status
- CI
Confidence interval
- HR
Hazard ratio
- Mb
Megabase
- OS
Overall survival
Authors' contributions
Xi Chen contributed to conceptualization, methodology, supervision, project administration, writing—original draft; Liangjie Fang contributed to methodology, data curation, resources; Yanping Zhu contributed to methodology, data curation, resources; Zhang Bao contributed to methodology, data curation, resources; Qing Wang contributed to methodology and resources; Rong Liu contributed to methodology and resources; Wenjia Sun contributed to methodology and resources; Haiwei Du contributed to writing—original draft, data curation; Jing Lin contributed to formal analysis, data curation, writing—review and editing; Bing Yu contributed to data curation, writing—review and editing; Songan Chen contributed to data curation, writing—review and editing; Jianya Zhou contributed to conceptualization, methodology, supervision, project administration, writing—review and editing; Jianying Zhou contributed to conceptualization, methodology, supervision, project administration.
Funding
This work was supported by the grants from the National Key R&D Program of China (grant number: 2016YFC1303800) and the National Natural Science Foundation of China (grant number: 81972179).
Declaration
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of College of Medicine of Zhejiang University.
Consent to participate
Informed consent was obtained from each patient for the use of their peripheral blood and tumor tissue samples.
Consent for publication
Obtained.
Availability of data and material
Data are not publicly available.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE, Carcereny Costa E, Patel JD, Chow LQM, Koczywas M, Ho C, Fruh M, van den Heuvel M, Rothenstein J, Reck M, Paz-Ares L, Shepherd FA, Kurata T, Li Z, Qiu J, Kowanetz M, Mocci S, Shankar G, Sandler A, Felip E. Phase II Trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH) J clin oncol : off J Am Soc Clin Oncol. 2017;35(24):2781–2789. doi: 10.1200/jco.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB, Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.Network NCC NCCN clinical practice guidelines in Oncology: Non-small cell lung cancer (Version 8.2020)
- 7.Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746–756. doi: 10.1002/cam4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/s1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall survival and long-term safety of nivolumab (Anti-Programmed Death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J clin oncol : off j Am Soc Clin Oncol. 2015;33(18):2004–2012. doi: 10.1200/jco.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansfield AS, Murphy SJ, Peikert T, Yi ES, Vasmatzis G, Wigle DA, Aubry MC. Heterogeneity of programmed cell death Ligand 1 expression in multifocal lung cancer. Clin cancer res : an off j Am Assoc Cancer Res. 2016;22(9):2177–2182. doi: 10.1158/1078-0432.ccr-15-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.mct-14-0983. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, NY) 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 14.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu WJ, Gajewski TF, Slingluff CL, Jr, Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom JW, Schneck JP, Horak CE, Weinhold N, Chan TA. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(4):934–949. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY) 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/s1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 18.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genom med. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, Hollmann T, Schalper KA, Gainor JF, Shen R, Ni A, Arbour KC, Merghoub T, Wolchok J, Snyder A, Chaft JE, Kris MG, Rudin CM, Socci ND, Berger MF, Taylor BS, Zehir A, Solit DB, Arcila ME, Ladanyi M, Riely GJ, Schultz N, Hellmann MD. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J clin oncol : off j Am Soc Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, Lieber DS, Lipson D, Silterra J, Amler L, Riehl T, Cummings CA, Hegde PS, Sandler A, Ballinger M, Fabrizio D, Mok T, Shames DS. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, Zhu B, Wang S, Zhuo M, Sun J, Wang Q, Bai H, Han J, Tian Y, Lu J, Xu T, Zhao X, Wang G, Cao X, Li F, Wang D, Chen Y, Bai Y, Zhao J, Zhao Z, Zhang Y, Xiong L, He J, Gao S, Wang J. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5(5):696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Wang Y, Shi W, Zhu M, Liu Z, Luo N, Zeng Y, He Y. Serial ultra-deep sequencing of circulating tumor DNA reveals the clonal evolution in non-small cell lung cancer patients treated with anti-PD1 immunotherapy. Cancer Med. 2019;8(18):7669–7678. doi: 10.1002/cam4.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Duan J, Wang G, Zhao J, Xu J, Han J, Zhao Z, Zhao J, Zhu B, Zhuo M, Sun J, Bai H, Wan R, Wang X, Fei K, Wang S, Zhao X, Zhang Y, Huang M, Huang D, Qi C, Gao C, Bai Y, Dong H, Xiong L, Tian Y, Wang D, Xu C, Wang W, Li J, Hu X, Cai S, Wang J. Allele frequency-adjusted blood-based tumor mutational burden as a predictor of overall survival for patients with NSCLC treated With PD-(L)1 inhibitors. J thorac oncol : off publ Int Assoc Stud Lung Cancer. 2019 doi: 10.1016/j.jtho.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Chae YK, Davis AA, Agte S, Pan A, Simon NI, Iams WT, Cruz MR, Tamragouri K, Rhee K, Mohindra N, Villaflor V, Park W, Lopes G, Giles FJ. Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist. 2019;24(6):820–828. doi: 10.1634/theoncologist.2018-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours revised RECIST. Eur j cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Xie Z, Liu L, Lin X, Xie X, Gu Y, Liu M, Zhang J, Ouyang M, Lizaso A, Zhang H, Feng W, Li B, Han-Zhang H, Chen S, Li S, Zhong N, Liu H, Zhou C, Qin Y. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Modern pathol : off j U S Canad Acad Pathol. 2019 doi: 10.1038/s41379-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics. 2014;70(1):212–223. doi: 10.1111/biom.12107. [DOI] [PubMed] [Google Scholar]
- 28.Stenzinger A, Endris V, Budczies J, Merkelbach-Bruse S, Kazdal D, Dietmaier W, Pfarr N, Siebolts U, Hummel M, Herold S, Andreas J, Zoche M, Togel L, Rempel E, Maas J, Merino D, Stewart M, Zaoui K, Schlesner M, Glimm H, Frohling S, Allen J, Horst D, Baretton G, Wickenhauser C, Tiemann M, Evert M, Moch H, Kirchner T, Buttner R, Schirmacher P, Jung A, Haller F, Weichert W, Dietel M. Harmonization and standardization of panel-based tumor mutational burden (TMB) measurement: real-world results and recommendations of the QuIP study. J thorac oncol : off publ Int Assoc Stud Lung Cancer. 2020 doi: 10.1016/j.jtho.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Wu HX, Wang ZX, Zhao Q, Wang F, Xu RH. Designing gene panels for tumor mutational burden estimation: the need to shift from 'correlation' to 'accuracy'. J Immun Cancer. 2019;7(1):206. doi: 10.1186/s40425-019-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazdal D, Endris V, Allgauer M, Kriegsmann M, Leichsenring J, Volckmar AL, Harms A, Kirchner M, Kriegsmann K, Neumann O, Brandt R, Talla SB, Rempel E, Ploeger C, von Winterfeld M, Christopoulos P, Merino DM, Stewart M, Allen J, Bischoff H, Meister M, Muley T, Herth F, Penzel R, Warth A, Winter H, Frohling S, Peters S, Swanton C, Thomas M, Schirmacher P, Budczies J, Stenzinger A. Spatial and temporal heterogeneity of panel-based tumor mutational burden in pulmonary adenocarcinoma: separating biology from technical artifacts. J thorac oncol : off publ Int Assoc Stud Lung Cancer. 2019;14(11):1935–1947. doi: 10.1016/j.jtho.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Buttner R, Longshore JW, Lopez-Rios F, Merkelbach-Bruse S, Normanno N, Rouleau E, Penault-Llorca F. Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO open. 2019;4(1):e000442. doi: 10.1136/esmoopen-2018-000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budczies JAM, Litchfield K, et al. Optimizing panel-based tumor mutational burden (TMB) measurement. Ann oncol: off j Eur Soc Med Oncol. 2019;30(9):1496–1506. doi: 10.1093/annonc/mdz205. [DOI] [PubMed] [Google Scholar]
- 33.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M, Horswell S, Escudero M, Matthews N, Rowan A, Chambers T, Moore DA, Turajlic S, Xu H, Lee SM, Forster MD, Ahmad T, Hiley CT, Abbosh C, Falzon M, Borg E, Marafioti T, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Shah R, Joseph L, Quinn AM, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Dentro S, Taniere P, O'Sullivan B, Lowe HL, Hartley JA, Iles N, Bell H, Ngai Y, Shaw JA, Herrero J, Szallasi Z, Schwarz RF, Stewart A, Quezada SA, Le Quesne J, Van Loo P, Dive C, Hackshaw A, Swanton C. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 34.Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA, Veeriah S, Rosenthal R, Marafioti T, Kirkizlar E, Watkins TBK, McGranahan N, Ward S, Martinson L, Riley J, Fraioli F, Al Bakir M, Gronroos E, Zambrana F, Endozo R, Bi WL, Fennessy FM, Sponer N, Johnson D, Laycock J, Shafi S, Czyzewska-Khan J, Rowan A, Chambers T, Matthews N, Turajlic S, Hiley C, Lee SM, Forster MD, Ahmad T, Falzon M, Borg E, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Hafez D, Naik A, Ganguly A, Kareht S, Shah R, Joseph L, Marie Quinn A, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Oukrif D, Akarca AU, Hartley JA, Lowe HL, Lock S, Iles N, Bell H, Ngai Y, Elgar G, Szallasi Z, Schwarz RF, Herrero J, Stewart A, Quezada SA, Peggs KS, Van Loo P, Dive C, Lin CJ, Rabinowitz M, Aerts H, Hackshaw A, Shaw JA, Zimmermann BG, Swanton C. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Chang L, Yang Y, Fang W, Guan Y, Wu A, Hong S, Zhou H, Chen G, Chen X, Zhao S, Zheng Q, Pan H, Zhang L, Long H, Yang H, Wang X, Wen Z, Wang J, Yang H, Xia X, Zhao Y, Hou X, Ma Y, Zhou T, Zhang Z, Zhan J, Huang Y, Zhao H, Zhou N, Yi X, Zhang L. The correlations of tumor mutational burden among single-region tissue, multi-region tissues and blood in non-small cell lung cancer. J Immun Cancer. 2019;7(1):98. doi: 10.1186/s40425-019-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, Chen Z, Ballman KV, Watson MA, Govindan R, Lanc I, Beer DG, Bueno R, Chirieac LR, Chui MH, Chen G, Franklin WA, Gandara DR, Genova C, Brovsky KA, Joshi MM, Merrick DT, Richards WG, Rivard CJ, Harpole DH, Tsao MS, van Bokhoven A, Shepherd FA, Hirsch FR. Correlation of PD-L1 expression with tumor mutation burden and gene signatures for prognosis in early-stage squamous cell lung carcinoma. J thorac oncol : off publ Int Assoc Stud Lung Cancer. 2019;14(1):25–36. doi: 10.1016/j.jtho.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, Gossai A, Frampton GM, Torres AZ, Lehnert EM, Bourque D, O'Connell C, Bowser B, Caron T, Baydur E, Seidl-Rathkopf K, Ivanov I, Alpha-Cobb G, Guria A, He J, Frank S, Nunnally AC, Bailey M, Jaskiw A, Feuchtbaum D, Nussbaum N, Abernethy AP, Miller VA. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA. 2019;321(14):1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcin Kowanetz WZ, Shames D, Cummings C, Rizvi N, Spira A, Frampton G, Leveque V, Flynn S, Mocci S, Shankar G, Funke R, Ballinger M, Waterkamp D, Chen D, Sandler A, Hampton G, Amler L, Hegde P, Hellmann M. Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J Thorac Oncol. 2017;12(1):S321–322. doi: 10.1016/j.jtho.2016.11.343. [DOI] [Google Scholar]
- 39.Singal GMP, Agarwala V, et al. Analyzing biomarkers of cancer immunotherapy (CIT) response using a real-world clinicogenomic database. Ann oncol: off j Eur Soc Med Oncol. 2017;28:v403–v427. doi: 10.1093/annonc/mdx376.005. [DOI] [Google Scholar]
- 40.Kowanetz MZW, Shames D, et al. OA20.01 Tumor Mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J thorac oncol: off publ Int Assoc Stud Lung Cancer. 2017;12:S321–S322. doi: 10.1016/j.jtho.2016.11.343. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.