Abstract
Purpose
To assess the antitumor activity of neoadjuvant chemotherapy in conjunction with PD-1 inhibitors (neoadjuvant chemoimmunotherapy) among patients with oropharyngeal and hypopharyngeal squamous cell carcinoma (OPHSCC) and compare its efficacy with neoadjuvant chemotherapy alone.
Methods
We conducted a retrospective analysis using data from patients diagnosed with OPHSCC and treated at the Sun Yat-sen University Cancer Center between September 2012 and August 2022. We included patients who received neoadjuvant chemotherapy alone or combined with PD-1 inhibitors. We assessed the clinical response using the Response Evaluation Criteria in Solid Tumors and evaluated progression-free survival (PFS) and overall survival (OS).
Results
Preliminary results demonstrate that neoadjuvant chemoimmunotherapy exhibited robust antitumor activity in OPHSCC, with an impressive overall response rate (ORR) of 81.0%. Complete response and partial response rates were 14.9% and 65.9%, respectively. Notably, neoadjuvant chemoimmunotherapy demonstrated superior PFS and OS to neoadjuvant chemotherapy alone. The 1-year PFS rate was 80.7%, and the 2-year rate was 61.1%. Additionally, the 1-year OS rate reached 92.3%. Finally, a multivariate analysis identified the American Joint Committee on Cancer stage reduction post-treatment as a favorable predictor of PFS.
Conclusion
Our results underscore the promising potential of neoadjuvant chemoimmunotherapy in enhancing antitumor activity in patients with OPHSCC. The robust ORR, along with improved PFS and OS, supports the utility of this combined approach. These results pave the way for further investigations to validate and refine the application of neoadjuvant chemoimmunotherapy in this challenging clinical context.
Keywords: Oropharynx, Laryngopharynx, PD-1 inhibitor, Progression-free survival, Overall survival, Retrospective study
Introduction
Head and neck squamous cell carcinoma (HNSCC) stands as the sixth most prevalent malignancy globally, contributing to an estimated half-million annual fatalities [1]. Its escalating prevalence portends an annual incidence of 1.08 million cases by 2030 [2]. Despite advancements in surgical interventions and multimodal therapeutic strategies, the aggregate 5-year survival rate remains around 40–50%, which is primarily attributed to elevated recurrence rates, invasive proclivity, and metastatic potential [3]. While oropharyngeal and hypopharyngeal squamous cell carcinoma (OPHSCC) constitute a smaller fraction within the spectrum of HNSCC, they are subject to relatively diminished scrutiny and are conventionally subsumed under the umbrella of HNSCC for investigative purposes [4]. However, due to their distinctive anatomical context, these subtypes warrant meticulous individual consideration and segregated analysis [5].
In recent years, the field of immunotherapy for HNSCC has made significant strides [6]. The notable success of programmed cell death protein 1 (PD-1) inhibitors in addressing metastatic/recurrent head and neck cancer [7] has engendered endeavors to substantiate its efficacy within the neoadjuvant therapeutic context [8]. Presently, we lack a standardized modality for the application and dosing of immunosuppressants in neoadjuvant settings. The realm of combination therapies intertwined with novel adjuvant immunotherapy, encompassing dual immunotherapies, immunoradiotherapies, and chemoimmunotherapies, has been subject to comprehensive investigation through clinical trials; most have revealed encouraging therapeutic effectiveness with a favorable balance of associated toxicity [8–10]. The fundamental objective underlying neoadjuvant therapy is twofold: to curtail the susceptibility to distant metastasis and to reshape the surgical landscape [11]. However, the reservoir of empirical evidence regarding the sustained survival benefits emanating from these neoadjuvant clinical trials is currently circumscribed. Furthermore, the relatively low sample sizes in these trials prevent us from definitively establishing the practical efficacy within clinical praxis.
Since 2019, our institution has adopted a tailored approach in the form of neoadjuvant chemoimmunotherapy for patients with resectable HNSCC. This therapeutic regimen involves a combination of paclitaxel, platinum agents, and PD-1 inhibitors. In the current landscape, established standards and definitive guidelines for the chemoimmunotherapy of HNSCC remain conspicuously absent. The selection of immunotherapy agents is based on a shared decision-making process between the attending physician and the patient, thereby engendering need for uniformity in the choice of PD-1 inhibitors. To furnish a more targeted evaluation of our treatment paradigm, we conducted an in-depth analysis within our institution, focusing on a patient cohort afflicted with OPHSCC who underwent neoadjuvant chemoimmunotherapy. We aimed to document the effectiveness and practicability of neoadjuvant chemoimmunotherapy and, concurrently, to present robust survival data gleaned from a substantially sized cohort. Concurrently, we compared this cohort with a parallel cohort of patients with OPHSCC subjected to neoadjuvant chemotherapy alone.
Materials and methods
Patients
We conducted a retrospective analysis employing data gathered from individuals diagnosed with conditions affecting the oropharynx and hypopharynx and treated at the Sun Yat-sen University Cancer Center between September 2012 and August 2022 (Fig. 1). Ethical endorsement for the investigation was obtained from the Institutional Ethical Committee, affirming its adherence to established ethical guidelines. Given the observational and retrospective design of this study, the necessity for explicit informed consent was deemed unnecessary, and thus waived. The cohort exclusively comprised patients who had undergone either neoadjuvant chemotherapy or a combined regimen of neoadjuvant chemotherapy and immunotherapy. Notably, individuals manifesting distant metastasis were deliberately excluded from this study.
Fig. 1.
Flowchart of sample selection
Procedure
Patients presenting with discernible disease at the point of their inclusion in the study were considered eligible for the evaluation of treatment response. Prior to enrollment, a mandatory prerequisite involved undergoing computed tomography (CT) and/or magnetic resonance imaging (MRI) of the head and neck region. Additionally, a chest CT scan was performed prior to treatment initiation to meticulously exclude the presence of metastatic lesions. All patients received treatment exclusively within the confines of the study hospital, wherein a conclusive pathological diagnosis, including expert consultative pathology assessments, was established, affirming the neoplasms’ classification as squamous cell carcinoma. After diagnosis, the cases were meticulously categorized according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system.
Patients were categorized into two distinct cohorts based on their treatment modalities: the chemotherapy group (Chemo group) and the chemoimmunotherapy group (CIT group). Within the Chemo group, therapeutic intervention comprised a minimum of two cycles, each lasting 3 weeks, of paclitaxel (either albumin-bound paclitaxel or docetaxel) and platinum agents (including cisplatin, nedaplatin, or lobaplatin), with or without 5-fluorouracil (TP regimen or TPF regimen). Meanwhile, the CIT group underwent treatment regimens consisting of a minimum of two 3-week cycles of albumin-bound paclitaxel, cisplatin, and a PD-1 inhibitor (The PD-1 inhibitor used was one of the following drugs: Camrelizumab, Sintilimab, Toripalimab, Pembrolizumab, Nivolumab, or Tislelizumab).
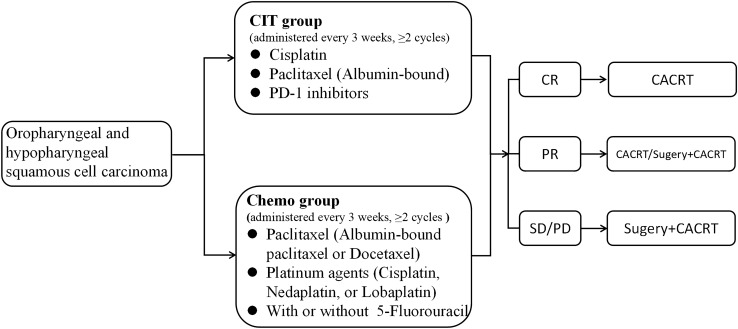
Following the completion of neoadjuvant therapy, the acquisition of CT or MRI scans targeting the head and neck region was mandatory. This post-therapy imaging protocol aimed to meticulously reassess alterations in the lesions. The treatment response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. In instances where patients demonstrated a partial or complete response, the recommended course of action entails the administration of adjuvant radiotherapy with platinum-based simultaneous chemotherapy in the absence of surgical intervention. Conversely, patients displaying stable or progressive disease are advised to undergo surgical procedures, followed by the subsequent application of adjuvant radiotherapy with simultaneous platinum-based chemotherapy (Fig. 2).
Fig. 2.
Grouping and flowchart. The individual drug dosages for a single administration were: Paclitaxel (albumin-bound), 260 mg/m2; docetaxel, 75 mg/m2; cisplatin, 60 mg/m2; nedaplatin, 90 mg/m2; lobaplatin, 50 mg/m2; 5-fluorouracil, 15 mg/kg. Abbreviations: CIT, chemoimmunotherapy; Chemo, chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; CACRT, concurrent adjuvant chemoradiotherapy
Follow-up
Three months after treatment completion, imaging assessments, specifically CT or MRI scans of the head and neck, in conjunction with chest CT, were carried out. This imaging protocol was then repeated at 6-month intervals over a 3-year span. Notably, patients diagnosed with hypopharynx cancer underwent additional laryngoscopy examinations. The determination of progression-free survival (PFS) was predicated upon the temporal interval stretching from the conclusion of radiotherapy to the point of either structural progression or the latest recorded follow-up date. Furthermore, overall survival (OS) was defined as the duration of survival post-radiotherapy. The definition of structural progression encompassed instances wherein metastatic lymph nodes were corroborated through biopsy or instances of distant metastasis were substantiated via CT scans or positron emission tomography (PET) imaging.
To keep follow-up duration homogeneous between the two cohorts, the maximum follow-up duration within the chemotherapy arm was restricted to 2 years. As for the CIT group, the termination date for follow-up assessments was set to July 2023.
Statistical analysis
Continuous variables are presented as the mean and standard deviation (SD) or, in cases of non-normally distributed data, as the median and interquartile range (IQR). Categorical variables are represented through numerical counts and corresponding percentages. Statistical analyses were performed using SPSS Statistics, version 21.0 (IBM, Armonk, NY, USA). For association analyses, a comprehensive approach was undertaken, encompassing both bivariate and multivariate COX regression models. PFS and OS were evaluated using the Kaplan–Meier (KM) method. Bar charts were plotted using PRISM, version 8.0 (GraphPad, San Diego, CA, USA). The survival package, integral to R, version 4.2.1, was harnessed to assess the hypothesis of proportional hazards and to model survival regression. Notably, the resultant outcomes were plotted using the survminer and ggplot2 packages.
Results
Baseline characteristics of the study populations
A total of 295 individuals were enrolled in this study; their average age was 57.2 years (ranging from 20 to 80 years). Among the participants, 271 (91.7%) were male, while the remaining 24 (8.2%) were female. Within this cohort, 90 (30.5%) patients were diagnosed with well-differentiated squamous cell carcinoma, encompassing focal carcinoma presentations. Moderately differentiated squamous cell carcinoma was identified in 105 (35.6%) cases, and 100 (33.9%) were classified as poorly differentiated squamous cell carcinoma. Since human papillomavirus (HPV) was not routinely tested, we only knew the HPV status of 31 patients, of whom 16 (51.6%) were positive. Furthermore, the distribution of primary tumor sites revealed that 194 patients (65.8%) exhibited hypopharyngeal tumors, while 101 patients (34.2%) had tumors localized in the oropharyngeal region. Regarding disease staging, most patients (282, 95.5%) were diagnosed at an advanced local stage. Specifically, within this subset, 268 patients (90.8%) were categorized as having stage IV disease, with the remaining 14 patients (4.7%) classified under stage III disease (Table 1).
Table 1.
Baseline characteristics of the study population
| Characteristic | CIT (range, %) (N = 147) | Chemo (range, %) (N = 148) | Total (range, %) (N = 295) |
|---|---|---|---|
| Age (year) | 58.0 ± 9.0 (35–80) | 56.3 ± 10.0 (20–77) | 57.2 ± 9.5 (20–80) |
| Sex (M/F) | 23.5 (95.9): 1 (4.1) | 7.2 (87.8): 1 (12.2) | 11.3 (91.9): 1 (8.1) |
| Tumor subsite (%) | |||
| Oropharynx | 37 (25.2) | 64 (43.2) | 101 (34.2) |
| Hypopharynx | 110 (74.8) | 84 (56.8) | 194 (65.8) |
| Differentiation | |||
| Well differentiated | 49 (33.3) | 41 (27.7) | 90 (30.5) |
| Moderately differentiated | 41 (27.9) | 64 (43.2) | 105 (35.6) |
| Poorly differentiated | 57 (38.8) | 43 (29.1) | 100 (33.9) |
| Pretreatment clinical T stage (%) | |||
| T1 | 15 (10.2) | 9 (6.0) | 24 (8.1) |
| T2 | 52 (35.4) | 55 (37.2) | 107 (36.3) |
| T3 | 23 (15.6) | 37 (25.0) | 60 (20.3) |
| T4a | 48 (32.7) | 38 (25.7) | 86 (29.2) |
| T4b | 9 (6.1) | 9 (6.1) | 18 (6.1) |
| Pretreatment clinical N stage (%) | |||
| N0 | 4 (2.7) | 5 (3.4) | 9 (3.1) |
| N1 | 12 (8.2) | 3 (2.0) | 15 (5.1) |
| N2 | 120 (81.6) | 128 (86.5) | 248 (84.1) |
| N3 | 11 (7.5) | 12 (8.1) | 23 (7.7) |
| Pretreatment AJCC stage (%) | |||
| I | 1 (0.7) | 1 (0.7) | 2 (0.7) |
| II | 8 (5.4) | 5 (3.4) | 13 (4.4) |
| III | 11 (7.5) | 3 (2.0) | 14 (4.7) |
| IV | 127 (86.4) | 139 (93.9) | 268 (90.8) |
| Therapy regimen | |||
| TP + Camrelizumab | 70 (47.6) | – | |
| TP + Sintilimab | 28 (19.0) | – | |
| TP + Toripalimab | 16 (10.9) | – | |
| TP + Pembrolizumab | 14 (9.5) | – | |
| TP + Nivolumab | 12 (8.2) | – | |
| TP + Tislelizumab | 7 (4.8) | – | 147 (49.8) |
| TP | – | 81 | 81 (27.5) |
| TPF | – | 67 | 67 (22.7) |
| Neoadjuvant cycle | |||
| 2 | 21 (14.3) | 34 (23.0) | 55 (18.6) |
| 3 | 102 (69.4) | 82 (55.4) | 184 (62.4) |
| 4 | 23 (15.6) | 26 (17.6) | 49 (16.6) |
| 5 | 1 (0.7) | 6 (4.0) | 7 (2.4) |
CIT chemoimmunotherapy, Chemo chemotherapy, TP Platinum + Taxol, TPF Platinum + Taxol + Fluorouracil
Treatments and outcomes
The Chemo group contained 148 patients, and the CIT group 147. All participants underwent a minimum of two cycles of neoadjuvant therapy. In both groups, a significant portion of patients received three cycles of neoadjuvant therapy (102 [69.4%] individuals in the Chemo group and 82 [55.4%] in the CIT group). Within the Chemo group, 81 patients (27.5%) received the TP regimen, and 67 (22.7%) received the TPF regimen. In contrast, within the CIT group, most patients (70, 47.6%) received camrelizumab; meanwhile, 28 patients (19.0%) received sintilimab, making it the second most used drug.
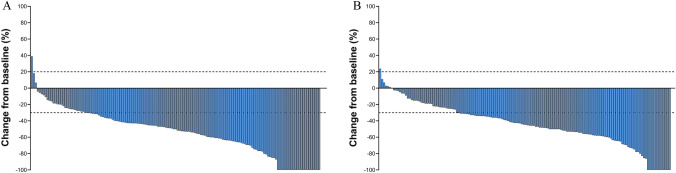
In the CIT group, 119 out of 147 patients exhibited a verified response, culminating in an overall response rate (ORR) of 81.0%. Within this cohort, 22 patients achieved a complete response, accounting for 14.9% of the group, while an impressive 97 patients (65.9%) demonstrated a partial response. Stable disease was reported in 13 patients (18.3%), whereas progressive disease manifested in only one patient (0.6%, Fig. 3A). In parallel, within the CIT group, 108 of the 148 patients responded to the treatment, yielding an ORR of 73%. Within this subset, 12 patients (8.1%) attained a complete response, and 96 (64.8%) experienced a partial response. Moreover, 39 patients (26.3%) were identified with stable disease, while a solitary patient (0.6%) demonstrated progressive disease (Fig. 3B). Noteworthily, the difference in ORR between the two groups was not statistically significant (81.0% vs 73.0%, P = 0.104, Table 2).
Fig. 3.
Waterfall plots constructed based on the RECIST v1.1 criteria to depict the objective radiological response rates in the CIT and Chemo groups
Table 2.
Summary of best overall response as per RECIST v1.1
| CIT (%) (N = 147) | Chemo (%) (N = 148) | P value | |
|---|---|---|---|
| Best overall response | |||
| Complete response | 22 (14.9) | 12 (8.1) | |
| Partial response | 97 (65.9) | 96 (64.8) | |
| Stable disease | 27 (18.3) | 39 (26.3) | |
| Progressive disease | 1 (0.6) | 1 (0.6) | 0.159 |
| ORR | 119 (81.0) | 108 (73.0) | 0.104 |
| Post-neoadjuvant therapy | |||
| Radiotherapy | 132 (89.8) | 126 (85.1) | |
| Surgery + radiotherapy | 15 (10.2) | 22 (14.9) | 0.227 |
| One-year PFS (95% CI) | 80.7 (74.0–88.0) | 67.0 (56.6–79.3) | < 0.001 |
| Two-year PFS (95% CI) | 61.1 (47.4–78.6) | 41.9 (34.5–50.9) | < 0.001 |
| One-year OS (95% CI) | 92.3 (87.6–97.3) | 80.5 (71.5–90.6) | < 0.001 |
CIT chemoimmunotherapy, Chemo chemotherapy, ORR objective response rate, PFS progression-free survival, CI confidence interval, OS overall survival
The mean follow-up durations were 15.0 ± 6.0 months for the CIT group and 18.8 ± 7.1 months for the Chemo group. During the study period, 34 CIT group patients experienced disease recurrence or progression, and there were 11 reported deaths. This led to the observation of a 1-year PFS rate of 80.7% and a 2-year rate of 61.1%. The 1-year OS rate for this group was 92.3% (Fig. 4A, B). Due to the relatively limited follow-up period, the prediction of a 2-year OS rate remained inconclusive. In comparison, the 83 Chemo group patients experienced disease recurrence or progression, and 62 died. Within this group, the 1-year PFS rate was 63.0%, which decreased to 41.9% by the end of the 2-year mark. Correspondingly, the 1-year and 2-year OS rates were 79.5% and 56.7%, respectively (Fig. 4A, B). Strikingly, the CIT group displayed significantly longer PFS than the Chemo group (log-rank test: = 12.362, hazard ratio [HR] = 2.01, P < 0.001). Moreover, the CIT group exhibited a notably superior OS to that of the Chemo group (log-rank test: = 26.245, HR = 4.30, P < 0.001).
Fig. 4.
Kaplan–Meier curves comparing the progression-free survival (PFS, 2A) and overall survival (OS, 2B) of the CIT and Chemo groups
Next, we conducted distinct survival analyses on various subgroups. Within the CIT group, the PFS and OS of patients who had undergone three cycles of neoadjuvant therapy were statistically similar to those of other patients (x2 = 0.059, HR = 0.913, P = 0.809 for PFS; x2 = 0.917, HR = 0.497, P = 0.372 for OS), as illustrated in Fig. 5A, B. Notably, when stratified by tumor differentiation level (low, moderate, and high), there were no noteworthy disparities in PFS (x2 = 2.120, P = 0.347) and OS (x2 = 1.376, P = 0.503), as depicted in Fig. 5C, D. Additionally, we compared the CIT group with the TP and TPF groups. Regarding PFS, the CIT group showcased a significantly better outcome than the TP group (HR = 2.312, P < 0.001), while the CIT and TPF groups had statistically similar PFS (HR = 1.561, P = 0.178). Interestingly, no significant difference in PFS emerged between the TP and TPF groups (HR = 0.696, P = 0.107), as demonstrated in Fig. 6A. Meanwhile, the OS advantage of the CIT group was apparent over both the TP (HR = 4.861, P < 0.001) and TPF (HR = 3.445, P = 0.001) groups. Notably, the OS disparity between the TP and TPF groups was not statistically significant (HR = 0.573, P = 0.107), as illustrated in Fig. 6B.
Fig. 5.
Kaplan–Meier curves comparing the progression-free survival (PFS) and overall survival (OS) according to the number of neoadjuvant cycles (3A, 3B) and degree of differentiation (3C, 3D)
Fig. 6.
Kaplan–Meier curves comparing progression-free survival (PFS, 4A) and overall survival (OS, 4B) in the CIT, platinum + taxol (TP), and platinum + taxol + fluorouracil (TPF) groups
We then searched for potential risk factors that could influence the survival outcomes of enrolled patients who underwent neoadjuvant chemoimmunotherapy (Table 3). Given the limited number of death events observed during the follow-up period, the survival analysis predominantly concentrated on PFS. In the preliminary univariate analysis, several factors emerged as significantly associated with PFS. It is worth noting that univariate Cox regression analysis revealed associations between failure to reduce T stage and shortened progression-free survival (HR = 2.29, P = 0.033) after neoadjuvant therapy. Similarly, there were associations observed between failure to reduce N stage and shortened progression-free survival (HR = 2.70, P = 0.042) as well as between failure to reduce N stage and shortened progression-free survival (HR = 3.15, P = 0.018) after neoadjuvant therapy. However, the multivariate COX regression analysis identified the failure to reduce AJCC stage post-neoadjuvant treatment as an independent risk factor for shorter PFS (HR = 3.15, P = 0.018).
Table 3.
Univariate and multivariate COX regression models for predicting progression-free survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender, male |
/ (/–/) |
0.385 | ||
| Tumor subsite | ||||
| Oropharynx | 1 (Reference) | |||
| Hypopharynx | 1.24 (0.56–2.74) | 0.601 | ||
| Neoadjuvant cycle | ||||
| 3 | 1 (Reference) | |||
| Others | 0.91(0.44–1.91) | 0.810 | ||
| Best overall response | ||||
| ORR | 1 (Reference) | |||
| Stable disease + progressive disease | 1.58 (0.75–3.30) | 0.226 | ||
| T stage change | ||||
| Down | 1 (Reference) | 1 (Reference) | ||
| Same | 2.29 (1.07–4.92) | 0.033 | 1.50 (0.63–3.58) | 0.364 |
| N-stage change | ||||
| Down | 1 (Reference) | 1 (Reference) | ||
| Same | 2.70 (1.04–7.02) | 0.042 | 1.15(0.22–6.10) | 0.874 |
| AJCC-stage change | ||||
| Down | 1 (Reference) | 1 (Reference) | ||
| Same | 3.15 (1.22–8.16) | 0.018 | 3.15 (1.22–8.16) | 0.018 |
ORR objective response rate
Discussion
The global burden of HNSCC is undeniably substantial, with an alarming prevalence that continues to rise. The current standard of care for locally advanced HNSCC entails the option of surgical resection, complemented by risk-adapted adjuvant radiotherapy with or without platinum-based chemotherapy [12]. Alternatively, a definitive approach involves concurrent chemoradiotherapy. Employing this robust and comprehensive combined modality therapy, the persistently suboptimal outcomes of elevated recurrence risk, distant metastasis occurrence, and mortality (as reflected by the 5-year survival rate) continue to plague patients afflicted with locally advanced HNSCC [13, 14]. This menacing trajectory has spurred urgent efforts to revolutionize therapeutic approaches. The advent of immunotherapy, epitomized by the success of PD-1 inhibitors, has ignited a renaissance in the management of HNSCC, prompting the exploration of neoadjuvant applications [15, 16].
Herein, we undertook an exhaustive investigation of neoadjuvant chemoimmunotherapy for resectable OPHSCC, contributing to the burgeoning literature aimed at delineating effective strategies to combat these formidable diseases. By leveraging a robust cohort size, we have generated compelling data that hold crucial implications for clinical practice and future research directions. Our findings reaffirm the premise that neoadjuvant chemoimmunotherapy represents a pivotal advancement in the treatment of OPHSCC. The substantial response rates observed in the chemoimmunotherapy group (ORR = 81.0%) underscore the potency of this therapeutic paradigm. This is further accentuated by the impressive complete response rate of 14.9% and partial response rate of 65.9%. Importantly, these outcomes are not only encouraging on their own but also substantiated by the statistically superior PFS and OS of the CIT group over the Chemo group. The robustness of our results is augmented by the inclusion of a parallel cohort undergoing neoadjuvant chemotherapy, allowing for a meaningful comparative analysis. Moreover, the ORR within the CIT cohort (81.0%) is consistent with other investigations (57–96.7%) focusing on neoadjuvant immunotherapeutic interventions for HNSCC [17–21]. Noteworthily, the CIT group had a higher—albeit not significant—ORR than the Chemo group.
Of particular interest is the observed PFS advantage conferred by neoadjuvant chemoimmunotherapy. This is reflected in the 1-year PFS rate of 80.7% and the 2-year rate of 61.1% in the CIT group, compared with 63.0% and 41.9% in the Chemo group. The HR of 2.01 unequivocally highlights the clinical significance of this finding. The disparity in PFS is not surprising, given the dual mechanism of action inherent to chemoimmunotherapy. The cytotoxic agents exert a direct impact on tumor cells, while the immune checkpoint inhibitors unleash the body’s immune system against the malignancy. The synergy between these modalities likely contributes to the more durable responses observed in the CIT group.
Equally striking is the substantial improvement in OS conferred by neoadjuvant chemoimmunotherapy. The chemoimmunotherapy group demonstrated a 1-year OS rate of 92.3%, underscoring the potential of this treatment regimen to confer significant survival benefits. Although the prediction of a 2-year OS rate remains inconclusive due to the limited follow-up duration, the magnitude of the observed effect is noteworthy. The HR of 4.30 reflects a profound impact on OS, further validating the therapeutic potential of neoadjuvant chemoimmunotherapy. This profound effect on both PFS and OS is a testament to the synergistic action of paclitaxel, platinum agents, and PD-1 inhibitors in combating HNSCC. Similarly, the data for PFS and OS align with findings from other investigations exploring neoadjuvant immunotherapeutic interventions [22–24]. An innovative facet of our investigation lies in the comparative analysis between the CIT and Chemo groups. Interestingly, while the ORR of the CIT and Chemo cohorts was not statistically different, it is noteworthy that the CIT group exhibited significantly improved PFS and OS over the Chemo group.
The toxicity of the chemotherapy and immunotherapy combination is a subject worthy of discussion. A comparative analysis of data from the literature encompassing a large sample size revealed that in squamous cell carcinoma of the head and neck, the incidence of grade 3–5 treatment-related adverse events in patients treated with chemotherapy combined with immunotherapy was 85%, and anemia was the only event with an occurrence rate above 20% (25%). Meanwhile, in the chemotherapy-only group, the incidence of grade 3–5 treatment-related adverse events was 83%, with two events exceeding a 20% occurrence rate, namely neutropenia (83%) and oral mucositis (21%). Notably, the occurrence rate of neutropenia was significantly lower in the chemotherapy combined with immunotherapy group (18%) than in the chemotherapy-only group (83%), possibly due to the immunomodulatory effects of the immunotherapy agents [25–27]. These results suggest that combining chemotherapy with immunotherapy does not increase toxicity, and patients exhibit favorable tolerance. A phase II clinical study by Zhang et al. [17] also provided compelling evidence: among the 30 enrolled patients with HNSCC undergoing neoadjuvant chemoimmunotherapy, there were no grade 4 or 5 neoadjuvant-related adverse events. The proportion of grade 3 neoadjuvant-related adverse events was also only 10% (one case of rash [3.3%], one case of pruritus [3.3%], and one case of thrombocytopenia [3.3%]). Importantly, no patients discontinued neoadjuvant therapy due to neoadjuvant-related adverse events. However, the retrospective and exploratory nature of this study limits its assessment of the toxicity differences between the CIT and the Chemo groups. Nevertheless, based on clinical experience and findings from other studies [17, 27], the combination of immunotherapy with chemotherapy for neoadjuvant treatment is considered a well-tolerated therapeutic approach.
On a separate note, the outcomes of our multifactorial regression analysis reveal a notable enhancement in PFS among patients experiencing a decline in AJCC staging after neoadjuvant treatment. This finding could become a tool for anticipating the immunotherapeutic efficacy of neoadjuvant chemotherapy, further aiding in the formulation of individualized patient treatment strategies: For patients who experience failure to downstage AJCC post-neoadjuvant treatment, a more proactive approach should be considered for their subsequent surgical or radiation therapy plans: higher radiation therapy doses or a larger extent of neck lymph node clearance should be considered. Additionally, the follow-up care for such patients should be more intensive, for example, by including shorter follow-up intervals and a more proactive approach to investigating suspicious lesions, such as more aggressive biopsies.
Immunotherapy has emerged as a promising avenue in the field of oncology, offering encouraging results in the treatment of various malignancies. However, despite the remarkable strides made, several challenges persist in the clinical application of immunotherapeutic agents, including immunotherapy resistance and acquired drug resistance. In response to these challenges, recent studies have proposed the utilization of bispecific antibodies targeting both TGF-β and PD-L1 (such as BiTP and YM101) [28–31]. These novel agents have demonstrated notable efficacy, potentially augmenting the immunotherapeutic landscape for OPHSCC.
Our study is not devoid of limitations. The observational and retrospective design inherently poses challenges in attributing causality, despite our rigorous statistical analyses. Additionally, the limited number of patients undergoing HPV testing has precluded us from conducting an analysis on the impact of HPV status on treatment outcomes. Furthermore, while the use of different PD-1 inhibitors did not influence outcomes in our study, the landscape of immunotherapy is rapidly evolving, and future studies could explore potential differences in response rates and outcomes associated with newer agents. Additionally, the relatively limited follow-up duration underscores the need for long-term data to corroborate our findings.
In conclusion, our study underscores the transformative potential of neoadjuvant chemoimmunotherapy in OPHSCC. The substantial improvement in PFS and OS observed in the CIT group, along with the impressive response rates, substantiates the notion that this approach holds promise in reshaping the therapeutic landscape of these malignancies. By contributing to the empirical evidence base, our study provides valuable insights that not only have immediate clinical relevance but also set the stage for future investigations. Prospective studies with extended follow-up duration and consideration of evolving immunotherapeutic agents are warranted to validate and refine these findings. Collectively, these endeavors will inevitably propel us toward a future where improved outcomes and enhanced survival become the hallmark of OPHSCC treatment strategies.
Acknowledgements
None.
Author contributions
PFX and QF designed the study, analyzed the data, and drafted the work. ZZ was involved in acquisition of data. FC interpreted the data. XKL and DW supervised the study and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Ethical endorsement for the investigation was obtained from the Institutional Ethical Committee, affirming its adherence to established ethical guidelines (ethical number: B2023-481-01). Given the observational and retrospective design of this study, the necessity for explicit informed consent was deemed unnecessary and thus waived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengfei Xu and Qi Fang have contributed equally to this work.
Di Wu and Xuekui Liu jointly supervised this work.
Contributor Information
Di Wu, Email: wudi1@sysucc.org.cn.
Xuekui Liu, Email: liuxk@sysucc.org.cn.
References
- 1.Psyrri A, Fayette J, Harrington K, et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: Kestrel, a randomized, open-label, phase III study. Ann Oncol. 2023;34:262–274. doi: 10.1016/j.annonc.2022.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Yang Y, Wang R, Fang J. Neoadjuvant PD-1/PD-L1 inhibitors combined with chemotherapy had a higher ORR than mono-immunotherapy in untreated HNSCC: Meta-analysis. Oral Oncol. 2023;145:106479. doi: 10.1016/j.oraloncology.2023.106479. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: a 2023 update. Drugs. 2023;83:217–248. doi: 10.1007/s40265-023-01835-2. [DOI] [PubMed] [Google Scholar]
- 5.Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 6.Stelmes JJ, Vu E, Grégoire V, et al. Quality assurance of radiotherapy in the ongoing EORTC 1420 “Best of” trial for early stage oropharyngeal, supraglottic and hypopharyngeal carcinoma: Results of the benchmark case procedure. Radiat Oncol. 2021;16:81. doi: 10.1186/s13014-021-01809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington KJ, Burtness B, Greil R, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the Phase III KEYNOTE-048 study. J Clin Oncol. 2023;41:790–802. doi: 10.1200/JCO.21.02508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darragh LB, Knitz MM, Hu J, et al. A phase I/Ib trial and biological correlate analysis of neoadjuvant SBRT with single-dose durvalumab in HPV-unrelated locally advanced HNSCC. Nat Cancer. 2022;3:1300–1317. doi: 10.1038/s43018-022-00450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal C, Prawira A, Antonia S, et al. Dual checkpoint targeting of B7–H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022 doi: 10.1136/jitc-2021-004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht M, Eckstein M, Rutzner S, et al. Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer. J Immunother Cancer. 2022 doi: 10.1136/jitc-2021-003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin N, Maroun CA, El Asmar M, et al. Neoadjuvant immunotherapy prior to surgery for mucosal head and neck squamous cell carcinoma: systematic review. Head Neck. 2022;44:562–571. doi: 10.1002/hed.26935. [DOI] [PubMed] [Google Scholar]
- 12.Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–683. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 14.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Wu B, Peng G, et al. Neoadjuvant chemoimmunotherapy for the treatment of locally advanced head and neck squamous cell carcinoma: a single-arm Phase 2 clinical trial. Clin Cancer Res. 2022;28:3268–3276. doi: 10.1158/1078-0432.CCR-22-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SA, Gibson MK, Deal A, et al. A phase 2 study of neoadjuvant chemotherapy plus durvalumab in resectable locally advanced head and neck squamous cell carcinoma. Cancer. 2023 doi: 10.1002/cncr.34930. [DOI] [PubMed] [Google Scholar]
- 19.Vos JL, Elbers JBW, Krijgsman O, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun. 2021;12:7348. doi: 10.1038/s41467-021-26472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris RL, Spanos WC, Leidner R, et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Immunother Cancer. 2021 doi: 10.1136/jitc-2021-002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leidner R, Crittenden M, Young K, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer. 2021 doi: 10.1136/jitc-2021-002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redman JM, Friedman J, Robbins Y, et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-β blockade in HPV-unrelated head and neck cancer. J Clin Invest. 2022 doi: 10.1172/JCI161400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uppaluri R, Campbell KM, Egloff AM, et al. Correction: Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter. Phase II trial Clin Cancer Res. 2021;27:357. doi: 10.1158/1078-0432.CCR-20-4484. [DOI] [PubMed] [Google Scholar]
- 24.Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a Phase 2 open-label randomized clinical trial. JAMA Oncol. 2020;6:1563–1570. doi: 10.1001/jamaoncol.2020.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 26.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 27.Yu WD, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66–70. doi: 10.1016/j.canlet.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Yi M, Zhang J, Li A, Niu M, Yan Y, Jiao Y, Luo S, Zhou P, Wu K. The construction, expression, and enhanced anti-tumor activity of YM101: A bispecific antibody simultaneously targeting TGF-beta and PD-L1. J Hematol Oncol. 2021;14:27. doi: 10.1186/s13045-021-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi M, Niu M, Zhang J, et al. Combine and conquer: Manganese synergizing anti-TGF-beta/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J Hematol Oncol. 2021;14:146. doi: 10.1186/s13045-021-01155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi M, Niu M, Wu Y, et al. Combination of oral STING agonist MSA-2 and anti-TGF-beta/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol. 2022;15:142. doi: 10.1186/s13045-022-01363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi M, Wu Y, Niu M, Zhu S, Zhang J, Yan Y, Zhou P, Dai Z, Wu K. Anti-TGF-beta/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer. 2022 doi: 10.1136/jitc-2022-005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.