Abstract
Objective
Chemoradiotherapy (CRT) is the standard treatment for limited-stage small cell lung cancer (LS-SCLC), which can exert anti-tumor effects by regulating immune cells. Different immune cell subsets are associated with a specific sensitivity to CRT. The purpose of this study was to characterize the proportion or composition of peripheral lymphocytes in patients with LS-SCLC before and after CRT, and evaluate their prognostic value.
Methods
A total of 98 patients with LS-SCLC were enrolled. The expression of CD3, CD4, CD8, CD45RA, CD45RO, CD38, CD56, and CD19 on the surface of peripheral blood cells was detected by flow cytometry and retrospectively analyzed. The relationship between the proportion of lymphocyte subsets, progression-free survival (PFS), and overall survival (OS) was evaluated using a log-rank test and Cox regression model.
Results
The median PFS was 12.3 months and the median OS was 21.7 months. Compared with the pre-treatment specimens, post-treatment lymphocytes had increased proportions of CD3+, CD3+CD8+, CD8+CD38+ T cells, and NKT cells, and a decreased proportion of CD3+CD4+ T cells, CD4+CD45RA+ T cells, B cells, NK cells, and CD4/CD8 ratio. Univariate and multivariate analyses showed that prophylactic cranial irradiation, high percentages of CD4+CD45RA+, CD8+CD38+ T cells after CRT independently predicted superior PFS. Male patients with a high baseline CD4+CD45RO+ T cell ratio predicted a poor OS.
Conclusions
CRT induced changes in the proportion of circulating lymphocyte subsets in LS-SCLC, which is helpful for designing a regimen of immune drugs to be combined with CRT. The prognostic value of the proportion of lymphocytes aids in understanding the role of peripheral immune profiles in LS-SCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02902-x.
Keywords: Small cell lung cancer, Lymphocyte subsets, Chemoradiotherapy
Introduction
Small cell lung cancer (SCLC) is the most malignant and aggressive subtype of lung cancer, exhibiting the characteristics of rapid proliferation, undifferentiated cells, and high invasiveness, accounting for 15% of all newly diagnosed lung cancer cases [1]. Although SCLC is sensitive to first-line chemoradiotherapy (CRT), 80% patients eventually relapse or develop tolerance to treatment. Moreover, the five-year overall survival rate of the patients with limited-stage SCLC (LS-SCLC) is approximately 15−20%, while that of patients with extensive-stage SCLC (ES-SCLC) is only 1−2% [2].
Although there have been multiple attempts to conduct targeted therapy in SCLC patients, it has been associated with poor outcomes. Recently, immunotherapy has achieved success as a treatment for different types of tumors. SCLC is a type of neuroendocrine carcinoma with a high tumor mutation burden, suggesting that patients may benefit more from immunotherapy. Two studies have changed the pattern of first-line treatment for ES-SCLC [3, 4]. However, at present, immunotherapy alone is insufficient to improve the clinical outcome in most patients, emphasizing the need to develop combination strategies [5–7]. The combination of immune checkpoint inhibitors (ICIs) and CRT is currently under investigation for LS-SCLC (NCT03811002; NCT02402920; NCT04189094; NCT03540420; NCT03585998; NCT03703297, https://www.clinicaltrials.gov). Unfortunately, due to tumor development and heterogeneity, the interaction between first-line therapy may affect the tumor's response to the subsequent administration of ICIs. Therefore, it is extremely important to understand the effects of first-line CRT on immune cells, especially regarding the composition of active cytotoxic cells. A key challenge in the study of SCLC is the difficulty in obtaining tumor tissue. Compared with obtaining a tumor biopsy, the peripheral blood is easier to be monitored and dynamically analyzed; however, the key question is whether circulating immune cells can replace the measurement of tumor-host immune cell interactions. Recently, Krieg et al. [8] showed that the frequency of specific peripheral blood immune cells can be used as a non-invasive pretreatment index of the immunotherapy response in patients with melanoma cancer. The study by Griffiths et al. [9] found that the phenotype of immune cells in the peripheral blood reflects the interaction between tumor and immune cells, and can reliably reveal the response of gastrointestinal cancer patients to immunotherapy. In addition, several studies have indicated that T cell clone sizes in the peripheral blood are significantly correlated with the intratumor clones, and blood-expanded specific clones may play a role in both tumor infiltration and differentiation into other T cell phenotypes [10, 11]. There has been increased development of peripheral immune-based biomarkers that may play a clinically important role.
In this study, we evaluated the changes in the proportion of circulating T, B, natural killer (NK), and natural killer T (NKT) cells after first-line chemoradiotherapy for LS-SCLC patients. This study also aimed to assess the prognostic value of the observed changes.
Materials and methods
Study population
The study population included patients who were diagnosed with LS-SCLC and received CRT at Zhejiang Cancer Hospital from December 2010 to July 2019. The determination of the proportion of lymphocyte subsets is a routine test, which is performed in almost all patients with lymphoma and in other tumor patients on a voluntary basis as informed by the doctor. Present study focused on the peripheral immune status of patients with LS-SCLC. Immune data before any treatment and three months after CRT were collected retrospectively. The following inclusion criteria were used for patients in this study: 1) LS-SCLC patients confirmed by pathology or cytology; 2) good physical condition (KPS ≥ 80); 3) normal heart, kidney, and liver function; 4) standard platinum-based induction chemotherapy and thoracic radiotherapy; 5) complete lymphocyte subsets and follow-up data. The exclusion criteria included patients who: 1) were suffering from other types of malignant tumors or 2) receiving hormone treatment and other treatment (targeted therapy or surgery); 3) had an additional chronic disease (especially hepatitis or HIV), inherent or acquired immunodeficiency, and autoimmune disorders. This study was approved by the Zhejiang Cancer Hospital Ethics Committee. The requirement of informed consent was waived by the committee as it was a retrospective research. All data collection was carried out in accordance with the approved guidelines.
Clinicopathological variables and laboratory parameters
The clinicopathological data collected for analysis included the age at diagnosis, gender, smoking history, tumor site, chemotherapy regimen and cycle, radiotherapy dose and frequency, prophylactic craniocerebral irradiation (PCI) treatment. Laboratory data collected for analysis were the proportion of pre- and post-CRT peripheral lymphocytes, including T cells defined by CD3 expression (CD3+ T cells), B cells by CD19 expression (CD19+ B cells). T-lymphocyte subsets were identified by the presence of CD4 (CD3+CD4+ T cells) and CD8 (CD3+CD8+ T cells). CD45RA and CD45RO were used to identify CD4+ naïve (CD4+CD45RA+) and memory (CD4+CD45RO+) T cells. CD3 and CD56 together facilitate identification of the NK (CD3−CD56+ cells) and NKT (CD3+CD56+ cells) lymphocyte population. Activated CD8+ T cells were labeled with CD38 positive (CD8+CD38+ T cells). The clinical efficacy of CRT was evaluated based on computed tomography (CT) or magnetic resonance imaging (MRI). The short-term clinical efficacy according to the solid tumor response evaluation criteria (RECIST) included the complete response (CR), partial response (PR), stable disease (SD) and disease progression (PD). The long-term effects were evaluated based on the OS through following up by telephone.
Immunophenotyping of the peripheral blood immune cells
According to the protocol, the steps of lymphocyte subgroups staining were performed as follows: EDTA anticoagulant vacuum vascular collection (#367841, BD Biosciences) was used to collect 2 mL peripheral blood. Five flow cytometry tubes (#352053, BD Biosciences) were prepared for each peripheral blood sample, and 20 uL CD3-FITC/CD56-PE, CD8-FITC/CD38-PE, CD4-FITC/CD45RA-PE/CD45RO-APC, CD19-FITC, FITC/PE/APC isotype controls were added into five tube separately. Then 100 uL blood was added to each tube, mixed with the antibodies, and incubated at room temperature in the dark. After 30 min, 2 mL of hemolytic agent (#70-LSB3, BD Biosciences) was added into each tube. After 5 min, the supernatant was removed by centrifugation (600 × g) for 5 min, washed twice with phosphate buffer saline (PBS) (#SH300256, Hyclone) and suspended in 0.5 mL 2% paraformaldehyde. The cells were detected by flow cytometry (FACSVia, BD Biosciences). For each tube, the flow cytometer collected more than 2000 cells within the lymphocyte gate. The percentages of positively labeled lymphocytes were analyzed using Cellquest software (BD Biosciences). The above staining procedures were completed and analyzed within 24 h after blood collection. Antibodies were purchased from BD Biosciences (San Jose, CA, USA): CD3-FITC (#555332), CD4-FITC (#550628), CD8-FITC (#555366), CD56-PE (#55664), CD19-FITC (#555412), CD45RA-PE (#555489), CD45RO-APC (#559865), CD38-PE (#555460), FITC/PE/APC isotype controls (#555748; #555749; #555576). According to the literature [12] and the flow cytometry detection platform of our hospital, the normal reference ranges were defined as follows: CD3+ T cells (60.0–84.0%), CD3+CD4+ T cells (29.0–60.0%), CD4+CD45RA+ T cells (15.5–25.0%), CD4+CD45RA− T cells (9.8–26.0%), CD4+CD45RO+ T cells (9.8–26.0%), CD45RA/CD45RO (1.2–5.8), CD3+CD8+ T cells (12.0–38.0%), CD8+CD38+ T cells (2.3–15.0%), CD4/CD8 (1.3–3.5), CD19+ B cells (7.0–22.0%), CD3−CD56+ NK cells (6.0–30.0%), CD3+CD56+ NKT cells (0.5–5.0%).
Statistical analysis
Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease recurrence or metastasis. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or the date of the last follow-up. Normality of distribution was tested by the Kolmogorov–Smirnov test. All results are presented as median percentages with interquartile ranges of positive lymphocytes. Wilcoxon test for associated samples was used to detect different percentages of immunocytes before and after CRT. Changes in the proportion of lymphocytes before and after CRT was defined as Δ lymphocyte = post-CRT lymphocyte proportion / baseline lymphocyte proportion. Mann–Whitney U tests were used to evaluate the relationship between Δ lymphocyte and CRT characteristics. In the prognostic analysis, we used the median proportion of lymphocyte subsets as the threshold to define the low and high level. The cox proportional hazards regression model was used to conduct univariate and multivariate analysis. A log-rank test was performed to compare Kaplan–Meier survival curves. All statistical analyses were calculated using SPSS software version 22.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 5 version 5.01 (Graph Pad Software Inc., San Diego, CA, USA). P value less than 0.05 was considered statistically significant.
Results
Patient characteristics
Of the 98 patients with LS-SCLC, 79 (80.6%) were male and 77 (78.6%) patients had a history of smoking. The median age was 62 years old and all patients completed CRT. The median number of chemotherapy cycles was 4.5 cycles (2–6 cycles). There were 86 (87.8%) patients who were treated with the cisplatin plus etoposide (EP) regimen, and 12 (12.2%) cases received carboplatin plus etoposide (EC). Three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) was used. The target volumes included post-chemotherapy primary tumor and positive lymph node regions. The treatment plan required a 95% prescription dose curve to include more than 95% planning target volume (PTV). A total of 49 (50%) patients received 1.5 Gy thoracic radiotherapy twice daily (Bid), 38 (38.8%) received 2.0 Gy once daily (Qd), and 11 (11.2%) received 3 Gy Qd. The patient characteristics are detailed in Table 1.
Table 1.
Clinical features of 98 patients with LS-SCLC
| Characteristic | N (%) or median range | Characteristic | N (%) or median range |
|---|---|---|---|
| Gender | 3.0 | 11 (11.2) | |
| Male | 79 (80.6) | RT frequency | |
| Female | 19 (19.4) | Qd | 49 (50.0) |
| Age (years) median (range) | 62 (38–75) | Bid | 49 (50.0) |
| Smoking history | RT fraction | ||
| Non-smoker | 21 (21.4) | Median (range) | 30 (15–36) |
| Smoker | 77 (78.6) | Total dose of RT (Gy) | |
| Primary tumor site | Median (range) | 45 (43.5–60) | |
| Left | 51 (51.0) | CRT method | |
| Right | 47 (48.0) | Concurrent | 64 (65.3) |
| Chemo-regimen | Sequential | 34 (34.7) | |
| EP | 86 (87.8) | CRT efficiency | |
| EC | 12 (12.2) | CR | 31 (31.6) |
| Chemo-cycle | PR | 60 (61.2) | |
| Median (range) | 4.5 (2–6) | SD | 7 (7.2) |
| RT dose/fraction (Gy) | PCI treatment | ||
| 1.5 | 49 (50.0) | No | 23 (23.5) |
| 2.0 | 38 (38.8) | Yes | 75 (76.5) |
Chemo-regimen Chemotherapy regimen; Chemo-cycle Chemotherapy cycle; EP Cisplatin plus Etoposide; EC Carboplatin plus Etoposide; RT Radiotherapy; Qd Once daily; Bid Twice daily; CRT Chemoradiotherapy; PCI Prophylactic cranial irradiation
Changes in the lymphocyte subpopulation in the peripheral blood after chemoradiotherapy
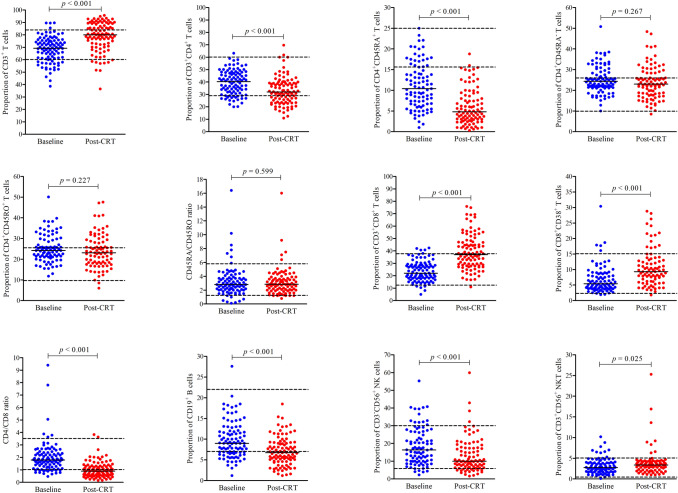
As shown in Fig. 1 and Table 2, compared with the baseline, the proportion of CD3+ T cells (p < 0.001), CD3+CD8+ T cells (p < 0.001), CD8+CD38+ T cells (p < 0.001), and CD3+CD56+ NKT cells (p = 0.025) were increased after CRT, while the percentage of CD3+CD4+ T cells (p < 0.001), CD4+CD45RA+ T cells (p < 0.001), CD4/CD8 ratio (p < 0.001), CD19+ B cells (p < 0.001), and CD3−CD56+ NK cells (p < 0.001) were decreased. No differences were observed in the proportion of CD4+CD45RA− T cells (p = 0.267), CD4+CD45RO+ T cells (p = 0.072), and CD45RA/CD45RO ratio (p = 0.337) before and after CRT. Moreover, we found that the median proportion of CD3+ T cells, CD4+ T cells, CD8+ T cells, CD8+CD38+ T cells, NK and NKT cells before and after CRT increased or decreased within the normal range. However, the percentages of baseline circulating naïve CD4+ (CD4+CD45RA+) T cells in 70/93 (75.3%) patients were lower than the reference range, after CRT, the proportion of naïve CD4+ T cells in 90/93 (96.8%) patients was low, and the median decreased further. The ratio of baseline CD4/CD8 in 65/98 (66.3%) patients was normal, but after treatment, the CD4/CD8 ratio of most patients (71/98, 72.4%) was lower than the normal value. Similar to the CD4/CD8 ratio, we observed that the B cell proportion was lower than normal in 52/98 (53.1%) patients after treatment. These results suggested that CRT leads to complex changes in the composition of peripheral lymphocytes in LS-SCLC.
Fig. 1.
Percentages of lymphocyte subsets in peripheral blood before and after chemoradiotherapy in LS-SCLC patients. A two related sample nonparametric test was used for statistical analysis and the charts represented the median % (min to max) deviation for the proportion of each lymphocyte during treatment. The baseline values represented those prior to any treatment. Post-CRT represented the values at three months after chemoradiotherapy. The dotted line represents the normal reference range
Table 2.
Chemoradiotherapy-induced alterations of the proportion of each circulating lymphocyte subpopulation for all patients
| Variables | Baseline | Post-CRT | p |
|---|---|---|---|
| T cells | |||
| CD3+ | 69.20 [60.98; 75.33] | 80.0 [72.28; 88.63] | .000** |
| CD3+CD4+ | 40.30 [31.70; 47.40] | 32.0 [24.53; 39.13] | .000** |
| CD4+CD45RA+ | 10.4 [6.70; 15.20] | 4.80 [2.90; 8.30] | .000** |
| CD4+CD45RA− | 24.30 [21.30; 28.90] | 23.0 [17.80; 28.40] | .267 |
| CD4+CD45RO+ | 24.20 [21.10; 29.40] | 23.10 [17.98; 28.40] | .227 |
| CD45RA/CD45RO | 2.80 [2.00; 3.70] | 2.85 [2.00; 3.70] | .599 |
| CD3+CD8+ | 22.05 [17.88; 28.76] | 37.00 [28.48; 47.15] | .000** |
| CD8+CD38+ | 5.35 [3.98; 8.60] | 9.30 [6.20; 13.05] | .000** |
| CD4/CD8 | 1.80 [1.20; 2.30] | 0.90 [0.60; 1.30] | .000** |
| CD19+ B cells | 9.00 [6.78; 11.85] | 6.75 [4.68; 9.05] | .000** |
| CD3−CD56+ NK cells | 16.35 [10.00; 26.50] | 10.00 [6.68; 17.45] | .000** |
| CD3+CD56+ NKT cells | 2.75 [1.78; 4.00] | 3.35 [2.20; 4.23] | .025* |
Data are shown as median [interquartile range] %; Baseline represents before any treatment; Post-CRT represents three months after chemoradiotherapy. *p ≤ 0.05; **p ≤ 0.001
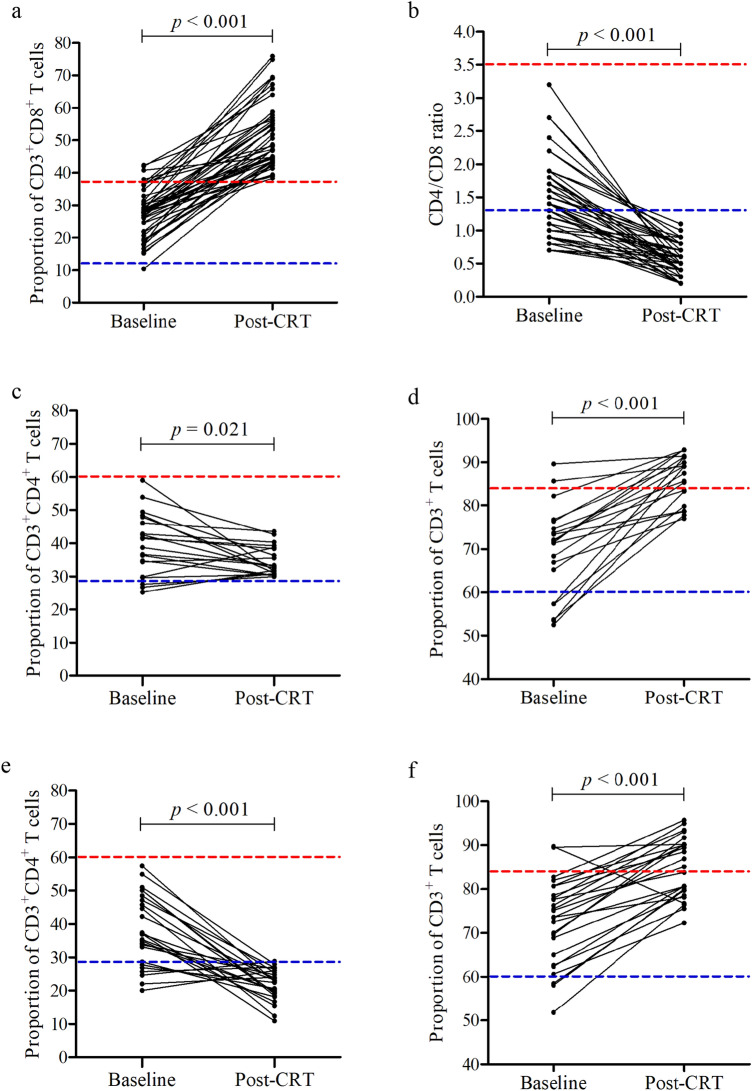
Of note, CD8+ T cells mainly differentiate into cytotoxic T cells (CTLs), which directly kill tumor cells, so we focused on the changes of CD8+ T cells after first-line therapy. After CRT, the proportion of CD8+ T cells in peripheral lymphocytes of 45/98 (45.9%) patients was higher than 38% (normal reference range, 12.0–38.0%), while the proportion of baseline CD8+ T cells in only 4/98 (4.1%) patients was over 38% (Fig. 2a). In addition, we found that the CD4/CD8 ratios of these 45 patients were all less than 1.3 (normal reference range, 1.3–3.5) after CRT (Fig. 2b). As we all know, the low CD4/CD8 ratio is due to the low proportion of CD4+ T cells and / or the high proportion of CD8+ T. Among the 45 patients with high proportion of CD8+ T cells after CRT, 20/45 (44.4%) patients had normal percentage of CD4+ T cells (Fig. 2c), the proportion of CD4+ T cells in the remaining 25/45 (55.6%) patients after CRT was less than 29.0% (normal reference range 29.0–60.0%) (Fig. 2e). Regardless of whether the CD4+ T cell ratio was normal or low, the total CD3+ T cell ratio of these patients was more than 60% after CRT (normal reference range, 60.0–84.0%) (Fig. 2d, f). The above results suggest that three months after CRT, the overall immune function of the patients is normal, the higher proportion of CD8+ T cells may be due to the production of more CTLs in the immune system response after treatment, which needs further study on the absolute counts of CD8+ T cells.
Fig. 2.
The proportion of circulating high CD8+ T cells in 45 patients. After chemoradiotherapy, a the proportion of peripheral CD8+ T cells in 45 patients was higher than the normal value (12.0–38.0%), and b the CD4/CD8 ratio was lower than reference range (1.3–3.5%). Among them, the percentages of CD4+ T cells were c normal in 20 cases and e low in 25 cases (normal range, 29.0–60.0%), d, f the proportion of CD3+ T cells in all patients was normal or higher than 84.0% (60.0–84.0%). The red dotted line represents the normal high value and the blue dotted line represents the normal low value
Correlation between the percentage of lymphocyte subsets and the characteristics of chemoradiotherapy
Changes in the lymphocytes before and after CRT may be affected by chemotherapy and/or radiotherapy. As shown in Table 3, the results indicated that the change in the percentage of circulating CD8+CD38+ T cells was related to different chemotherapy regimens. Compared with the EC chemo-regimen, the percentage of CD8+CD38+ T cells in patients treated with EP regimen increased more significantly after CRT (EP vs EC, p = 0.047). Compared with sequential CRT, concurrent CRT caused more substantial damage to circulating CD19+ B cells, resulting in a significant decrease in the percentage of B cells after CRT (Concurrent CRT vs sequential CRT, p < 0.001). However, no significant correlations were observed between the fractionated dose and frequency of thoracic radiotherapy with the changes in the composition of circulating lymphocyte subsets. Figure 3 further showed the relationship between the changes of CD8+CD38+ T cells or CD19+ B cells and the features of CRT.
Table 3.
Correlation between lymphocyte subpopulation changes and chemoradiotherapy characteristics
| Variables | Chemo-regimen (EP vs EC) | Chemo-cycle (≤ 4 vs > 4) | RT dose/fraction (1.5 vs 2.0 Gy) | RT frequency (Qd vs Bid) | CRT method (concurrent vs sequential) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| z | p | z | p | z | p | z | p | z | p | |
| Δ CD3+ T cell | − .932 | .351 | − .728 | .466 | − .043 | .966 | − .082 | .935 | − .582 | .560 |
| Δ CD3+CD4+ T cell | − .921 | .357 | − .359 | .720 | − 1.623 | .105 | − 1.311 | .190 | − .291 | .771 |
| Δ CD4+CD45RA+ T cell | − .238 | .812 | − .815 | .415 | − .363 | .716 | − .088 | .929 | − .251 | .802 |
| Δ CD3+CD8+ T cell | − .314 | .753 | − .828 | .408 | − 1.099 | .363 | − 1.091 | .275 | − .649 | .516 |
| Δ CD8+CD38+ T cell | − 1.985 | .047* | − .334 | .738 | − .207 | .836 | − .044 | .965 | − .146 | .884 |
| Δ CD4/CD8 ratio | − .580 | .562 | − .837 | .403 | − 1.352 | .176 | − 1.284 | .199 | − .636 | .525 |
| Δ CD19+ B cell | − 1.763 | .083 | − .434 | .664 | − .559 | .576 | − .185 | .853 | − .352 | .000** |
| Δ CD3−CD56+ NK cell | − .464 | .643 | − .762 | .446 | − .396 | .692 | − .227 | .821 | − 1.355 | .175 |
| Δ CD3+CD56+ NKT cell | − .743 | .458 | − .1609 | .108 | − .070 | − 1.221 | − .822 | .411 | − 1.001 | .317 |
Chemo-regimen Chemotherapy regimen; Chemo-cycle Chemotherapy cycle; RT Radiotherapy; CRT Chemoradiotherapy; EP Cisplatin plus Etoposide; EC Carboplatin plus Etoposide; Qd Once daily; Bid Twice daily. *p ≤ 0.05; **p ≤ 0.001
Fig. 3.
The relationship between the changes of a CD8+CD38+ T cells and b CD19+ B cells and the factors of chemoradiotherapy
Correlation between immune parameters and PFS
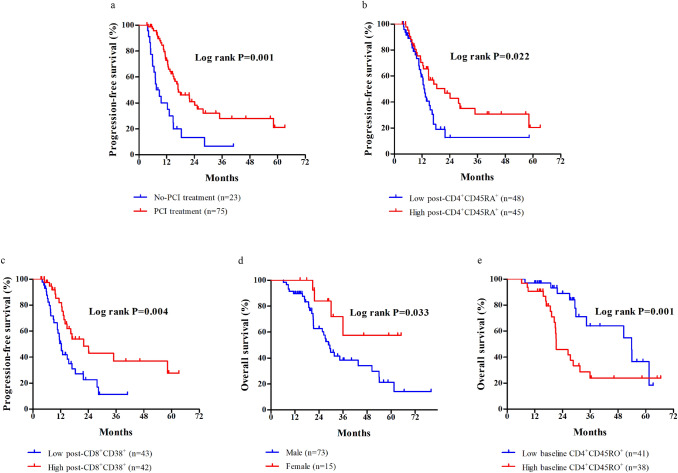
The median PFS was 12.3 months (range: 3.5−63.2 months). Local recurrence was observed with a median time to recurrence of 13.3 months (range: 4−35 months) in 28 (28.6%) patients. Distant metastasis occurred in 32 (32.7%) cases, of which 19 cases were brain metastasis. The median time of metastasis is 9.2 months (range: 4−58 months). In the univariate analysis, PCI treatment (p < 0.001), post-CD4+CD45RA+ T cells (p = 0.024), post-CD8+CD38+ T cells (p = 0.006), and post-CD3−CD56+ NK cells (p = 0.043) were associated with PFS (Table S1). However, only PCI (p = 0.003), the proportion of post-CD4+CD45RA+ T cells (p = 0.004), and post-CD8+CD38+ T cells (p = 0.015) were independent predictors of PFS according to the multivariate analysis (Table S2). Patients with high percentages of CD4+CD45RA+ (≥ 4.8%) and CD8+CD38+ (≥ 9.3%) T cells after CRT and received PCI treatment had a significant longer median PFS than those without PCI treatment and had low proportion of CD4+CD45RA+ and CD8+CD38+ T cells (Fig. 4a−c).
Fig. 4.
Progression-free survival (PFS) and overall survival (OS) of patients with LS-SCLC. Stratifications were based on the median proportation of lymphocytes and patient clinical characteristics. a Kaplan–Meier curve of PFS for patients with or without PCI treatment. b Kaplan–Meier curve of PFS for patients with the CD4+CD45RA+ T cell ratio after CRT. c Kaplan–Meier curve of PFS for patients with the CD8+CD38+ T cell ratio after CRT. d Kaplan–Meier curve of OS for male and female patients. e Kaplan–Meier curve of OS for patients with the baseline CD4+CD45RO+ T cell ratio
Correlation between immune parameters and OS
The median OS was 21.7 months (range: 6.2−79.9 months). In the univariate analysis, gender (p = 0.038), smoking (p = 0.036), baseline CD3+CD4+ T cells (p = 0.044), post-CD4+CD45RA+ T cells (p = 0.044), baseline CD4+CD45RO+ T cells (p = 0.014), baseline CD19+ B cells (p = 0.075), and Δ CD4+CD45RA+ T cells (p = 0.034) were associated with the OS (Table S3). However, only gender (p = 0.022) and the percentage of baseline CD4+CD45RO+ T cells (p = 0.009) were independent predictors of OS in the multivariate analysis (Table S4). Male patients had shorter survival compared with that of the female patients (Fig. 4d). In addition, an increased baseline CD4+CD45RO+ T cell ratio was associated with an inferior OS (Fig. 4e).
Discussion
In the present work, we focused on changes in the composition of circulating lymphocyte subsets before and after CRT to assess their potential role in predicting survival in patients with LS-SCLC. The results showed that after CRT, the proportion of circulating CD3+ T cells, CD8+ T cells, CD8+CD38+ T cells and NKT cells increased, while the proportion of CD4+ T cells, CD4+CD45RA+ T cells, CD4/CD8 ratio, B cells, and NK cells decreased. Moreover, percentages of CD4+CD45RA+ and CD8+CD38+ T cells detected three months after CRT can predict disease progression, baseline CD4+CD45RO+ T cell ratio was associated with long survival. These data suggest that CRT is involved in the regulation of peripheral immune cell composition in LS-SCLC.
A previous investigation showed that compared with healthy individuals, the proliferation of CD8+ T cells was inhibited in the peripheral blood, and the proportion of peripheral CD3+ and CD4+ T cells was decreased in patients with ES-SCLC [13]. Our results complement the study of peripheral immune cell spectrum of SCLC by showing that percentages of CD8+ T cells and its subpopulation CD38+ T cells were higher in lymphocytes after patients received CRT, and reductions in the proportion of CD4+ T cells were more concentrated in the naïve subsets (CD4+CD45RA+). The decrease in naïve CD4+ T cells ratio is in concordance with the study by Nakayama et al. [14], which suggests that naïve CD4+ T cells in LS-SCLC patients are more selectively damaged than memory CD4+ T cells by CRT, which may be due to variations in the recirculation behavior. CD4+ T cells secrete cytokines to recruit and activate other immune cells, whereas CD8+ T cells are associated with cytotoxic functions that can directly kill tumor cells [15]. Changes of CD4+ and CD8+ T cells in LS-SCLC are consistent with the observation of patients with locally advanced esophageal squamous cell carcinoma lung cancer (ESCC) [16]. The decrease in the proportion of CD4+ T cells may reveal that cell-mediated immunity is weakened after CRT. Elevated proportion of CD8+ T cells may be stimulated by the release of tumor-associated antigens induced by CRT. The level of CD38 expression on the surface of CD8+ T cells is a biological marker of immune activation, and is especially involved in the progression of HIV-1 infection [17, 18], rarely reported in tumors. The study by Wang et al. [19] showed that the ratio of CD8+CD38+/CD8+ cells was positively correlated with the TNM stage, tumor size, lymph node metastasis, and distant metastasis in pan-cancers.
Besides T cell immunity, humoral immunity and innate immunity also play an important role in anti-tumor. B cells secrete antibodies under antigen stimulation to perform humoral immunity. Consistent with previous findings in breast cancer and ESCC [16, 20], our results indicate that CD19+ B cells were decreased after CRT. In addition, we found that concurrent CRT caused more damage to B cells than sequential CRT. NK cells have the inherent ability to kill malignant tumor cells without prior sensitization. Hyperactivation of NK cell activity by enhancing IL-15 or TGF-β signal pathway can reduce the metastasis of SCLC, and has a synergistic effect with PD-1 inhibitor [21]. Waidhauser et al. [22] found that while there was a decrease in B lymphocytes during systemic chemotherapy, the NK lymphocyte count exhibited no significant changes before and after chemotherapy in pan-cancer patients (including 2 cases of SCLC). However, in breast cancer patients, researchers found a decrease in circulating NK cells after radiation [23]. In LS-SCLC patients, we revealed that the proportion of peripheral CD3−CD56+ NK cells decreased after CRT.
NKT cells are a special subgroup of T cells that express both T cell receptor (TCR) and NK cell receptor. Many cancers exhibit a decreased number and functional activity of NKT cells [24, 25]. Al et al. [26] showed that compared with the healthy controls, NKT cells were increased in SCLC patients; however, they appear to be functionally impaired. In this study, we noted that there was a significant increase in the proportion of NKT cells after CRT, but no functional test was performed and thus further research is required in the future.
Increasing evidence has shown that lymphocyte subgroups play an important role in the pathophysiology of SCLC [27]. A higher number of tumor infiltrating CD45+ T cells were found to be predictive of a better OS in SCLC, as well as independent of stage and performance status [28]. The proliferation potential of peripheral CD8+ T cells is a significant predicator of PFS in patients with untreated ES-SCLC [13]. Our results suggest for the first time that in LS-SCLC, the proportion of naïve CD4+ T cells and CD8+CD38+ T cells in peripheral lymphocyte subsets after treatment is related to disease progression, while the proportion of baseline memory CD4+ T cells is related to long-term survival, which needs further verification.
Based on the findings of this study, the proportion of total T lymphocytes in most LS-SCLC patients is in the normal range or higher, and the proportion of CD8+ T cell subsets in 46% of the patients is higher than the normal value, suggesting that the immune function tends to be enhanced three months after first-line treatment, which provides a basis for CRT combined with ICIs. In addition, whether screening patients with normal or activated immune function to receive ICIs will be more effective, which is worthy of further study. Furthermore, we observed that the proportion of peripheral B cells in 53.1% of patients was lower than normal after CRT, suggesting that humoral immunity was impaired, but B cells also express inhibitory surface markers, such as PD-1 and CTLA-4 [29], which may affect subsequent immunotherapy. NKT cells also have cytotoxic functions that were initially thought to play an important regulatory role by secreting either pro-inflammatory or anti-inflammatory cytokines following activation [30], clinical trials have been conducted to explore the adoptive transfer of NKT cells as an immunotherapy option for patients with advanced melanoma [31], which may provide potential opportunities for the treatment of SCLC in the future.
The absolute count of lymphocyte subsets was not obtained in our work, in the future studies, prospective collection of samples to analyze the percentage and absolute count of lymphocyte subsets will be more valuable and informative. Moreover, we acknowledge that the retrospective evaluation of lymphocyte subsets is limitted, and there is no analysis of the number and function of lymphocytes at various time points during CRT are Potential calculation bias cannot be excluded, primarily due to the limited sample size.
In conclusion, we comprehensively analyzed the proportion of peripheral blood lymphocyte subsets in LS-SCLC patients who received standard CRT. CRT was found to induce changes in the composition of peripheral lymphocyte profiles. After CRT, the percentage of CD4+CD45RA+ and CD8+CD38+ T cells may be indicators for predicting PFS. Baseline CD4+CD45RO+ T cell ratio may be able to predict OS. These findings provide additional evidence for patients with LS-SCLC treated with chemoradiotherapy combined with immunotherapeutic drugs, and suggest the potential use of circulating lymphocytes as biomarkers for the prognosis of LS-SCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contributions
MC conceived and designed the study. YJ and XH collected the data. YC analyzed the data and wrote the paper. All the authors read and approved the manuscript.
Funding
This research project was supported by the National Natural Science Foundation of China (Grant No. 81672972) and the Medical and Health Science Foundation of Zhe Jiang Province (Grant No. 2018255396).
Compliance with ethical standards
Conflict of interest
None of the authors had any conflicts of interest to declare.
Ethics approval
All data were obtained after approval by the Ethics Committee of Zhejiang Cancer Hospital (the ethical approval number: IRB-2020–229). The Committee waived the requirement for informed consent as it was a retrospective research. All experiments were performed in accordance with the approved guidelines.
Footnotes
The original online version of this article was revised: The presentation of Figure 1 and Table 1 was incorrect.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/16/2021
A Correction to this paper has been published: 10.1007/s00262-021-02929-0
References
- 1.Altan M, Chiang AC. Management of small cell lung cancer: progress and updates. Cancer J. 2015;21:425–433. doi: 10.1097/PPO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Zimmermann S, Parikh K, et al. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94:1599–1622. doi: 10.1016/j.mayocp.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 5.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invet. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W, Wolchok JD, Chen L. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:324r–328r. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messenheimer DJ, Jensen SM, Afentoulis ME, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. 2017;23:6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths JI, Wallet P, Pflieger LT, et al. Circulating immune cell phenotype dynamics reflect the strength of tumor-immune cell interactions in patients during immunotherapy. Proc Natl Acad Sci USA. 2020;117:16072–16082. doi: 10.1073/pnas.1918937117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaide O, Emerson RO, Jiang X, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015;21:647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu TD, Madireddi S, de Almeida PE, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579:274–278. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 12.Qin L, Jing X, Qiu Z, et al. Aging of immune system: Immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging Albany NY. 2016;8(5):848–859. doi: 10.18632/aging.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An N, Wang H, Jia W, et al. The prognostic role of circulating CD8(+) T cell proliferation in patients with untreated extensive stage small cell lung cancer. J Transl Med. 2019;17:402. doi: 10.1186/s12967-019-02160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama Y, Makino S, Fukuda Y, et al. Varied effects of thoracic irradiation on peripheral lymphocyte subsets in lung cancer patients. Intern Med. 1995;34:959–965. doi: 10.2169/internalmedicine.34.959. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann TR, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Zhang W, Qian D, et al. Chemoradiotherapy-induced CD4(+) and CD8(+) T-Cell alterations to predict patient outcomes in esophageal squamous cell carcinoma. Front Oncol. 2019;9:73. doi: 10.3389/fonc.2019.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resino S, Bellon JM, Gurbindo MD, et al. CD38 expression in CD8+ T cells predicts virological failure in HIV type 1-infected children receiving antiretroviral therapy. Clin Infect Dis. 2004;38:412–417. doi: 10.1086/380793. [DOI] [PubMed] [Google Scholar]
- 18.Kou W, Banerjee S, Eudy J, et al. CD38 regulation in activated astrocytes: implications for neuroinflammation and HIV-1 brain infection. J Neurosci Res. 2009;87:2326–2339. doi: 10.1002/jnr.22060. [DOI] [PubMed] [Google Scholar]
- 19.Wang YY, Zhou N, Liu HS, et al. Circulating activated lymphocyte subsets as potential blood biomarkers of cancer progression. Cancer Med. 2020;9:5086–5094. doi: 10.1002/cam4.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016;18:10. doi: 10.1186/s13058-015-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best SA, Hess JB, Souza-Fonseca-Guimaraes F, et al. Harnessing natural killer immunity in metastatic SCLC. J Thorac Oncol. 2020;15:1507–1521. doi: 10.1016/j.jtho.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Waidhauser J, Schuh A, Trepel M, et al. Chemotherapy markedly reduces B cells but not T cells and NK cells in patients with cancer. Cancer Immunol Immunother. 2020;69:147–157. doi: 10.1007/s00262-019-02449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sage EK, Schmid TE, Sedelmayr M, et al. Comparative analysis of the effects of radiotherapy versus radiotherapy after adjuvant chemotherapy on the composition of lymphocyte subpopulations in breast cancer patients. Radiother Oocol. 2016;118:176–180. doi: 10.1016/j.radonc.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Molling JW, Kolgen W, van der Vliet HJ, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 25.Krijgsman D, de Vries NL, Skovbo A, et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. 2019;68:1011–1024. doi: 10.1007/s00262-019-02343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al OS, Marshall E, Middleton D. Increased numbers but functional defects of CD56+CD3+ cells in lung cancer. Int Immunol. 2012;24:409–415. doi: 10.1093/intimm/dxr122. [DOI] [PubMed] [Google Scholar]
- 27.Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Hodkinson P, McLaren F, et al. Histologic assessment of tumor-associated CD45(+) cell numbers is an independent predictor of prognosis in small cell lung cancer. Chest. 2013;143:146–151. doi: 10.1378/chest.12-0681. [DOI] [PubMed] [Google Scholar]
- 29.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krijgsman D, Hokland M, Kuppen P. The role of natural killer T cells in cancer-A phenotypical and functional approach. Front Immunol. 2018;9:367. doi: 10.3389/fimmu.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Exley MA, Friedlander P, Alatrakchi N, et al. adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a phase I clinical trial. Clin Cancer Res. 2017;23:3510–3519. doi: 10.1158/1078-0432.CCR-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.