Abstract
Immunotherapy has been one of the great advances in the recent years for the treatment of advanced tumors, with nonsmall-cell lung cancer (NSCLC) being one of the cancers that has benefited most from this approach. Currently, the only validated companion diagnostic test for first-line immunotherapy in metastatic NSCLC patients is testing for programmed death ligand 1 (PD-L1) expression in tumor tissues. However, not all patients experience an effective response with the established selection criteria and immune checkpoint inhibitors (ICIs). Liquid biopsy offers a noninvasive opportunity to monitor disease in patients with cancer and identify those who would benefit the most from immunotherapy. This review focuses on the use of liquid biopsy in immunotherapy treatment of NSCLC patients. Circulating tumor cells (CTCs), cell-free DNA (cfDNA) and exosomes are promising tools for developing new biomarkers. We discuss the current application and future implementation of these parameters to improve therapeutic decision-making and identify the patients who will benefit most from immunotherapy.
Keywords: Lung cancer, NSCLC, Liquid biopsy, Immunotherapy, CTC, cfDNA
Introduction
Lung cancer is currently the most frequently diagnosed malignant tumor, representing the leading cause of cancer death worldwide [1]. Nonsmall-cell lung cancer (NSCLC) accounts for approximately 85% of lung malignancies, and approximately 70% of them are diagnosed with a nonsquamous histology such adenocarcinoma or large cell carcinoma [2]. More than 60% of newly diagnosed patients present locoregional or distant metastases at the time of detection.
Over the past 20 years, the treatment landscape and prognosis of advanced NSCLC patients have changed [3]. First, the introduction of target therapies for oncogene-addicted tumors improved patient survival outcomes. However, only 15% of patients with advanced NSCLC present genetic alterations in EGFR, ALK, ROS1, or BRAF [4]. More recently, immune checkpoint inhibitor (ICI) agents targeting both programmed cell death protein 1 (PD1) or programmed cell death ligand 1 (PD-L1) have been established as a standard treatment in the NSCLC setting. Unfortunately, not all patients respond, and only a subset of them benefit from immunotherapy. Hence, there is a need for new biomarkers. Identifying optimal responsive biomarkers to anti-PD-1/PD-L1 therapies in NSCLC is essential for selecting patients who will benefit from immunotherapy while limiting ineffective therapy that can produce adverse reactions in patients.
It is within this context that there has been interest in liquid biopsy, a concept that refers to any tumor-derived material circulating through the blood or nonblood body fluids [5]. Liquid biopsy has been developed as an attractive approach to detect biomarkers in a minimally invasive, cost-effective, and rapid manner. Circulating tumor cells (CTCs), cell-free DNA (cfDNA) and extracellular vesicles are the most widely studied components in the field of NSCLC. This review focuses on the use of liquid biopsy in immunotherapy treatment of NSCLC patients, highlighting its potential to be applied in a clinical setting.
Immune checkpoint inhibitors in the treatment of NSCLC
The European Medicines Agency (EMA) and the U.S. The Food and Drug Administration (FDA) have approved two classes of ICIs for the treatment of malignancies, including NSCLC [6, 7]: Inhibitors of PD-1 or its ligand PD-L1 and inhibitors of cytotoxic lymphocyte-associated protein 4 (CTLA-4). Table 1 summarizes the different ICIs employed in the treatment of NSCLC.
Table 1.
Immune checkpoint inhibitors in the treatment of NSCLC
| Drug | Target | Approved use for NSCLC | Line of treatment | Biomarker for patient selection | Comments | References |
|---|---|---|---|---|---|---|
| Nivolumab | PD1 | Metastatic | 2nd line | None | Monotherapy | [8, 9] |
| Pembrolizumab | PD-1 | Metastatic | 2nd line | PD-L1 IC ≥ 1% | Monotherapy | [11] |
| 1st line | PD-L1 IC ≥ 50% | Monotherapy | [12] | |||
| None | In combination with platinum-based chemotherapy | [13, 14] | ||||
| Atezolizumab | PD-L1 | Metastatic | 2nd line | None | Monotherapy | [15] |
| 1st line | Nonsquamous NSCLC, independent of the PD-L1 expression level | In combination with bevacizumab, carboplatin, and paclitaxel | [17] | |||
| Durvalumab | PD-L1 | Unresectable, stage III | Maintenance | None | [20] | |
| Ipilimumab | CTLA-4 | Metastatic | 1st line | PD-L1 ≥ 1% | Nivolumab plus Ipilimumab | [22] |
| None | Nivolumab plus Ipilimumab in combination with platinum-based chemotherapy | [23] |
TMB tumor mutational burden, PD-L1 IC PD-L1 expression in immune cells in the tumor area
Nivolumab plus Ipilimumab for first-line treatment of metastatic NSCLC alredy has the approval of the European Medicines Agency (EMA)
Nivolumab, a human immunoglobulin G4 (IgG4) monoclonal antibody (mAb) that targets PD-1, has shown improved overall survival (OS), progression-free survival (PFS) and objective response rate in previously treated advanced squamous cell lung carcinoma in comparison with docetaxel according to the results of the CheckMate 017 trial [8]. In nonsquamous NSCLC, the results of the CheckMate 057 trial demonstrated that nivolumab significantly improved OS and objective response rate (ORR) compared to docetaxel [9]. However, as compared to chemotherapy, nivolumab monotherapy has not shown significant effects on improving PFS or OS in previously untreated advanced NSCLC patients with PD-L1 expression on at least 5% of tumor cells [10].
Pembrolizumab, a humanized IgG4 mAb directed against PD-1, was approved for previously treated metastatic NSCLC patients with a PD-L1 tumor expression level of 1% or more, taking into consideration the benefits in the KEYNOTE 10 trial [11]. Recently, the use of pembrolizumab has moved into the first-line setting for recurrent or metastatic NSCLC. The results from the KEYNOTE-024 trial demonstrated that compared with platinum-based chemotherapy, pembrolizumab led to significant improvements in OS, PFS, and ORR in patients with PD-L1 expression on at least 50% of tumor cells [12]. Moreover, the addition of pembrolizumab to platinum-based chemotherapy in previously untreated metastatic NSCLC has recently demonstrated a significant improvement in survival results independent of PD-L1 status [13, 14].
Atezolizumab is a humanized IgG1 mAb that specifically targets PD-L1, blocking its receptors PD-1 and B7.1. In previously treated NSCLC patients, atezolizumab significantly prolonged OS and ORR when compared with docetaxel according to the results of the OAK trial [15]. Consequently, the efficacy of atezolizumab, alone [16] or in combination with chemotherapy [17–19], has been studied in chemotherapy-naïve advanced NSCLC patients in several trials.
Durvalumab, a human IgG1 mAb directed against PD-L1, has been recently approved as consolidation therapy following concurrent chemoradiation in unresectable stage III NSCLC patients [20].
Ipilimumab, a fully human IgG1 mAb directed against CTLA-4, has also been studied in combination with nivolumab in first-line treatment of advanced NSCLC in CheckMate 227, Part 1 [21, 22]. In this trial, in previously untreated NSCLC patients and with high-tumor mutational burden (TMB), PFS was significantly longer with nivolumab plus ipilimumab than with platinum-based chemotherapy, irrespective of PD-L1 expression level [21]. Moreover, in this trial, the combination of nivolumab plus ipilimumab resulted in a longer duration of OS than chemotherapy in patients with NSCLC, independent of the PD-L1 expression level [22]. Finally, the CheckMate 9LA trial has shown that adding 2 cycles of platinum-based chemotherapy to the combination of Nivolumab plus Ipilimumab prolongs the survival of patients with stage IV or recurrent NSCLC [23].
Biomarkers in immunotherapy
Intensive work has been carried out in the recent years in the search for biomarkers that identify groups of patients who could benefit most from PD-1/PD-L1 inhibitors. The best-known biomarker in this scenario is PD-L1 expression in tumor or immune cells: a higher probability of response with higher expression of PD-L1 has been described [24, 25]. More than PD-L1 expression on more than 50% of tumor cell NSCLC patients predicts higher effectiveness of pembrolizumab than chemotherapy as a first-line treatment in the metastatic setting [12]. In clinical practice, this marker has limited utility because patients who do not express PD-L1 may respond to PD-1 or PD-L1 checkpoint inhibitors, and in contrast, some patients with elevated expression of PD-L1 do not benefit from the use of these drugs.
High TMB has been linked to the effectiveness of immunotherapy, but given the conflicting results among several studies, this biomarker has not yet been translated into the clinic [21, 22] or authorized by regulatory agencies. The variability among the techniques used for the analysis and interpretation of TMB may influence the value of this biomarker as a predictive factor of response to immunotherapy. As a result, there is an ongoing initiative for increased standardization of TMB interpretation, a subject of active pursuit by the TMB Harmonization Project [26, 27].
In a meta-analysis of 14,395 patients, Yu et al. concluded that the combined use of PD-L1 expression in tissue and TMB is a promising biomarker to evaluate patient survival and response to precision immunotherapy [28]. The further combination of CD8+ tumor-infiltrating lymphocytes, PD-L1 expression, and TMB was associated with reliable prognosis, but these results need to be validated in large-scale prospective trials.
Liquid biopsy
Tumor diagnosis is made based on tissue biopsy, as well as complementary techniques that can add any genomic information for therapy selection. However, tumor lesions are sometimes difficult to biopsy, and invasive procedures are not the best way to monitor the evolution of the disease. In addition, tumor heterogeneity originates from the appearance of mutations that cause resistance or progression to treatments by selection of more aggressive clones. Considering the risks and logistical challenges, and even high cost, that limit the application of interventional biopsies, liquid biopsy presents an attractive opportunity for minimally invasive genomic diagnostics.
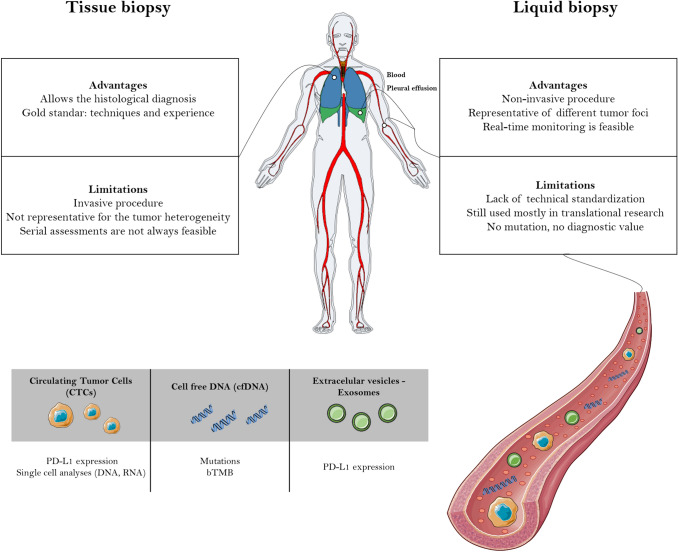
Tissue provides a snapshot of the tumor at a given time and location, while liquid biopsy has the potential to establish a tumor molecular profile at the beginning of the treatment and during the course of the disease (Fig. 1). In addition, liquid biopsy can capture dynamic intrapatient genomic heterogeneity [5]. These characteristics are especially important in the management of patients with lung cancer due to the great difficulty in obtaining a tissue sample in some cases [29].
Fig. 1.
Comparison of the advantages and limitations between tissue and liquid biopsy. Plot generated by the gganatogram R package (https://github.com/jespermaag/gganatogram)
Currently, there are limitations that prevent us from putting into clinical practice the use of the biomarkers used by liquid biopsy. The most relevant is that there is no clear agreement on the usefulness or threshold to be considered for most of the biomarkers studied (notably TMB and PD-L1). Initiatives are underway to harmonize and standardize methods of analysis through universal protocols. An agreement in the scientific community about the ideal biomarker and cut off to define this parameter is needed. The minimally invasive nature of liquid biopsy sampling offers a clear opportunity in immunotherapy. In this work, we will focus on the potential clinical applications of CTCs and cfDNA, the most widely studied substrates in the field of NSCLC.
Cell-free DNA
Different types of biological samples are used for liquid biopsies, but cfDNA is currently the material with the greatest potential in clinical practice. The short half-life of cfDNA, approximately 2 h, permits a rapid assessment of tumor-related changes and enables real-time monitoring of molecular biomarkers of response or relapse. Composed of double-stranded fragments of 150–200 base pairs, cfDNA derives from normal physiological tissue remodeling events [30], although a portion of cfDNA results from necrosis and apoptosis of cancer cells, and active cfDNA secretion has also been suspected [31]. The fraction of circulating tumor DNA (ctDNA) in overall cfDNA in patients with cancer varies greatly, from less than 0.1% to more than 90% [30], with ctDNA being distinguished solely through characteristic somatic genomic alterations and its trend to be more fragmented, with sizes ranging from 90 to 150 base pairs.
Therapies for NSCLC patients targeting EGFR, ALK or ROS1 molecular alterations have been proven effective, inhibiting molecules that are pivotal for cell proliferation. Circulating free DNA is now regularly used to manage tyrosine kinase inhibitor treatment in EGFR-mutated NSCLC, and a large number of studies have demonstrated that EGFR gene mutations detected in cfDNA are highly concordant with those detected in tumor tissue of NSCLC patients [32]. What is clear is that cfDNA has been widely studied in the field of targeted therapy. Two FDA- and EMA-approved assay kits to detect somatic mutations in the EGFR oncogene in patients with NSCLC are now available: the Cobas EGFR Mutation Test v2 CE-IVD (Roche, Basel, Switzerland) and therascreen mutation kits (Qiagen, Hilden, Germany). In contrast, the potential of cfDNA to guide the choice of and follow the response to immune therapy is just beginning to be evaluated (Table 2).
Table 2.
Summary of liquid biopsy studies in NSCLC treated with immunotherapy
| cfDNA studies | Sample number | Biomarker | Method | Conclusions | Limitations |
|---|---|---|---|---|---|
| Gandara et al. 2018 [33] | 259 | bTMB | NGS 394 genes |
bTMB was validated as a clinical biomarker for PFS in patients under immunotherapy 64% correlation between tTMB and bTMB |
The tTMB computational algorithm counted both indels and SNVs, whereas the bTMB counted only SNVs |
| Wang et al. 2019 [34] | 50 | bTMB | NGS 150 genes |
bTMB was validated as a clinical biomarker for PFS in patients under immunotherapy 62% correlation between tTMB and bTMB |
The clinical cohort was obtained from different trials |
| Chen et al. 2019 [35] | 853 | MSAF | NGS | MSAF alone or in combination with bTMB can effectively distinguish NSCLC patients with or without OS and PFS benefit from atezolizumab compared with docetaxel | The results require validation from external cohorts |
| Koeppel et al. 2017 [37] | 32 | bTMB | WES |
cfDNA-WES TML is feasible for patients with good ctDNA fraction There was a weak correlation between variant frequencies of tDNA and cfDNA |
The cohort size of the study was small |
| Guibert et al. 2019 [40] | 97 | cfDNA | NGS 36 genes |
Correlation of cfDNA mutations with survival outcomes: PTEN and STK11 are correlated with poor prognosis Tv KRAS and p53 are correlated with better prognosis Early changes in AF can predict response |
Patients were not treated with a homogeneous regimen Validation in prospective studies is required |
| Chaudhuri et al. 2017 [41] | 40 | cfDNA | CAPP-seq | Molecular residual disease was detected earlier with analyses of cfDNA than imaging (72% patients) | Patients were treated with different therapies, mostly radiotherapy |
| Cabel et al. 2017 [44] | 15 | cfDNA | NGS, ddPCR or Bi-PAP | Monitoring of cfDNA levels concluded that undetectable cfDNA at week 8 was associated with superior PFS and OS | The cohort size of the study was small |
| Giroux Leprieur et al. 2018 [45] | 23 | cfDNA | NGS 22 genes | cfDNA changes correlated with tumor response, clinical benefit and PFS | Only 15 of 23 patients were eligible for NGS |
| Goldberg et al. 2018 [46] | 28 | cfDNA | NGS 24 genes | A cfDNA response (cfDNA decrease < 50%) was associated with superior PFS and OS | Patients with undetectable cfDNA levels were excluded |
| Alama et al. 2019 [47] | 89 | cfDNA | qPCR | cfDNA is significantly associated with OS in patients under nivolumab treatment | The cohort size of the study was small |
| Passiglia et al. 2019 [57] | 45 | cfDNA variations | qPCR | cfDNA > 20% at week 6 correlated with poor outcomes (OS and TTP) |
Retrospective design Small number of patients |
| CTCs studies | Sample | Biomarker | Platform | Conclusions | Limitations |
|---|---|---|---|---|---|
| Alama et al. 2019 [47] | 89 | CTC count | ScreenCell CYTO | ≥ 2 CTCs was significantly associated with worse OS in patients under nivolumab treatment | The cohort size of the study was small |
| Guibert et al. 2018 [50] | 96 | PD-L1 on CTCs | ISET | No correlation was found between PD-L1 expression in tissue and CTCs | Only 72% of the patients had enough tissue to analyze |
| Boffa et al. 2017 [51] | 112 | PD-L1 on CTCs | EPIC science | PD-L1+ was correlated with better OS (OS-2y: PD-L1+ 31.2% vs PD-L1− 78.8%, (p = 0.00159)) | Counted nonconventional CTCs (CK negative) |
| Nicolazzo et al. 2016 [52] | 24 | PD-L1 on CTCs | CellSearch | PD-L1+ was correlated with progression disease in 100% of samples | Only 15 patients had available samples after 3 months of treatment |
| Ilié et al. 2018 [53] | 106 | PD-L1 on CTCs and white blood cells | ISET | 93% concordance between PD-L1 expression in tissue and CTCs | Only 67% of patients presented CTCs in blood |
| Dhar et al. 2018 [54] | 22 | PD-L1 on CTCs | Vortex-isolated | 100% correlation between PD-L1 expression in tissue and CTCs | Only 4 tissue samples available for correlation analysis |
| Jainning et al. 2019 [55] | 127 | PD-L1 on CTCs | CellSearch/Parsortix | No correlation between PD-L1 expression in tissue and CTCs | Not all samples were recruited before the start the treatment |
| Koh et al. 2019 [56] | 67 | PD-L1 on CTCs | Microcavity array | No correlation between PD-L1 expression in tissue and CTCs | - |
| Chen et al. 2019 [58] | 51 | PD-L1 on CTCs | CMx microfluidic platform | 57% concordance between PD-L1 expression in tissue and CTCs | – |
| Tamminga et al. 2019 [59] | 104 | CTC count and tdEV | CellSearch | The presence of CTCs was associated with worse PFS and OS in patients under ICI treatment | CTCs detected in only 30% of patients |
Bi-PAP bidirectional-pyrophosphorolysis-activated polymerization, bTMB blood tumor mutational burden, CAPP-seq cancer personalized profiling by deep sequencing, CK cytokeratin, ddPCR droplet digital PCR, MSAF maximum somatic allele frequency, NGS next-generation sequencing, OS overall-survival, PFS progression-free survival, qPCR quantitative polymerase chain reaction, SNVs single-nucleotide variants, tdEV tumor derived extracellular vesicles, TML tumor mutation load, tTMB tissue tumor mutational burden, TTP time to progression, WES whole exome sequencing
Blood tumor mutational burden (bTMB)
One of the biomarkers that could drive the choice of treatment is the blood tumor mutational burden (bTMB) because it can be estimated from cfDNA next-generation sequencing (NGS). Recently, Gandara et al. demonstrated the possibility of using cfDNA to determine TMB [33], also reporting an association with superior results in terms of efficacy in NSCLC treated with immunotherapy. In this regard, a gene panel and algorithm were also optimized for bTMB [34], suggesting that bTMB may serve as a potential biomarker of clinical benefit in patients with NSCLC treated with anti-PD-1 and anti-PD-L1 agents. bTMB may be related to the ability of each tumor to produce antigens that are detected by the immune system, producing an antigenic reaction that triggers an immune response to that tumor. However, studies focused on the relationship between TMB in tissue and the immune response have produced conflicting results [35–37]. In general, further studies are needed to clarify the conditions required to use bTMB in routine clinical practice. The methods for its determination, as well as the threshold to be adopted as a cutoff point for high, intermediate and/or low classification, have yet to be unified and standardized by the scientific community [38]. Moreover, there is a high cost coming from whole-exome sequencing or broad panels, which confers another challenge for clinical implementation. Perhaps it is necessary to move beyond TMB and move to identifying specific somatic alterations detectable in cfDNA that can facilitate selection of patients for immunotherapy. Not all point mutations participate in the generation of highly immunogenic peptides [39]. Guibert et al. showed that targeted NGS of plasma cfDNA and monitoring its early variations could predict the response to immunotherapy [40]. The study revealed the detrimental effect of STK11 and PTEN mutations in cfDNA on the ICI outcomes reported in tissue. In contrast, mutational transversion (Tv, substitution of a purine by a pyrimidine or vice versa) in KRAS and p53 predicts superior results in terms of PFS. Moreover, the combination of all these specific markers in a predictive model, together with known targetable oncogenic drivers, allows guided patient treatment towards either targeted therapy or ICI.
Minimal residual disease
Detection of minimal residual disease (MRD) following chemoradiation therapy (CRT) predicts the development of progressive disease in NSCLC with high sensitivity and specificity [41]. Immunotherapy is used in the adjuvant setting for patients with unresectable locoregionally advanced NSCLC. The optimal use of immunotherapy requires the identification of patients with MRD to maximize the opportunity for cure, and cfDNA might enable approaches for disease progression monitoring [42]. Antonia SJ et al. showed a survival advantage with durvalumab consolidation therapy after concurrent chemoradiation therapy in patients with stage III [20], unresectable NSCLC. Recently, Moding EJ et al. showed that patients with MRD after CRT receiving ICIs had significantly better outcomes than patients who did not receive consolidation ICI therapy [43]. cfDNA analysis may guide the decision to administer consolidation ICI therapy based on the presence of MRD.
Changes in cfDNA concentration
There are a few studies that propose the cfDNA concentration as a predictive marker of immunotherapy response. In a proof-of-concept study, changes in cfDNA concentration were reported as a valuable tool to assess tumor response in patients treated with anti-PD-1 drugs [44]. Giroux Leprieur et al. reported that low cfDNA concentration at first evaluation (at 2 months) had a relationship with long-term benefit of nivolumab [45]. In addition, a decrease in cfDNA level was correlated with radiological response to immunotherapy [46], suggesting that cfDNA levels may be an early marker of therapeutic efficacy and predict prolonged survival in patients treated with immune checkpoint inhibitors for NSCLC. In another study, NSCLC patients treated with nivolumab and with a cfDNA below their median values survived significantly longer than those with a cfDNA value above their median values [47]. Moreover, the joint analysis of cfDNA with CTCs helped to discriminate a low-risk population that might benefit from continuing nivolumab beyond progression. This suggests that the use of cfDNA and CTCs could help to select those patients who will benefit most from immunotherapy.
Circulating tumor cells
Circulating tumor cells (CTCs) are released from the primary tumor into the blood and have a very short half-life (1–2.4 h). The proportion of CTCs in the bloodstream is very low, approximately 1 CTC per 106–107 leukocytes. Initially, CTCs have an epithelial phenotype; however, the bloodstream is not an ideal place for epithelial tumor cells, and for this reason, they can undergo epithelial-to-mesenchymal transition (EMT). This transition reduces the expression of epithelial markers and increases plasticity and capacity for migration, invasion, and dissemination, being the CTCs isolation a challenge for their total isolation and capture.
CTCs isolation platforms
In most of the current assays for the detection of CTCs, the platforms are based on the expression of epithelial markers such as EPCAM and cytokeratins. Both biomarkers are not expressed on mesenchymal cells; therefore, new CTC isolation platforms were developed [48]. Currently, the methods to detect and isolate CTCs are based on different aspects: (1) the surface presence of specific antigens (EpCAM is the most common), such as what is used in the CellSearch® system (Menarini), Epic platform (Epic sciences) or GILUPI CellColector (GILUPI); or (2) size and deformability, such as what is used in Parsortix (Angle), ISET (ISET, Rarecells Diagnostics), Vortex VTX-1 (Vortex Bioscience), and the ClearCell FX device (Biolidics).
CTCs and PD-L1 expression
In the field of immunotherapy, the expression of PD-L1 in tissue samples is the gold-standard biomarker to choose the treatment. Pilot studies in lung cancer have shown PD-L1 as a predictive factor. However, the PD-L1 status can be underestimated in small biopsies, which may not be representative of the entire tumor, causing some to not receive ICI treatment due to tissue sampling bias [49]. PD-L1 analyses in CTCs could be a good option to solve this problem.
The possibility of measuring the expression of PD-L1 in CTCs before and during treatment has been analyzed in several studies (Table 2). Some authors have studied the relationship between baseline CTCs and overall survival, finding an association between a high number of CTCs and an increased risk of death and progression [50]. In addition, the presence of CTCs with positive PD-L1 expression (≥ 1% of cells) before ICI treatment was correlated with poor prognosis and survival [50, 51], and the persistence of PD-L1 on CTCs after 6 months of ICI therapy may represent the occurrence of tumor escape, which results in disease progression. It may also be a reflection of the aggressiveness of these CTCs, which may show characteristics of the mesenchymal phenotype (via EMT) [52]. In a stage-adjusted Cox model, it was concluded that a high proportion of PD-L1-positive cells was a predictor of mortality [51].
CTCs vs. tissue
The concordance between PD-L1 expression by CTCs and PD-L1 expression in tissue has been evaluated in several studies, showing controversial results [50, 53]. Iliè et al. showed a concordance of 93% between tissue and CTCs regarding PD-L1 expression in a cohort of 106 patients treated with chemotherapy [53], using the platform ISET. Dhar et al. also showed a good correlation between tissue and CTCs using a different isolation platform (Vortex isolation). However, the sample size in this study was only 4 patients [54]. Other studies reveal the opposite situation. Guibert et al., reported no correlation between CTCs and tissue in a cohort of 96 patients treated with nivolumab in 2nd line, isolating the CTCs with the ISET platform [50]. Recently, other two studies reported the same conclusion using two different approaches to isolate the CTCs (Microcavity array and Parsortix system) [55, 56]. It is important to remark that in these studies, the expression of PD-L1 was analyzed using different methods, therefore, different phenotypes of CTCs could be obtained in each study. Also, different antibodies and unbalanced populations were employed, so comparisons between them must be interpreted with caution. More studies are needed to assess a standardized method and PD-L1 antibody to detect and analyze CTCs.
Other blood biomarkers
There are other markers in the field of liquid biopsy with great potential for future clinical applications. Extracellular vesicles (EVs) are nanosized particles with membranes released by any cell type, including cancer cells. The most common subtypes of EVs are exosomes. Exosomes have a size between 50 and 150 nm, are very abundant, express proteins on their surface and contain several particles, such as proteins, nucleic acids, and lipids [60]. For this reason, exosomes are also a promising biomarker for analyzing the expression of PD-L1. One study in NSCLC patients concluded that PD-L1 expression in exosomes was correlated with disease progression, tumor size, lymph node status, metastasis, and TNM stage. However, no association was demonstrated between PD-L1 expression in exosomes and tissue [61]. In addition, the standardization of methodologies for exosome isolation remains a challenge [49].
Numerous studies have also reported a correlation between immunotherapy and the neutrophil-to-lymphocyte ratio (NLR) [62]. The NLR is defined as the neutrophil count in blood divided by the lymphocyte count in blood and is a marker for the general immune response to distinct stress stimuli. Recently, a meta-analysis concluded that high NLR values were correlated with shorter PFS and OS in NSCLC patients under ICI treatment [63].
Other elements, such as circulating microRNA (miRNA), circulating RNA, platelets, and circulating proteins, have not been included in this review because they are at the early stages of use for tumor genotyping and more specifically for the use in immunotherapy of NSCLC patients [62].
Conclusion and future perspectives
Liquid biopsy is being incorporated as a useful procedure in many situations in oncology due to its minimal invasiveness and its great capacity to indicate cancer tumorigenesis. The search for new biomarkers will help us to manage our patients in a personalized way, something that constitutes a clear objective for the implementation of precision oncology.
Over the past few years, several integrated biological strategies have been used to find noninvasive markers to predict response to the administration of immunotherapy in NSCLC. The existence of promising results from observational studies, together with the continued development of analysis platforms, has propelled cfDNA-based liquid biopsy diagnostics into the next developmental phase. Because of its potential to select populations for specific immunotherapy (alone or combined) and predict responders/nonresponders or the appearance of resistance, we foresee that cfDNA analysis will become increasingly important. In the case of lung cancer, cfDNA methods are particularly useful because they minimize the need for tumor tissue biopsy. The capability of cfDNA to guide the choice and follow the response to immune therapy is just beginning to be assessed. Recently, Chabon et al. established the potential of cfDNA for lung cancer screening, underlining the importance of risk-matching cases, and controls in cfDNA-based screening studies [64]. However, we cannot ignore that cfDNA isolation and analysis are still a challenge. Plasma cfDNA is a complex mixture of DNA from many sources, including germline, fetal, infectious, and malignant [31], but there are also mutations in the cfDNA of hematopoietic origin with risk being mistaken as tumor-derived mutations [65]. Because a large proportion of cfDNA is derived from peripheral blood cells, the impact of clonal haematopoiesis represents a relevant limitation in the implementation of liquid biopsy for analysing cfDNA. Paired plasma–peripheral blood cells sequencing should be implemented as the standard practice for NGS genomic analysis of cfDNA to prevent misinterpretation of results [66]. Moreover, cfDNA is not tumor-specific and its measurement is prone to false positive results. In fact, the level of cfDNA can increase under some conditions, such as inflammation, infection, and even exercise [30]. The sensitivity and specificity of cfDNA measurement are also affected by the variability of ctDNA, which may vary through tumor evolution, have different origins (primary tumor vs. metastasis; tumor heterogeneity) and be affected by different treatments. We are in a situation in which there are rapidly evolving technologies supporting the use of liquid biopsy, while there is a need for standardization procedures for collecting and processing cfDNA samples. Another aspect to standardize should be the size of the panels for appraising specific cancer mutations and genomic metrics (MRD and TMB), since they vary greatly across assays.
On the other hand, CTCs are intact viable cells with tumor-specific information and can also enable cancer diagnosis and treatment evaluation. Analyses of the presence or absence of proteins, such as PD-L1 or PD-1 on the surface promise to be good options in the future to guide and select treatment, but the strategies will need to be validated in robust studies [49]. In addition, due to methods that allow the preservation and capture of intact CTCs, single-cell analyses or cluster analyses are possible. Single- or cluster-cell characterization of CTCs will allow more precise characterization of the entire cancer in each patient. RNA and DNA analyses are possible and could show the intertumoral and intratumoral heterogeneity in each patient [67]. In contrast, a good and standardized platform to capture CTCs and rigorous validation remain a challenge. Owing to the effects of EMT, a new marker to detect tumor cells undergoing this transition is needed.
Recent studies have focused on the peripheral blood T-cell receptor (TCR) repertoire, as an emergent biomarker to select the most suitable ICI treatment [68]. The analysis of T-cell clonality may reveal the degree of tumor-antigen driven T-cell expansions and help to understand mechanisms underlying T-cell tolerance to cancer antigens. Recently, Reuben et al. showed the heterogeneity of tissue T-cell repertoire in localized NSCLC [69], proposing a positive relation between T-cell density and clonality. They demonstrated an association between higher T-cell density in the blood and improved outcome following surgery. These findings suggest that the peripheral T cell repertoire in NSCLC patients may be reflective of increased systemic immunity. TCR sequencing might be applicable for the treatment selection in patients with EGFR mutations by evaluating the proportions of TCRβ clones in the tumor. Miyauchi et al. provided relevant information for understanding the molecular mechanism behind EGFR-mutant patients [70], showing that the low clonal T-cell expansion in NSCLC with EGFR mutations might be a critical factor related to the unfavorable response to ICI. T-cell clonality in the circulation may have predictive value for antitumor responses from checkpoint inhibition. However, since there are very preliminary results, continue this line of research is essential in order to know if it has practical application in the clinic.
In conclusion, liquid biopsy through CTCs and cfDNA analyses could be a promising tool for diagnosis, selection of ICI treatment and monitoring of NSCLC patients under immunotherapy.
Author contributions
EMBV and RDP designed the manuscript, and collected the material and data necessary to construct the article. JGG, LLM, PMM, MPC, and RLL provided supervisory support and constructive critique of the article to enable revisions. All authors helped to write the manuscript.
Funding
This study was financed by all the donors who participated in the Liquid Biopsy Crowdfunding campaign in 2017.
Compliance with ethical standards
Conflict of interest
Rafael López-López has received honoraria for participation in Advisory Boards from Roche, AstraZeneca, Merck, MSD, Bayer, BMS, Novartis, Janssen, Lilly, Pfizer and Leo; travel, accommodations and expenses from Pharmamar, Roche, BMS and Pierre Fabre; research funding from Roche and Merck; and is co-founder and shareholder in Nasasbiotech, S.L., Mtrap Inc. Luis León-Mateos reports personal fees from AstraZeneca, Boehringer-Ingelheim, Novartis, Jansen, Astellas and Sanofi; and personal fees and nonfinancial support from Bristol-Myers Squibb, Lilly, MSD and Roche, outside the submitted work. Jorge García-González reports personal fees from AstraZeneca, Boehringer-Ingelheim, Novartis, Pierre Fabre, Rovi and Sanofi; and personal fees and nonfinancial support from Bristol-Myers Squibb, Lilly, MSD and Roche, outside the submitted work. Elena Brozos-Vázquez reports personal fees from Leo Pharma, Rovi, and travel grants from Merck, Sanofi, Roche, Pierre-Frabre and Amgen, outside the submitted work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elena María Brozos-Vázquez and Roberto Díaz-Peña Contributed equally.
Contributor Information
Elena María Brozos-Vázquez, Email: elena.maria.brozos.vazquez@sergas.es.
Roberto Díaz-Peña, Email: roberto.diaz.pena@sergas.es.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Houston KA, Henley SJ, Li J, et al. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer (Amsterdam, Netherlands) 2014;86:22–28. doi: 10.1016/j.lungcan.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doroshow DB, Herbst RS. Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol. 2018;4:569. doi: 10.1001/jamaoncol.2017.5190. [DOI] [PubMed] [Google Scholar]
- 4.Barlesi F, Mazieres J, Merlio J-P, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet (Lond, Engl) 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 5.Muinelo-Romay L, García-González J, León-Mateos L. Lung cancer and liquid biopsy: realities and challenges in routine clinical practice. Arch Bronconeumol. 2019;55:289–290. doi: 10.1016/j.arbres.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remon J, Passiglia F, Ahn M-J, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;S1556–0864:30198–30202. doi: 10.1016/j.jtho.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 15.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spigel D, de Marinis F, Giaccone G, et al. IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol. 2019;30:v915. [Google Scholar]
- 17.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 18.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 19.Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous non-small-cell lung cancer (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;S1556–0864:30292–30296. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 21.Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 23.Reck M, Ciuleanu T, Cobo Dols M, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38:9501–9501. [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 26.Merino DM, McShane L, Butler M, et al. TMB standardization by alignment to reference standards: phase II of the friends of cancer research TMB harmonization project. J Clin Oncol. 2019;37:2624–2624. doi: 10.1200/JCO.2019.37.15_suppl.2624. [DOI] [Google Scholar]
- 27.Wood MA, Weeder BR, David JK, et al. Burden of tumor mutations, neoepitopes, and other variants are weak predictors of cancer immunotherapy response and overall survival. Genome Med. 2020;12:33. doi: 10.1186/s13073-020-00729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Zeng D, Ou Q, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open. 2019;2:e196879. doi: 10.1001/jamanetworkopen.2019.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. 2018;13:1248–1268. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379:1754–1765. doi: 10.1056/NEJMra1706174. [DOI] [PubMed] [Google Scholar]
- 31.Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 32.Blons H, Garinet S, Laurent-Puig P, Oudart JB. Molecular markers and prediction of response to immunotherapy in non-small cell lung cancer, an update. J Thorac Dis. 2019;11:S25–S36. doi: 10.21037/jtd.2018.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5:696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YT, Seeruttun SR, Wu XY, Wang ZX. Maximum somatic allele frequency in combination with blood-based tumor mutational burden to predict the efficacy of atezolizumab in advanced non-small cell lung cancer: a pooled analysis of the randomized POPLAR and OAK studies. Front Oncol. 2019;9:1432. doi: 10.3389/fonc.2019.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenizia F, Pasquale R, Roma C, et al. Measuring tumor mutation burden in non-small cell lung cancer: tissue versus liquid biopsy. Transl Lung Cancer Res. 2018;7:668–677. doi: 10.21037/tlcr.2018.09.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koeppel F, Blanchard S, Jovelet C, et al. Whole exome sequencing for determination of tumor mutation load in liquid biopsy from advanced cancer patients. PLoS ONE. 2017;12:e0188174. doi: 10.1371/journal.pone.0188174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenzinger A, Allen JD, Maas J, et al. Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosom Cancer. 2019;58:578–588. doi: 10.1002/gcc.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 40.Guibert N, Jones G, Beeler JF, et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2019;137:1–6. doi: 10.1016/j.lungcan.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.CD-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC—challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol. 2018;15:577–586. doi: 10.1038/s41571-018-0058-3. [DOI] [PubMed] [Google Scholar]
- 43.Moding EJ, Liu Y, Nabet BY, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer. 2020;1:176–183. doi: 10.1038/s43018-019-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabel L, Riva F, Servois V, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28:1996–2001. doi: 10.1093/annonc/mdx212. [DOI] [PubMed] [Google Scholar]
- 45.Giroux Leprieur E, Herbretau G, Dumenil C, et al. Circulating tumor DNA evaluated by next-generation sequencing is predictive of tumor response and prolonged clinical benefit with nivolumab in advanced non-small cell lung cancer. OncoImmunology. 2018;7:e1424675. doi: 10.1080/2162402X.2018.1424675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg SB, Narayan A, Kole AJ, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res. 2018;24:1872–1880. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alama A, Coco S, Genova C, et al. Prognostic relevance of circulating tumor cells and circulating cell-free DNA association in metastatic non-small cell lung cancer treated with nivolumab. J Clin Med. 2019;8:1011. doi: 10.3390/jcm8071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 49.Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30:1448–1459. doi: 10.1093/annonc/mdz196. [DOI] [PubMed] [Google Scholar]
- 50.Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–112. doi: 10.1016/j.lungcan.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Boffa DJ, Graf RP, Salazar MC, et al. Cellular expression of PD-L1 in the peripheral blood of lung cancer patients is associated with worse survival. Cancer Epidemiol Biomark Prev. 2017;26:1139–1145. doi: 10.1158/1055-9965.EPI-17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicolazzo C, Raimondi C, Mancini M, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6:31726. doi: 10.1038/srep31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ilié M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29:193–199. doi: 10.1093/annonc/mdx636. [DOI] [PubMed] [Google Scholar]
- 54.Dhar M, Wong J, Che J, et al. Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci Rep. 2018;8:2592. doi: 10.1038/s41598-018-19245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janning M, Kobus F, Babayan A, et al. Determination of PD-L1 expression in circulating tumor cells of NSCLC patients and correlation with response to PD-1/PD-L1 inhibitors. Cancers. 2019;11:835. doi: 10.3390/cancers11060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koh Y, Yagi S, Akamatsu H, et al. Heterogeneous expression of programmed death receptor-ligand 1 on circulating tumor cells in patients with lung cancer. Clin Lung Cancer. 2019;20:270–277. doi: 10.1016/j.cllc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Passiglia F, Galvano A, Castiglia M, et al. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther Adv Med Oncol. 2019;11:1758835919839928. doi: 10.1177/1758835919839928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen YL, Huang WC, Lin FM, et al. Novel circulating tumor cell-based blood test for the assessment of PD-L1 protein expression in treatment-naïve, newly diagnosed patients with non-small cell lung cancer. Cancer Immunol Immunother. 2019;68:1087–1094. doi: 10.1007/s00262-019-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamminga M, De Wit S, Hiltermann TJN, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immuno Therapy Cancer. 2019;7:173. doi: 10.1186/s40425-019-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasini L, Ulivi P. Extracellular vesicles in non-small-cell lung cancer: functional role and involvement in resistance to targeted treatment and immunotherapy. Cancers. 2020;12:40. doi: 10.3390/cancers12010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Li C, Zhi C, et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med. 2019;17:355. doi: 10.1186/s12967-019-2101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasini L, Ulivi P. Liquid biopsy for the detection of resistance mechanisms in NSCLC: comparison of different blood biomarkers. J Clin Med. 2019;8:998. doi: 10.3390/jcm8070998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang T, Bai Y, Zhou F, et al. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer. 2019;130:76–83. doi: 10.1016/j.lungcan.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Chabon JJ, Hamilton EG, Kurtz DM, et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580:245–251. doi: 10.1038/s41586-020-2140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu Y, Ulrich BC, Supplee J, et al. False-positive plasma genotyping due to clonal hematopoiesis. Clin Cancer Res. 2018;24:4437–4443. doi: 10.1158/1078-0432.CCR-18-0143. [DOI] [PubMed] [Google Scholar]
- 66.Chan HT, Nagayama S, Chin YM, et al. Clinical significance of clonal hematopoiesis in the interpretation of blood liquid biopsy. Mol Oncol. 2020;14:1719–1730. doi: 10.1002/1878-0261.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cortés-Hernández LE, Eslami-S Z, Pantel K, Alix-Panabières C. Molecular and functional characterization of circulating tumor cells: from discovery to clinical application. Clin Chem. 2019;66:97–104. doi: 10.1373/clinchem.2019.303586. [DOI] [PubMed] [Google Scholar]
- 68.Rossi G, Russo A, Tagliamento M, et al. Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers. 2020;12:1125. doi: 10.3390/cancers12051125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reuben A, Zhang J, Chiou SH, et al. Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun. 2020;11:603. doi: 10.1038/s41467-019-14273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyauchi E, Matsuda T, Kiyotani K, et al. Significant differences in T cell receptor repertoires in lung adenocarcinomas with and without epidermal growth factor receptor mutations. Cancer Sci. 2019;110:867–874. doi: 10.1111/cas.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]