Abstract
Background
CKLF-like MARVEL transmembrane domain-containing 6 (CMTM6) is a critical regulator of tumor immunology among various cancers. However, the role and underlying molecular mechanism of CMTM6 in oral squamous cell carcinoma (OSCC) progression remains unclear.
Methods
The expression of CMTM6, PD-L1 and CD163 in OSCC tissues were detected by immunohistochemistry on tissue microarray. The effect of CMTM6 knockdown on OSCC cells and macrophage polarization were analyzed by CCK-8 assay, apoptotic assay, would-healing assay, transwell assay and qPCR. OSCC cell derived exosomes were obtained by ultracentrifugation and the mechanistic studies were conducted by qPCR and Western Blot. 4-Nitroquinoline N-oxide (4NQO) induced OSCC mice were used for verifying the effect of CMTM6 downregulation on M2 macrophage infiltration and tumor growth.
Results
In OSCC samples, higher CMTM6 expression has been obviously associated with higher pathological stage of OSCC patients, CD163 + macrophages infiltration and PD-L1 expression. CMTM6 knockdown of OSCC cells inhibited proliferative, migrative and invasive abilities of OSCC cells, as well as inhibited M2 macrophage polarization in vitro with downregulating PD-L1 expression. Importantly, exosomes from OSCC cells shuttled CMTM6 to macrophages and promoted M2-like macrophage polarization through activating ERK1/2 signaling. In addition, in 4NQO-induced OSCC mice, CMTM6 level was positively associated with CD163, CD206 and PD-L1 as well as M2-like macrophage infiltration.
Conclusion
OSCC cell-secreted exosomal CMTM6 induces M2-like macrophages polarization to promote malignant progression via ERK1/2 signaling pathway, revealing a novel crosstalk between cancer cells and immune cells in OSCC microenvironment.
Keywords: Oral squamous cell carcinoma, CKLF-like MARVEL transmembrane domain-containing 6, Macrophage, Exosome, PD-L1
Introduction
Oral squamous cell carcinoma (OSCC) is the most common malignancy of oral cancers, and results in more than 145 thousand mortality annually overworld [1]. Traditional treatment options such as surgery, radiotherapy and chemotherapy failed to improve the 5 year survival rate yet, which is about 50% [2, 3]. In the United States, the estimated numbers of new cases and deaths of OSCC have increased in 2019 [4], emphasizing the need to better understand molecular mechanisms underlying the progression of OSCC to identify new therapeutic targets.
CKLF-like MARVEL transmembrane domain-containing 6 (CMTM6), a protein at the plasma membrane, recently has been identified to regulate PD-L1 expression of tumor cells and limit antitumor immunity [5, 6]. And in the cancer genome atlas (TCGA) databases, CMTM6 is overexpressed in all samples from 30 cancer types, including cervical squamous cell carcinoma, oesophageal cancer, lung squamous cell carcinoma and head and neck squamous cell carcinoma (HNSCC) [6]. Recent evidence shows that CMTM6 expression was associated with high malignant gliomas as its expression was positively related to malignant characteristics with frequently genomic aberrations of driver oncogenes in gliomas [7]. And CMTM6 was also highly expressed in type II and III renal clear cell carcinoma, of which the overall survival was smaller than type I [8]. And polymorphisms of cmtm6 gene have also observed to in HCC of a southern Chinese population, which may contribute to genetic susceptibility of HCC [9]. However, CMTM6 was downregulated in hepatocellular carcinoma (HCC) tissues and correlated with metastasis and prognosis of HCC patients [10]. Hence, much is needed to address the molecular mechanisms of CMTM6 regulating cancer cells, especially OSCC cells.
Macrophages are pivotal drivers of tumor permissive inflammatory microenvironment that contribute to the proliferation and invasion of tumor cells, induce angiogenesis and block anti-tumor immune responses [11]. Tumor-associated macrophages (TAM), which are polarized from M1-like antitumor phenotype to M2-like pro-tumor phenotype, have been demonstrated to increase within many human solid tumors and are related with prognosis [12]. Especially, the infiltration of M2 polarized macrophages has been suggested to be positively correlated with poor prognosis in early-stage OSCC [13, 14]. M2 macrophages produce immunosuppressive cytokines such as interleukin-10 (IL-10) and arginase-1 (Arg-1) to block antitumor immunity, and express a high level of programmed death ligand 1 (PD-L1, also known as CD274) in many cancers such as HCC, pancreatic cancer as well as OSCC, thus triggering checkpoint blockade of T cells and contributing to immunosuppression towards tumor cells [15–18]. However, the role and significance of CMTM6 in OSCC contributing to M2-like macrophage polarization remains absent.
Here, we demonstrated that CMTM6 expression was positively associated with CD163+ macrophages infiltration, PD-L1 expression and tumor stage in OSCC clinical samples and 4NQO-induced oral carcinoma model of mice. And knockdown of CMTM6 inhibiting proliferation, migration and invasion of OSCC cells was also observed. In addition, a coculture system was established to demonstrate that CMTM6 increased M2-like macrophage polarization through OSCC-derived exosomes dependent on ERK1/2 signaling in macrophages. Taken together, our results showed that CMTM6 contributed to OSCC progression, partly by inducing M2 macrophage polarization, which may offer a new insight into OSCC treatment.
Materials and methods
Patients and tissue microarray (TMA)
Paraffin-embedded tumor samples were from patients diagnosed as OSCC from department of oral and maxillofacial surgery, West China Hospital of Stomatology, Sichuan University between 2010 and 2015 and without any preoperative treatments including surgery, radiotherapy or chemotherapy before surgery. Informed consent was obtained from all the patients. 5 normal oral epithelial tissues and 45 OSCC tissues were cored and transferred to the recipient master block.
Animals and ethics statement
All animal experiments were processed as per procedures which approved by the subcommittee on research and animal care (SRAC) of Sichuan University. 6-week-old female wide-type C57BL/6 mice (Dashuo, China) were housed under standard laboratory conditions in state key laboratory of oral diseases, West China Hospital of Stomatology. 4NQO (Sigma, United States) was dissolved to the drinking water at 100 μg/mL for 10 weeks and changed to distilled water for another 10 weeks. Then, the mice were randomly divided into two groups for intratumor injections every 3 days.
Before injection, mice were anesthetized by intraperitoneally injecting a solution with ketamine (80 mg/ml) and xylazine (10 mg/ml). For each local injection, 5 μg CMTM6 siRNA or negative control siRNA was mixed with 5 μl transfection reagent (Entranster-in vivo, Engreen, China). siRNA target sequence for mouse CMTM6: 5′-UGCCUAACAGAAAGCGUGUTT-3′. After 3 weeks injection, mice were sacrificed by cervical vertebra luxation and the tumor volumes were measured by a caliper. Tumor volume = (length × width2)/2.
Immunohistochemistry
Immunohistochemistry staining of OSCC tissues was performed as described previously [9]. Briefly, samples were incubated with hydrogen peroxide and serum in turn after dewaxing and dehydration with gradient ethanol. Then, specimens were incubated with anti-CMTM6 antibody (ZENBIO, China, 1:100), anti-CD163 antibody (Proteintech, China, 1: 1500) and anti-PD-L1 (Proteintech, China, 1:300) at 4 °C overnight, washed and then incubated with horseradish peroxidase labeled streptavidin and biotin labeled secondary antibody. All the sections were stained with DAB and were hematoxylin counterstained.
The immunohistochemical result quantification was performed and the subcellular localization (nuclear, cytoplasm, cell membrane) was identified, respectively. 5 microscopic fields at 400 × magnification per section were observed and the positive cell ratio in tumor cells or stromal cells was estimated. For CMTM6, PD-L1 and CD163, the strongly positive expression, moderately positive expression, weakly positive expression and negative expression were categorized into (+ + +), > 50%; (+ +), 25–50%; ( +), 5–25%; (−), < 5%.
Cell culture and treatment
The human OSCC cell line Cal-27 and SCC25, the human monocytes cell line THP-1 were from state key laboratory of oral diseases (West China Hospital of Stomatology, Sichuan University). Cal-27 and SCC25 cells were cultured in DMEM medium (HyClone, USA) and F12 medium (HyClone, USA) respectively, and THP-1 cells were maintained in complete RPMI1640 medium (HyClone, USA). All these cells were cultured with 10% FBS and kept under 5% CO2 at 37 °C.
Macrophage polarization and coculture
THP-1 cells were seeded at the lower compartment with 100 ng/ml phorbol myristate acetate (PMA). After 48 h, macrophages were washed with PBS in prepare. The Cal-27 or SCC25 cells were seeded into the upper compartment of the Transwell (Millipore, USA) coculture system. Then, the upper and lower compartments were combined with complete RPMI1640 medium with 10% FBS for another 48 h in a humidified chamber. In order to generate well-differentiated M1 macrophages, PMA-differentiated THP-1 cells were cultured with IFN-γ (20 ng/mL) and LPS (100 ng/mL) for another 48 h. And to obtain well-differentiated M2 macrophages, PMA-differentiated THP-1 cells were cultured with IL-4 (20 ng/mL) and IL-13 (20 ng/mL) for another 48 h.
Cell transfection
The siRNAs sequence 5′-GCUGCAAUUGUGUUUGGAUTT-3′ targeting human cmtm6 or control siRNA were transiently transfected using Lipofectamine 2000 transfection reagent (GeneCopoeia, US) according to the manufacturer’s instructions for siRNA transfection. 24 h later, the transfected cells were collected for further research.
CCK-8 assays
The proliferation of Cal-27 and SCC25 cells was evaluated with CCK-8 assays (Dojindo, China) after transfection. In brief, the plate was washed and added with a 100 μL serum-free medium with 10 μL CCK-8 reagent at 24 h, 48 h and 72 h after transfection, respectively. The absorbance was measured at 450 nm after incubation for 30 min.
Would healing assays
Cal-27 or SCC25 cells were seeded on six-well plates and transfected. Then, a wound across the wall was introduced. After washing with PBS, the plate was incubated with serum-free medium for 24 h. Cell migration was observed and photographed by microscopy.
Apoptosis assays
Cal-27 or SCC25 cells were collected at the time of 24 h and 48 h after transfection. 5 μL Annexin V-FITC (KeyGEN, China) was added to the cell suspension for staining in the dark. After that, 5 μL PI (KeyGEN, China) was applied. Cells were then analyzed by Flow Cytometer.
Invasion assays
Invasion assays were conducted using an 8 μm pore Transwell filter (Corning, US) coated with Matrigel matrix. Cal-27 or SCC25 cells were seeded in the upper chamber with DMEM/F12 serum-free medium. And medium with 20% FBS was added to the lower chamber. After incubation for 48 h, the Transwell filter was washed, fixed with 5% glutaraldehyde and in turn stained with 0.1% crystal violet staining solution.
RNA extraction and reverse transcription-PCR (qPCR) analysis
Total RNA of OSCC cells and THP-1-derived macrophages were extracted using total RNA extraction kit (Solarbio, China). Synthesis of cDNA was performed using PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan) according to manufacturer’s instructions. Then, the samples were analyzed in PowerUp SYBR green master mix system (ABI, US). GADPH mRNA levels were used to normalize relative mRNA levels. Primer sets used were as follows: GAPDH (human), forward 5′-ACAACTTTGGTATCGTGGAAGG-3′, reverse 5′-GCCATCACGCCACAGTTTC-3′; CMTM6 (human), forward 5′-ATGAAGGCCAGCAGAGACAG-3′, reverse 5′-GTGTACAGCCCCACTACGGA-3′; CD163 (human), forward 5′-TTTGTCAACTTGAGTCCCTTCAC-3′, reverse 5′-TCCCGCTACACTTGTTTTCAC-3′; CD86 (human), forward 5′-TGGTGCTGCTCCTCTGAAGATTC-3′, reverse 5′-ATCATTCCTGTGGGCTTTTTGTG-3′; TNF-α, forward 5′-GACAAGCCTGTAGCCCATGTTGTA-3′, reverse 5′-CAGCCTTGGCCCTTGAAGA-3′; IL-12p40, forward 5′- CGGTCATCTGCCGCAA-3′, reverse 5′-AACCTAACTGCAGGGCACAG-3′; IL-10, forward 5′- GAGATGCCTTCAGCAGAGTGAAGA-3′, reverse 5′-AGGCTTGGCAACCCAGGTAAC-3′; Arg-1, forward 5′- TGGACAGACTAGGAATTGGCA-3′, reverse 5′-CCAGTCCGTCAACATCAAAACT-3′; SOCS3, forward 5′- GCTCCAAAAGCGAGTACCAGC-3′, reverse 5′-AGTAGAATCCGCTCTCCTGCAG-3′; STAT3, forward 5′-ATCACGCCTTCTACAGACTGC-3′, reverse 5′-CATCCTGGAGATTCTCTACCACT-3′; ERK1, forward 5′- CTACACGCAGTTGCAGTACAT-3′, reverse 5′- CAGCAGGATCTGGATCTCCC-3′; ERK2, forward 5′- TACACCAACCTCTCGTACATCG-3′, reverse 5′- CATGTCTGAAGCGCAGTAAGATT-3′; MIF, forward 5′- AGCAGCTGGCGCAGGCCAC-3′, reverse 5′- CTCGCTGGAGCCGCCGAAGG-3′; GADPH (mouse), forward 5′-GATCCGGGTCCTCAGAGGTTT-3′, reverse 5′-ATCAGGTGGTAGCATAGGCTT-3′; CMTM6 (mouse), forward 5′-GTGAGCTGTAGCGCCTTTCTC-3′, reverse 5′-TCCGATGACTTGACTTTTCCAG-3′; CD206 (mouse), forward 5′-CTCTGTTCAGCTATTGGACGC-3′, reverse 5′-CGGAATTTCTGGGATTCAGCTTC-3′; CD86 (mouse), forward 5′-TCAATGGGACTGCATATCTGCC-3′, reverse 5′-GCCAAAATACTACCAGCTCACT-3′.
Western blotting
Cell or exosomes were lysed with RIPA buffer (Pierce, US) and then centrifuged at 14,000 g for 15 min at 4 °C to collect the supernatants. After mixed with SDS sample buffer, the proteins were heated to 99 °C for 10 min, separated on 10% SDS–polyacrylamide gels, transferred to PVDF membranes, and probed with antibodies against PD-L1 (1:3000; proteintech, China), p-ERK1/2 (1:1000, Cell Signaling, US), calnexin (1:1000; Affinity, China), CD63 (1:1000; Affinity, China) and α-tublin (1:2000, Proteintech, China) at 4 °C overnight and blots were visualized using gel imaging systems (Bio-Rad).
Exosome isolation
Cal-27 cells were cultured in DMEM medium with 10% exosome-free FBS. The conditioned medium was collected after 48 h. Then, the exosomes in conditioned medium were isolated by ultracentrifugation. In brief, 40 mL conditioned medium was centrifuged at 300 g for 10 min, 2000 g for 10 min, 10,000 g for 30 min, 100,000 g for 70 min and 100,000 g for another 30 min successively to pellet the exosomes. The final pellet was resuspended in PBS and frozen at − 80 °C.
Exosome uptake
Purified exosomes from supernatant of Cal-27 cells were re-suspended in PBS and labeled by a PKH26 fluorescent kit (Sigma, USA). Briefly, 4 μl PKH26 dye was added to 25 μg exosomes followed by 0.5 ml diluent C and incubated for 5 min at room temperature. They were then mixed with an equal volume of 0.5% BSA. Exosomes labeled with PKH26 were re-isolated as stated above. Finally, the labeled exosomes were incubated with PMA-differentiated THP-1 cells at 37 °C for 12 h and the fluorescence uptake was observed by the inverted fluorescence microscope. Cal-27 cells incubated with non-labeled exosomes (PBS was added to 25 μg exosomes instead of PKH26) and Cal-27 cells with only PKH26 (PKH26 was added to PBS instead of exosomes and was re-isolated as stated) were used as controls.
Statistical analyses
All experiments were repeated three times independently. Data were analyzed with SPSS 13.0. Statistical significance was designated as p < 0.05.
Results
CMTM6 expression is positively associated with CD163 + macrophages infiltration and clinical characteristics in OSCC patients
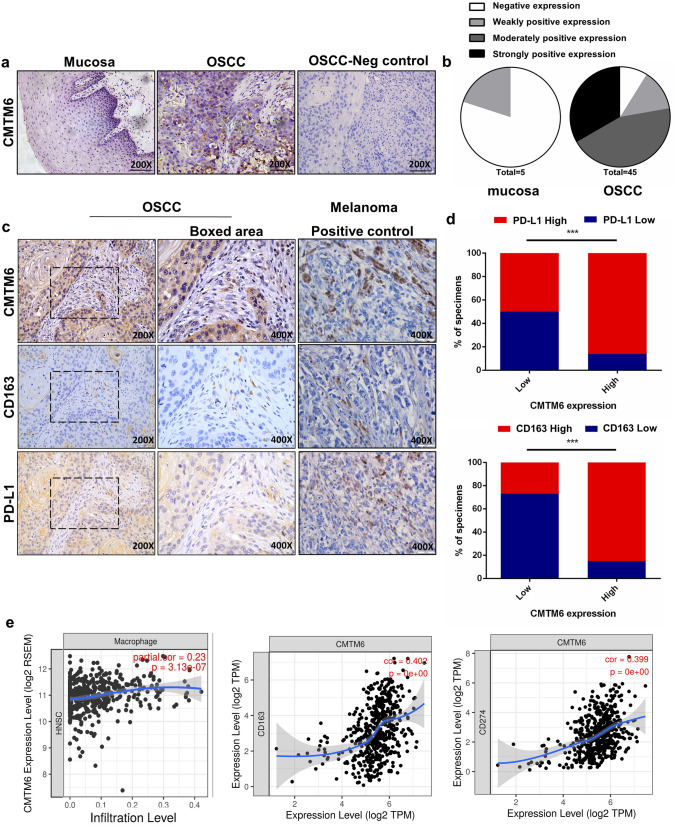
A tissue microarray containing 5 normal oral mucosa and 45 OSCC tissues was used for CMTM6, CD163 and PD-L1 immunochemistry staining. Clinic characteristics of 45 OSCC patients were shown in Table 1. The data showed that CMTM6 were positively stained in plasma membrane or cytoplasm of tumor cells, and was markedly overexpressed in 91.1% cases of OSCC (41/45), compared with 20% (1/5) cases of normal oral mucosa (Fig. 1a). And the strongly, moderately and weakly positive rate was 33.3% (15/45), 44.4% (20/45) and 13.3% (6/45), respectively (Table. 1 and Fig. 1b). And the expression of CMTM6 was positively related to T stage, pathological grade and lymph node metastasis of OSCC patients (p<0.05), but not to the age or gender of patients (p > 0.05) (Table 2).
Table 1 .
The expression of CMTM6 in normal oral mucosa and OSCC
| Cases | Normal (n = 5) |
OSCC (n = 45) |
p value | |
|---|---|---|---|---|
| CMTM6 | − | 4 | 4 | 0.001 |
| + | 1 | 6 | ||
| + + | 0 | 20 | ||
| + + + | 0 | 15 |
( −), < 5% negative expression; ( +), 5–25% weakly positive expression; (+ +), 25–50% moderately positive expression; (+ + +), > 50% strongly positive expression
*p < 0.05 was regarded as statistically significant in rank sum test
Fig. 1 .
Representative immunohistochemical images of oral mucosa and OSCC sections. a Representative images of CMTM6 immunohistochemical expression and negative control (PBS was added instead of primary antibodies in OSCC tissue microarrays (scale bar: 100 µm). b Histoscores for CMTM6 expression in oral mucosa and OSCC sections. c Representative images of OSCC tissues stained for CMTM6, PD-L1 and CD163 (scale bar: 100um and boxed area scale bar: 50 um) and melanoma tissues stained for CMTM6, PD-L1 and CD163 for positive control (scale bar: 50 um). d Percentage of specimens showing low or high CMTM6 expression in relation to the expression levels of PD-L1 and CD163. The Chi square test was used to analyze statistical significance. e Analyzes of Tumor IMmune Estimation Resource (TIMER) databases showed the association between CMTM6 expression with macrophages infiltration and CD163, PD-L1 expression based on RNA-seq in head and neck squamous cell carcinoma (HNSCC). *p < 0.05, **p < 0.01, ***p < 0.001
Table 2 .
Clinicopathological features of OSCC patients and their relationship with CMTM6 expression (n = 45)
| Characteristics | Cases | CMTM6 | p value | |
|---|---|---|---|---|
| Higher (≥ 50%) | Lower (< 50%) | |||
| Age (years) | ||||
| < 60 | 23 | 18 | 5 | 0.780 |
| ≥ 60 | 22 | 17 | 5 | |
| Gender | ||||
| Female | 16 | 14 | 2 | 0.244 |
| Male | 29 | 21 | 8 | |
| Tumor size | ||||
| T1–T2 | 16 | 9 | 7 | 0.027* |
| T3–T4 | 29 | 26 | 3 | |
| Differentiation | ||||
| Well or moderate | 20 | 12 | 8 | 0.027* |
| Poor | 25 | 23 | 2 | |
| Clinical stage | ||||
| I–II | 13 | 7 | 6 | 0.033* |
| III–IV | 32 | 28 | 4 | |
| Nodal metastasis | ||||
| Yes | 19 | 18 | 1 | 0.048* |
| No | 26 | 17 | 9 | |
*p < 0.05 was regarded as statistically significant in Chi-square test
Next, the relationship between CMTM6 with CD163 + macrophages infiltration and PD-L1 expression was evaluated. CD163 + macrophages were heterogeneously located at the tumor stroma, with partial expression at the epithelium. Higher CD163 + macrophages distribution was significantly detected in CMTM6 positive OSCC compared to CMTM6 negative OSCC (Fig. 1c). Furthermore, the intensity of CD163 + macrophages of CMTM6-positive OSCC was also stronger in CMTM6-positive OSCC compared to CMTM6-negative OSCC (moderate to dense, 84.85% vs. 27.27%) (Fig. 1d). Spearman correlation coefficient test suggested that the intratumoral CD163 + macrophages density was closely related to CMTM6 expression (r = 0.595, p<0.001) (Fig. 1c). And the positive expression of PD-L1 was in the cytoplasm of tumor cells, and was associated with CMTM6 expression (r = 0.288, p<0.05) (Fig. 1c and 1d). The data were in accordance with the results in tumor immune estimation resource (TIMER) databases that the infiltration of macrophages as well as the expression of CD163 and PD-L1 were positively associated with CMTM6 expression in HNSCC cases through RNA-seq (Fig. 1e). Together, these data implicated that CMTM6 overexpression indicated poor clinicopathological characteristics, and was positively related to CD163 + macrophage infiltration and PD-L1 expression.
CMTM6 knockdown in OSCC cells mitigates OSCC cancer progress with PD-L1 downregulation
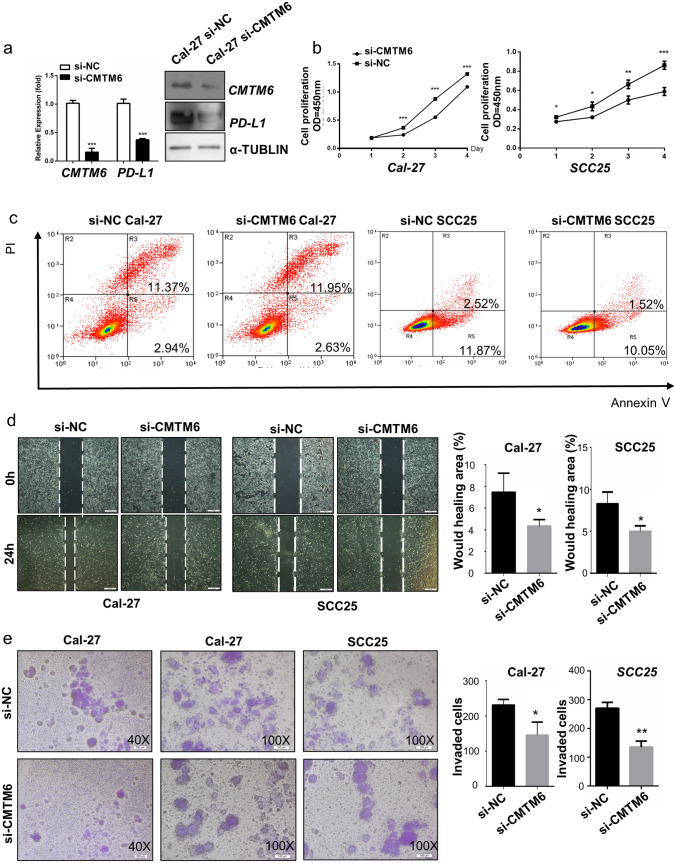
To investigate the potential function of CMTM6 in OSCC cells, we established three independent siRNAs to knockdown CMTM6 in Cal-27 and SCC25 cells and siRNA 3 stably downregulated the mRNA and protein levels of CMTM6 at 24 h after transfection (Fig. 2a). As shown in Fig. 2b, Cal-27 and SCC25 cells proliferation were decreased after silencing CMTM6. Cellular migration and invasion were inhibited in both Cal-27 and SCC25 cells after transfection with si-CMTM6 (Fig. 2d, e). However, the apoptosis of Cal-27 and SCC25 cells was not affected by transfection of si-CMTM6 (Fig. 2c). Moreover, CMTM6 knockdown dramatically reduced the protein level of PD-L1 in OSCC cells (Fig. 2a). Thus, these indicated that CMTM6 knockdown could dramatically inhibit the proliferation, migration and invasion of OSCC cells to mitigate OSCC progression with downregulating PD-L1 expression.
Fig. 2 .
CMTM6 knockdown inhibited proliferation, migration and invasion of OSCC cells. a qPCR and Western blot showing CMTM6 knockout in Cal-27 cells and PD-L1 expression in CMTM6 knockout Cal-27 cells. b Cell proliferation of OSCC cells transfected with CMTM6 siRNA and control siRNA quantified by CCK8 assays. c Cell apoptosis of OSCC cells transfected with CMTM6 siRNA and control siRNA quantified by flow cytometry. d Cell migration of OSCC cells transfected with CMTM6 siRNA and control siRNA quantified by Wound healing assays (scale bar: 200 um). e Cell invasion of OSCC cells transfected with CMTM6 siRNA and control siRNA quantified by Transwell assays (scale bar: 200um). *p < 0.05, **p < 0.01, ***p < 0.001
CMTM6 in OSCC cells contributes to M2-like macrophage polarization
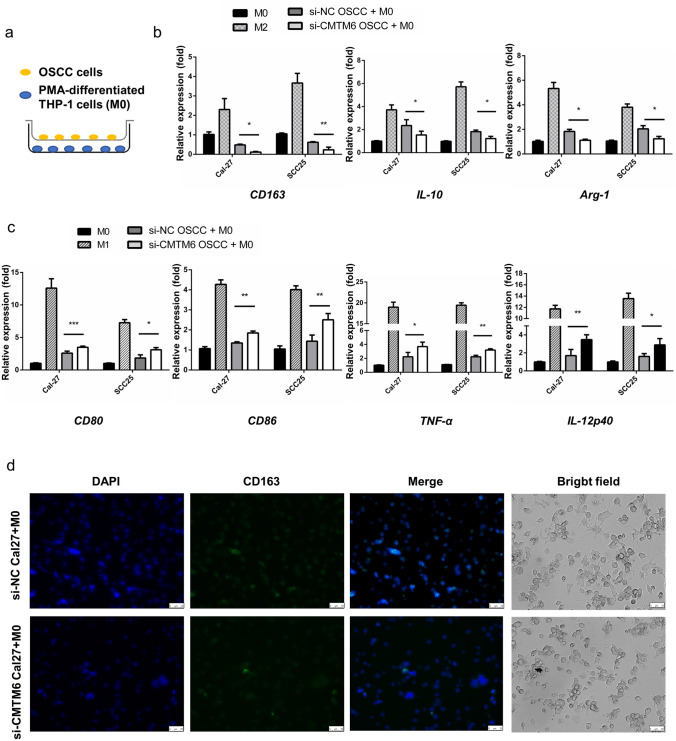
After obtaining the effect of CMTM6 on promoting OSCC malignancy, we then analyzed whether it could promote the M2-polarized macrophages, which played an important role in immunity microenvironment facilitating OSCC progression [13]. PMA-differentiated human THP-1 monocytes (M0 macrophages) were cocultured with OSCC cells using a Transwell system (Fig. 3a), and the expression of M1 and M2 macrophage markers were examined by qPCR. The expression of CD163 was lower in M0 macrophages with si-CMTM6 OSCC cells than that with si-NC OSCC cells (Fig. 3b); meanwhile the M1 markers such as CD80 and CD86 were increased (Fig. 3c). The mRNA levels of tumor necrosis factor α (TNF-α) and IL-12p40 (M1 cytokines) were markedly upregulated in M0 macrophages in responses to coculture with CMTM6-silencing OSCC cells in a variable extent (Fig. 3c). While CD163 + macrophages were decreased in M0 with si-CMTM6 Cal-27 cells compared to that with si-NC Cal-27 cells as shown in Fig. 3d (Fig. 3d). And M2 representative gene IL-10 and Arg-1 were lower in macrophages induced by si-CMTM6 OSCC cells than that induced by si-NC OSCC cells (Fig. 3b). Together, these findings indicated that CMTM6 may be an inducer of M2-like macrophages polarization in OSCC microenvironment.
Fig. 3 .
CMTM6 inhibition resulted M1-like phenotype polarized in vitro. THP-1 cells were pre-treated with 100 ng/ml PMA for 48 h before cocultured with Cal-27 cells. a A sketch for the Transwell coculture system for PMA-induced THP-1 cells and Cal-27 cells. b, c qPCR analyses showing mRNA levels of M1 markers (CD80, CD86, TNF-α and IL-12p40) and M2 macrophage markers (CD163, IL-10 and Arg-1) of M0 cells cocultured with OSCC cells. Results are showed as the relative fold change compared with M0. d Immunofluorescent DAPI (blue) and CD163 (green) staining and the morphology of M0 after coculture (scale bar: 25um). *p < 0.05, **p < 0.01, ***p < 0.001
OSCC cells shuttle CMTM6 to macrophages through exosomes
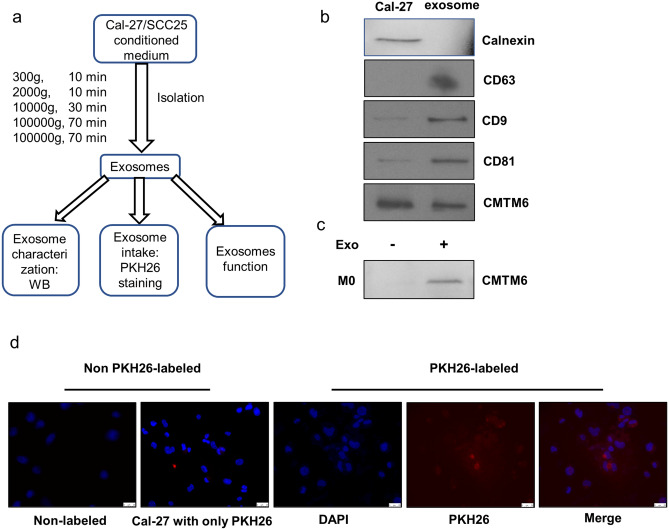
We next explored the molecular mechanisms that OSCC cells employed to modulate macrophages polarization. Considering the indirect coculture system conducted, the direct cell-to-cell contacts dependent on cytoskeletal remodeling is excluded. Then, we investigated whether OSCC cells could regulate macrophages polarization through exosomes, which is a crucial mediator in intercellular communication [19]. The exosomes from OSCC cell culture supernatants were extracted through ultracentrifugation (Fig. 4a). Subsequently, we detected the expression of exosome marker CD63, CD9 and CD81, as well as endoplasmic reticulum protein calnexin by Western blot (Fig. 4b). CD63, CD9 and CD81 were positively expressed in exosomes, while calnexin was confirmed absent. Additional, Cal-27 cell lysates were positive for calnexin, suggesting that the exosomes from Cal-27 cells were purified without endoplasmic reticulum vesicles. Thus, we concluded the vesicles we extracted from Cal-27 cell supernatant were purified exosomes.
Fig. 4 .
The isolation and identification of Cal-27 exosomes. a A sketch of isolation path and identification of exosomes. b Western blot analyzed of exosomes for CD63, CD9 and CD81 (exosome biomarker), Calnexin (negative marker) and CMTM6. c Western blot analyzed of M0 cells and exosomes incubated M0 cells for CMTM6. d Representative fluorescent images for PKH26-labeled exosomes taken by M0 after 12 h incubation. Exosomes were stained red and M0 nuclei were stained blue by DAPI (scale bar: 25um). Cal-27 cells incubated with non-labeled exosomes (PBS was added to 25 μg exosomes instead of PKH26) and Cal-27 cells with only PKH26 (PKH26 was added to PBS and was re-isolated as stated) were used as controls
Then, we explored the possibility that CMTM6 could be shuttled to macrophages via OSCC cell secreted exosomes. The absorption of OSCC cell-secreted exosomes by macrophages was demonstrated by fluorescence microscopy. We labeled the exosomes with PKH26 and incubated them with M0 for 12 h. Figure 4d shows that the fluorescence signals of PKH26 were dispersed in the cytoplasm of macrophages, demonstrating the OSCC cell-derived exosomes could be efficiently internalization and diffusion in macrophages. Importantly, using Western blot, we found that CMTM6 was contained in exosomes derived from OSCC cells and could be detected in M0 cells after incubating with OSCC-exosomes (Fig. 4b, c). These results implied CMTM6 could be shuttled to macrophages through OSCC cells-derived exosomes.
CMTM6 in OSCC cells modulates M2-like macrophage polarization through activating ERK1/2 signaling
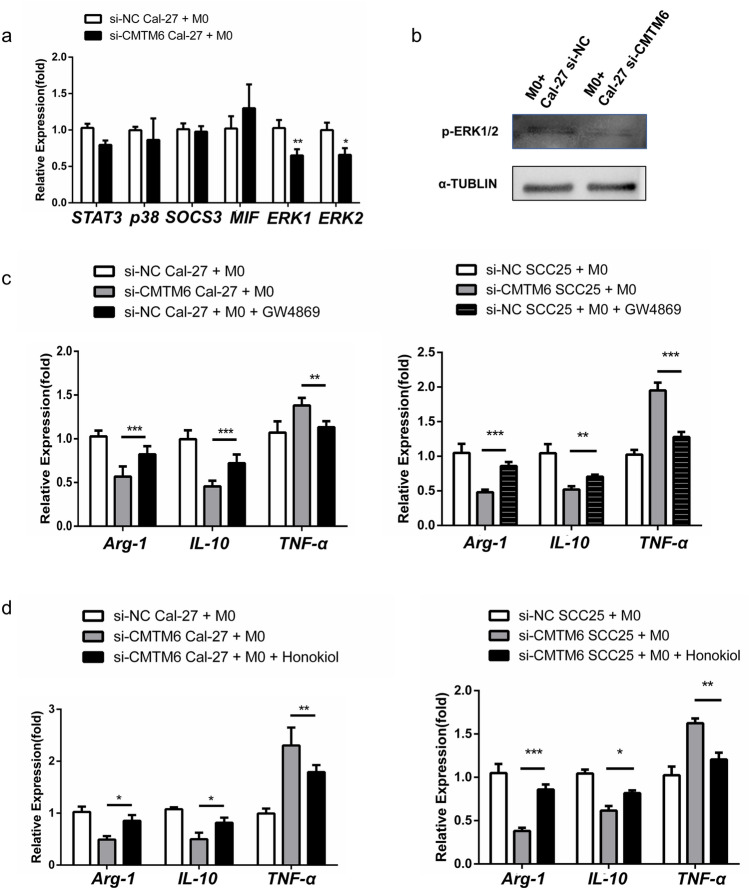
ERK1/2 signaling has been known to be related with the polarization of macrophages [20]. Therefore, we asked whether ERK1/2 signaling is involved in the regulation of macrophages by CMTM6 in OSCC cells. Intriguingly, M0 cocultured with si-CMTM6 Cal-27 resulted in downregulation of both total and phosphorylated ERK1/2 (Fig. 5b). But the mRNA levels of SOCS3 (inhibitor of STAT3 signaling), p38 and macrophage migration inhibitory factor (MIF) were not affected (Fig. 5a). Besides, pre-treatment of OSCC cell with GW4869 (the inhibitor of exosomes) could reduce M2-like macrophages (Fig. 5c). To investigate whether ERK1/2 signaling resulted in M2 polarization, Honokiol, an ERK1/2 activator, was applied to M0 with si-CMTM6 OSCC cells coculture. As seen in Fig. 5d, M1-like macrophage polarization was largely blocked by honokiol as assessed by the mRNA levels of M1 representative genes TNF-α and IL-12p40, and the mRNA levels of M2 representative genes IL-10 and Arg-1 were increased. Taken together, the results showed that exosomal CMTM6 of OSCC cells promotes M2 polarization through activating ERK1/2 signaling in macrophage.
Fig. 5 .
OSCC exosomes promote M2-like phenotype through activating ERK1/2 signaling. M0 macrophages were cocultured with Cal-27 cells transfected with si-CMTM6 or control siRNA. a The mRNA levels of STAT3, SOCS3, MIF, p38 and ERK1/2 analyzed by qPCR. b The protein levels of p-ERK1/2 analyzed by Western blot. c The mRNA levels of M1 markers (TNF-α and IL-12p40) and M2 markers (IL-10 and Arg-1) after a 48 h coculturing with 10 μM GW4869 pre-treated OSCC cells. d The mRNA levels of M1 markers (TNF-α and IL-12p40) and M2 markers (IL-10 and Arg-1) after a 48-h coculture with or without 5 μM Honokiol
CMTM6 silencing inhibits tumor growth and decreases TAM infiltration in 4NQO-treated mice
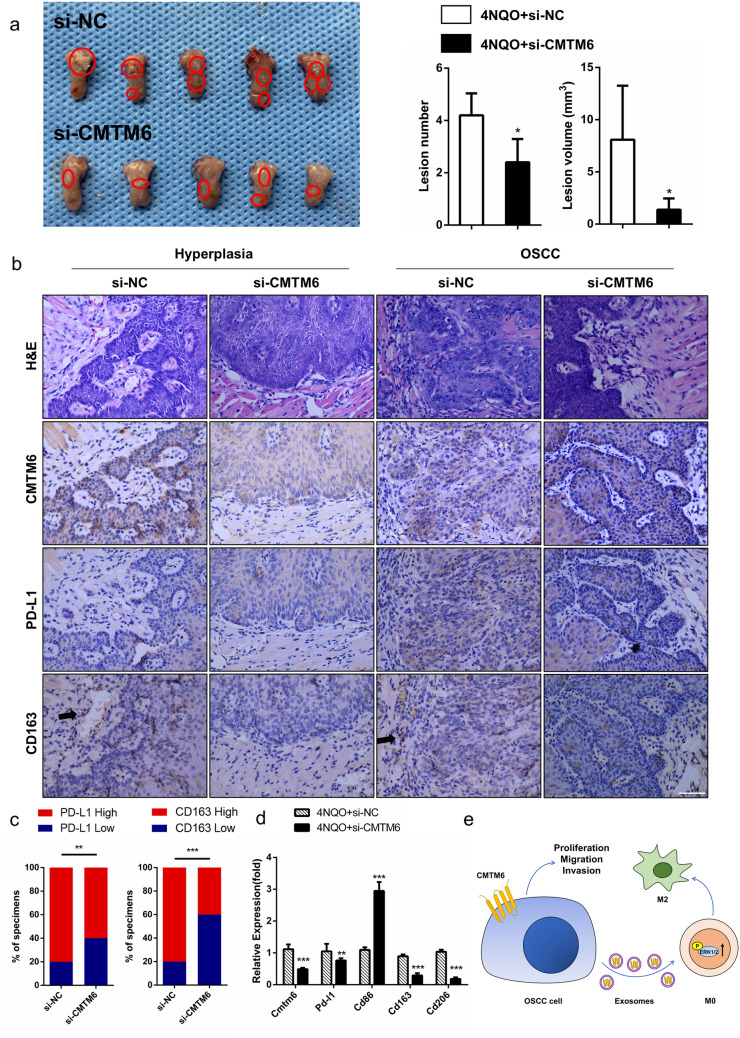
To confirm the in vitro findings shown above, a 4NQO-induced oral carcinoma model was performed as described previously [21]. si-CMTM6 or si-NC was mixed with in vivo transfection reagent, and locally injected into the tumor mass every 5 days for 3 weeks. We observed that the tumor volume at the time of sacrifice injected with si-CMTM6 was markedly less than si-NC (p = 0.0097, Fig. 6a). In si-CMTM6 injection group, the average tumor lesion volume was (1.39 ± 0.48) mm3 and the average lesion number was 2.4 ± 0.4, while the average tumor lesion volume was (8.09 ± 2.31) mm3 and average lesion number was 4.2 ± 0.4 in si-NC injection group (Fig. 6a). As expected, a lower mRNA levels of Cmtm6 was found in tumor tissue of si-CMTM6 injection group compared to control (Fig. 6d). Next, we investigated whether reduced CMTM6 expression was associated with impaired M2-like macrophage and PD-L1 expression. Immunohistochemistry analysis showed that tumor samples of si-CMTM6 injection group exhibited less CD163 + cells than those in control tumor counterparts, reflecting a smaller number of M2-like macrophages in tumor microenvironment (Fig. 6b, c). And the mRNA levels of M2 macrophage markers CD163, CD206 and PD-L1 was lower in the tumor samples of si-CMTM6 injection mice (Fig. 6d). The results also showed that knockdown of CMTM6 significantly decreased PD-L1 expression in 4NQO-treated mice. Therefore, these indicated that disruption of CMTM6 could block M2-like macrophages in OSCC microenvironment.
Fig. 6 .
CMTM6 knockdown blocked OSCC progression and decreased PD-L1 and CD163 expression in vivo. a Image of tongue tissues (scale bar 1 cm) and the quantitative data of tumor lesion size and lesion number per mouse. b Representative HE and immunohistochemical images of tongue tissues, including hyperplasia and carcinoma (scale bar 50 μm). c Percentage of specimens showing the expression levels of PD-L1 and CD163 in si-NC and si-CMTM6 group. The Chi-square test was used to analyze statistical significance. d The mRNA levels of CMTM6, PD-L1, CD86 (M1 marker), CD163 and CD206 (M2 marker) in tongue tissues in 4NQO induced oral carcinogenesis mice injected with si-CMTM6 and control siRNA. e Schematic cartoon illustrating that CMTM6 promoted proliferation, migration and invasion of OSCC cells and induced M2 macrophage polarization through exosomes
Discussion
It is now becoming clear that exosomes travel between cell populations and modify phenotypes of recipient cells in tumor microenvironment. Here, we found that the vesicles we extracted from OSCC cell supernatant were purified exosomes and CMTM6 could be shuttled to macrophages through OSCC cells-derived exosomes. OSCC-exosomal CMTM6 facilitated M2 macrophage polarization by activating ERK1/2 signaling. And CMTM6 promoted migratory and invasive capabilities through enhancing PD-L1 expression. CMTM6 was overexpressed in OSCC tissues and was positively related to CD163 + macrophage infiltration, PD-L1 expression, as well as clinical pathology. As far as we know, this is the first study to report the roles of CMTM6 in the crosstalk between cancer cells and immune cells in OSCC microenvironment, which may provide novels insights into tumor treatment on targeting CMTM6.
First, we evaluated CMTM6 expression in OSCC TMA, and found that CMTM6 was overexpressed in OSCC cells and was positively associated with more advanced stage of OSCC patients. In line with our results, evidences have suggested that CMTM6 was increased in gliomas and renal clear cell carcinoma [7, 8]. And the highly expression of CMTM6 has been verified to be associated with high WHO Grade, the IDH wildtype status, as well as predicts poor prognosis in gliomas [7]. Hence, we speculated that CMTM6 could interact with OSCC cells and facilitate the malignant progression of OSCC. Then, the effect of CMTM6 on OSCC cells has also been explored. Our results revealed that CMTM6 depletion resulted in decreased proliferation, migration and invasion of OSCC cells, while the apoptosis of OSCC cells was not affected. In accordance with this, we found that inhibition of CMTM6 suppressed tumor growth in vivo. And, CMTM6 interference resulted in impaired PD-L1 expression of OSCC cancers, which is consistent with Mezzadra et al., who indicated that CMTM6 silencing significantly impaired PD-L1 protein expression. These suggested that CMTM6 could promote OSCC progression through increasing the proliferation, migration and invasion of OSCC cells, with promoting PD-L1 expression.
It is well known that macrophages are characterized by their plasticity and can mutually transform in response to stimulus in tumor microenvironment. M1 macrophages are generally considered to exert anti-tumor effects in cancers, while M2 macrophages, which are in the majority of TAM, have an opposite function to block antitumor immunity [22]. In gastric and breast cancers, tumor recruiting M2 macrophages was suggested to contribute to tumor progression and metastasis [23, 24]. Here, we demonstrated that CD163+ macrophages were highly infiltrated in CMTM6 overexpressed OSCC samples. Furthermore, CMTM6 could induced M0–M2-like macrophages dependently on ERK1/2 signaling when cocultured with OSCC cells. In support of these results, inhibition of CMTM6 resulted in less M2 infiltration in the tumor microenvironment of 4-NQO-induced OSCC tumorigenesis mice. Researches also demonstrated a profound effect of cancer cells on macrophages to effectively evade immune surveillance. For example, Weng et al. showed that oncogene Multiple Copies in T cell Malignancy 1 (MCT-1) expression in tumor cells drove M2 macrophage polarization in triple-negative breast cancer [25]. Mu et al. also indicated tumor derived lactate was a pivotal metabolite that promoted M2-like polarization and breast cancer progression [20]. Similarly, pancreatic cancer cells could also potently induce M2 phenotype of RAW264.7 macrophages [26]. Together with our results, we showed that OSCC cells could induce M2-like phenotype through CMTM6 expression.
Exosomes once were thought to remove redundancy from cells. While this has been highlighted recently with increased researches that exosomes were capable to travel between cells and transport signals to recipient cells, thus participating in cancer progression [27]. We found that CMTM6 could be shuttled by OSCC derived exosomes to M0 and resulted in M2-like polarization in vitro. In accordance with our results, Wang et al. founded that hypoxic exosomal miR-301a-3p from pancreatic cancer cells induced M2 polarization through activating PTEN/PI3Kγ signaling pathway [28]. Similarly, Chen et al. indicated that hypoxia induced high expression of miR-940 in epithelial ovarian cancer (EOC)-derived exosomes to stimulate M2 phenotype polarization, which promoted EOC proliferation and migration [29]. While others suggested that melanoma exosomes did not exclusively polarize macrophages, but induced a mixed M1 and M2 polarization [30]. This indicated CMTM6 of OSCC cells modulate M2 macrophage polarization through exosomes.
In summary, OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway to contribute to the malignant progression, which involved in mediation of crosstalk between cancer cells and macrophages. Although this signaling pathway concerns complicated immune networks that need to be studied further in detail, targeting CMTM6 combined with PD-L1 inhibition or immunotherapy might be a new strategy for OSCC.
Acknowledgements
This work was supported by National Natural Science Foundation of China grants (Nos.8207300, 81672672, 81972542 and 81902779) and National Science Foundation of Sichuan Province (Nos.2020JDRC0018 and 2020YFS0171).
Author contributions
Xin Pang, Sha-sha Wang and Mei Zhang performed most of the experiments and wrote the manuscript. Hua-yang Fan and Jia-shun Wu performed the mice materials. Hao-fan Wang analyzed data and assisted in manuscript writing. Xin-hua Liang and Ya-ling Tang conceived the study and performed the final corrections.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Pang, Sha-sha Wang and Mei Zhang contributed equally to this work.
Change history
11/25/2021
A Correction to this paper has been published: 10.1007/s00262-021-03113-0
Contributor Information
Xin-hua Liang, Email: lxh88866@scu.edu.cn.
Ya-ling Tang, Email: tangyaling@scu.edu.cn.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Krstevska V. Evolution of treatment and high-risk features in resectable locally advanced Head and Neck squamous cell carcinoma with special reference to extracapsular extension of nodal disease. J BUON. 2015;20(4):943–953. [PubMed] [Google Scholar]
- 3.Pring M, Prime S, Parkinson E, Paterson I. Dysregulated TGF-beta1-induced smad signalling occurs as a result of defects in multiple components of the TGF-beta signalling pathway in human head and neck carcinoma cell lines. Int J Oncol. 2006;28(5):1279–1285. [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 5.Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, Lam EYN, Henderson MA, Bell CC, Stolzenburg S, Gilan O, Bloor S, Noori T, Morgens DW, Bassik MC, Neeson PJ, Behren A, Darcy PK, Dawson SJ, Voskoboinik I, Trapani JA, Cebon J, Lehner PJ, Dawson MA. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–105. doi: 10.1038/nature23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland I, Broeks A, Horlings HM, Wessels LFA, Blank CU, Xiao Y, Heck AJR, Borst J, Brummelkamp TR, Schumacher TNM. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549(7670):106–110. doi: 10.1038/nature23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan X, Zhang C, Zhao J, Sun G, Song Q, Jia W. CMTM6 overexpression is associated with molecular and clinical characteristics of malignancy and predicts poor prognosis in gliomas. EBioMedicine. 2018;35:233–243. doi: 10.1016/j.ebiom.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W, Zhao F, Yang K, Lu Y, Zhang Y, Wang W, Xie H, Deng K, Yang C, Rong Z, Hou Y, Li K. An immunophenotyping of renal clear cell carcinoma with characteristics and a potential therapeutic target for patients insensitive to immune checkpoint blockade. J Cell Biochem. 2019;120(8):13330–13341. doi: 10.1002/jcb.28607. [DOI] [PubMed] [Google Scholar]
- 9.Yafune A, Kawai M, Itahashi M, Kimura M, Nakane F, Mitsumori K, Shibutani M. Global DNA methylation screening of liver in piperonyl butoxide-treated mice in a two-stage hepatocarcinogenesis model. Toxicol Lett. 2013;222(3):295–302. doi: 10.1016/j.toxlet.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Qi G, Li C, Bei C, Tan C, Zhang Y, Shi W, Zeng W, Kong J, Fu Y, Tan S. Expression and clinical significance of CMTM6 in hepatocellular carcinoma. DNA Cell Biol. 2019;38(2):193–197. doi: 10.1089/dna.2018.4513. [DOI] [PubMed] [Google Scholar]
- 11.Singh Y, Pawar VK, Meher JG, Raval K, Kumar A, Shrivastava R, Bhadauria S, Chourasia MK. Targeting tumor associated macrophages (TAMs) via nanocarriers. J Control Release. 2017;254:92–106. doi: 10.1016/j.jconrel.2017.03.395. [DOI] [PubMed] [Google Scholar]
- 12.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 13.Weber M, Iliopoulos C, Moebius P, Buttner-Herold M, Amann K, Ries J, Preidl R, Neukam FW, Wehrhan F. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016;52:75–84. doi: 10.1016/j.oraloncology.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180(4):2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 15.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7–H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3(4):399–411. doi: 10.1158/2326-6066.Cir-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C, Yuan F, Wang J, Wu L. Oral squamous cell carcinoma suppressed antitumor immunity through induction of PD-L1 expression on tumor-associated macrophages. Immunobiology. 2017;222(4):651–657. doi: 10.1016/j.imbio.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Wen ZF, Liu H, Gao R, Zhou M, Ma J, Zhang Y, Zhao J, Chen Y, Zhang T, Huang F, Pan N, Zhang J, Fox BA, Hu HM, Wang LX. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. J Immunother Cancer. 2018;6(1):151. doi: 10.1186/s40425-018-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J, Liu JL, Li KY, Meng Z, Zhang B. Cancerassociated fibroblastderived exosomal miR3825p promotes the migration and invasion of oral squamous cell carcinoma. Oncol Rep. 2019;42(4):1319–1328. doi: 10.3892/or.2019.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, Yang J, Pan J, Hu S, Zhang C, Zhang J, Wang C, Shen J, Che Y, Liu Z, Lv Y, Wen H, You Q. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17(4):428–438. doi: 10.1080/15384101.2018.1444305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JS, Zheng M, Zhang M, Pang X, Li L, Wang SS, Yang X, Wu JB, Tang YJ, Tang YL, Liang XH. Porphyromonas gingivalis promotes 4-Nitroquinoline-1-Oxide-induced oral carcinogenesis with an alteration of fatty acid metabolism. Frontiers in microbiology. 2018;9:2081. doi: 10.3389/fmicb.2018.02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong H, Ke JQ, Jiang FZ, Wang XJ, Wang FY, Li YR, Lu W, Wan XP. Tumor-associated macrophage-derived CXCL8 could induce ERalpha suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016;376(1):127–136. doi: 10.1016/j.canlet.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, Harashima A, Harada S, Yamamoto H, Ohta T. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19(4):1052–1065. doi: 10.1007/s10120-015-0579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncology. 2017;10(1):36. doi: 10.1186/s13045-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM, Tung YC, Hsu HL. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Can. 2019;18(1):42. doi: 10.1186/s12943-019-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khabipov A, Kading A, Liedtke KR, Freund E, Partecke LI, Bekeschus S. RAW 264.7 macrophage polarization by pancreatic cancer cells—a model for studying tumour-promoting macrophages. Anticancer Res. 2019;39(6):2871–2882. doi: 10.2187/anticanres.13416. [DOI] [PubMed] [Google Scholar]
- 27.Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Moller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int J Cancer. 2017;141(3):614–620. doi: 10.1002/ijc.30752. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Can Res. 2018;78(16):4586–4598. doi: 10.1158/0008-5472.Can-17-3841. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38(1):522–528. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 30.Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]