Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment. Since clinical benefits are limited to a subset of patients, we aimed to identify peripheral blood biomarkers that predict the efficacy of the anti-programmed cell death protein 1 (PD-1) antibody (nivolumab) in patients with gastric cancer.
Methods
We collected peripheral blood samples from gastric cancer patients (n = 29) before and after treatment with nivolumab and investigated the relationship between the frequency of surface or intracellular markers among nivolumab-binding PD-1+CD8+ T cells and treatment responses using multicolor flow cytometry. The tumors, lymph nodes, and peripheral blood of gastric cancer patients who underwent gastrectomy following nivolumab treatment were collected, and nivolumab-binding PD-1+CD8+ T cells in these tissue samples were characterized.
Results
Patients with a high frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of treatment had significantly better progression-free survival than the low group (P = 0.032). This CD103+PD-1+CD8+ T cell population mainly consisted of central memory T cells, showing the high expression of Ki-67 and few cytotoxic granules. In contrast, effector memory T cells were more frequently observed among CD103+PD-1+CD8+ T cells in tumors, which implied a change in the differentiated status of central memory T cells in lymph nodes and peripheral blood to effector memory T cells in tumors during the treatment with ICIs.
Conclusions
A high frequency of CD103 among PD-1+CD8+ T cells 2 weeks after nivolumab treatment in patients with advanced gastric cancer may be a useful biomarker for predicting the efficacy of anti-PD-1 therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03240-2.
Keywords: CD103, Clinical response, Gastric cancer, Nivolumab, Predictive biomarker
Introduction
Antibodies that block the programmed cell death protein 1 (PD-1) pathway have demonstrated potent anti-tumor activity in cancer patients and are now an approved treatment for several cancers [1]. Although a significant number of patients benefit from immune checkpoint inhibitors (ICIs), clinical efficacy is limited to a subset of patients. Therefore, extensive efforts have been made to identify biomarkers that predict responses to this therapy [2]. As predictive markers in the tumor microenvironment, the tumor expression of programmed death ligand 1 (PD-L1), the intensity of intratumoral CD8+ T cell infiltration, and the tumor mutational burden (TMB) have been proposed as biomarkers for responses to ICIs [2]. Tumor PD-L1 expression is now used as the indication for anti-PD-1 therapy for patients with non-small cell lung cancer because of its proven relationship with clinical responses to this treatment [3, 4]. However, tumor PD-L1 expression was not identified as a predictor of the therapeutic effects of anti-PD-1 therapy in the ATTRACTION-2 trial, which assessed the clinical efficacy of anti-PD-1 therapy for gastric cancer, and there is currently no clinically available biomarker that predicts the therapeutic efficacy of ICIs for gastric cancer [5].
Since there are limitations to the use of tumor factors in terms of frequent sample availability, particularly for visceral tumors, more easily applicable markers need to be considered for routine clinical use. Peripheral blood samples are more easily available than tumor samples and offer serial information on dynamic changes before and after treatment. Therefore, a blood analysis may provide valuable insights into dynamic changes in T cell responses during treatment with ICIs [6–8].
The mechanisms underlying the efficacy of anti-PD-1 therapy, namely, what cells function for anti-tumor immunity and when are these cells attracted to the tumor microenvironment during treatment with ICIs, have not yet been elucidated. Since the anti-PD-1 antibody binds to PD-1-expressing cells, it appears to be important to focus on the characteristics of anti-PD-1 antibody-binding T cells, which may play a major role in responses to ICIs [9]. Anti-PD-1 antibody-binding T cells have been detected more than 20 weeks after the last infusion of anti-PD-1 therapy; therefore, changes in this cell population may be examined over time after the initiation of treatment with ICIs [10].
We herein aimed to identify peripheral blood biomarkers that predict the efficacy of anti-PD-1 therapy (nivolumab) and characterize cell populations by analyzing nivolumab-binding T cells in patients with advanced gastric cancer.
Materials and methods
Study design and patients
The present study was conducted to identify peripheral blood biomarkers that predict the efficacy of nivolumab using serial analyses of peripheral blood lymphocytes before and after nivolumab therapy in patients with gastric cancer. We enrolled 29 consecutive patients with recurrent or metastatic gastric cancer who were refractory to standard therapy and treated with nivolumab between April 2017 and March 2019 at Osaka University Hospital. These patients received 3 mg/kg nivolumab every 2 weeks in 6-week cycles (Fig. 1a). Treatment was continued until disease progression, death, unacceptable toxic effects, or a patient’s request to discontinue. All patients provided written informed consent before tissue sampling according to the guidelines of the Declaration of Helsinki. The present study was approved by the Institutional Ethics Committee of Osaka University Hospital (Osaka, Japan) (approval number; #13266).
Fig. 1.
Study design and expression frequency of each marker. a Peripheral blood samples were collected from gastric cancer patients before treatment and 2 and 6 weeks after the start of the nivolumab treatment. b The frequency of each marker among PD-1+CD8+ T cells or PD-1−CD8+ T cells before treatment and 2 and 6 weeks after the start of treatment (0 weeks: n = 29, 2 weeks: n = 25, 6 weeks: n = 19). Values are indicated as medians. The significance of differences was calculated using the nonparametric Wilcoxon matched-pairs signed-rank test. (*P < 0.05, **P < 0.01, ***P < 0.001)
Evaluation
A physical examination and laboratory tests were performed every 2 weeks, and tumor responses were assessed by computed tomography (CT) every 6 weeks. The best overall response (BOR) was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) based on the results of CT examinations using the following categories: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and not evaluable (NE). Progression-free survival (PFS) was defined as the time from the start of nivolumab to either disease progression or death from any cause. Overall survival (OS) was defined as the time from the start of nivolumab to death from any cause.
Peripheral blood and tissue samples
Peripheral blood samples were collected before treatment and 2 and 6 weeks after the initiation of nivolumab. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples and stored in the N2 bank. Tumor and lymph node samples in addition to blood samples were collected from two patients who had undergone surgical resection of the stomach after treatment with nivolumab. To collect tissue-infiltrating lymphocytes, fresh tumor and lymph node tissues were minced and treated with the gentleMACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) as previously described [8]. Extracted lymphocytes were stored in the N2 bank and analyzed with a LSR Fortessa cytometer (BD Biosciences, San Jose, USA) after thawing and washing.
Antibody and multicolor flow cytometry
The fluorescence-labeled antibodies used in the present study were as follows: anti-CD3 (clone UCHT1; BioLegend, San Diego, CA), CD4 (clone OKT4; BioLegend), CD8 (clone RPA-T8; BioLegend), CD27 (clone L128; BD Biosciences), CD45RA (clone HI100; BioLegend), ICOS (clone ISA-3; eBioscience), PD-1 (clone EH12.1; BD Biosciences), CD103 (clone Ber-ACT8; BioLegend), T cell immunoglobulin mucin domain 3 (Tim-3, clone F38-2E2; BioLegend), TNFα (clone Mab11; Invitrogen), IL-2 (clone MQ1-17H12; eBioscience), IFN-γ (clone 4S.B3; BioLegend), Granzyme B (clone GB12; Invitrogen), Perforin (clone B-D48; BioLegend), Ki-67 (clone Ki-67; BioLegend), and the anti-human IgG4 antibody, biotin (clone HP6025; Invitrogen) followed by secondary staining with streptavidin. Dead cells were identified using the LIVE/DEAD Fixable Red Dead Cell Stain Kit (Invitrogen). Corresponding isotype control antibodies were purchased from the same manufacturer. To detect PD-1+ T cells after the nivolumab treatment, the antibody was substituted by the anti-IgG4 antibody because PD-1 molecules were completely and stably covered by nivolumab (human IgG4). We confirmed that the detection rate of the anti-IgG4 antibody against PD-1+CD8+ T cells treated with nivolumab in vitro was similar to that of the anti-PD-1 antibody against untreated PD-1+CD8+ T cells (Supplementary Fig. 1a).
In the flow cytometric analysis, thawed cells were incubated with antibodies at 4 °C for 30 min after washing. Washed cells were analyzed with a LSR Fortessa cytometer (BD Biosciences) and the data obtained were analyzed using BD FACS Diva software. Regarding intracellular staining, thawed cells were stained with antibodies to surface antigens and a fixable viability dye (eBioscience) at 4 °C for 30 min. After being incubated, cells were washed, fixed, and permeabilized with fix/perm solution (BD Biosciences) at 4 °C for 15 min. Cells were then stained with antibodies for intracellular molecules at 4 °C for 30 min.
Assessment of cytokine production
Cytokine production was assessed in a sample from one patient 2 weeks after the initiation of nivolumab. PBMCs were separated into nivolumab-binding PD-1+cells and PD-1−cells with a cell sorter (MACSQuant® Analyzer; Miltenyi Biotec, Bergisch Gladbach, Germany) and separated cells were then stimulated with 50 ng/ml PMA (Sigma-Aldrich, Saint Louis, MO), 1 μg/ml ionomycin (Sigma-Aldrich), and Golgi Plug reagent (BD Biosciences) at 37 °C for 5 h. Harvested cells were washed and used for intracellular staining.
Statistical analysis
Differences between the two groups in each experiment were analyzed using the Student’s t-test or Mann–Whitney U test, where appropriate. Categorical variables and continuous variables were compared as indicated in the figure captions. All FCS files were uploaded and evaluated using Cytobank software, a web-based platform for storing, exploring, and sharing cytometry data [http://www.cytobank.org]. To assess whether biomarkers had an independent significant effect on survival, we performed univariate and multivariate Cox regression analyses with adjustments for age, sex, previous gastrectomy, blood test results before the nivolumab treatment, and the ratio of the frequency of each surface or intracellular marker among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of the nivolumab treatment to that before treatment. Survival curves were calculated using the Kaplan–Meier method and differences were assessed using the Log-rank test. The high and low groups were divided by the median values of each factor. A value of P < 0.05 was considered to be significant. All statistical analyses were performed using JMP Pro 14 Discovery™ (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
Patient characteristics at baseline are summarized in Table 1. Eighteen out of 29 patients (62.1%) had recurrence after gastrectomy. All patients received two or more lines of previous systemic chemotherapy before the nivolumab treatment and had no previous treatment with other ICIs.
Table 1.
Patient characteristics at baseline (n = 29)
| n = 29 (%) | |
|---|---|
| Age, median (range) | 64 (31–77) |
| Sex | |
| Male | 19 (65.5%) |
| Female | 10 (34.5%) |
| ECOG performance status | |
| 0 | 13 (44.8%) |
| 1 | 16 (55.2%) |
| Histology | |
| Differentiated | 8 (27.6%) |
| Undifferentiated | 18 (62.1%) |
| Unknown | 3 (10.3%) |
| HER2 status | |
| Positive | 4 (13.8%) |
| Negative | 24 (82.8%) |
| Unknown | 1 (3.4%) |
| MMR expression status | |
| Proficient | 22 (75.9%) |
| Deficient | 1 (3.4%) |
| Unknown | 6 (20.7%) |
| Site of metastasis | |
| Lymph node | 13 (44.8%) |
| Liver | 11 (37.9%) |
| Peritoneum | 8 (27.6%) |
| Lung | 2 (6.9%) |
| Bone | 2 (6.9%) |
| Pleura | 1 (3.4%) |
| Adrenal | 1 (3.4%) |
| Other | 3 (10.3%) |
| Previous gastrectomy | |
| Yes | 18 (62.1%) |
| No | 11 (37.9%) |
| Previous treatment regimens* | |
| Two | 12 (41.4%) |
| Three | 8 (27.6%) |
| More than four | 9 (31.0%) |
| Previous chemotherapy | |
| Pyrimidine analogues | 29 (100%) |
| Platinum | 28 (96.6%) |
| Taxane | 28 (96.6%) |
| Irinotecan | 12 (41.4%) |
| Ramucirumab | 29 (100%) |
| Previous radiation | |
| Yes | 2 (6.9%) |
| No | 27 (93.1%) |
ECOG Eastern Cooperative Oncology Group, HER2 human epidermal growth factor receptor 2, MMR mismatch repair
*Includes treatments received in the adjuvant setting
Clinical responses
The median duration of the nivolumab treatment was 1.9 months (range; 0.4–43.9 months). Median PFS and OS were 1.8 months (range; 0.4–45.5 months) and 6.7 months (range; 0.7–45.5 months), respectively. BOR was PR in 3 patients (10.3%), SD in 2 (6.9%), PD in 21 (72.4%), and NE in 3 (10.3%) (Table 2). All three cases of NE were clinically diagnosed as PD. Patients were classified into the responder group of CR, PR, and SD and the non-responder group of PD and NE for further analyses.
Table 2.
Treatment results
| n = 29 | ||
|---|---|---|
| Administration | ||
| Months | Median (range) | 1.9 (0.4–43.9) |
| Number of nivolumab infusions | Median (range) | 5 (1–88) |
| Best overall response | CR/PR/SD/PD/NE | 0/3/2/21/3 |
| Progression-free survival (months) | Median (range) | 1.8 (0.4–45.5) |
| Overall survival (months) | Median (range) | 6.7 (0.7–45.5) |
CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluated
The expression frequency of each marker among PD-1+CD8+T cells in peripheral blood after the nivolumab treatment
Peripheral blood samples were collected from 29 patients before treatment, 25 patients after 2 weeks of treatment, and 19 patients after 6 weeks of treatment. PD-1+ T cells before and after the nivolumab treatment were detected with the anti-PD-1 antibody and anti-IgG4 antibody, respectively (Supplementary Fig. 1b). We initially evaluated the expression frequency of surface or intracellular markers among PD-1+CD8+ T cells or PD-1−CD8+ T cells in peripheral blood before and after the nivolumab treatment, including the activation marker CD103, naive marker CD45RA, immune checkpoint molecules Tim-3, ICOS, and CD27, and proliferative marker Ki-67 (Fig. 1b, Supplementary Fig. 1c, 2). The frequency of each marker, except for CD45RA, was higher among PD-1+CD8+ T cells than among PD-1−CD8+ T cells at all time points. Furthermore, the frequencies of CD103, Tim-3, ICOS, and Ki-67 among PD-1+CD8+ T cells rapidly increased after the start of the nivolumab treatment and were sustained for 6 weeks (Fig. 1b, Supplementary Fig. 1c). On the other hand, changes in the frequency of each marker among PD-1−CD8+ T cells were smaller than those observed among PD-1+CD8+ T cells. Since a larger change was observed in the frequency of each marker, particularly among PD-1+CD8+ T cells, the ratio of the frequency of each marker among PD-1+CD8+ T cells 2 or 6 weeks after the start of the nivolumab treatment to that before treatment (% of each marker 2w or 6w/pre) was compared between the responder and non-responder groups (Fig. 2a, b). The ratio of the frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of the nivolumab treatment to that before treatment was significantly higher in the responder group than in the non-responder group (P = 0.038). No significant differences were observed in the ratio of the frequencies of other markers among PD-1+CD8+ T cells in peripheral blood 2 or 6 weeks after the start of the nivolumab treatment to that before treatment between the responder and non-responder groups (Fig. 2a, b). When changes in the expression of markers on PD-1+CD8+ T cells before and after the nivolumab treatment were detected with the t-SNE analysis in one representative responder and one non-responder, CD103 expression on PD-1+CD8+ T cells 2 weeks after the start of the nivolumab treatment was higher in the responder than in the non-responder, and this cell population highly co-expressed ICOS, Ki-67, and CD27 with the lower expression of Tim-3 and CD45RA (Supplementary Fig. 3).
Fig. 2.
The relationship between changes in the expression frequency of each marker and therapeutic effects. The ratio of the frequency of each marker among PD-1+CD8+ T cells 2 weeks (a responder n = 4, non-responder n = 21) and 6 weeks (b responder n = 4, non-responder n = 15) after the start of treatment to the pre-treatment rate between the responder and non-responder groups based on the best overall response. Values are indicated as medians. The significance of differences was calculated using the nonparametric Mann–Whitney U test
Analysis of functional characteristics of CD103+PD-1+CD8+ T cells
To characterize the phenotype and functions of the CD103+PD-1+CD8+ T cell subset, we analyzed proliferative activity and cytotoxicity in the following subsets: CD103+PD-1+CD8+ T cells, CD103−PD-1+CD8+ T cells, and PD-1−CD8+ T cells. Among the three groups, the highest frequency of Ki-67 was observed in CD103+PD-1+CD8+ T cells before the nivolumab treatment. Moreover, the frequency of Ki-67 increased 2 weeks and decreased 6 weeks after the start of the nivolumab treatment in all three subsets, with the largest increase in the frequency of Ki-67 occurring among CD103+PD-1+CD8+ T cells (Fig. 3a). The majority of CD103+PD-1+CD8+ T cells expressed Ki-67 in the tSNE-analysis and a strong correlation was observed between the expression of CD103 and Ki-67 on PD-1+CD8+ T cells (Supplementary Figs. 3, 4a, b). Regarding cytotoxicity and the activation status, CD103+PD-1+CD8+ T cells had lower frequencies of perforin and granzyme than the other subsets at all time points, but higher frequencies of INF-γ, TNF-α, and IL-2 2 weeks after the start of the nivolumab treatment (Fig. 3b, c). These results suggest that the CD103+PD-1+CD8+ T cell subsets were highly proliferative and less cytotoxic, but strongly activated.
Fig. 3.
Analysis of functional characteristics of CD103+PD-1+CD8+ T cells. Ki-67 (a), perforin, and granzyme (b) frequencies among CD103+PD-1+ CD8+ T cells, CD103−PD-1+CD8+ T cells, and PD-1−CD8+ T cells before treatment and 2 and 6 weeks after the start of the nivolumab treatment (0 weeks: n = 29, 2 weeks: n = 25, 6 weeks: n = 19). c Cytokine production in CD103+PD-1+CD8+ T cells, CD103−PD-1+CD8+ T cells, and PD-1−CD8+ T cells 2 weeks after the start of the nivolumab treatment (n = 1). d The percentages of each differentiation subset in CD103+PD-1+CD8+ T cells, CD103−PD-1+CD8+ T cells, and PD-1−CD8+ T cells 2 weeks after the start of the nivolumab treatment (n = 25). Values are indicated as medians. The significance of differences was calculated using the nonparametric Wilcoxon matched-pairs signed-rank test. (*P < 0.05, **P < 0.01, ***P < 0.001)
We examined the differentiation status of T cells characterized by the naïve T cell marker, CD45RA, and the differentiation T cell marker, CD27 among the naive (CD45RA+CD27+), central memory (CD45RA−CD27+), effector memory (CD45RA−CD27−), and terminally differentiated (CD45RA+CD27−) subsets (Supplementary Fig. 5a) [8, 11–16]. In this classification, the central memory subset was dominant in PD-1+CD8+ T cells, particularly in CD103+PD-1+CD8+ T cells, and the percentage of this subset significantly increased after the nivolumab treatment (Fig. 3d, Supplementary Fig. 5b).
The relationship between an increase in the subset of CD103+PD-1+CD8+ T cells and patient prognosis
When patients were divided into the high and low groups based on the median value of the frequency of CD103 among PD-1+CD8+ T cells 2 weeks after treatment, the high group had significantly better PFS than the low group (P = 0.032, Fig. 4a, Supplementary Fig. 6). In the analysis of the ratio of the frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of the nivolumab treatment to that before treatment (%CD103 2w/pre), the high group also had significantly better PFS than the low group (P = 0.007; Fig. 4b). Similar results were observed in the analysis of the actual cell number of CD103+PD-1+CD8+ T cells/µl 2 weeks after the start of treatment (Supplementary Fig. 7a, b). No significant differences were observed in OS between the high and low groups (Supplementary Fig. 8a, b). Furthermore, a multivariate analysis of clinicopathological variables revealed that the ratio of the frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after treatment to that before treatment (%CD103 2w/pre) was an independent prognostic factor for PFS (HR: 3.09, 95% CI: 1.19–8.79; P = 0.020, Supplementary Table S1).
Fig. 4.

The relationship between an increase in the subset of CD103+PD-1+CD8+ T cells and patient prognosis. Kaplan–Meier curves of PFS in patients with a high or low frequency of CD103 among PD-1+CD8+ T cells 2 weeks after the start of the nivolumab treatment (a n = 25), and those with a high or low ratio of the frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of the nivolumab treatment to that before treatment (b n = 25). The median of each value was used as the cut-off. The significance of differences was calculated using the Log-rank test
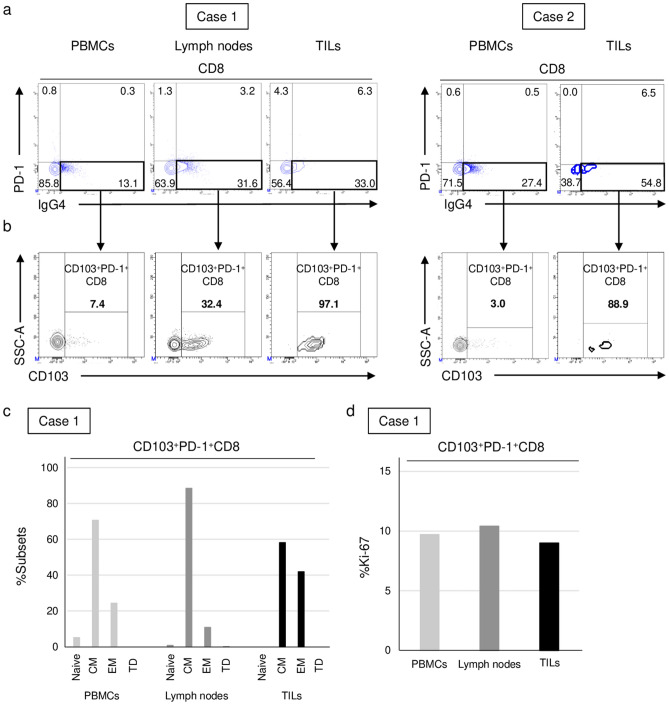
Evaluation of nivolumab-binding T cells in tumors and lymph nodes resected after the nivolumab treatment
Tumors, lymph nodes, and peripheral blood were collected from two individual patients (cases 1 and 2) who were treated with nivolumab, showed a durable response, and subsequently underwent gastrectomy. We detected the presence of nivolumab-binding CD8+ T cells in tumors, lymph nodes, and peripheral blood (Fig. 5a). The majority of nivolumab-binding CD8+ tumor-infiltrating lymphocytes (TILs) expressed CD103, while a relatively high percentage of nivolumab-binding T cells from lymph nodes expressed CD103, in contrast to a lower percentage in peripheral blood (Fig. 5b). Regarding the differentiation status, CD103+PD-1+CD8+ T cells in lymph nodes consisted of the highest percentage of central memory T cells, whereas effector memory T cells were more frequently observed among the CD103+PD-1+CD8+ T cells of TILs (Fig. 5c). The differentiation status of CD103+PD-1+CD8+ T cells in peripheral blood was between that of the lymph nodes and tumors. The frequency of Ki-67 among CD103+PD-1+CD8+ T cells was similar between the three samples (Fig. 5d).
Fig. 5.
Evaluation of nivolumab-binding T cells in tumors and lymph nodes resected after the nivolumab treatment. Marker expression frequency in samples from two patients who underwent surgical resection after the nivolumab treatment. a PD-1 frequency among CD8+ T cells in PBMCs, lymphocytes in lymph nodes, and TILs. b CD103 frequency among PD-1+CD8+ T cells (nivolumab-binding T cells) in PBMCs, lymphocytes in lymph nodes, and TILs. Subsets of differentiation markers (c) and Ki-67 (d) on CD103+PD-1+CD8+ T cells in PBMCs, lymphocytes in lymph nodes, and TILs (n = 1)
Discussion
Although immunotherapy has evolved as a cancer treatment with the development of ICIs, therapeutic efficacy is limited to a few patients. Therefore, there is an urgent need to identify biomarkers that predict the therapeutic efficacy of ICIs. Intensive investigations of biomarkers of ICIs in gastric cancer, including PD-L1 expression, microsatellite instability (MSI), TMB, and Epstein-Barr virus (EBV) infection, have been performed on tumor tissue [17]. Although the utility of PD-L1 expression as a predictive factor for the efficacy of pembrolizumab has been suggested, its predictive value was not confirmed in some trials and, thus, is not definite in gastric cancer [5, 18–20]. The FDA approved pembrolizumab for patients with MSI-high or mismatch repair-deficient (MMR-d) solid tumors, and a high clinical response for pembrolizumab was confirmed for MSI-high or MMR-d gastric cancer in some trials [18–20]. Although a trial on pembrolizumab reported an extremely high clinical response in patients with EBV-positive gastric cancer, the impact of the EBV status on the efficacy of anti-PD-1 therapy remains controversial [17, 21]. ICIs have been developed for HER2-negative gastric cancer, and combination therapy with ICIs and an anti-HER2 antibody is also being investigated for HER2-positive gastric cancer [22]. However, definite markers in tumor tissues that predict the therapeutic efficacy of ICIs or the prognosis of gastric cancer have not yet been identified. Therefore, we focused on the host response to anti-PD-1 therapy, and identified a peripheral blood-based biomarker that predicts responses to anti-PD-1 therapy in gastric cancer in the present study. The ratio of the frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of treatment with nivolumab to that before treatment predicted responses to and survival after anti-PD-1 therapy. To the best of our knowledge, this is the first study to describe the importance of CD103 expression on PD-1+CD8+ T cells after anti-PD-1 therapy in peripheral blood.
CD103, also known as integrin αEβ7, is a well-known marker for tissue-resident memory T cells, which are poised to rapidly respond to local pathogen re-encounters in peripheral tissues [23]. This molecule is a transmembrane heterodimer complex that mediates the cell adhesion, migration, and homing of lymphocytes through their binding to E-cadherin on the surface of epithelial cells [24, 25]. Therefore, CD103 favors the location and retention of resident memory T cells close to malignant cells in tumors by binding to E-cadherin on malignant cells, and the CD103-E-cadherin interaction is required for the polarized exocytosis of lytic granules causing target cell death [26]. Since tissue-resident memory T cells are considered to enhance tumor immunity, an abundance of CD103+CD8+ TILs was identified as a superior prognostic factor in several cancers [25, 27, 28]. Moreover, anti-PD-1 therapy significantly increased the production of IFN-γ in CD103+CD8+ TILs, and the elevated number of CD103+CD8+ TILs after anti-PD-1 therapy correlated with a good response by patients with malignant melanoma [29, 30], suggesting the importance of CD103+CD8+ T cell infiltration into tumors treated with ICIs. However, the function of CD103+CD8+ T cells in peripheral blood related to ICIs remains unclear. In the present study, CD103-expressing CD8+ T cells were rarely detected in peripheral blood before the treatment with nivolumab, whereas CD103-expressing CD8+ T cells increased after the treatment, particularly among PD-1+CD8+ T cells. The increased frequency of CD103 in PD-1+CD8+ T cells in peripheral blood after the nivolumab treatment correlated with a good response and better prognosis after anti-PD-1 therapy. Therefore, increases in CD103+PD-1+CD8+ T cells in peripheral blood may be used as a marker to predict good treatment efficacy.
The efficacy of ICIs depends on the existence of tumor antigen-specific T cells [31]. Two major studies proved the activation of tumor antigen-specific T cells in peripheral blood after treatment with ICIs [32, 33]. Although some markers were used to capture tumor antigen-specific T cells, including CD107a, CD39, CD103, CD154, and CD137, there has been no definite marker for tumor antigen-specific T cells [34–37]. Nevertheless, some studies showed that CD103+CD8+ T cells in tumors contained tumor-reactive CD8+ T cells in several cancers [28, 38, 39]. Consistent with previous findings, the present results suggest that CD103+PD-1+CD8+ T cells in peripheral blood contained tumor-reactive CD8+ T cells because this cell population correlated with a good clinical response to and better survival after treatment with ICIs. Tumor antigen-specific CD8+ T cells were identified from PD-1+CD8+ T cells in the peripheral blood of melanoma patients, and this is consistent with our hypothesis that PD-1+ T cells contain tumor antigen-specific CD8+ T cells [40]. Although experiments were not performed to assess the tumor cell-killing activity of CD103+PD-1+CD8+ T cells, the present results support the hypothesis that tumor antigen-specific T cells are present in CD103+PD-1+CD8+ T cells in peripheral blood after treatment with nivolumab and activate anti-tumor immunity.
Memory T cells facilitate immunosurveillance and recall responses to reinvading pathogens. Two types of memory T cells have been defined in peripheral blood: central memory T cells residing in secondary lymphoid tissues with a high potential for self-renewal and effector memory T cells abundant in non-lymphoid tissues with cytotoxic properties. Central memory T cells become effector memory T cells by a re-stimulation with various antigens and ultimately become effector T cells that exhibit killing activity [41]. Regarding the relationship between memory T cells and the anti-tumor effects of ICIs, an increased subset of central memory T cells in peripheral blood predicted a good clinical response to ICIs, while the effector memory T cell subset in tumors was the major phenotype that expanded in patients who responded to ICIs [42, 43]. The present results demonstrated that the population of central memory T cells in CD103+PD-1+CD8+ T cells after the nivolumab treatment was higher in lymph nodes than in peripheral blood or tumors, whereas effector memory T cells increased in the CD103+PD-1+CD8+ T cells of tumors. Therefore, central memory T cells among CD103+PD-1+CD8+ T cells in lymph nodes may be reactivated by tumor antigens after treatment with ICIs, differentiate to effector memory T cells, migrate through peripheral blood to tumors, and then target tumor cells with cytotoxic functions. A recent study on tumor antigen-specific T cells after treatment with ICIs showed that the dominant population in peripheral blood changed from central memory to effector memory T cells over time after the initiation of treatment with a serial sample analysis [44]. Therefore, the abundance of the central memory subtype in CD103+PD-1+CD8+ T cells in peripheral blood may be a marker that predicts the efficacy of ICIs. Further studies are needed to investigate the fate of CD103+PD-1+CD8+ T cells during treatment with ICIs.
Since the anti-PD-1 antibody binds to PD-1-expressing cells, we focused on nivolumab-binding T cells, which have been suggested to play a major role in responses to ICIs, and these cells may be detected by a method using the anti-IgG4 antibody. Previous studies using this method indicated that the early proliferative (Ki-67+) response of peripheral blood PD-1+CD8+ T cells after anti-PD-1 therapy was associated with clinical outcomes in patients with non-small cell lung cancer and thymic epithelial tumors [6, 7]. In the present study, Ki-67+-proliferating CD8+ T cells were more frequently observed in CD103+PD-1+CD8+ T cells than in CD103−PD-1+CD8+ T cells or PD-1−CD8+ T cells and 2 weeks after treatment initiation than before or 6 weeks after treatment initiation. Therefore, CD103+PD-1+CD8+ T cells were the most proliferative and observed at an early time point after the initial treatment. These results support the use of peripheral blood monitoring for CD103+PD-1+CD8+ T cells at early time points after treatment initiation. Considering the mode of action of PD-1-blocking antibodies, measurements of CD103-expressing PD-1+CD8+ T cells may be applicable to several cancer types. An approach to expand CD103-expressing PD-1+CD8+ T cells may be important for overcoming unresponsiveness to anti-PD-1 therapy.
The present study had a number of limitations. It was performed at a single institution with a relatively small sample size and only one type of cancer was examined. Therefore, the present results need to be validated in a larger cohort and in another type of cancer. Furthermore, different antibodies were used to detect PD-1+ T cells before and after the nivolumab treatment, namely, anti-PD-1 antibodies before treatment and anti-IgG4 antibodies after treatment. Therefore, pre- and post-treatment comparisons may not be consistent. However, since no significant differences were observed in the frequency of PD-1 among CD8+ T cells in in vitro experiments with anti-PD-1 and anti-IgG4 antibodies, the majority of PD-1+CD8+ T cells were considered to be accurately detected (Supplementary Fig. 1a, b).
In conclusion, the present results suggest that the ratio of the frequency of CD103 among PD-1+CD8+ T cells in peripheral blood 2 weeks after the start of the nivolumab treatment to that before treatment reflects responses to and survival by patients receiving anti-PD-1 therapy. Further studies with a larger number of patients and various cancer types are needed to validate this parameter as a useful biomarker for anti-PD-1 therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the patients who contributed to this study. We thank M. Matsumoto and K. Goto for their help.
Author Contributions
YN, TS, and HW contributed to the conception or design of the work. YN, TS, and KY performed the experiments. MH and AU helped perform the experiments. YN, TS, YD, and HW collected data and all authors analyzed data. YN drafted the article. TS, YD, and HW helped finalize the article. All authors have read and approved the final article. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures were in accordance with the Helsinki Declaration. The Human Ethics Review Committee of Osaka University Graduate School of Medicine approved the protocol for this study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.Ccr-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/s0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 6.Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KH, Cho J, Ku BM, et al. The first-week proliferative response of peripheral blood PD-1(+)CD8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25:2144–2154. doi: 10.1158/1078-0432.Ccr-18-1449. [DOI] [PubMed] [Google Scholar]
- 8.Kato R, Yamasaki M, Urakawa S, et al. Increased Tim-3(+) T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother. 2018;67:1673–1683. doi: 10.1007/s00262-018-2225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol. 2018;9:14. doi: 10.3389/fimmu.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osa A, Uenami T, Koyama S et al (2018) Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight 3. 10.1172/jci.insight.59125 [DOI] [PMC free article] [PubMed]
- 11.Romero P, Zippelius A, Kurth I, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 12.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caccamo N, Meraviglia S, Ferlazzo V, Angelini D, Borsellino G, Poccia F, Battistini L, Dieli F, Salerno A. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol. 2005;35:1764–1772. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 15.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 16.Odaira K, Kimura SN, Fujieda N, Kobayashi Y, Kambara K, Takahashi T, Izumi T, Matsushita H, Kakimi K. CD27(-)CD45(+) γδ T cells can be divided into two populations, CD27(-)CD45(int) and CD27(-)CD45(hi) with little proliferation potential. Biochem Biophys Res Commun. 2016;478:1298–1303. doi: 10.1016/j.bbrc.2016.08.115. [DOI] [PubMed] [Google Scholar]
- 17.Kawazoe A, Shitara K, Boku N, Yoshikawa T, Terashima M. Current status of immunotherapy for advanced gastric cancer. Jpn J Clin Oncol. 2021;51:20–27. doi: 10.1093/jjco/hyaa202. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/s0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 20.Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 Phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 22.Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–831. doi: 10.1016/s1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 24.Boutet M, Gauthier L, Leclerc M, Gros G, de Montpreville V, Théret N, Donnadieu E, Mami-Chouaib F. TGFβ signaling intersects with CD103 integrin signaling to promote T-lymphocyte accumulation and antitumor activity in the lung tumor microenvironment. Cancer Res. 2016;76:1757–1769. doi: 10.1158/0008-5472.Can-15-1545. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Liu H, Cao Y, et al. Identification and validation of an immunogenic subtype of gastric cancer with abundant intratumoural CD103(+)CD8(+) T cells conferring favourable prognosis. Br J Cancer. 2020;122:1525–1534. doi: 10.1038/s41416-020-0813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, Tihy I, Tartour E. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6:87. doi: 10.1186/s40425-018-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.Ccr-13-1877. [DOI] [PubMed] [Google Scholar]
- 28.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Huang B, Gao Y, Yang J, Liang Z, Zhang N, Fu X, Li L. CD103(+)CD8(+) T lymphocytes in non-small cell lung cancer are phenotypically and functionally primed to respond to PD-1 blockade. Cell Immunol. 2018;325:48–55. doi: 10.1016/j.cellimm.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Edwards J, Wilmott JS, Madore J, et al. CD103(+) Tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during Anti-PD-1 treatment. Clin Cancer Res. 2018;24:3036–3045. doi: 10.1158/1078-0432.Ccr-17-2257. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell JS, Long GV, Scolyer RA, Teng MW, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duhen T, Duhen R, Montler R, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balança CC, Salvioni A, Scarlata CM et al (2021) PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells. JCI Insight 6. 10.1172/jci.insight.142513 [DOI] [PMC free article] [PubMed]
- 36.Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ., Jr CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014;20:44–55. doi: 10.1158/1078-0432.Ccr-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumgaertner P, Jandus C, Rivals JP, et al. Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int J Cancer. 2012;130:2607–2617. doi: 10.1002/ijc.26297. [DOI] [PubMed] [Google Scholar]
- 38.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015;3:926–935. doi: 10.1158/2326-6066.Cir-14-0239. [DOI] [PubMed] [Google Scholar]
- 39.Yang R, Cheng S, Luo N, et al. Distinct epigenetic features of tumor-reactive CD8+ T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol. 2019;21:2. doi: 10.1186/s13059-019-1921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ando M, Ito M, Srirat T, Kondo T, Yoshimura A. Memory T cell, exhaustion, and tumor immunity. Immunol Med. 2020;43:1–9. doi: 10.1080/25785826.2019.1698261. [DOI] [PubMed] [Google Scholar]
- 42.Ribas A, Shin DS, Zaretsky J, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. 2016;4:194–203. doi: 10.1158/2326-6066.Cir-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi Y, Tanemura A, Tada Y, Katayama I, Kumanogoh A, Nishikawa H. Clinical response to PD-1 blockade correlates with a sub-fraction of peripheral central memory CD4+ T cells in patients with malignant melanoma. Int Immunol. 2018;30:13–22. doi: 10.1093/intimm/dxx073. [DOI] [PubMed] [Google Scholar]
- 44.Caushi JX, Zhang J, Ji Z, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596:126–132. doi: 10.1038/s41586-021-03752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.