Abstract
The nanoparticle complex of cholesteryl pullulan (CHP) and NY-ESO-1 antigen protein (CHP-NY-ESO-1) presents multiple epitope peptides to MHC class I and II pathways, leading to CD8+ and CD4+ T cell responses. Poly-ICLC is a synthetic, double-stranded RNA, an agonist of toll-like receptor (TLR)-3, and a cytoplasmic receptor of melanoma differentiation-associated gene (MDA)-5. It should be a suitable immune adjuvant of cancer vaccine to overcome the inhibitory tumor microenvironment. We conducted a phase 1 clinical trial of CHP-NY-ESO-1 with poly-ICLC in patients with advanced or recurrent esophageal cancer. CHP-NY-ESO-1/poly-ICLC (μg/mg) was administered at a dose of 200/0.5 or 200/1.0 (cohorts 1 and 2, respectively) every 2 weeks for a total of six doses. The primary endpoints were safety and immune response. The secondary endpoint was tumor response. In total, 16 patients were enrolled, and six patients in each cohort completed the trial. The most common adverse event (AE) was injection site skin reaction (86.7%). No grade 3 or higher drug-related AEs were observed. No tumor responses were observed, and three patients (30%) had stable disease. The immune response was comparable between the two cohorts, and all patients (100%) achieved antibody responses with a median of 2.5 vaccinations. Comparing CHP-NY-ESO-1 alone to the poly-ICLC combination, all patients in both groups exhibited antibody responses, but the titers were higher in the combination group. In a mouse model, adding anti-PD-1 antibody to the combination of CHP-NY-ESO-1/poly-ICLC suppressed the growth of NY-ESO-1-expressing tumors. Combining the vaccine with PD-1 blockade holds promise in human trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02892-w.
Keywords: Cancer vaccine, Esophageal cancer, NY-ESO-1 antigen, Poly-ICLC
Introduction
Much progress has been made regarding the clinical efficacy of immune checkpoint inhibitors (ICIs), including anti-PD-1 antibody, in recurrent and metastatic esophageal cancer patients [1, 2]. This success demonstrates that esophageal cancer is a tumor type that is sensitive to immunotherapy and suggests the value of developing other immunotherapeutic options, especially for ICI-resistant esophageal tumors.
Cancer vaccines are one such option. However, their clinical efficacy has not yet been proved in large-scale clinical trials [3–5]. It is supposed that a vaccine administered as monotherapy would be hampered by the inhibitory mechanisms in tumor microenvironments, including regulatory T cells and PD-L1 expression. To overcome this immunosuppression, a suitable immune-adjuvant combination should be used in conjunction with a cancer vaccine. Incomplete Freund's adjuvant (IFA) is one of the most common adjuvants administered with peptide vaccines [6]. However, a previous report indicated that IFA-based vaccination caused T cells to accumulate at the vaccination site rather than at the tumor site, and also induced T cell apoptosis [7]. Thus, caution should be used when considering IFA as a vaccine adjuvant.
Poly-ICLC is a double-stranded RNA complex that consists of poly(I:C) (polyinosinic:polycytidylic acid) stabilized by poly-L-lysine. It is recognized by toll-like receptor (TLR)-3 and is a cytoplasmic receptor of melanoma differentiation-associated gene (MDA)-5 [8, 9]. To date, several clinical trials have evaluated poly-ICLC as an immune-adjuvant in terms of safety and immune responses [10–12], but it has not been tested in a comparative analysis without an adjuvant.
We have been developing the cholesteryl pullulan (CHP)-antigen protein nanoparticle as a cancer vaccine for esophageal cancer [13, 14]. A CHP nanoparticle that contains a tumor antigen is a new type of cancer vaccine with a novel antigen delivery system for both the MHC class I and class II pathways. It induces antigen-specific CD8+ and CD4+ T cell immunity as well as humoral immunity [15]. NY-ESO-1 antigen is a cancer-testis antigen that is expressed only in tumor tissues and normal testis and placenta [16, 17].
A previous phase I clinical trial using a nanoparticle complex of CHP and NY-ESO-1 protein (CHP-NY-ESO-1) vaccine in patients with advanced or recurrent esophageal tumors showed that the 200-µg dose of NY-ESO-1 protein more efficiently induced immune responses than the 100-µg dose, and might therefore lead to better survival benefits [14].
Here, we conducted a single-institute, phase 1 clinical trial of CHP-NY-ESO-1 vaccine with the 200-µg dose of NY-ESO-1 protein in combination with an escalating dose of poly-ICLC in advanced or recurrent esophageal cancer patients. We evaluated the safety and antibody immune responses to the NY-ESO-1 antigen. Specifically, we analyzed the antibody reaction in comparison with clinical samples collected from a vaccine trial that did not use poly-ICLC [14]. Furthermore, we used a mouse model to investigate the in vivo anti-tumor efficacy of CHP-NY-ESO-1/poly-ICLC in combination with an anti-PD-1 antibody to evaluate the feasibility of human combination vaccine treatment.
Material and methods
Patients
Patients with histologically proven esophageal cancer at stages III/IV (Union for International Cancer Control < UICC > TNM classification system, 7th edition) or disease recurrence were recruited between February 2012 and January 2017. The inclusion criteria were as follows: confirmed expression of NY-ESO-1 antigen in tumor cells determined by immunohistochemistry or PCR; successful patient recovery from all acute adverse effects of prior therapy; adequate organ function (hemoglobin ≥ 8.0 g/dL, WBC count ≥ 2.0 × 109/L, ANC ≥ 1.0 × 109/L, platelet count ≥ 75 × 109/L, serum creatinine ≤ 2.0 mg/dL, AST and ALT ≤ 2.5 × ULN, serum total bilirubin ≤ 1.5 × ULN); ECOG performance status of 0, 1 or 2; life expectancy ≥ 4 months; and age 20 years or over. The exclusion criteria were as follows: clinically significant heart disease (NYHA Class III or IV); serious active infection requiring antibiotics; bleeding disorders; unstable metastatic disease in the central nervous system; concomitant systemic treatment with corticosteroids (topical steroids were permitted); history of any severe or life-threatening hypersensitivity or allergic reaction; known HIV infection; history of immunodeficiency disease or autoimmune disease; history of anticancer chemotherapy, immunotherapy, radiotherapy, or any other investigational agent within 4 weeks (6 weeks for nitrosoureas or mitomycin C) prior to enrollment; and pregnant or lactating women. Concomitant immunosuppressive or anticancer therapy was not permitted.
Study design
This was a non-randomized, open label, phase I clinical trial to investigate the safety of CHP-NY-ESO-1 in combination with three doses of poly-ICLC administered to patients with esophageal cancer expressing NY-ESO-1 antigen at stage III/IV or during disease recurrence. The study assessed the NY-ESO-1-specific antibody immune response as an indicator of biological activity, and this measure was used along with the safety profile to determine the optimal dose of poly-ICLC. As a secondary objective, tumor responses were assessed. This study was approved by the ethics committee of Kyoto Prefectural University of Medicine. This trial was registered in the University Hospital Medical Information Network (UMIN) Clinical Trial Registry as ID: UMIN000007961.
Expression of NY-ESO-1
Archival tumor samples or those newly obtained from patients were screened for NY-ESO-I expression. Eligible patients were those with NY-ESO-1 expression in ≥ 1% of tumor cells according to immunohistochemical staining with an E978 monoclonal antibody [17], or ≥ 1 copy NY-ESO-1 per 104 copies glyceraldehyde-3-phosphate dehydrogenase (GAPDH) according to quantitative real-time PCR analysis using specific primers [18].
Treatment protocol
CHP-NY-ESO-1 vaccine (IMF-001) was provided by ImmunoFrontier, Inc. (Osaka, Japan), while poly-ICLC (Hiltonol) was purchased from Oncovir, Inc. (Washington, D.C.) and provided by Cancer Research Institute (New York, NY). Both compounds were manufactured according to good manufacturing practices. In manufacturing NY-ESO-1 protein, full-length NY-ESO-1 cDNA was introduced into Escherichia coli cells, and the His-tagged NY-ESO-1 protein was recovered and highly purified using a combination of chromatographic techniques. CHP-NY-ESO-1 vaccine was subcutaneously injected into the upper arm or thigh every 2 weeks for a total of six injections at a fixed dose level of 200 μg. Poly-ICLC was administered concurrently with CHP-NY-ESO-1 every 2 weeks for a total of six injections, at three dose levels (0.5 mg, 1 mg, and 2 mg) in six patients each. Poly-ICLC was subcutaneously injected at a site 2 cm away from the periphery of the CHP-NY-ESO-1 injection bulge. Mixing the two drugs under the skin was prohibited. Each patient received six cycles of CHP-NY-ESO-1/poly-ICLC over 12 weeks, unless there was disease progression or development of unacceptable toxicity that required discontinuation of the study therapy. Intra-patient dose escalation was not allowed and dose reductions were not planned.
Adverse events (AEs) and efficacy assessment
Safety and toxicity were determined based on regular patient interviews, physical examination, and laboratory tests on days 15, 29, 43, 57, and 71. Safety was assessed and reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). The following were considered to be dose-limiting toxicities (DLTs): grade 3 or higher injection site reaction, allergic reaction, pruritus, chills, and fever. We used an accelerated titration design to assess the safety of the three poly-ICLC dose levels (0.5 mg, 1 mg, and 2 mg). If ≤ 1 DLT was observed in the first (or second) cohort of patients, the next cohort of six patients was treated at the next higher dose. If ≥ 2 DLTs were noted in the initial or expanded cohort, no further dose escalations were performed and the maximum tolerated dose (MTD) was considered to have been exceeded. Thus, the MTD was defined as the highest dose administered to six patients of whom no more than one experienced a DLT during the treatment cycle. Initially, we planned to enroll six patients in cohort 3 (2 mg poly-ICLC), but enrollment was slower than expected. Thus, patient accrual was terminated when the evaluation of cohort 2 (1 mg poly-ICLC) was completed.
Objective tumor response was assessed by computed tomography scans in accordance with the Response Evaluation Criteria in Solid Tumor (RECIST version 1.1) criteria. Disease assessment was performed by computed tomography at baseline, 8 weeks, and 14 weeks after the initial treatment.
Antibody responses
To analyze antigen-specific immunological responses, serial serum samples were collected before treatment and at days 15, 29, 43, 57, and 71. All samples were stored at -80 °C until analysis. Anti-NY-ESO-1 antibody serum titers were measured by enzyme-linked immunosorbent assay (ELISA) as previously described [19]. Briefly, recombinant NY-ESO-1 proteins (GST-tagged) were absorbed onto immunoplates (442,404; Nunc, Roskilde, Denmark) at a concentration of 10 ng/50 μL/well at 4 °C. The collected serum samples were diluted from 1:400 to 1:102,400. After washing and blocking the plate, sera were added and incubated for 10 h. After washing, goat anti-human IgG (H + L chain) (MBL, Nagoya, Japan) conjugated with peroxidase (The Binding Site, San Diego, CA) was added. After adding the TMB substrate (Pierce, Rockford, IL), the plate was read using a Microplate Reader (model 550; Bio-Rad, Hercules, CA). The cutoff level selected for the anti-NY-ESO-1 IgG antibody was defined as the mean optical density (OD) 450–550 absorption value + 1.645 × the standard deviation as 0.254. An OD 450 absorption value of at least 0.254 was considered to indicate a positive reaction at a serum dilution of 1:400. In patients who were antibody positive at the baseline, antibody titers were judged to be “augmented” if they changed by fourfold or more compared with baseline. To compare antibody responses with serum samples from the previous clinical trial of CHP-NY-ESO-1 vaccine that did not use an adjuvant, we collected 12 ELISA results from the database in our laboratory [14].
Mice, cell lines, and anti-PD-1 monoclonal antibody
Female BALB/c mice aged 6–10 weeks were used. Mouse colon cancer cell line CT26 was transfected with the human NY-ESO-1 gene [20]. Tumor volume was calculated as follows: 0.5 × length (mm) × width (mm)2. An anti-mouse PD-1 (clone RMP1-14) monoclonal antibody (mAb) was used in the mouse experiments [21].
Statistical analysis
Student’s t test was used to assess changes in IgG titers between the CHP-NY-ESO-1/poly-ICLC and the CHP-NY-ESO-1 vaccine groups. The Mann–Whitney U test was used to assess changes in tumor sizes in the three groups in in vivo mouse experiments. Calculations were performed with Excel (Microsoft Japan, Tokyo, Japan) for Student’s t test and with SPSS Statistics version 25 (IBM Japan, Ltd., Tokyo, Japan) for the Mann–Whitney U test. p-values below 0.05 were considered statistically significant.
Results
Demographics and clinical characteristics
A total of 16 patients were sequentially enrolled into the two cohorts of the phase 1 study between February 2012 and January 2017 (Supplementary Fig. 1). The demographic characteristics of the enrolled patients are listed in Table 1. Of the 16 patients in this study, 15 were male and the median age of all patients was 68.5 years (range: 53–84). Five of six patients (83.3%) in cohort 1 and four of 10 patients (40%) in cohort 2 had an ECOG performance status of 0. Most patients had recurrent disease (n = 9), and of these, three had UICC stage III and four had stage IV (Supplementary Table 1). All patients had received prior treatment (i.e., chemotherapy, radiotherapy, and surgery) before entering this study. Tumor expression of NY-ESO-1 was mandatory for study entry and this was confirmed by immunohistochemistry in all patients. Study treatment was discontinued in two patients (No. 9 and No. 13) due to uncontrolled symptoms induced by cancer progression: one after three doses of vaccine and the other after four doses. One patient (No. 15) experienced worsening radiation pneumonitis resulting from prior radiation therapy, and steroid administration was started. In this patient, study treatment was discontinued after one dose of vaccine. Finally, one patient (No. 8) died of a myocardial infarction during travel at day 8, after one dose of vaccine.
Table 1.
Demographic characteristics of the enrolled patients allocated to two Poly-ICLC doses
| Poly-ICLC 0.5 mg | Poly-ICLC 1 mg | |
|---|---|---|
| No. patients enrolled | 6 | 10 |
| Sex | ||
| Male | 5 | 10 |
| Female | 1 | 0 |
| Age | ||
| Median | 67 | 70 |
| Range | 53–84 | 55–81 |
| ECOG/PS | ||
| 0/1 | 5/1 | 4/6 |
| Prior therapy | ||
| Surgery | 2 | 4 |
| Radiotherapy | 6 | 10 |
| Chemotherapy | 6 | 10 |
ECOG eastern cooperative oncology group; PS performance status
AEs
All 16 patients enrolled in the study were evaluated for AEs. Injection site reactions were evaluated in all patients except No. 8. The overall AEs reported in our study are summarized in Table 2. The most common AEs were injection site reaction (86.7%), usually characterized by pain, itching, redness, and swelling. Most patients experienced these symptoms after a single vaccine dose (Supplementary Table 2). Eight patients (53.3%) experienced fever after vaccination, but the fever was grade 2 or less in all cases. No DLT was observed. Grade 3 or higher AEs were observed in four patients (26.7%). Bronchial stenosis and esophagobronchial fistula, both caused by cancer progression, were observed in one patient each during the treatment period. One patient demonstrated grade 3 AST elevation and one died of myocardial infarction. None of these more severe AEs were considered to be related to the vaccine treatment. Laboratory testing showed that at least 20% of patients exhibited anemia (n = 4), elevated ALT (n = 4), and hyponatremia (n = 5). Each of these abnormalities was grade 2 or lower, and was considered to be unrelated or not likely to be related to the vaccination. There were no serious AEs that were potentially related to vaccine treatment.
Table 2.
Maximum grade of adverse events per patient
| Adverse event | Cohort 1, N = 6 | Cohort 2, N = 10 | All cohort, N = 16 (%) | |||
|---|---|---|---|---|---|---|
| Grade | Grade | Grade | ||||
| 1–2 | ≥ 3 | 1–2 | ≥ 3 | 1–2 | ≥ 3 | |
| Laboratory data | ||||||
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphopenia | 0 | 0 | 2 | 0 | 2 (12.5) | 0 |
| Anemia | 2 | 0 | 2 | 0 | 4 (25.0) | 0 |
| Thrombocytopenia | 0 | 0 | 3 | 0 | 3 (18.8) | 0 |
| Elevated AST | 1 | 0 | 1 | 1* | 2 (12.5) | 1* (6.3) |
| Elevated ALT | 1 | 0 | 3 | 0 | 4 (25.0) | 0 |
| Elevated ALP | 0 | 0 | 1 | 0 | 1 (6.3) | 0 |
| Elevated total bilirubin | 0 | 0 | 1 | 0 | 1 (6.3) | 0 |
| Elevated creatinine | 0 | 0 | 3 | 0 | 3 (18.8) | 0 |
| Hyponatremia | 2 | 0 | 3 | 0 | 5 (31.3) | 0 |
| Hypokalemia | 0 | 0 | 1 | 0 | 1 (6.3) | 0 |
| Hypocalcemia | 0 | 0 | 1 | 0 | 1 (6.3) | 0 |
| Clinical findings | ||||||
| Injection site reaction† | 5 | 0 | 8 | 0 | 13 (86.7) | 0 |
| Fatigue | 3 | 0 | 3 | 0 | 6 (37.5) | 0 |
| Fever | 4 | 0 | 4 | 0 | 8 (50.0) | 0 |
| Anorexia | 0 | 0 | 3 | 0 | 3 (18.8) | 0 |
| Nausea | 2 | 0 | 2 | 0 | 4 (25.0) | 0 |
| Diarrhea | 2 | 0 | 3 | 0 | 5 (31.3) | 0 |
| Constipation | 3 | 0 | 3 | 0 | 6 (37.5) | 0 |
| Headache | 2 | 0 | 0 | 0 | 2 (12.5) | 0 |
| Mucositis | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 1 | 0 | 0 | 0 |
| Bronchial stenosis | 0 | 1* | 0 | 0 | 0 | 1* (6.3) |
| Esophagobronchial fistula | 0 | 1* | 0 | 0 | 0 | 1* (6.3) |
| Acute coronary syndrome | 0 | 0 | 0 | 1* | 0 | 1* (6.3) |
| Bullous dermatitis | 0 | 0 | 2 | 0 | 2 (12.5) | 0 |
*These grade 3 or over events were considered unrelated to the vaccine treatment. †Evaluation in 15 cases other than No.8 patient
Clinical outcomes
Twelve patients who each completed six doses of the vaccine were evaluated for efficacy. Ten patients had measurable lesions by the RECIST criteria. Of the 10 evaluable patients, three had stable disease and seven had progressive disease (Supplementary Table 3). Neither complete nor partial response was observed in this study. According to survival analysis from the per-protocol population, the median OS was 6.03 months (95% CI, 2.8–17.07) (Supplementary Fig. 2).
Antibody responses
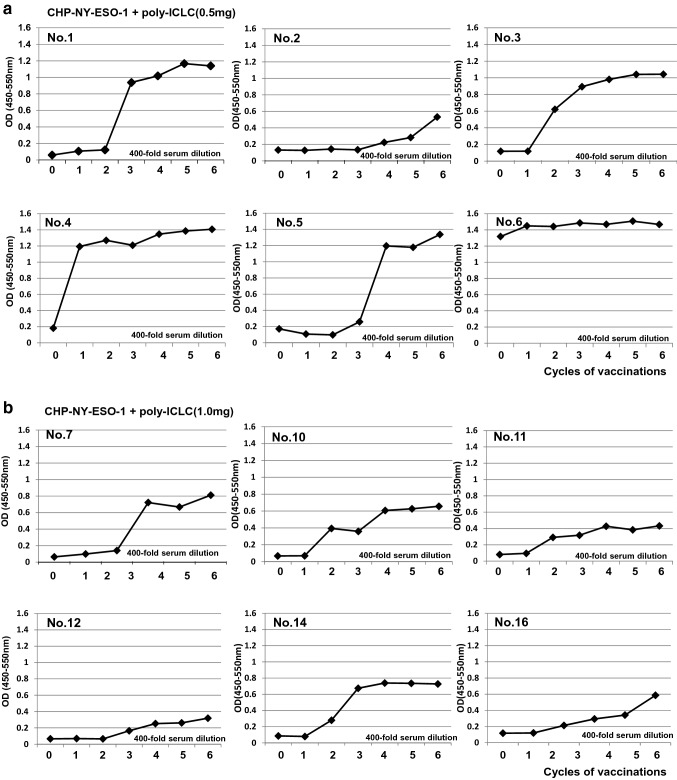
Only one of the 12 patients, No. 6, had pre-existing antibodies to NY-ESO-1 before the vaccinations. All patients who received 0.5-mg or 1.0-mg doses of poly-ICLC demonstrated seroconversion or augmentation of the NY-ESO-1 antibody (Fig. 1, Table 3). The median number of vaccine cycles to achieve an antibody response was 2.5 in both poly-ICLC groups, ranging from one to five cycles in the 0.5-mg-dose group and from one to four cycles in the 1.0-mg-dose group (Table 3).
Fig. 1.
Serial levels of serum anti-NY-ESO-1 antibody in patients vaccinated with CHP-NY-ESO-1/poly-ICLC. a. Serum IgG levels against NY-ESO-1 protein in six patients vaccinated CHP-NY-ESO-1 (200 μg) and poly-ICLC (0.5 mg). The patients were vaccinated every 2 weeks, and serum was collected prior to each vaccination. ELISA was used to analyze 400-fold diluted serum. IgG levels are displayed at an optical density value of 450 nm. b. Serum levels of IgG against NY-ESO-1 protein in six patients vaccinated with CHP-NY-ESO-1 (100 μg) and poly-ICLC (1.0 mg)
Table 3.
Antibody titers during vaccinations of CHP-NY-ESO-1/poly-ICLC
| Lab-ID | Patient No | Poly-ICLC dose | Baseline | 1 cycle | 2 cycles | 3 cycles | 4 cycles | 5 cycles | 6 cycles |
|---|---|---|---|---|---|---|---|---|---|
| Lab-1572 | 1 | 0.5 mg | – | – | – | × 6400 | × 25,600 | × 25,600 | × 25,600 |
| Lab-1567 | 2 | 0.5 mg | – | – | – | – | – | × 400 | × 400 |
| Lab-1393 | 3 | 0.5 mg | – | – | × 6400 | × 25,600 | × 25,600 | × 25,600 | × 25,600 |
| Lab-1434 | 4 | 0.5 mg | – | × 6400 | × 25,600 | × 102,400 | × 102,400 | × 102,400 | × 102,400 |
| Lab-2154 | 5 | 0.5 mg | – | – | – | – | – | × 25,600 | × 25,600 |
| Lab-2155 | 6 | 0.5 mg | × 6400 | × 6400 | × 25,600 | × 25,600 | × 25,600 | × 25,600 | × 25,600 |
| Lab-2156 | 7 | 1.0 mg | – | – | – | × 6400 | × 6400 | × 25,600 | NA |
| Lab-2159 | 10 | 1.0 mg | – | × 400 | × 400 | × 6400 | × 25,600 | × 25,600 | × 25,600 |
| Lab-2160 | 11 | 1.0 mg | – | × 400 | × 1600 | × 6400 | × 6400 | × 6400 | × 6400 |
| Lab-2161 | 12 | 1.0 mg | – | – | – | – | × 400 | × 1600 | × 1600 |
| Lab-2006 | 14 | 1.0 mg | – | – | × 400 | × 1600 | × 6400 | × 6400 | × 25,600 |
| Lab-2296 | 16 | 1.0 mg | – | – | – | × 400 | × 400 | × 1600 | NA |
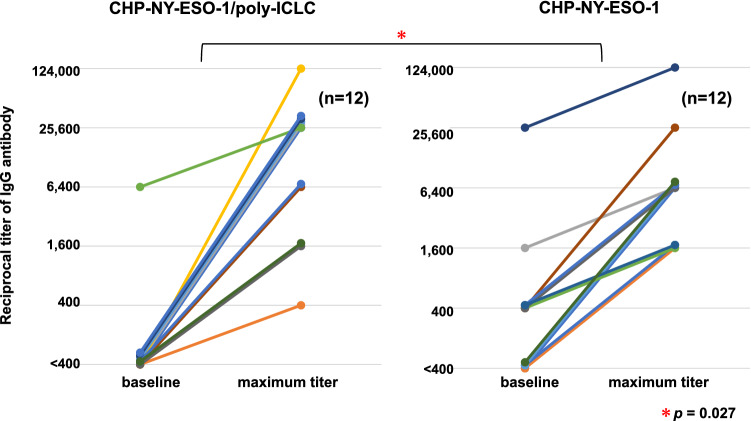
The immune responses were comparable between the two groups, and all patients demonstrated NY-ESO-1-specific antibody immune responses within five vaccinations. To compare the immune responses following the combination treatment with those following administration of the same dose (200 µg) of CHP-NY-ESO-1 vaccine alone, we collected IgG ELISA data from a previous phase 1 trial in advanced or recurrent esophageal cancer patients [14]. Twelve patients were vaccinated with CHP-NY-ESO-1 alone. Both groups showed immune responses in all patients, but antibody titers were significantly higher in the CHP-NY-ESO1/poly-ICLC group than in the CHP-NY-ESO-1 alone group (p = 0.027) (Fig. 2).
Fig. 2.
Titers of serum anti-NY-ESO-1 antibody at baseline and maximum titers after CHP-NY-ESO-1 vaccinations. Six esophageal cancer patients were enrolled in cohort 1. They were all vaccinated with 6 cycles of vaccination, and were evaluated for immune responses. In cohort 2, 10 patients were enrolled, in whom six were given six cycles of vaccination. They were evaluated for immune responses. In total, 12 patients were evaluated for immune responses. The left panel displays the changes in the 12 patients vaccinated with CHP-NY-ESO-1 (200 μg) and poly-ICLC (0.5 mg or 1.0 mg). The right panel displays the changes in 12 esophageal cancer patients vaccinated with CHP-NY-ESO-1 (200 μg) alone. The amplification of antibody titers was significantly higher in the CHP-NY-ESO1/poly-ICLC group than those in the CHP-NY-ESO-1 alone group (p = 0.027). Changes in the multiplier number (400 × 4n) of serum dilutions were statistically tested (Student t test)
Preclinical study of CHP-NY-ESO-1 / poly-ICLC with a PD-1 inhibitor
To determine whether CHP-NY-ESO-1 exerts tumor protection activity in an animal model, we assessed vaccine efficacy after administering an anti-PD-1 monoclonal antibody to mice treated with CHP-NY-ESO-1/poly-ICLC. Treatment with CHP-NY-ESO-1/poly-ICLC did not suppress tumor growth, and anti-PD-1 antibody marginally suppressed tumor growth; in contrast, treatment with both CHP-NY-ESO-1/poly-ICLC and anti-PD-1 antibody suppressed tumor growth, but not significantly compared to either of the other groups (Fig. 3). It presents tumor protection activity in a NY-ESO-1-transfected tumor inoculation model.
Fig. 3.
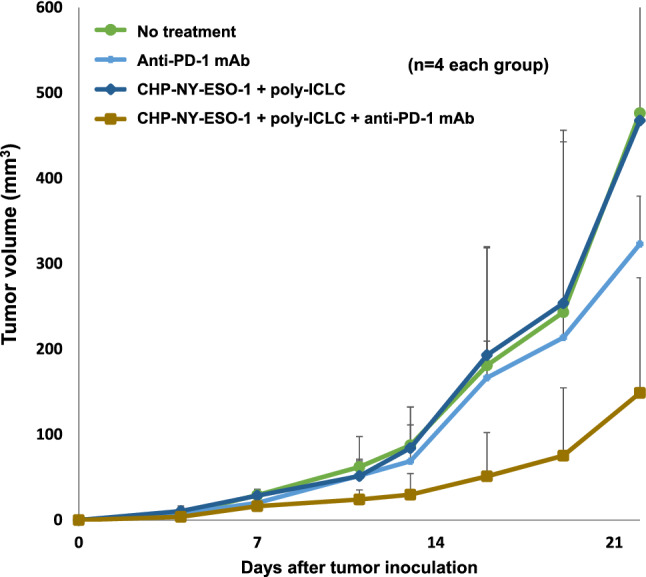
Addition of a PD-1 inhibitor to CHP-NY-ESO-1/poly-ICLC in the NY-ESO-1-transfected tumor inoculation mouse model. BALB/c mice subcutaneously inoculated with 1 × 106 human NY-ESO-1-transfected murine colon cancer CT26 cells were treated with anti-PD-1 mAb (clone RMP1-14) alone, CHP-NY-ESO-1/poly-ICLC, or CHP-NY-ESO-1/poly-ICLC/anti-PD-1 mAb (n = 4 per group). CHP-NY-ESO-1 (40 μg/mouse) and poly-ICLC (50 μg/mouse) were administered subcutaneously on days 1 and 7. The anti-PD-1 monoclonal antibody (clone RMP1-14, 150 μg/mouse) was administered intraperitoneally on days 1, 4, 7, 9, 13, 16, and 19. Compared with the non-treatment group, only the CHP-NY-ESO-1/poly-ICLC/anti-PD-1 mAb group showed tumor growth suppression (not statistically significant by the Mann–Whitney U test). The mouse model shows tumor protection activity in NY-ESO-1-expressing tumor inoculation
Discussion
This clinical trial showed that NY-ESO-1 vaccination was safe when administered with both 0.5-mg and 1.0-mg doses of poly-ICLC, and combination treatment with both doses exhibited complete antibody responses to NY-ESO-1 antigen. The magnitudes of the titers were higher than with CHP-NY-ESO-1 vaccination alone.
We monitored immune responses by analyzing IgG antibody to NY-ESO-1 protein. A previous study demonstrated that CD4+ T cell responses were clearly correlated with antibody responses to NY-ESO-1 in unvaccinated patients with NY-ESO-1-expressing melanoma and other cancers [22]. Since the CHP antigen vaccine can boost the activity of CD4+ T cells, it is important to analyze NY-ESO-1-specific antibody responses to determine the immune responses of NY-ESO-1-specific helper CD4+ T cells and B lymphocytes. T cell responses could not be quantitatively evaluated in this study because no standardized CD4+ T cell assay has been established. Instead, the IgG titer measured by ELISA can be assessed quantitatively in real time, and it is possible to monitor the vaccine-specific immune status in vaccinated patients. We found that both doses of poly-ICLC in combination with CHP-NY-ESO-1-induced definite antibody immune responses to NY-ESO-1 antigen. All 12 patients achieved IgG responses with a median of 2.5 cycles. These immune responses were stronger than those achieved with CHP-NY-ESO-1 alone (Fig. 2) [14]. It was reported that 30% of NY-ESO-1-expressing esophageal cancer patients have pre-existing antibody to NY-ESO-1 [23]. As shown in Fig. 2, Seven (58%) out of the 12 patients in the previous study (without poly-ICLC) were sero-positive. In the current trial, 1 (8%) was sero-positive. They all had recurrent esophageal tumors, and both groups were comparable in the clinical background. The number of sero-positive cases may have happened to be different in between the two groups. In a clinical trial of CHP-NY-ESO-1 combined with two different doses of MIS416, a non-toxic microparticle adjuvant, MIS416 did not augment cellular NY-ESO-1-specific immune responses at either dose [24]. We should therefore be cautious about the possible negative effect of adjuvant treatment on the immune response. Nonetheless, the combination of CHP-NY-ESO-1 with a 200-µg dose of poly-ICLC showed a stronger immune response than CHP-NY-ESO-1 alone.
In initial clinical trials, poly-ICLC doses ranged from 0.5 mg to 27.0 mg. Poly-ICLC is a type-1 IFN inducer, and toxic reactions in the initial trials included fever (in 100% of patients), nausea (44%), hypotension (28%), thrombocytopenia and leukopenia (68%), erythema (12%), and polyarthralgia plus myalgia (16%) [25]. Recent clinical studies reported that a 0.35-mg dose of poly-ICLC was tolerable in doses ranging from 0.35 to 1.4 mg in combination with NY-ESO-1 protein vaccine in melanoma patients, as well as at doses of 0.25 mg or 30 μg/kg when injected intra-tumorally with a dendritic cell vaccine [10–12]. In the current trial, as expected, we observed grade 1 or 2 fever (53.3%) and skin reaction at the injection site (86.7%). Both doses of poly-ICLC were tolerable and demonstrated stable immune reactions. Therefore, we recommend that further clinical trials administer a 1.0-mg dose of poly-ICLC in combination with a 200-μg dose of NY-ESO-1 protein vaccine.
In the current trial, we subcutaneously administered poly-ICLC at a separate location from the CHP-NY-ESO-1 vaccination site. Some trials showed that intra-tumoral injections enhanced anti-tumor immunity [10, 11], while in another trial, systemic administration induced T cell infiltration into the tumor site [12]. It was recently reported that in a mouse model, systemic administration of poly-ICLC enhanced T cell infiltration into the tumor site more significantly than intra-tumoral injection [26]. Our approach of administering poly-ICLC systemically may thus be particularly effective at augmenting T cell infiltration into tumors.
Research into cancer vaccines began with the discovery in 1991 that the MAGE-A3 cytotoxic T lymphocyte (CTL) epitope was presented to MHC class 1 molecules [27]. In the past 20 years, a number of clinical trials, including a large phase 3 randomized trial, have been conducted [3–5]. However, the clinical efficacy of cancer vaccines has not yet been proved. It is supposed that a vaccine as a single agent may be hampered by the inhibitory mechanisms in tumor microenvironments, such as regulatory T cells and PD-L1 expression. Poly-ICLC is a synthetic poly-IC that can induce tumor-specific natural killer (NK) cell, CTL, and NK-T cell-mediated immune responses. In combination with CHP-NY-ESO-1, poly-ICLC has the potential to overcome the inhibitory tumor microenvironment. In the current human trial, we demonstrated robust immune responses without toxicities.
Since several human trials failed to show the effectiveness of vaccines as single-treatment modalities, it is worthwhile exploring their use as combination treatments. In preclinical research using a mouse model, we demonstrated the efficacy of combination treatment involving CHP-NY-ESO-1/poly-ICLC with anti-PD-L1 antibody. The CT26 tumor cell line responded marginally to anti-PD-1 antibody, and was refractory to NY-ESO-1 vaccine/poly-ICLC. However, simultaneous administration of all three components successfully suppressed the NY-ESO-1-expressing tumor. This finding is a proof-of-concept supporting further human trials of combination vaccines with anti-PD-1 antibody, nivolumab, or pembrolizumab.
Immunotherapy with anti-PD-1 antibody has shown definite clinical benefits in esophageal cancer [1, 2]. As a second-line therapy, nivolumab treatment prolonged overall survival for 2.5 months, from 8.0 to 10.5 months [1]. However, regarding tumor response, fewer than 25% of patients showed clinical benefits even when nivolumab was administered together with ipilimumab [28], and the OS prolongation was unsatisfactory. One strategy to improve the clinical efficacy of PD-1 blockade is to augment the immunotherapy with a vaccine. Our mouse in vivo model suggested tumor protection activity in NY-ESO-1-expressing tumor inoculation when PD-1 antibody was given with the vaccine. Thus, the combination of PD-1/PD-L1 blockade with CHP-NY-ESO-1/poly-ICLC may be an effective second-line therapy in esophageal cancer patients.
In conclusion, CHP-NY-ESO-1 vaccine was safely administered with a 1.0-mg dose of poly-ICLC, and the combination induced a robust antibody immune response. These results warrant future clinical trials to examine the use of CHP-NY-ESO-1/poly-ICLC in conjunction with an anti-PD1 antibody for advanced or recurrent esophageal tumors that are resistant to anti-PD-1 blockade.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Tadashi Hishida (former CEO of ImmunoFrontier, Inc.) for manufacturing CHP-NY-ESO-1, and Ms. Sahoko Sugino and Ms. Junko Nakamura (Mie University) for technical assistance with ELISA. We are also grateful to Ms. Saeko Tsuchiya (Kyoto Prefectural University of Medicine) for diligently overseeing the patients in this study.
Abbreviations
- AE
Adverse event
- ALT
Alanine aminotransferase
- ANC
Absolute neutrophil count
- AST
Aspartate aminotransferase
- CHP
Cholesteryl pullulan
- CR
Complete response
- CTL
Cytotoxic T lymphocyte
- DLT
Dose-limiting toxicity
- ECOG
Eastern cooperative oncology group
- ELISA
Enzyme-linked immunosorbent assay
- GAPDH
Gyceraldehyde-3-phosphate dehydrogenase
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- IFA
Incomplete Freund's adjuvant
- IFN
Interferon
- mAb
Monoclonal antibody
- MAGE
Melanoma-associated gene
- MDA-5
Melanoma differentiation-associated gene-5
- MHC
Major histocompatibility complex
- MTD
Maximum tolerated dose
- NYHA
New York heart association
- OS
Overall survival
- PCR
Polymerase chain reaction
- PD
Progressive disease
- PD-1
Programmed cell death-1
- Poly(I:C)
Polyinosinic:polycytidylic acid
- PR
Partial response
- RECIST
Response evaluation criteria in solid tumor
- SD
Stable disease
- TLR-3
Toll-like receptor-3
- WBC
White blood cell
Author contributions
TI, SK (Mie), SK (Kyoto) and HS contributed to the design of the study. TI, TO, SK (Kyoto) and TI MI contributed to patient recruitment, treatment and clinical data collection. YM contributed to immune reaction data. LW and HY contributed to preclinical data collection and analysis. TI, SK (Mie), YM and HS interpreted the data. TI and SK (Mie) performed the statistical analyses. TI and SK (Mie) wrote the manuscript. All authors contributed to draft revisions and approved the final manuscript.
Funding
This study was supported by JSPS KAKENHI Grant Number JP15K10093 and research funds of Kyoto Prefectural University of Medicine and Mie University.
Data availability
To protect patient information in the clinical trial database, the datasets generated and/or analyzed in the present study are not publicly available, but they are available from the corresponding author on request.
Declarations
Conflict of interest
CHP-NY-ESO-1 was supplied by ImmunoFrontier Inc. (Osaka, Japan). Hiroshi Shiku is a stockholder of ImmunoFrontier Inc. Shinichi Kageyama, Yoshihiro Miyahara, and Hiroshi Shiku received research funds from ImmunoFrontier Inc. The other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in our studies involving human participants were conducted in accordance with Japanese Ethical Guidelines for Clinical Research, Japanese Guidelines for Medical and Health Research Involving Human Subjects, and the Helsinki declaration. The Institutional Review Board reviewed and approved the protocol and the informed consent documents and their amendments before use (Kyoto Prefectural University of Medicine approval number RBMR-C-941–2). All animal experiments were conducted in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions and approved by Mie University (approval number 24–14).
Animal source
Female BALB/c mice aged 6–10 weeks were purchased from Japan SLC, Inc. (Shizuoka, Japan).
Cell line authentication
The CT26 cell line was purchased from the American Type Culture Collection (Virginia, USA). A human NY-ESO-1 transfected CT26 cell line was established as described previously [21].
Informed consent
Written informed consent to participate in the study and for the use of clinical data for research and publication was obtained from all patients included in the studies: Kyoto Prefectural University of Medicine approval number: RBMR-C-941–2.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takeshi Ishikawa, Email: iskw-t@koto.kpu-m.ac.jp.
Shinichi Kageyama, Email: kageyama@kaisehp.com.
References
- 1.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 2.Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020 doi: 10.1200/jco.20.01888. [DOI] [PubMed] [Google Scholar]
- 3.Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–835. doi: 10.1016/S1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 4.Dreno B, Thompson JF, Smithers BM, et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:916–929. doi: 10.1016/S1470-2045(18)30254-7. [DOI] [PubMed] [Google Scholar]
- 5.Mittendorf EA, Lu B, Melisko M, et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25:4248–4254. doi: 10.1158/1078-0432.CCR-18-2867. [DOI] [PubMed] [Google Scholar]
- 6.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. 2016;28(7):329–338. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins KAO, Bavari S, Salazar AM. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev Vaccines. 2015;14(3):447–459. doi: 10.1586/14760584.2015.966085. [DOI] [PubMed] [Google Scholar]
- 9.Sultan H, Salazar AM, Celis E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin Immunol. 2020;30:101414. doi: 10.1016/j.smim.2020.101414. [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra S, Britten CD, Chin S, et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017;10(1):82. doi: 10.1186/s13045-017-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlick A, Blazquez AB, Meseck M, et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves antibody and cellular immune responses in patients with high-risk melanoma. Cancer Immunol Res. 2020;8(1):70–80. doi: 10.1158/2326-6066.CIR-19-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Ruiz ME, Perez-Gracia JL, Rodríguez I, et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol. 2018;29(5):1312–1319. doi: 10.1093/annonc/mdy089. [DOI] [PubMed] [Google Scholar]
- 13.Aoki M, Ueda S, Nishikawa H, et al. Antibody responses against NY-ESO-1 and HER2 antigens in patients vaccinated with combinations of cholesteryl pullulan (CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine. 2009;27:6854–6861. doi: 10.1016/j.vaccine.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama S, Wada H, Muro K, et al. Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med. 2013 doi: 10.1186/1479-5876-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitano S, Kageyama S, Nagata Y, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006;12:7397–7405. doi: 10.1158/1078-0432.CCR-06-1546. [DOI] [PubMed] [Google Scholar]
- 16.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 18.Soga N, Hori Y, Yamakado K, et al. Limited expression of cancer-testis antigens in renal cell carcinoma patients. Mol Clin Oncol. 2013;1:326–330. doi: 10.3892/mco.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockert E, Jäger E, Chen Y-T, et al. A survey of the antibody immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187(8):1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraoka D, Kato T, Wang L, et al. Peptide vaccine induces enhanced tumor growth associated with apoptosis induction in CD8+ T cells. J Immunol. 2010;185(6):3768–3776. doi: 10.4049/jimmunol.0903649. [DOI] [PubMed] [Google Scholar]
- 21.Muraoka D, Seo N, Hayashi T, et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J Clin Invest. 2019;129(3):1278–1294. doi: 10.1172/JCI97642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnjatic S, Atanackovict D, Jäger E, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci U S A. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshima Y, Shimada H, Yajima S, et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: screening in 1969 patients with various cancers. J Gastroenterol. 2016;51(1):30–34. doi: 10.1007/s00535-015-1078-8. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara M, Tono Y, Miyahara Y, et al. First-in-human phase I clinical trial of the NY-ESO-1 protein cancer vaccine with NOD2 and TLR9 stimulants in patients with NY-ESO-1-expressing refractory solid tumors. Cancer Immunol Immunother. 2020;69(4):663–675. doi: 10.1007/s00262-020-02483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine AS, Sivulich M, Wiernik PH, Levy HB. Initial clinical trials in cancer patients of polyriboinosinic-polyribocytidylic acid stabilized with poly-L-lysine, in carboxymethylcellulose [Poly(ICLC)], a highly effective interferon inducer. Cancer Res. 1979;39(5):1645–1650. [PubMed] [Google Scholar]
- 26.Sultan H, Wu J, Fesenkova VI, et al. Poly-IC enhances the effectiveness of cancer immunotherapy by promoting T cell tumor infiltration. J Immunother Cancer. 2020;8(2):e001224. doi: 10.1136/jitc-2020-001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;178(5):2617–2621. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 28.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–2844. doi: 10.1200/JCO.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To protect patient information in the clinical trial database, the datasets generated and/or analyzed in the present study are not publicly available, but they are available from the corresponding author on request.