Abstract
Background
SCLC is an aggressive malignancy where immunotherapies show limited efficacy. We aimed to characterize the SCLC microenvironment according to the expression patterns of SCLC subtype markers and novel immune checkpoints to identify therapeutic vulnerabilities.
Methods
We included SCLC tissue samples from 219 surgically resected, limited-stage patients in this cross-sectional study. We performed immunohistochemistry for STING and MHCII, as well as for the novel subtype markers (ASCL1, NEUROD1, POU2F3, YAP1). Moreover, we assessed CD45 + , CD8 + and CD68 + immune cell infiltration.
Results
36% of SCLC tumors showed significant stromal or intraepithelial CD45 + immune cell infiltration. These patients exhibited significantly increased overall survival (OS) (vs. patients with immune-deserted tumors). High CD8 expression was associated with increased median OS. We found STING expression on cancer-associated fibroblasts in the stroma and on T-cells and macrophages in both tumorous and stromal compartments. STING expression positively correlated with immune cell infiltration. Increased STING-positivity in tumor nests was an independent favorable prognosticator for OS. ASCL1 was the most frequently expressed subtype-specific protein. Concomitant expression of three or four subtype-defining markers was seen in 13.8% of the included samples, whereas 24.1% of the cases were classified as quadruple negative tumors. YAP1 expression was associated with increased immune infiltrates. Tumor cell MHCII expression positively correlated with immune cell infiltration and with STING- and YAP1 expressions.
Conclusions
STING and MHCII are expressed in SCLC. The majority of immune-infiltrated SCLCs exhibit increased STING expression. Immune infiltration and STING expression are prognostic in limited-stage SCLC, making STING a potential therapeutic target.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03270-w.
Keywords: SCLC, STING, MHCII, CD45, CD8, Tumor microenvironment
Introduction
Small cell lung cancer (SCLC) is one of the most aggressive types of malignancies, where conventional attempts to repurpose known biomarkers and clinical trials have sequentially failed. In contrast to non-small cell lung cancer (NSCLC), many biomarkers (such as smoking status and PD-L1) are not predictive for immune checkpoint inhibitor (ICI) response, indicating that SCLC tumors might be associated with different immune microenvironments. The 5-year-survival of immunotherapy-treated, advanced-stage NSCLC patients recently increased from 5% to 23.2% [1]. This contrasts with SCLC, with many therapy approval failures and 5-year OS still below 1% in patients with advanced-stage disease. Many ICI-related trials in other cancers showed great success, including the phase 3 KEYNOTE-426 trial, with previously untreated advanced clear-cell renal-cell carcinoma, where the median progression-free survival (PFS) in the pembrolizumab–axitinib group was much higher (15.1 months) than those in SCLC [2]. In contrast to other cancer types, there is no ICI combination therapy approved in SCLC, and trials on checkpoint inhibitors showed only minor increases in PFS compared to the standard of care chemotherapy [3, 4].
To date, our concepts of the critical immunological mechanisms concerning the tumor microenvironment (TME) such as tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs) are still developing [5]. Still, immune cell infiltration and increased CD8 + T-cells were reported to be independent positive prognostic markers and could enhance the response to anti-PD1-immunotherapy in a multitude of malignancies, including colorectal- [6], prostate- [7] and breast cancers [8], melanoma [9] and NSCLC [9, 10]. A more controversial element of the TME, the tumor-related activity of TAMs, can differ between tumor types and compartments [11]. TAMs and other inflammatory cells are drawn to the tumor stroma by the release of cytokines and chemokines produced by tumor cells executing pro-tumorigenic and immunosuppressive functions [12].
Recently, our group showed that the expression of immune checkpoints, such as indoleamine 2,3-dioxygenase (IDO), T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), poliovirus receptor (PVR) and major histocompatibility complex (MHCII) are significantly higher in neuroendocrine (NE)-low SCLC [13]. While constitutive expression of MHCII in antigen-presenting cells (APCs) is well known, certain types of cancers are also capable of expressing the molecule [14]. It was also reported that tumor-specific expression of the MHCII molecule might increase tumor recognition by immune cells [15] and harboring high numbers of immunosuppressive M2-macrophages [16] rising obstacles against prospects for future immunotherapies. Another immune checkpoint, the stimulator of interferon genes (STING), acts as an essential innate immune sensor for tumor detection [17]. STING signaling drives interferon type-I production upon activation in APCs within the tumor microenvironment (TME), thus enhancing the cross-priming of effector (CD8 +) T cells [18, 19]. Others discovered reduced STING/cGAS expression in lung cancer with worse prognosis [20], [21].
In recent years, a novel SCLC subtype typology seems to emerge defined by the distinct gene expression pattern of four key transcription regulators. This novel classification scheme is based on both the results of preclinical models [22] and on individual protein expression profiles of human tumor samples [23, 24]. NE-high tumors are characterized by high achaete-scute homolog 1 (ASCL1) expression, whereas NE-low tumors express neurogenic differentiation factor 1 (NEUROD1). Non-NE tumors can be subclassified into yes-associated protein 1 (YAP1)-high or POU class 2 homeobox 3 (POU2F3)-high categories [22], [25]. ASCL1 is essential for the survival of pulmonary neuroendocrine cells (PNECs, [26]. NEUROD1 overexpression may promote invasiveness and contribute to the formation of metastasis in SCLC [27], whereas YAP1 promotes gene transcription activity by binding to transcription factors [28] responsible for apoptosis suppression and growth promotion. POU2F3-expressing SCLC cell lines outside their microenvironment (i.e., in cell cultures) generally lack other NE subtype markers and exhibit an expression profile similar to that of tuft cells [29, 30].
This study performed high-throughput immunohistochemistry (IHC) screening on TMA specimens of 219 SCLC patients. We evaluated the expression pattern and prognostic role of emerging immune checkpoints STING and MHCII in the context of immune cell density and the four major SCLC subtype markers in distinct tumor compartments, including stroma and tumor nests.
Materials and methods
Ethical statement
We conducted the study according to the guidelines of the Helsinki Declaration of the World Medical Association, and with the national level ethics committee (ETTTUKEB-7214–1/2016/EKU). The need for individual informed consent was waived for this retrospective study. After obtaining the clinical information, the patients were de-identified; therefore, the patients cannot be identified directly or indirectly.
Study population
In this retrospective study, surgically resected and histologically confirmed consecutive SCLC patients were included between 1975 and 2013 at the National Koranyi Institute of Pulmonology. The study and all treatments were conducted based on the institutional guidelines. The date of the last follow-up included in this analysis was April 2021.
Tissue processing
SCLC patient tumors were fixed and processed into paraffin blocks after surgery. TMA construction from FFPE blocks was performed as previously described [31]. Briefly, 4-micron sections were cut from each tissue block using an HM-315 microtome (Microm) and placed on charged glass slides (Colorfrost Plus, #22-230-890, Fisher). The slides were stained for H&E on an automated Tissue-Tek Prisma staining platform (Sukura). A board-certified pathologist with thoracic oncology experience inspected H&E slides for the tumor area, where its borders were marked. Two 1-mm punches of tissue were taken from each donor tissue block and seated into a recipient paraffin block in a positionally encoded array format (MP10 1.0-mm tissue punch on a manual TMA instrument, Beecher Instruments).
Immunohistochemistry and immunofluorescence
For IHC staining, five-micron-thick sections were cut. The staining process was carried out on a Leica Bond RX autostainer using rabbit monoclonal antibody for CD45 (#13917), CD8 (#8112), CD68 (#201340), STING (#13647) and MHCII (#68258S) from Cell Signaling and diluted in 1:200 prior to staining as previously described [13]. For SCLC subtype markers, we used rabbit monoclonal antibodies for ASCL1 (Sigma-Aldrich, #32355, diluted in 1:300), POU2F3 (Sigma-Aldrich, #32537, diluted in 1:300) and YAP1 (Invitrogen, #PA1-46,189, diluted in 1:300) and a mouse monoclonal antibody for NEUROD1 (abcam, #60704, diluted in 1:300). We visualized the binding spots of the primary antibodies, and the slides were stained using the Bond Polymer Refine Detection kit (#DS9800) with Leica IHC Protocol F and exposed to epitope retrieval 1 (low pH) for 20 min. For double IHC stainings, antibodies for CD45 (#13917), CD3 (#17143), CD68 (#201340) and SMA (#7817) were used from abcam diluted in 1:300. ImmPRESS® Duet Double Staining Polymer Kit (HRP Anti-Mouse IgG-brown, AP Anti-Rabbit IgG-magenta, MP-7724, VectorLabs) was used as secondary antibodies and chromogenic substrates. For anti-POU2F3 staining, AP anti-rabbit IgG (Vector) was used as a secondary antibody, diluted 1:200 in 1% PBS/BSA. Endogenous alkaline phosphatase activity was quenched by a 5% Levamisole solution prior to the visualization of antigen-binding spots with VectaRed AP kit (Vector). Counterstaining was performed with hematoxylin. For double immunofluorescence (IF), Alexa IgG anti-rabbit A488 and IgG anti-mouse A546 fluorescent secondaries were used (Invitrogen) for signal detection of CD45, CD3, CD68 and SMA antibodies. We used TrueBlack® Lipofuscin Autofluorescence Quencher (Biotium) to eliminate autofluorescence on FFPE samples. The detection of protein expression was optimized in human tonsil and lung adenocarcinoma tissue as a positive control.
Scoring, cell counting and morphometry
Images of TMA sections were captured via a BX53 upright Olympus microscope and a DP74 CMOS camera in 20MP resolution for scoring, cell counting and representative images from tumor tissues. The STING and MHCII expression and staining intensity for SCLC subtype markers (ASCL1, NEUROD1, POU2F3 and YAP1) were evaluated using a semiquantitative scoring system. For MHCII, scores were determined according to the expression pattern of the immune checkpoint, where 0 = no positive tumor cells, 1 = scattered clusters of tumor cells are positive (1–50 cells), 2 = multiple clusters of tumor cells are positive (> 50 cells), and 3 = diffuse positive staining on most tumor cells. Positive staining on the TME (stromal cells, endothelium, immune cells) was not included in the scoring. For STING, we applied scoring based on the number of positive cells per TMA core, where scores 0 = zero cells, 1 = 1–20 cells, 2 = 20–40 cells and 3 = > 40 cells were determined for each section and compartment (stroma and tumor nest). Scores for SCLC subtype marker expression on tumor cells were determined by the intensity of staining according to the Allred score, where 0 = negative, 1 = weak, 2 = intermediate and 3 = strong scores were given. Stromal expression of SCLC subtype markers was not scored and evaluated. We calculated STING total scores, stroma and tumor nest scores averaged for every section.
Morphometry and cell counting for cell density measurements of tumor-infiltrating immune cells were carried out by Olympus CellSens Dimensions Software package and manual annotation of measured areas (stroma and tumor nest), as previously described [13]. Briefly, positive cells for immune markers CD45, CD8 and CD68 were identified by software-assisted, manual cell counting by two independent observers with the “cell counter” plugin of the ImageJ software package [32]. In the case of discrepancy below 20%, values were averaged,otherwise, the samples were rescored. Three separate five-micron-thick sections (with a minimum of 100 μm distance in Z between the sections were scored or quantified for every core and every used biomarker. Square micrometers (μm2 were converted to square millimeters (mm2 for the calculation of cell density parameters in statistical analyses. The results from separate sections of the identical TMA scores were averaged.
Quantitative assessment of colocalization
We assessed the ratio of STING-positive versus STING-negative immune cells; total cell numbers were counted for all immune-cell markers (CD45, CD68, and CD3), then the total number of STING-CD45, STING-CD68 and STING-CD3 double-positive cells was counted separately in the stroma, and the tumor compartment. For the quantification of colocalization, we included only CD45 stroma oasis or oasis tumors (Fig. 1). Cell counting was performed with the "cell counter" plugin of the ImageJ software package on IF-stained slides, where secondary antibodies and fluorochromes Alexa A488 (for STING) and Alexa A546 (for immune cell markers) were used. Colocalization was assessed upon the occurrence of a double expression color signal (yellow).
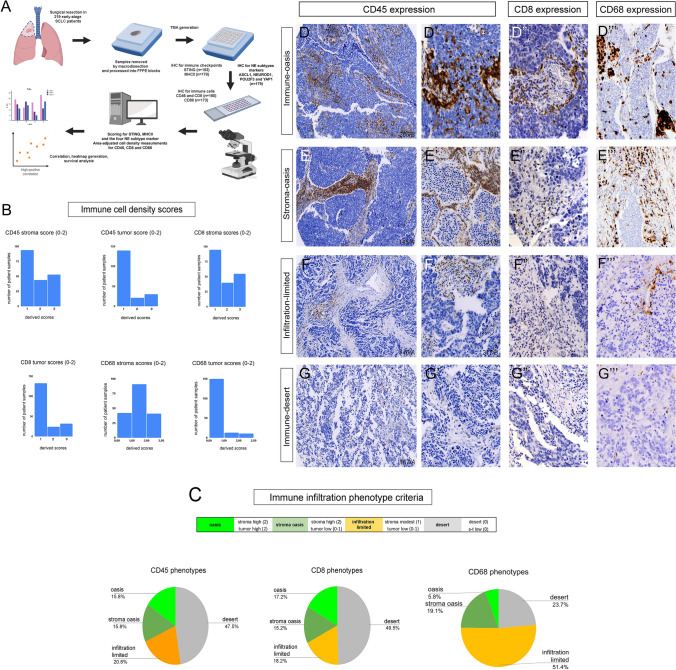
Fig. 1.
Immune infiltration phenotypes according to CD45 + immune cell, CD8 + cytotoxic T-cell and CD68 + macrophage densities. Study workflow is shown in panel A. A total of n = 219 patients underwent surgical resection. IHC stainings for CD45 and CD8 were available in n = 190 cases, for CD68 in n = 173 cases, for STING in n = 193 cases, while for MHCII in n = 178 cases. 2 TMA cores were scored (3 sections every core) for every patient with area-adjusted cell counting. Scores were derived from cell density values (cells/mm2) separately in the stroma and in tumor nests described in methods. Relative distribution of tumors exhibiting extensive (2), modest (1) or minimal (0) CD45 + , CD8 + or CD68 + cellular infiltration in the stroma and tumor compartments are shown in panel B. Based on the morphological features and infiltration scores in different compartments, we established 4 TME phenotypes for immune cell populations (C). Representative tissue samples show CD45, CD8 and CD68 immunolabeling on TMA specimens (D-G’’’). Immune oasis tumors are defined as strongly infiltrated tumors both in stroma and tumor nests with CD45 (D-D’), CD8 (D”) and CD68 (D”’) immunolabeling. Stroma oasis tumors have extensive stromal immune cell infiltration with relatively low numbers of CD45 + (E-E’), CD8 + (E”) or CD68 + (E”’) cells entering tumor nests. In contrast, immune cells occur sparsely and exclusively in the stroma compartment in infiltration-limited tumors with virtually no intratumoral presence of these cells (F-F”’). Immune desert tumors are totally negative or exhibit minimal expression of CD45, CD8 or CD68 (G-G”’)
Cutoff values and determination of TME phenotypes
We calculated different TME phenotypes according to cellular densities (cell/mm2) in the stroma and tumor compartments. The k-means R plugin generated 3 mean-centered groups for stroma- and tumor nest infiltration densities for every biomarker (0-1-2). Then, we merged the separate stroma and tumor scores to determine an overall "infiltration phenotype" for every patient sample. Cutoff values, scores and classification criteria for different TME phenotypes are shown in STable 1.
Statistical methods
Normality was evaluated with the Kolmogorov–Smirnov test. Tumor core A and B biomarker expressions were compared with the Wilcoxon matched pair test and found no significant differences in any comparison; therefore, we used average tumor core A and B values for further statistical analyses. Statistical analyses were performed using the PASW Statistics 22.0 package (SPSS Inc., Chicago, IL, USA). Data preprocessing and cluster analysis were performed with R packages, including ggplot2 for PCA plot and pheatmap (R package version 0.7.7) for plotting heatmaps. For data preprocessing, no scaling method was used. Using the Oncomine database, relative expression values for TMEM173 (STING) RNA (transcript per read, TPM) were evaluated in SCLC (n = 54) and NSCLC (n = 136) cell lines. Survival analysis was performed with Kaplan–Meier curves and a comparison of survival curves with the log-rank test. Cox-proportional hazard regression was used to screen for significant predictor variables. The analysis was two-sided with a level of significance of α = 0.05. To assess the goodness of fit of our multivariate model, Harrel’s C-index (> 0.7) was calculated. Survival analyses were performed using the SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and Statistica 13.05 (TIBCO Software Inc.) software packages.
Results
Immune cell infiltration in SCLC samples and the implementation of TME phenotype
In our study, we identified a total of 219 patients diagnosed with SCLC who underwent surgical resection. The study workflow is shown in Fig. 1A. IHC stainings for CD45 and CD8 were available in n = 190 cases, for CD68 in n = 173 cases, for STING in n = 193 cases, and for MHCII in n = 178 cases. Relevant clinicopathological data, including stage, OS, and adjuvant chemotherapy were available for n = 129 patients.
Figure 1B shows the relative distribution of tumors exhibiting extensive (2), modest (1) or minimal (0) CD45 + , CD8 + or CD68 + cellular infiltration in the stroma and tumor compartments. Figure 1C shows the relative distribution of the four major infiltration phenotypes derived from stroma and tumor cell-density scores, as previously described Cut-off values and scores for different TME phenotypes are shown in STable 1. Representative tissue samples show CD45, CD8 and CD68 immunolabeling on TMA specimens. Based on the morphological features and area-adjusted cell counting, we established 4 TME phenotypes regarding CD45 + immune cell-, CD8 + T-cell- and CD68 + macrophage infiltration. Immune oasis tumors are defined as strongly infiltrated tumors both in stroma and tumor nests with CD45 (Fig. 1D, D’), CD8 (Fig. 1D”) and CD68 (Fig. 1D’’’) immunolabeling. Stroma oasis tumors have extensive stromal immune cell infiltration with a relatively low number of CD45 + (Fig. 1E, E’), CD8 + (Fig. 1E”) or CD68 + (Fig. 1E’’’) cells entering the tumor nests. In contrast, immune cells occur sparsely and exclusively in the stroma compartment in infiltration-limited tumors with virtually no intratumoral presence of the aforementioned cell types (Fig. 1F-F’’’). Immune desert tumors are totally negative for or exhibit minimal expression of CD45, CD8 or CD68 (Fig. 1G-G’’’).
Expression of STING and MHCII proteins in the context of the immune TME
Immune checkpoint protein STING was expressed on a variety of cells in SCLC. Figure 2 shows the STING expression pattern on representative tissue samples (Fig. 2A–B’). IHC stainings showed that STING protein expression is present mostly in stromal cells (Fig. 2A and B) but also occurs in non-malignant cells of ramified or round-shaped morphology, scattered inside tumor nests, identified by colocalization studies as CD68 + macrophages and CD3 + T-lymphocytes (Fig. 2B’ arrowheads, Fig. 3). Cancer cells express STING only in 4% of patients, mostly in scattered clusters (Fig. 2C–C’). This is underpinned by RNAseq data from the Oncomine database, where STING expression is significantly lower in SCLC- (n = 54) compared to NSCLC cell lines (n = 136) (0.043 vs 2.41 TPM, p = 0.012) (Fig. 2C”). Histograms show the scoring outcome and relative distribution of STING expression scores (Fig. 2E–E”) and a pie chart shows the ratio of STING high vs. low tumors in the whole cohort (Fig. 2F).
Fig. 2.
STING immune checkpoint protein expression in SCLC. Panel A-B’ shows the expression pattern of STING in representative sections of TMA specimens. STING is expressed in a large number of stromal cells (B) and occasionally in non-cancer cells in tumor nests (B’). Tumor cell expression of STING (C) was found only in 4% of patients (C’). According to RNAseq data obtained from Oncomine, STING expression is significantly lower in SCLC- (n = 54) compared to NSCLC cell lines (n = 136) (p = 0.012) (C’’). Histograms show the scoring outcome and relative distribution of STING expression scores in the stroma and tumor nests (D-D”). Pie chart shows the ratio of STING-high vs low tumors in the whole cohort (F)
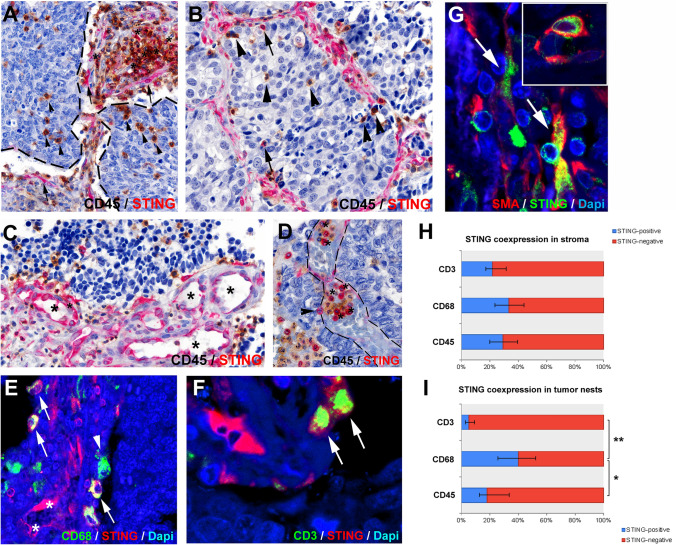
Fig. 3.
Colocalization of STING protein with CD45 + immune cells, CD68 + macrophages, CD3 + T-cells and SMA + CAFs. In the stroma compartment, STING is expressed in CD45 + cells of various morphology (A, asterisks) and in CD45-negative elongated, ramified cells (A, arrows). Majority of intraepithelial CD45 + cells do not express STING (A-B, arrowhead), only a limited number (B, arrows). STING is also expressed in stromal endothelial cells (C, asterisks) and in intravascular, round-shaped blood cells (D, asterisks) during extravasation to tumor tissues (D, arrowhead). Pan-macrophage marker CD68 is coexpressed in multiple STING-expressing cells (E, arrows), though many macrophages do not express STING (D, arrows, H-I). STING is also detected in CD3 + T-cells, mostly in the stroma compartment (F, arrows, H-I). SMA-staining shows CAFs expressing STING in the stroma (G, arrows, inset). In tumor nests, a significantly higher percentage of CD68 + macrophages express STING compared to CD3 + lymphocytes (p = 0.008) and CD45 + (p = 0.022) immune cells. Blue bars show the percentage of STING double-positive cells with corresponding immune cell markers. Error bars indicate SD. Statistical significance *P < 0.05; **P < 0.01
To identify immune cell populations that express STING, we performed double IHC stainings for CD45 (leukocytes) and double immunofluorescence (IF) for CD3 (T-cells) and CD68 (tumor-associated macrophages, TAMs). Quantification of coexpression was performed in immune-oasis or stroma-oasis tumors. Colocalization studies showed that multiple stromal CD45 + cells express the STING protein (Fig. 3A, asterisk), but 71% of immune cells are still STING-negative (Fig. 3H). Instead, STING is expressed in many CD45-negative elongated stromal cells (Fig. 3A, arrows) and in endothelial cells (Fig. 3C). Interestingly STING-expression also occurs in extravasating CD45 + immune cells in the lumen of tumor-associated vessels (Fig. 3D asterisks and arrowhead). In tumor nests, 81% of CD45 + immune cells are STING-negative (Fig. 3A–B, arrowheads and I), only a few cells coexpress the STING protein (Fig. 3B, arrows and I). To elaborate on the immunophenotype of STING-expressing immune cells, we performed double immunofluorescence for pan-macrophage marker CD68 and T-cell marker CD3 (Fig. 3E–F). In the stroma compartment, 33% of CD68 + cells coexpress STING protein (Fig. 3E, arrows and H), whereas, in tumor nests, 40% of TAMs are STING-positive (Fig. 3I). Arrowhead shows STING-negative CD68 + TAM in Fig. 3E, and asterisks show STING + CD68- cells with a ramified, elongated morphology. CD3-STING double IF shows that multiple T-cells express STING protein (22%) in the stroma (Fig. 3F, arrows and H). In contrast, only 5% of CD3-immunopositive T-cells are positive for STING inside tumor nests (Fig. 3I). STING protein is expressed by a significantly higher percentage of CD68 + TAMs than CD3 + T-cells (p = 0.008) in tumor nests. Elongated cells, positive for STING but negative for CD68 and CD3, express SMA, a general maker for cancer-associated fibroblasts (CAFs) (Fig. 3G).
According to our IHC analyses, the MHCII molecule is also expressed in cancer cells. Certain tumors display MHCII expression in the stroma and on the surface of immune cells entering in tumor nests, but cancer cells show no MHCII positivity (Fig. 4A–A’). However, some tumors exhibit diffuse MHCII expression in their tumor cells (Fig. 4B–B’). As an intermediate phenotype, mosaic expression of MHCII also exists, where only clusters of tumor cells are favorable for the molecule (Fig. 4C–C’). The distribution of MHCII expression scores is shown in Fig. 4D. Pie charts show the ratio of MHCII high vs. low tumors and MHCII/STING double-high vs. double-low tumors (Fig. 4E) in the whole cohort.
Fig. 4.
Expression of MHCII in SCLC. In certain tumors, MHCII expression in the stroma and in tumor nests occur in the TME, but cancer cells lack MHCII expression (score = 0, A-A’). In contrast, some tumors exhibit diffuse MHCII expression in their cancer cells (score = 3, B-B’), and in some cases separate clusters of tumor cells express the molecule (score = 1–2, C–C’). Histogram shows the average expression scores (Allred) concerning MHCII in the patient cohort (D). Pie charts show the ratio of MHCII-high and -low patients and the ratio of MHCII-STING double-high and -low patients in the whole cohort (E) (color figure online)
SCLC subtypes and their correlation with the TME
IHC staining for ASCL1, POU2F3, YAP1 and NEUROD1 was available for n = 179 patients. First, we analyzed the expression pattern of the four key subtype-defining proteins with IHC and scored the samples according to staining intensity on tumor cells. We found that ASCL1 and YAP1 proteins show expression in both stromal and tumor cells, whereas NEUROD1 and POU2F3 expression is dominant in cancer cells of tumor nests (Fig. 5A–D). Immunostainings on consecutive sections showed double- triple- and quadruple in situ expression of subtype proteins in a representative patient sample (Fig. 5E–E’’’, ID of samples in italics). Correlation matrix summarizes the association of subtype markers with immune cell densities and immune checkpoint expressions in different tumor compartments in Fig. 5F. CD45 + and CD8 + immune cell densities correlated strongly (r = 0.65–0.94) in all comparisons, CD68 + TAM-density is only moderately correlated with CD45 + and CD8 + cellular densities in tumor nests (r = 0.48–0.49). Immune cell markers showed moderate positive correlation with both STING and MHCII expression (r = 0.41–0.6), similarly as the expression of the two checkpoints with each other (r = 0.38–0.42) (Fig. 5F).
Fig. 5.
SCLC subtype-specific characteristics of the TME in SCLC. ASCL1 and YAP1 proteins are expressed in both stromal and tumor cells, whereas NEUROD1 and POU2F3 expression is prominent in cancer cells of tumor nests (A-D). Immunostainings on serial sections indicate double-, triple- and quadruple in situ expression of subtype proteins in a representative patient sample (E-E’’’). Correlation matrix highlights the associations of subtype markers with immune cell densities and immune checkpoint expression in different tumor compartments (F). CD45 + and CD8 + immune cell densities are strongly correlated with each other irrespective of tumor compartment (r = 0.65–0.94), whereas CD68 + TAM-density is only moderately correlated with CD45 + and CD8 + cellular densities in tumor nests (r = 0.48–0.49). Densities of infiltrating CD45 + and CD8 + immune cells show a moderate correlation with STING expression in stroma and tumor nest and with MHCII expression in tumor nests (r = 0.41–0.6). MHCII expression shows a moderate correlation with STING expression both in stroma and in tumor nests (r = 0.38–0.42) (F). ASCL1, NEUROD1 and POU2F3 protein expression showed no significant correlation with any of the immune cell markers, whereas ASCL1 and NEUROD1 showed a significant, but weak correlation with STING expression in stroma (r = 0.22–0.27) and POU2F3 with STING expression in tumor nests (r = 0.22). YAP1 showed a significant moderate positive linear correlation with CD45 + and CD8 + cellular densities, which was more prominent in tumor nests (r = 0.43–0.44), but only a weak correlation concerning CD68 (r = 0.26–0.30) (F). Subtype score shows a significant moderate association with STING expression in the stroma and tumor nest (r = 0.36–0.39) and a weak significant correlation with MHCII expression on tumor cells (r = 0.26) (F). ASCL1 (1.632) was the most expressed of the four subtype markers, (1.632 vs 0.939, vs 0.982, vs 0.855, p < 0.001, respectively, G). POU2F3 (0.939 vs 0.855, p = 0.041) and YAP1 (0.982 vs 0.855, p = 0.021) had considerably higher expression than NEUROD1, and there was no significant difference between POU2F3 and YAP1 expression (p = 0.733, G). YAP1-high (≥ 2) patients have significantly higher CD45 + and CD8 + cellular densities in both in stroma (1506 vs 820 cells/mm [15], p < 0.001 and 1499 vs 1276 cells/mm [15], p = 0.001, respectively) and tumor nests (106 vs 15 cells/mm [15] and 21.2 vs 2.5 cells/mm. [15], p < 0.001, respectively). YAP1-high (≥ 2) patients have significantly higher MHCII expression on tumor cells (p = 0.0148, I), and YAP1 expression correlates with MHCII (r = 0.37, p < 0.001, J). Panels K-K" demonstrate colocalization of YAP1 and MHCII in tumor cells and STING protein in stromal cells on serial sections. Pie chart in L shows the distribution of patients with high subtype score (> 6, 25.4%), and low subtype score (≤ 6, 74.6%). Patients with high score have significantly greater levels of STING expression in both stroma and tumor nests (p = 0.001, respectively, M-M'). Error bars indicate SD. Statistical significance *p < 0.05,**p < 0.01,***p < 0.001
Out of all subtype markers, ASCL1 showed the strongest expression and its expression levels were significantly higher than in the case of all other markers (Fig. 5G). ASCL1, NEUROD1 and POU2F3 protein expression showed no significant correlation with any of the immune cell markers and only a negligible level of correlations with the expression of immune checkpoints (Fig. 5F). However, YAP1 showed a significant moderate correlation with CD45 + and CD8 + cellular densities which were more pronounced in tumor nests (r = 0.43–0.44), but only a weak correlation with CD68 (r = 0.26–0.30) (Fig. 5F). The latter is underpinned by the fact that YAP1-high patients exhibited significantly increased CD45 + and CD8 + cellular densities both in stroma and tumor nests (Fig. 5H-H’’’). YAP1-high patients showed significantly increased MHCII expression on tumor cells (p = 0.148, Fig. 5I) and YAP1 expression significantly correlated with MHCII (r = 0.37, p < 0.001, Fig. 5J). Regression plots display significantly correlated biomarkers in SFig 1. Figure 5K–K” show colocalization of YAP1 and MHCII in tumor cells and STING protein in stromal cells on consecutive sections of the same patient sample.
We also implemented a “subtype score,” where scores for every subtype protein were summed per patient. This might be useful because of the conspicuous overlap between subtype-specific marker expression in a significant number of patients (Fig. 5L). Subtype score showed a significant moderate correlation with stromal and tumor nest expression of STING (r = 0.36–0.39) and a weak significant correlation with MHCII expression on tumor cells (r = 0.26) (Fig. 5F). Subtype-high patients exhibited significantly higher expression of STING (compared to subtype-low patients) in both stroma and tumor nests (p < 0.001, respectively, Fig. 5 M–M’).
Cluster-analysis according to immune cell infiltration, SCLC subtypes and checkpoint expression
Heatmaps were generated, to perform cluster-analysis and reveal patient groups according to the extent of immune cell infiltration (in stroma and tumor nests, respectively), immune checkpoint expression and SCLC subtype profiles. Regarding stromal infiltration (Fig. 6A), patients can be classified into two main clusters: with high- (cluster A) and with low- (cluster B) immune cell infiltrates and associated checkpoint expression. Interestingly, subcluster A2 shows high MHCII expression but low STING expression, while subcluster A3 is characterized by the opposite. Some patients exhibit low- or minimal immune cell infiltrations with increased STING (subcluster Bs) or with increased STING and MHCII expression (subcluster B1). The existence of this cluster underlines the stromal expression of STING on non-immune cells (CAFs). B2 subcluster represents immune- and checkpoint desert tumors. In tumor nests (Fig. 6B), cluster A includes tumors with increased- (subcluster A1) and lacking (subcluster A2) CD68 + TAM infiltration, with CD45- CD8- and checkpoint expression scores. Checkpoint—high and immune infiltrate—low cluster also occur (subcluster B1 and B2). Subcluster C1 represents tumors with no intratumoral CD8 + T-cell infiltration, but with a variable level from other immune infiltrates and checkpoint expression, whereas subcluster C2 represents totally immune- and checkpoint desert tumors.
Fig. 6.
Cluster analysis according to checkpoint expression, immune cell infiltration and SCLC subtypes. Heatmaps and cluster analysis represent groups of patients with similar immune infiltrate- checkpoint and SCLC subtype expression characteristics. Z-score (0–6) was generated for every cell using the k-means method (CD45, CD8 and CD68) and linear transformation (checkpoints, SCLC subtypes). In terms of stromal infiltration (A), patients are divided into two groups: those with high (cluster A) and those with low (cluster B) immune cell infiltrates and associated checkpoint expression. Surprisingly, subcluster A2 has strong MHCII expression but low STING expression, whereas subcluster A3 has the opposite. Some patients have modest or negligible immune cell infiltrations with elevated STING- (subcluster Bs) or STING and MHCII expression (subcluster B1). Subcluster B2 represents immune- and checkpoint desert tumors. Cluster A in tumor nests (B) contains immune-infiltrated and STING and MHCII—high tumors with high (subcluster A1) or low (subcluster A2) CD68 + TAM infiltration. There is also a high checkpoint and a low immune infiltration cluster (subcluster B1 and B2). Subcluster C1 tumors present no intratumoral CD8 + T-cell infiltration but varying levels of other immune infiltrates and checkpoint expression, whereas subcluster C2 tumors are completely immune- and checkpoint deserted. We identified six patient clusters based on NE subtype markers (C). Cluster A1 tumors include concurrent expression of multiple subtype markers (quadruple and triple positive tumors), including ASCL1 and NEUROD1 and, to a lesser extent, POU2F3 and YAP1. Cluster A2 contains tumors that are both ASCL1-positive and NEUROD1-positive. POU2F3 expression is prominent in cluster B1; however, on a small percentage of tumors, ASCL1 and/or YAP1 expression coexist. Cluster B2 tumors are YAP1-dominant, with some expressing ASCL1. Cluster B3 has only ASCL1-positive tumors, whereas Cluster B4 contains quadruple negative tumors. Pie chart (D) shows the distribution of patient clusters based on their SCLC subtype phenotype
Regarding SCLC subtype markers, we detected 6 major patient clusters (Fig. 6C). Cluster A1 includes tumors with simultaneous expression of multiple subtype markers all including ASCL1 and NEUROD1 and to a variable extent POU2F3 and YAP1. This is the so-called high-subtype score patient group. Cluster A2 represents ASCL1/NEUROD1 double-positive tumors or only NEUROD1-expressing tumors. In cluster B1, POU2F3 expression is dominant, but in a certain number of tumors simultaneous ASCL1 and / or YAP1 expression occur as well. Cluster B2 is YAP1- dominant, with some tumors expressing ASCL1 too. Cluster B3 is exclusively ASCL1—positive, whereas cluster B4 includes quadruple negative tumors, showing no expression for any protein subtype marker. Figure 6D shows the proportion of different subtype clusters based on their dominant subtype.
Clinicopathological characteristics and prognostic factors for OS
Survival analysis was performed to evaluate the prognostic role of immune cell infiltration and immune checkpoints STING and MHCII. OS data were available for n = 129 patients, n = 96 with an early-stage (I–II), and n = 33 with stage III disease. A total of n = 51 patients received adjuvant chemotherapy (CT). Patients with a stage (I-II) disease had improved OS (vs. stage III, p < 0.001, Fig. 7A), whereas adjuvant CT had no significant effect on survival (p = 0.062, SFig 2A).
Fig. 7.
Prognostic role of immune cell infiltration and STING expression in early-stage SCLC. Early-stage patients with (I-II) exhibited improved OS (vs. stage III, p < 0.001, A). KM curves demonstrate OS for oasis, stroma-oasis, infiltration-limited and desert infiltration-phenotypes according to CD45 + immune cell and CD8 + T-cell infiltration in early-stage patients (B and C), where oasis and stroma oasis tumors exhibited significantly increased OS compared to desert and infiltration limited tumors in every comparison, except for the case of CD45 oasis vs CD45 infiltration limited tumors (p = 0.145, B). There was no significant difference in OS between desert and infiltration limited tumors in any comparison. Regarding CD68, stroma oasis and oasis tumors exhibited significantly increased survival compared to desert tumors (p < 0.001, p = 0.011, respectively), but not compared to infiltration limited tumors (p = 0.094, p = 0.291, respectively) (D). When patients with only infiltrated tumors were selected (CD45 stroma oasis and oasis), stroma oasis and infiltration limited tumors showed the best prognosis (172.3 and 59.8 months, respectively) and controversially, immune oasis tumors showed the worst (21 months) (E). With the same selection (only CD45 stroma oasis and oasis tumors), patients with high tumor nest STING expression (≥ 2) showed significantly increased survival (compared to STING-low (< 2) patients, p = 0.002, G), whereas there was no significant difference in OS regarding stromal expression of STING (p = 0.125, F)
Figure 7B–E show divergent KM curves for the oasis, stroma-oasis, infiltration-limited, and desert tumors according to CD45 + immune cell, CD8 + T-cell infiltration, and CD68 + TAM infiltration. Because there was a significant difference between OS of early-stage (I-II) and stage III patients, we performed KM analyses selected for early-stage patients. Concerning CD45 and CD8 infiltration phenotypes, there were no significant differences in survival between desert and infiltration-limited tumors (CD45: p = 0.613; CD8: p = 0.922), or between stroma oasis and oasis tumors (CD45: p = 0.854; CD8: p = 0.27) (Fig. 7B–C). However, patients with CD45 or CD8 stroma oasis tumors exhibited significantly increased OS compared to CD45 and CD8 desert (p = 0.001, p < 0.001, respectively) or CD45 and CD8 infiltration limited tumors (p = 0.049, p < 0.001). CD8 oasis tumors conferred significantly better OS than its desert or infiltration limited counterparts (p = 0.01, p = 0.004, respectively) (Fig. 7B–C). Of note, the more modest difference in the case of CD45 (OS is not significantly better for CD45 oasis tumors vs infiltration limited tumors (p = 0.145)) suggests that CD8 infiltration is a more reliable prognostic factor. When pooling CD45 stroma oasis and oasis tumors vs. infiltration-limited and desert phenotypes, patients with stroma oasis or oasis tumors show significantly improved OS (Median OS: 59.9 vs. 13.3 months; p = 0.0019). In the same comparison, patients with CD8 stroma oasis and oasis tumors show similarly increased survival vs. infiltration-limited and desert tumors (Median OS: 74.4 vs. 12.5 months; p = 0.0001). The survival benefit for more infiltrated tumors is not present in the case of late-stage disease (SFig 2B-C).
Regarding CD68, patients with desert and oasis tumors had the worst, whereas those with stroma oasis tumors had the best OS (Fig. 7D). To place CD68 + TAM infiltration into context, we performed KM analysis in selected patients with a stroma oasis or oasis (infiltrated) phenotype. Interestingly, both CD68 stroma oasis and infiltration limited tumors exhibited significantly increased OS compared to CD68 oasis tumors (p < 0.001, p = 0.016, respectively) (Fig. 7E). Thus, TAM-infiltration in tumor nests might mean a detrimental effect for OS.
Because of the significant correlation between the expression of immune checkpoints and immune cell infiltration, the prognostic role of STING protein was evaluated in infiltrated tumors. While the stromal expression of STING showed no significant effect on OS (Fig. 7F), STING expression in tumor nests contributed to significantly better OS (p = 0.002), even among immune-infiltrated tumors (Fig. 7G). KM analysis for tumor cell MHCII expression showed no significant difference in OS neither among infiltrated tumors nor in the whole cohort (p = 0.14, p = 0.862, respectively, SFig 2D-E).
Next, we performed multivariate analysis (MVA) using the Cox model to assess the prognostic value of STING expression in the stroma/tumor nests and MHCII expression on tumor cells. Stroma oasis/oasis and the infiltration limited/desert phenotypes were assessed separately for the MVA. We performed a chi-square test to rule out the skewness of any parameter between the MVA group (n = 129) and the whole IHC-stained cohort (n = 193), where none of the parameters showed significant difference. There were no significant difference regarding any parameter in CD45 and CD8 infiltration-limited and desert tumors, no immune checkpoint, stage or adjuvant CT had predictive prognostic survival values (STable 2–3), but in CD45-infiltrated tumors, stage proved to be a significant prognosticator of OS (p = 0.0266, [HR]: 0.1444, Table 1) and there was a trend for decreased OS in Stage III patients (p = 0.0579, [HR]: 0.1964) with CD8-immune-oasis and stroma oasis tumors. Interestingly, concerning CD8 stroma oasis and oasis tumors, STING expression in tumor nests had a significant positive prognostic value (p = 0.0429, [HR]: 0.4571). Neither MHCII expression in tumor cells (p = 0.5367) nor STING expression in the stroma (p = 0.6594) showed any prognostic value regarding survival (Table 2). Altogether, high levels of CD45 + immune cell- and CD8 + cytotoxic T-cell infiltration confers significantly improved survival for stage I-II SCLC patients. Furthermore, multivariate analysis demonstrated that increased tumor nest STING expression in stroma oasis and oasis tumors had a positive prognostic value for OS.
Table 1.
Cox-model for CD45 Oasis and Stroma oasis tumors
| Parameter Estimate | Standard Error | Chi-square | P value | 95% Lower CL | 95% Upper CL | Hazard Ratio | 95% Hazard Ratio Lower CL | 95% Hazard Ratio Upper CL | |
|---|---|---|---|---|---|---|---|---|---|
| MHCII tumor score | – 0.0694 | 0.2906 | 0.0570 | 0.8111 | – 0.639 | 0.5002 | 0.9329 | 0.5277 | 1.6491 |
| STING stroma score | – 0.2820 | 0.4089 | 0.4757 | 0.4903 | – 1.083 | 0.5194 | 0.7542 | 0.3384 | 1.6810 |
| STING tumor nest score | – 0.6291 | 0.4217 | 2.2249 | 0.1357 | – 1.455 | 0.1975 | 0.5330 | 0.2331 | 1.2184 |
| Stage | – 0.9672 | 0.4362 | 4.9153 | 0.0266 | – 1.822 | – 0.1121 | 0.1444 | 0.0261 | 0.7990 |
| Adjuvant CT | – 0.3402 | 0.2280 | 2.2261 | 0.1356 | – 0.787 | 0.1067 | 0.5063 | 0.2071 | 1.2379 |
Bold values signify predictors according to multivariate Coxregression
Table 2.
Cox-model for CD8 Oasis and Stroma oasis tumors
| Parameter Estimate | Standard Error | Chi-square | P value | 95% Lower CL | 95% Upper CL | Hazard Ratio | 95% Hazard Ratio Lower CL | 95% Hazard Ratio Upper CL | |
|---|---|---|---|---|---|---|---|---|---|
| MHCII tumor score | – 0.1779 | 0.2881 | 0.3815 | 0.5367 | – 0.742 | 0.3867 | 0.8369 | 0.4757 | 1.4722 |
| STING stroma score | – 0.1617 | 0.3670 | 0.1942 | 0.6594 | – 0.881 | 0.5575 | 0.8506 | 0.4143 | 1.7464 |
| STING tumor nest score | – 0.7827 | 0.3866 | 4.0988 | 0.0429* | – 1.540 | – 0.0249 | 0.4571 | 0.2142 | 0.9753 |
| Stage | – 0.8137 | 0.4292 | 3.5937 | 0.0579 | – 1.655 | 0.0275 | 0.1964 | 0.0365 | 1.0567 |
| Adjuvant CT | – 0.3444 | 0.2306 | 2.2299 | 0.1353 | – 0.796 | 0.1076 | 0.5021 | 0.2033 | 1.2401 |
Bold values signify predictors according to multivariate Coxregression
Discussion
There is an unmet need for therapeutic advancements in SCLC, however, there is a lack of new treatment options. Targeting immunostimulatory molecules is a critical asset in future immunotherapies that can fundamentally enhance antitumor immunity, including T-cell priming, proliferation and development. Therefore our current study characterized the in situ protein expression landscape of immune checkpoints STING and MHCII according to CD45 + , CD8 + and CD68 + immune cell infiltration and SCLC subtypes using a large TMA set of SCLC tumors. Our key finding is that 36% of SCLC tumors harbor high CD45 + immune cell infiltrations (oasis and stroma oasis). In contrast, more than two-third of SCLC tumors are limited or deserted in immune cell infiltration. CD45 + cellular density shows a strong positive correlation with CD8 + T-cell density both in the stroma and in tumor nests, whereas CD68 + TAM-density is moderately correlated with CD8 + T-cell infiltration. This is in line with our previous findings in limited patient subsets [5, 13]).
In SCLC, however, due to its aggressive behavior and limited accessibility to tumor tissue, in situ evaluation of immune cell infiltration happened only in smaller cohorts of primary tumors (Sun et al. [33]; [13], [34] or brain metastases [35], without conclusive data on clinical outcomes. To our knowledge, this is the first comprehensive study on a large dataset that characterized different immune checkpoints according to immune infiltration phenotypes, molecular subtypes and clinical outcomes in SCLC. Here, we report that increased CD45 + immune cell densities are associated with significantly increased survival (Median OS, 59 vs. 13 months), and this benefit is even more significant regarding CD8 + T-cell densities. Additionally, there was no difference in survival between patients with stroma immune-infiltrated (stroma oasis) or tumor-infiltrated, where tumor nests are also colonized by immune cells (oasis). We hypothesize that the underlying cause is a temporal divergence that the two phenotypes can be interpreted as consequent stages of tumor evolution, where the invasion of tumor nests is always preceded by solid stromal infiltration. This is underlined by the fact that every tumor with a high intraepithelial immune cell ratio has its stroma heavily infiltrated as well. In contrast, the presence of CD68 + TAMs in tumor nests was significantly detrimental to OS, also in otherwise immune-infiltrated tumors. This underpins the potential immunosuppressive effect of intratumoral macrophage colonization that we showed in our previous study [16] and may partly explain the limited success of immunotherapy in SCLC.
Recent SCLC profiling studies on various preclinical models [22, 36] and primary human tumors [23, 24] led to the refinement of the SCLC classification scheme defined by the distinct expression of four key transcription regulators (ASCL1, NEUROD1, POU2F3 and YAP1) or by an inflammatory genetic signature (SCLC-I, [37]). Multiple studies suggested recently that human tumors exhibit a more complex phenotype compared to cell lines regarding SCLC subtypes and found that all human and mouse tumors are composites of subtypes [38]. This functional intratumoral heterogeneity may promote the emergence of a rich microenvironment and adaptive response to treatment [39]. Here, we showed that ASCL1 is the most expressed subtype marker in SCLC. Surprisingly, we found that 13.8% of patients displayed triple- or even quadruple positivity for multiple subtype markers and that ASCL1 expression may overlap with the expression of the “Non-NE” markers POU2F3 or YAP1. Additionally, 24.1% of all included patients were considered quadruple negative with no detectable protein expression of any subtype marker.
In line with our overlapping subtype characteristics, Borromeo and colleagues reported co-expressing subsets on 81 human primary SCLC tumor samples, profiled by single-cell RNA-seq analysis earlier [40]. In their study, they clustered tumors into ASCL1 and NEUROD1 double-high and double-low subgroups. In further support of our data, the same study also revealed that ASCL1-high tumors are much more common than NEUROD1-high tumors [36]. In our study, out of all subtype markers, only YAP1 showed a meaningful correlation with immune cell infiltration, especially with CD45 + and CD8 + cells in tumor nests. This is in line with the current state of knowledge regarding SCLC biology [36, 37, 41].
Next, we analyzed the expression pattern of the immune checkpoint STING. Others showed that intratumoral STING activation with its agonist cGAMP normalized tumor vasculatures in xenografts and spontaneous cancers, reaching maximal therapeutic efficacy when mutually expressed in stromal hematopoietic cells as well [42]. In our study, increased STING expression was associated with high CD45 + and CD8 + immune cell densities in both compartments. This was also confirmed in previous reports, where endothelial STING expression was a predictive biomarker for therapy and correlated with enhanced T cell infiltration and prolonged survival in colon- [43] and breast cancers [44]. According to our results, STING protein expression is increased in 40% of SCLC tumors, but in contrast with NSCLC (Della Corte et al. [45], [46], tumor cells express STING only in 4% of patients, leaving the TME to produce the immunostimulatory molecule. This might hamper subsequent immune activation in small-cell histology and lead to decreased TIL colonization or efficacy.
Nonetheless, even in immune oasis- STING-high- SCLC tumors, only a fraction of immune cells express the checkpoint molecule with a low number of STING-positive lymphocytes in tumor nests. However, increased STING expression in tumor nest-infiltrating immune cells is an independent favorable prognostic factor for survival. In contrast, high stromal expression of STING alone provides no survival benefit at all. This might be because many STING-expressing cells in the stroma are CAFs, known about their immunosuppressive, tumorigenic role in the TME [47]–[49]. Out of the four subtype markers, only YAP1 showed a weak positive correlation with STING expression both in the stroma and tumor nests. However, there was a significant moderate correlation between STING expression and aggregate subtype score, suggesting that the stronger and more overlapping the subtype molecule expression of a tumor is, the higher is its STING expression.
It was shown previously that in lung cancer cell lines [50] and tissue samples [51], some tumor cells display MHCII expression mainly in the vicinity of TILs in highly infiltrated tumors. This suggests that immune cell infiltration might induce the expression of MHCII molecule in tumor cells in the case of a permissive microenvironment [52]. MHCII is critical for antigen presentation to CD4 + T-lymphocytes, whose role in antitumor immunity is becoming increasingly appreciated [52]. Accordingly, tumor-specific MHCII expression may increase recognition of a tumor by the immune system and therefore may play an important role in immunotherapy [52]. Furthermore, tumor-specific MHCII associates with superior prognosis and improved response to immunotherapy in melanoma and breast cancer patients and, moreover, with increased tumor rejection in murine models [53], [54], [55], [56, 57]. Therefore, MHCII holds promise as a biomarker of inflamed tumors and of a higher likelihood of response to anti-PD-1/anti-PD-L1 agents [52]. An unanswered consideration is what effect MHCII has on CD4 + Tregs which are abundant in many solid tumor types and can potently suppress immunity and inflammation [58]. This controversy concerning the role of MHCII in Treg activation mainly exists because MHC-II expression is linked with improved immune-mediated outcomes, whereas Treg activation has anti-inflammatory effects [52, 59]. Here we revealed that MHCII expression in tumor cells shows a moderate correlation with immune cell infiltration, including CD8 + T-cells, and a moderate-to-weak correlation with STING-expression. MHCII expression was associated with YAP1 from the four transcriptional driver genes and YAP1-high patients expressed significantly increased amount of MHCII. MHCII-high tumors represent 22.5% of the whole patient cohort and in contrast with NSCLC [60], our multivariate analysis showed no survival benefit or detriment in terms of MHCII expression for SCLC patients that might be due to different TME biology in SCLC.
Similar to our findings concerning MHCII expression, a recent study by Cai et al. revealed that NE-low, “variant”-type SCLCs are associated with pronounced MHCI expression, a more immune-infiltrated TME, chemoresistance and an increased MYC expression (vs. the NE-high tumors) even when no changes in ASCL1 expression occurs [61]. Given that these tumors usually express a series of immunosuppressive genes [16], it will be critical to understand which of these gene functions should be targeted in order to achieve prolonged antitumor immune responses in NE-low SCLCs. Notably, others have also observed that a specific SCLC subpopulations with a NE-low phenotype is characterized by strong MHCI expression and are especially sensitive to STING agonism which leads to the enhancement of T-cell recognition in syngeneic murine models [62].
This study has limitations. First of all, this is a retrospective cross-sectional study with limited clinicopathological data available. Second, this is a predominantly early-stage cohort, and the patients were not treated with specific therapies. Accordingly, we cannot assess the predictive or prognostic value of the biomarkers. Since the recruitment period was fairly long, the therapeutic guidelines (including the surgical techniques) might have changed over time, thus influencing the survival. In our study, most intraepithelial immune cells show no STING expression that can limit effective antitumor immune responses in otherwise heavily infiltrated tumors. Overcoming this issue might help in boosting host immunity against cancer cells and provide better long-term outcomes in this recalcitrant malignancy.
Conclusion
STING and MHCII are expressed in SCLC. Definition of immune infiltration according to tumor compartments are critical for SCLC progression. Immune cell infiltration, particularly CD8 + cytotoxic T-cell density, is prognostic in limited-stage SCLC patients. Moreover, the expression of STING in tumor nests is prognostic in immune-infiltrated tumors and therefore STING is a potential therapeutic target in SCLC. Further prospective studies are needed to define the subtype-specific therapeutic vulnerabilities in extensive-stage SCLC patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and the clinical teams involved. We thank Leslie Rozeboom, UCD, for her assistance in creating TMA blocks. ZL acknowledges funding from the Hungarian National Research, Development and Innovation Office (OTKA #124652 and OTKA #129664). BD acknowledges funding from the Austrian Science Fund (FWF I3522, FWF I3977 and I4677) and from the Hungarian National Research, Development and Innovation Office (KH130356; 2020-1.1.6-JÖVŐ and TKP2021-EGA-33). VL is a recipient of the Bolyai Research Scholarship of the Hungarian Academy of Sciences and the UNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology. ZM was supported by the UNKP-20-3 and UNKP-21-3 New National Excellence Program of the Ministry for Innovation and Technology of Hungary and by the Hungarian Respiratory Society (MPA #2020).
Authors’ contributions
Conception and design: ZL, DD. Development of methodology: ZL, DD, CR, HY, ZM. Acquisition of data: DD, CR, HY, SLP. Analysis and interpretation of data: DD, ZL, ED, KH, ZM. Administrative, technical or material support: CR, VL, TH, JM, BD, FH, ZM. Study supervision: ZL, DD, BD. Writing and reviewing the manuscript: all authors.
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
Declarations
Conflict of Interest
The authors declare no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zoltan Lohinai and Balazs Dome: these authors contributed equally to this work.
Contributor Information
Zoltan Lohinai, Email: zoltan.lohinai@koranyi.hu.
Balazs Dome, Email: balazs.dome@meduniwien.ac.at.
References
- 1.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Felip E, Goldman JW, Scalzo C, Jensen E, Kush DA, Hui R. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T (2019) KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 380(12):1116–1127. doi: 10.1056/NEJMoa1816714
- 3.Horn L, Mansfield AS, Szczȩsna A, Havel L, Krzakowski M, Hochmair MJ, Liu SV. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Williamson M. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. The Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52(1):55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 7.Vesalainen S, Lipponen P, Talja M, Syrjänen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A(12):1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 8.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 9.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Gu J, Cui X, Huang L, Li S, Feng J, Liu B, Zhou Y. Deciphering microenvironment of NSCLC based on CD8+ TIL density and PD-1/PD-L1 expression. J Cancer. 2019;10(1):211–222. doi: 10.7150/jca.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61. 10.1016/j.immuni.2014.06.010. Erratum in: Immunity. 2014 Nov 20;41(5):866 [DOI] [PMC free article] [PubMed]
- 12.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 13.Dora D, Rivard C, Yu H, Bunn P, Suda K, Ren S, Lueke Pickard S, Laszlo V, Harko T, Megyesfalvi Z, Moldvay J, Hirsch FR, Dome B, Lohinai Z. Neuroendocrine subtypes of small cell lung cancer differ in terms of immune microenvironment and checkpoint molecule distribution. Mol Oncol. 2020;14(9):1947–1965. doi: 10.1002/1878-0261.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? nature reviews immunology. Nature Publishing Group; 2014. [DOI] [PubMed] [Google Scholar]
- 15.Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, Schreiber RD. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574(7780):696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dora D, Rivard C, Yu H, Pickard SL, Laszlo V, Harko T, Megyesfalvi Z, Dinya E, Gerdan C, Szegvari G, Hirsch FR, Dome B, Lohinai Z. Characterization of tumor-associated macrophages and the immune microenvironment in limited-stage neuroendocrine-high and -low small cell lung cancer. Biology (Basel) 2021;10(6):502. doi: 10.3390/biology10060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber GN. STING: Infection, inflammation, and cancer nature reviews immunology. Nature Publishing Group; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrales L, Gajewski TF. Molecular pathways: targeting the stimulator of interferon genes (STING) in the immunotherapy of cancer. Clin Cancer Res. 2015;21(21):4774–4779. doi: 10.1158/1078-0432.CCR-15-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera Vargas, T., Benoit-Lizon, I., & Apetoh, L. (2017) Rationale for stimulator of interferon genes targeted cancer immunotherapy European Journal of Cancer. Elsevier Ltd, Amsterdam [DOI] [PMC free article] [PubMed]
- 20.Kitajima S, Ivanova E, Guo S, Yoshida R, Campisi M, Sundararaman SK, Tange S, Mitsuishi Y, Thai TC, Masuda S, Piel BP, Sholl LM, Kirschmeier PT, Paweletz CP, Watanabe H, Yajima M, Barbie DA. Suppression of STING associated with LKB1 loss in KRAS-driven lung cancer. Cancer Discov. 2019;9(1):34–45. doi: 10.1158/2159-8290.CD-18-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohinai Z, Dora D, Caldwell C, Rivard CJ, Suda K, Yu H, Rivalland G, Ellison K, Rozeboom L, Dziadziuszko R, Mitchell P, John T, Millan IS, Ren S, Hirsch FR. Loss of STING expression is prognostic in non-small cell lung cancer. J Surg Oncol. 2022;125(6):1042–1052. doi: 10.1002/jso.26804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson D, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, Beras A, Spencer R, Lopardo J, Bodd F, Montecalvo J, Sauter JL, Chang JC, Buonocore DJ, Travis WD, Sen T, Poirier JT, Rudin CM, Rekhtman N. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15(12):1823–1835. doi: 10.1016/j.jtho.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Megyesfalvi Z, Barany N, Lantos A, Valko Z, Pipek O, Lang C, Schwendenwein A, Oberndorfer F, Paku S, Ferencz B, Dezso K, Fillinger J, Lohinai Z, Moldvay J, Galffy G, Szeitz B, Rezeli M, Rivard C, Hirsch FR, Brcic L, Popper H, Kern I, Kovacevic M, Skarda J, Mittak M, Marko-Varga G, Bogos K, Renyi-Vamos F, Hoda MA, Klikovits T, Hoetzenecker K, Schelch K, Laszlo V, Dome B. Expression patterns and prognostic relevance of subtype-specific transcription factors in surgically resected small-cell lung cancer: an international multicenter study. J Pathol. 2022 doi: 10.1002/path.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwendenwein A, Megyesfalvi Z, Barany N, Valko Z, Bugyik E, Lang C, Ferencz B, Paku S, Lantos A, Fillinger J, Rezeli M, Marko-Varga G, Bogos K, Galffy G, Renyi-Vamos F, Hoda MA, Klepetko W, Hoetzenecker K, Laszlo V, Dome B. Molecular profiles of small cell lung cancer subtypes: therapeutic implications. Mol Ther Oncolytics. 2021;6(20):470–483. doi: 10.1016/j.omto.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- 27.Ikematsu Y, Tanaka K, Toyokawa G, Ijichi K, Ando N, Yoneshima Y, Iwama E, Inoue H, Tagawa T, Nakanishi Y, Okamoto I. NEUROD1 is highly expressed in extensive-disease small cell lung cancer and promotes tumor cell migration. Lung Cancer. 2020;146:97–104. doi: 10.1016/j.lungcan.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I, Hirota J. Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One. 2017;12(12):e0189340. doi: 10.1371/journal.pone.0189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YH, Klingbeil O, He XY, Wu XS, Arun G, Lu B, Somerville TDD, Milazzo JP, Wilkinson JE, Demerdash DE, Spector DL, Egeblad M, Shi J, Vakoc CR. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32(13–14):915–928. doi: 10.1101/gad.314815.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battifora H. Methods in laboratory investigation. the multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986;55(2):244–248. [PubMed] [Google Scholar]
- 32.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. Image J2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017;18(1):7. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Zhai C, Chen X, Dong Z, Hou L, Zhou C, Jiang T. Characterization of PD-L1 protein expression and CD8+ tumor-infiltrating lymphocyte density, and their associations with clinical outcome in small-cell lung cancer. Transl Lung Cancer Res. 2019;8(6):748–759. doi: 10.21037/tlcr.2019.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, Schalper KA. Expression and clinical significance of PD-L1, B7–H3, B7–H4 and TILs in human small cell lung Cancer (SCLC) J Immunother Cancer. 2019;7(1):65. doi: 10.1186/s40425-019-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berghoff AS, Ricken G, Wilhelm D, Rajky O, Widhalm G, Dieckmann K, Birner P, Bartsch R, Preusser M. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC) J Neurooncol. 2016;130(1):19–29. doi: 10.1007/s11060-016-2216-8. [DOI] [PubMed] [Google Scholar]
- 36.George J, Lim JS, Jang SJ, Cun Y, Ozretia L, Kong G, Thomas RK. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, Nabet BY, Fujimoto J, Solis LM, Lu W, Xi Y, Cardnell RJ, Wang Q, Fabbri G, Cargill KR, Vokes NI, Ramkumar K, Zhang B, Della Corte CM, Robson P, Swisher SG, Roth JA, Glisson BS, Shames DS, Wistuba II, Wang J, Quaranta V, Minna J, Heymach JV, Byers LA. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346–360. doi: 10.1016/j.ccell.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirier JT, Gardner EE, Connis N, Moreira AL, de Stanchina E, Hann CL, Rudin CM. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene. 2015;34(48):5869–5878. doi: 10.1038/onc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wooten DJ, Groves SM, Tyson DR, Liu Q, Lim JS, Albert R, Quaranta V. Systems-level network modeling of Small Cell Lung Cancer subtypes identifies master regulators and destabilizers. PLoS Comput Biol. 2019;15(10):e1007343. doi: 10.1371/journal.pcbi.1007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borromeo MD, Savage TK, Kollipara RK, He M, Augustyn A, Osborne JK, Johnson JE. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 2016;16(5):1259–1272. doi: 10.1016/j.celrep.2016.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Girard L, Zhang YA, Haruki T, Papari-Zareei M, Stastny V, Gazdar AF. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Trans Lung Cancer Res. 2018;7(1):32–49. doi: 10.21037/tlcr.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, Kim C. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Investig. 2019;129(10):4350–4364. doi: 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chon HJ, Kim H, Noh JH, Yang H, Lee WS, Kong SJ, Kim C. STING signaling is a potential immunotherapeutic target in colorectal cancer. J Cancer. 2019;10(20):4932–4938. doi: 10.7150/jca.32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaston J, Cheradame L, Yvonnet V, Deas O, Poupon MF, Judde JG, Goffin V. Intracellular STING inactivation sensitizes breast cancer cells to genotoxic agents. Oncotarget. 2016;7(47):77205–77224. doi: 10.18632/oncotarget.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Della Corte CM, Byers LA. Evading the STING: LKB1 loss leads to STING silencing and immune escape in KRAS-mutant lung cancers. Cancer Discov. 2019;9(1):16–18. doi: 10.1158/2159-8290.CD-18-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raaby Gammelgaard K, Sandfeld-Paulsen B, Godsk SH, Demuth C, Meldgaard P, Sorensen BS, Jakobsen MR. cGAS-STING pathway expression as a prognostic tool in NSCLC. Translational lung cancer research. 2021;10(1):340–354. doi: 10.21037/tlcr-20-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, Yin R. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett RL, Puré E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. Elife. 2020;28(9):e57243. doi: 10.7554/eLife.57243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen PY, Wei WF, Wu HZ, Fan LS, Wang W. Cancer-associated fibroblast heterogeneity: a factor that cannot be ignored in immune microenvironment remodeling. Front Immunol. 2021;8(12):671595. doi: 10.3389/fimmu.2021.671595.PMID:34305902;PMCID:PMC8297463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Y, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Yu H, Zhou C, Hirsch FR. MHC class II expression in lung cancer. Lung Cancer. 2017;112:75–80. doi: 10.1016/j.lungcan.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Kamma H, Yazawa T, Ogata T, Horiguchi H, Iijima T. Expression of MHC class II antigens in human lung cancer cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(6):407–412. doi: 10.1007/BF02899573. [DOI] [PubMed] [Google Scholar]
- 52.Axelrod ML, Cook RS, Johnson DB, Balko JM. Biological consequences of MHC-II expression by tumor cells in cancer. Clin Cancer Res. 2019;25(8):2392–2402. doi: 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class IItransfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA. 1997;94(13):6886–6891. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS, Greenplate AR, Sanders ME, Lovly CM, Frederick DT, Kelley MC, Richmond A, Irish JM, Shyr Y, Sullivan RJ, Puzanov I, Sosman JA, Balko JM. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;29(7):10582. doi: 10.1038/ncomms10582.PMID:26822383;PMCID:PMC4740184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forero A, Li Y, Chen D, Grizzle WE, Updike KL, Merz ND, Downs-Kelly E, Burwell TC, Vaklavas C, Buchsbaum DJ, Myers RM, LoBuglio AF, Varley KE. Expression of the MHC class II pathway in triple-negative breast cancer tumor cells is associated with a good prognosis and infiltrating lymphocytes. Cancer Immunol Res. 2016;4(5):390–399. doi: 10.1158/2326-6066.CIR-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andres F, Yufeng L, Dongquan C, William EG, Katherine LU, Natalie DM, Katherine EV. Expression of the MHC class II pathway in triple-negative breast cancer tumor cells is associated with a good prognosis and infiltrating lymphocytes. Cancer Immunol Res. 2016;4(5):390–399. doi: 10.1158/2326-6066.CIR-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Hodi FS. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Trans Med. 2018;10:450. doi: 10.1126/scitranslmed.aar3342. [DOI] [PubMed] [Google Scholar]
- 58.Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45(5):1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;11:1070–8. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson AM, Bullock BL, Neuwelt AJ, Poczobutt JM, Kaspar RE, Li HY, Kwak JW, Hopp K, Weiser-Evans MCM, Heasley LE, Schenk EL, Clambey ET, Nemenoff RA. Cancer cell-intrinsic expression of MHC class II regulates the immune microenvironment and response to anti-PD-1 therapy in lung adenocarcinoma. J Immunol. 2020;204(8):2295–2307. doi: 10.4049/jimmunol.1900778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai L, Liu H, Huang F, Fujimoto J, Girard L, Chen J, Li Y, Zhang YA, Deb D, Stastny V, Pozo K, Kuo CS, Jia G, Yang C, Zou W, Alomar A, Huffman K, Papari-Zareei M, Yang L, Drapkin B, Akbay EA, Shames DS, Wistuba II, Wang T, Johnson JE, Xiao G, DeBerardinis RJ, Minna JD, Xie Y, Gazdar AF. Cell-autonomous immune gene expression is repressed in pulmonary neuroendocrine cells and small cell lung cancer. Commun Biol. 2021;4(1):314. doi: 10.1038/s42003-021-01842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M, Hong D, Thai TC, Piel B, Han S, Reinhold BB, Duke-Cohan JS, Poitras MJ, Taus LJ, Lizotte PH, Portell A, Quadros V, Santucci AD, Murayama T, Cañadas I, Kitajima S, Akitsu A, Fridrikh M, Watanabe H, Reardon B, Gokhale PC, Paweletz CP, Awad MM, Van Allen EM, Lako A, Wang XT, Chen B, Hong F, Sholl LM, Tolstorukov MY, Pfaff K, Jänne PA, Gjini E, Edwards R, Rodig S, Reinherz EL, Oser MG, Barbie DA. Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov. 2021;11(8):1952–1969. doi: 10.1158/2159-8290.CD-20-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.