Abstract
Chimeric antigen receptor (CAR) T cells remain unsatisfactory in treating solid tumors. The frequency of tumor-infiltrating T cells is closely related to the good prognosis of patients. Augmenting T cell accumulation in the tumor microenvironment is essential for tumor clearance. To overcome insufficient immune cell infiltration, innovative CAR designs need to be developed immediately. CXCL9 plays a pivotal role in regulating T cell migration and inhibiting tumor angiogenesis. Therefore, we engineered CAR T cells expressing CXCL9 (CART-CXCL9). The addition of CXCL9 enhanced cytokine secretion and cytotoxicity of CAR T cells and endowed CAR T cells with the ability to recruit activated T cells and antiangiogenic effect. In tumor-bearing mice, CART-CXCL9 cells attracted more T cell trafficking to the tumor site and inhibited angiogenesis than conventional CAR T cells. Additionally, CART-CXCL9 cell therapy slowed tumor growth and prolonged mouse survival, displaying superior antitumor activity. Briefly, modifying CAR T cells to express CXCL9 could effectively improve CAR T cell efficacy against solid tumors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03193-6.
Keywords: CAR T cells, CXCL9, Solid tumor, Infiltration, Angiogenesis
Introduction
CAR T cell immunotherapy has shown clinical efficacy in treating hematologic malignancies. Kymriah and Yescarta, CD19-targeting CAR T cell products, have been approved for the treatment of CD19-positive lymphoma or leukemia [1, 2]. However, CAR T cell therapy against solid tumors is hampered by multiple challenges, so the expected curative effect has not been achieved [3, 4]. The heterogeneity of tumor antigens, immunosuppressive tumor microenvironment (TME), infiltration, proliferation, and persistence of T cells severely restrict their therapeutic effects [5]. Among them, the migration and accumulation of T cells at tumor site are the first steps to realize effector functions. However, the complex location of solid tumors and the microenvironment make it difficult for immune cells to access [6]. Therefore, there is an urgent need to improve intratumoral T cell infiltration in solid tumors.
Chemokines and their receptors play a vital role in T cell migration. The expression of chemokines at tumor site is closely associated with T cell infiltration, tumor control, and patient prognosis [5]. Studies have shown that tumors cause immune desertification and tumor escape by disrupting the chemokine–chemokine receptor network that supports T cell recruitment, thereby inhibiting effector T cell infiltration and increasing tumor progression [7, 8]. Therefore, introducing an appropriate chemokine–chemokine receptor network to promote T cell migration is a feasible strategy. CXCL9, also known as monokine induced by gamma interferon (MIG), is a member of the CXC family. It can be induced by IFN-γ, but is not affected by IFN-α/β [9–11]. CXCL9 plays an essential role in immune cell chemotaxis and recruits cells expressing CXCR3 receptors, which consist mainly of activated T and natural killer (NK) cells [12]. Furthermore, CXCL9 can promote the polarization of effector Th cells, thereby enhancing the immune response and antitumor effects [13, 14]. In addition, CXCL9, which does not contain the ELR (Glu-Lue-Arg) motif, generally inhibits angiogenesis and delays tumor progression [9, 15]. CXCL9 can be used as a biomarker to predict the immune response of patients, and its expression is associated with better prognosis in patients with tumors [16–18]. Thus, studies have focused on strategies to improve immunotherapy by increasing CXCL9 expression [19].
So far, the main study focused on constructing the tumor cell line overexpressing CXCL9, inducing CXCL9 expression of tumor cells by low-dose irradiation, intratumoral injection of recombinant CXCL9, or delivering CXCL9 to tumor site by mesenchymal stem cells to inhibit tumor growth [20–23]. However, the combination of CXCL9 and CAR T cells has not yet been reported. In this study, we engineered mesothelin-targeting CAR T cells co-expressing CXCL9 and explored the immune function and antitumor efficacy of CAR T cells overexpressing CXCL9 both in vitro and in vivo.
Materials and methods
Cell lines
The human lung cancer cell lines (H322 and A549), human umbilical vein endothelial cells (HUVECs), and human embryonic kidney cell line 293 T were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) or RPMI-1640 medium (Gibco) containing 10% fetal bovine serum (FBS, HyClone), 100 U/mL penicillin, and 100 μg/ml streptomycin (Invitrogen), and maintained at 37 °C in a humidified incubator with 5% CO2.
CAR construction
The CAR design targeting mesothelin was based on the second generation of CD28, as described in a previous report [24]. Human CXCL9 (Uniprot: Q07325) was connected to the meso-CAR structure with a P2A linker and synthesized by Sangon Biotech (Shanghai, China). The coding sequence was inserted into the lentiviral vector pCDH-EF1 (Systembio) expressing green fluorescent protein (GFP).
CAR T cell generation
The generation and transduction of CAR T cells were performed as reported [25]. Briefly, the 293 T cells were co-transfected with the pCDH lentiviral and packaging (psPAX2 and pMD.2G) plasmids. The supernatant containing the lentivirus was harvested 48 and 72 h after transduction. CD3+ T cells from healthy donors were activated with anti-CD3/CD28 Dynabeads (Miltenyi Biotec, Germany). The virus supernatant collected was concentrated and infected activated CD3+ T cells. Transfection efficiency was detected 5 days after virus infection, and function assays were performed.
T cell function analysis and flow cytometry
The flow cytometry antibodies used in this study were purchased from BioLegend. The collected cells were washed with phosphate-buffered saline (PBS) containing 2% FBS and stained with fluorescence-conjugated antibodies. The cells were incubated at 4 °C in the dark for 20 min to detect the expression of T cell surface molecules. To analyze the expression of functional cytokines, cells were stimulated with 12-myristate 13-acetate (PMA, 1 mg/mL; Sigma-Aldrich), ionomycin (1 mg/mL; Sigma-Aldrich) and brefeldin A (BioLegend), or antigen-stimulation (H322 cells) for 6 h. Cytokines were first surface-stained and then intracellularly stained. The cells were incubated with 4% paraformaldehyde and permeation buffer for 30 min at 4 °C. Next, the cells were stained with human cytokine antibodies. All samples were analyzed using C6 or FACSCanto II flow cytometer (BD Biosciences).
Cytotoxicity assay
The target cells H322 and A549 were incubated with control untransduced T cells (UTD) or CAR T cells at different effector-to-target ratios in a 96-well plate for 6 h. The supernatants and cells were harvested and stained with annexin V (BioLegend) and propidium iodide (PI, BioLegend). The percentage of annexin V single-positive (early apoptosis) plus PI and annexin V double-positive (late apoptosis) was calculated as the apoptosis rate of tumor cells. All samples were analyzed using C6 or FACSCanto II flow cytometer (BD Biosciences).
Chemokine and chemokine receptor analysis
To study the expression of CXCL9 and its receptors, collecting UTD and CARTmeso or CARTmeso-CXCL9 cells transduced for 5 days were stained with CD3 (BioLegend, Cat. No. 300316), CXCL9 (BioLegend, Cat. No. 357906) and CXCR3 (BioLegend, Cat. No. 353706) antibodies, and performed flow cytometry. To measure the level of chemokine production, the supernatants of T cells, CARTmeso, or CARTmeso-CXCL9 cells were harvested, and the secretion of T cell chemokines was detected using the LEGENDplex™ Human Proinflammatory Chemokine Panel (BioLegend, Cat. No. 740003).
Mesothelin expression in tumor cell lines
To explore the expression of mesothelin in different lung cancer cell lines, the digested tumor cells were, respectively, labeled with mesothelin (Miltenyi Biotec, Order no. 130-118-168) and its isotype antibody (Miltenyi Biotec, Order no. 130-113-438). The samples were incubated for 20 min at 4 °C in the dark and detected by flow cytometry.
Transwell assay
As previously reported, the migration ability of CD3+ T cells was evaluated in 24-well plates with 5-μm pore size polycarbonate filters (Corning Inc., Corning, NY, USA) [26]. Recombinant human CXCL9 (Pepro-Tech) or the culture supernatant of CAR T cells was added to the lower chambers. CD3+ T cells were activated with anti-CD3/CD28 Dynabeads (Miltenyi Biotec, Germany) for 48 h. Activated CD3+ T cells were added to the upper chambers (5 × 105 cells per well). The 24-well plate was incubated at 37 °C in a 5% CO2 incubator for 3 h. The cells in the lower chambers were calculated to determine differences in T cell migration.
Enzyme-linked immunosorbent assay (ELISA)
The supernatant was collected after co-culture of T cells and H322 cells for 24 h without exogenous cytokines. Human IFN-γ ELISA kit (Dakewe Biotech, 1,110,002) was used to test the secretion of IFN-γ in T cells. Duplicate wells were provided for each sample. The concentration of protein was calculated by measuring the optical density (OD) value of the absorbance at 450 nm.
Tube formation assay
Fifty microliters of Matrigel (BD Biosciences, Bedford, MA, USA) was added to each well of a precooled 96-well plate and incubated at 37 °C for 30 min. HUVECs (3 × 104) suspended in 100 μL medium were seeded into each well. Tube formation was observed for 4 h at 37 °C. The tubes that formed were imaged and counted the number of loops and nodes under a microscope for statistical analysis.
VEGFR2 expression in HUVECs
To detect the expression of vascular endothelial growth factor receptor 2 (VEGFR2), HUVECs were incubated with different concentrations of recombinant human CXCL9 (Pepro-Tech) or culture supernatants of CAR T cells. The collected HUVECs washed with PBS containing 2% FBS and stained with VEGFR2 antibodies (BioLegend, Cat. No. 393003) at 4 °C in the dark for 20 min. Flow cytometry analyzed VEGFR2 expression.
Matrigel plug assay
Female NOD/SCID mice aged 5 weeks were obtained from the Vital River (Beijing, China). All mice were fed in the Henan Key Laboratory for Pharmacology of Liver Diseases. One hundred microliters of cell suspension (2 × 106, HUVECs: H322 = 1:1) was mixed with 400 μL Matrigel (R&D Systems, 3632-001-02). The mixture was inoculated subcutaneously into each mouse. Then, the mice were intravenously infused with 100 μL UTD, CARTmeso, or CARTmeso-CXCL9 (5 × 106). After 7 days, the Matrigel plugs were removed and processed for histology.
Immunohistochemistry
Tumor tissues from the animals were embedded in paraffin-embedded sections. The tissue sections were deparaffinized and incubated with hydrogen peroxide blocking solution and protein-blocking agent. They were stained with anti-CD3 (Abcam, ab16669) or anti-CD31 antibody (Abcam, ab28364) at 4 °C overnight and counterstained with hematoxylin. The CD3 immunohistochemical score was evaluated based on the intensity of cell staining and the frequency of positive cells. The intensity was scored as follows: 0, negative; 1, light yellow; 2, brown; and 3, deep brown. The frequency of positive cells was defined as follows: 0, 0%; 1, 1% to 25%; 2, 26% to 50%; 3, 51% to 75%; and 4, greater than 75%. The positive expression of CD31 under microscopic observation indicated the presence of vascular endothelial cells. The measurements of CD31 staining were evaluated based on Weidner’s method (Weidner 1995) and were obtained at X 200 magnification in each area. Staining of brown blood vessels with lumen was positive, as was staining of individual endothelial cells.
Xenograft tumor model
Female NOD/SCID mice aged 5 weeks were subcutaneously injected with 1 × 105 luciferase-expressing H322 cells. After 3 days, the mean total flux of tumors reached 1 × 109 p/s, and mice were intravenously infused with 6 × 106 UTD, CARTmeso cells, CARTmeso-CXCL9 cells, and equal volumes of PBS, respectively. Mice were killed when the values of total flux were over 2 × 1011. Bioluminescence images were recorded weekly to monitor tumor growth.
T cell trafficking and functional evaluation in vivo
Seven days after CAR T cell injection, the tumor, spleen, and peripheral blood were harvested, and single-cell suspensions were prepared. The tumor tissues were cut into pieces and digested with three-step enzymatic digestion (1 g/L trypsin, 1 g/L hyaluronidase and 2 g/L collagenase). The spleens were cut and ground to obtain cells. Peripheral blood mononuclear cells were isolated by density gradient centrifugation. The single-cell suspensions were washed with PBS containing 2% FBS and stained with fluorescence-conjugated antibodies specific to human CD3 (BioLegend, Cat. No. 300316), CD4 (BioLegend, Cat. No. 357414), CD69 (BioLegend, Cat. No. 310906), PD-1 (BioLegend, Cat. No. 621608), Tim-3 (BioLegend, Cat. No. 345012) at 4 °C for 20 min. The analysis of functional molecular IFN-γ (BioLegend, Cat. No. 502512), TNF-α (BioLegend, Cat. No. 502909), and Ki67 (BioLegend, Cat. No. 350514) was consistent with the description of intrinsic factor detection above. Protein-L was used for the CAR staining in CAR T cells. All samples were analyzed using the BD FACSCanto II flow cytometer (BD Biosciences).
Bioinformatics analysis
The correlation of CXCL9 and CD8A in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) was obtained from online website cbioportal (https://www.cbioportal.org/).
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Animal studies were approved by the Animal Care and Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Statistical analysis
All in vitro experiments were performed in at least three independent replicates, with five mice in each group in the in vivo studies. The data were analyzed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA) and presented as mean ± SEM. Statistical differences among groups were performed using the t test and ANOVA comparison. The survival curve was determined by the log-rank test. Statistical significance was set at p < 0.05.
Results
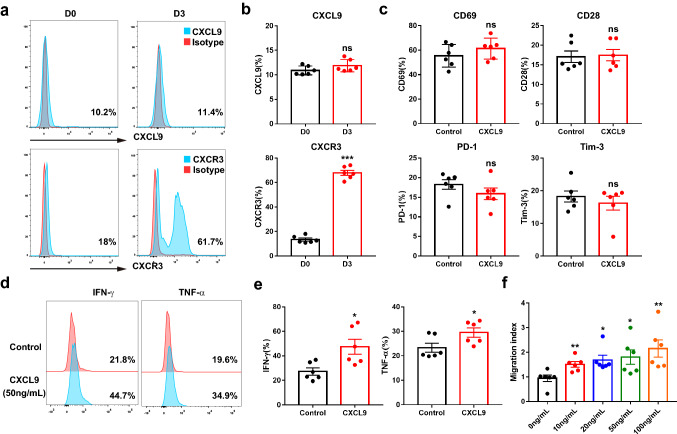
CXCL9 augmented effector function and migration of T cells
CXCL9 as a chemokine was positively correlated with the expression of CD8A, CD3E and CD4 in tumors (Fig. S1). To further understand the role of CXCL9, we investigated how this chemokine regulated T cell function. We added 50 ng/mL recombinant human CXCL9 protein to T cells with anti-CD3/CD28 beads activation for 3 days and found that CXCL9 induced the formation of effector Th1 cells (Fig. S2), which was consistent with previous reports [27]. However, CXCL9 showed low expression in T cells before and after activation; the expression of its receptor CXCR3 was upregulated in activated T cells (Fig. 1a, b). To further assess the function of CXCL9, CXCL9 was added to CD3+ T cells, and the activation (CD69, CD28) and inhibitory (PD-1, Tim-3) markers remained the same (Fig. 1c). Of note, cytokine secretion (IFN-γ and TNF-α) of CD3+ T cells treated with CXCL9 was increased (Fig. 1d, e), suggesting that CXCL9 could promote the expression of effector molecules in CD3+ T cells. In addition, there was no difference in the composition of CD4 and CD8 subsets in CD3+ T cells (Fig. S3a). Similarly, the effects of CXCL9 on CD8+ T cell subsets were tested. CXCL9 did not alter the phenotype of CD8+ T cells, but augmented the secretion of functional molecules (Fig. S3b, c). Transwell assay results revealed that T cell migration increased in a CXCL9 concentration-dependent manner (Fig. 1f). Collectively, these results indicate that CXCL9 can promote the effector function and migration ability of T cells.
Fig. 1.
CXCL9-treated T cells exhibited enhanced effector function and movement ability. a, b Flow cytometric analysis of CXCL9 and CXCR3 in CD3+ T cells before and after activation. c Expression of CD69, CD28, PD-1, Tim-3 in CD3+ T cells from healthy donors treated or not with recombinant human CXCL9 protein (50 ng/mL). d, e Analysis of intracellular IFN-γ and TNF-α in CD3+ T cells from healthy donors cultured with or without CXCL9 protein (50 ng/mL) by flow cytometry. f Activated CD3+ T cells incubated for 3 h under different dose of CXCL9 protein. The migrated cells in the lower chamber were counted by flow cytometry to calculate migration index. Results are representative of at least 3 independent experiments. *p < 0.05, **p < 0.01 (repeated-measures one-way ANOVA or student t test)
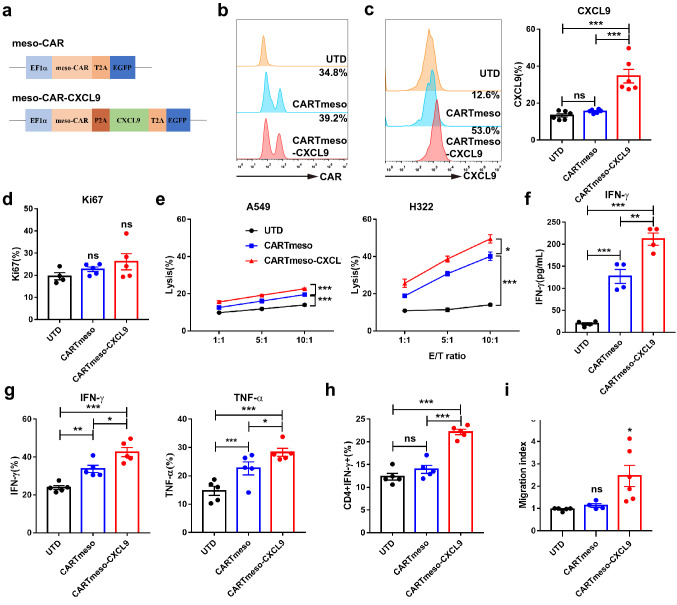
CXCL9 enhanced CAR T cell function in vitro
We then generated mesothelin-targeted CAR T cells overexpressing CXCL9 (CARTmeso-CXCL9), which connected the encoded CAR gene with the CXCL9 gene through the 2A peptide. Mesothelin-specific CAR T cells (CARTmeso) served as controls (Fig. 2a). The flow cytometry analysis showed that the transduction efficiency of CAR T cells was not affected by adding CXCL9, but the CXCL9 expression was increased in the cytoplasm and cultured supernatant of CARTmeso-CXCL9 cells (Fig. 2b, c, and S4a). Moreover, there was no significant difference in the Ki67 expression in T cells, and the transduction of CXCL9 did not influence CAR T cell proliferation (Fig. 2d). To verify whether CXCL9 can enhance the function of CAR T cells, the cytotoxicity assay was performed at different effector-to-target (E/T) ratios. H322 (high mesothelin expression, 66.2%) and A549 (low mesothelin expression, 7.09%) were used as target cells (Fig. S4b). As expected, CXCL9 enhanced the cytotoxicity of CAR T cells and IFN-γ secretion against tumor cells (Fig. 2e, f and S5). Consistent with the previous results, the secretion of effector cytokines IFN-γ and TNF-α was augmented, and the T cell phenotype remained unchanged in CARTmeso-CXCL9, compared to UTD and CARTmeso cells (Fig. 2g and S4c). Furthermore, 5 days after constructing CAR T cells, the polarization of CD4+ T cells was detected, and the proportion of Th1 subpopulations increased in CARTmeso-CXCL9 cells (Fig. 2h). To explore whether CARTmeso-CXCL9 cells can recruit activated T cells, cell culture supernatants of UTD, CARTmeso, and CARTmeso-CXCL9 were collected and subjected to transwell experiments with activated T cells. There was obvious T cell accumulation in the cell culture supernatant of CARTmeso-CXCL9, suggesting that CARTmeso-CXCL9 cells have the ability to direct activated T cell movement (Fig. 2i). Therefore, these data reveal that CARTmeso-CXCL9 cells have the immune function superior to traditional CAR T cells and the ability to recruit activated T cells.
Fig. 2.
CARTmeso-CXCL9 cells displayed better cytotoxicity and capacity in recruiting activated T cells than CARTmeso cells. a Schematic diagram of engineered meso-CAR and meso-CAR-CXCL9 constructs. b The transfection efficiency of CAR T cells was tested by protein-L staining using flow cytometry. c, d Flow cytometric detection of CXCL9 (c) and Ki67 (d) expression 5 days after CAR T cell transduction. e Cytotoxicity assay using A549 or H322 cells as targets. UTD or CAR T cells were co-cultured with target cells at the indicated effector-to-target (E/T) ratios for 6 h. f IFN-γ secretion in supernatant of UTD or CAR T cells co-cultured with tumor cells was measured by ELISA assay. g UTD or CAR T cells were stimulated with H322 cells for 6 h. The effector molecules (IFN-γ and TNF-α) of T cells were measured. h The percentage of CD4+IFN-γ+ cells in CAR T cells constructed for 5 d were analyzed by flow cytometry. i Transwell assay was used to explore the recruitment ability of CARTmeso-CXCL9. Activated T cells were added in the upper chamber, and the collected UTD or CAR T cell supernatant was placed in the lower chamber, incubating for 3 h. Data represent 3 independent repeats. *p < 0.05, **p < 0.01, ***p < 0.001 (repeated-measures one-way ANOVA or Student t test)
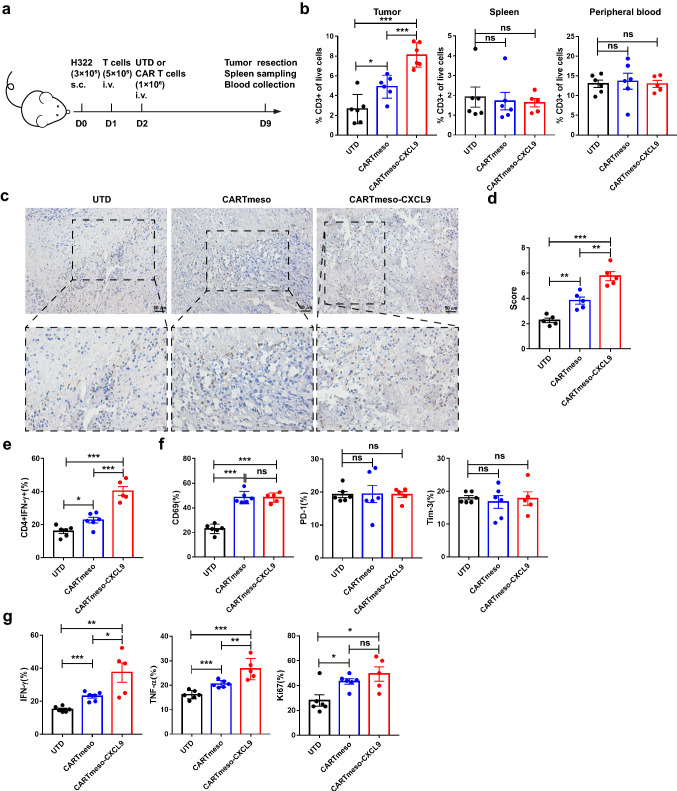
CXCL9 enhanced CAR T cell cytokine secretion and attracted T cells to the tumor site in vivo.
To clarify the efficacy of modified CAR T cells in vivo, H322 cells were subcutaneously injected into NOD/SCID mice. Activated CD3+ T cells were infused intravenously on the first day, to verify whether CARTmeso-CXCL9 cells could recruit these activated T cells in vivo. Then, CAR T cells sorted by flow cytometry were intravenously injected the next day. After 7 days, tumor, spleen, and peripheral blood samples were collected to detect T cell infiltration and function (Fig. 3a). The results showed that compared with mice treated with UTD and CARTmeso cells, the proportion of CD3+ T cells in the CARTmeso-CXCL9 group doubled in the tumor, but with no statistical difference between the spleen and peripheral blood (Fig. 3b–d), suggesting that CARTmeso-CXCL9 could make more T cells homing to tumor site, which was consistent with the experimental results in vitro. Then, the polarization of CD4+ T cells in UTD or CAR T cells infiltrated by the tumor was detected. The percentage of Th1 (CD4+IFN-γ+) in the CARTmeso-CXCL9 increased compared to that in traditional CAR T cells (Fig. 3e), indicating that CARTmeso-CXCL9 cells may improve the immune response. The expression of phenotype markers and Ki67 in CAR T cells was not significantly different between the CAR T cell treatment groups (Fig. 3f, g). In addition, functional molecule expression in UTD or CAR T cells in tumors was analyzed by flow cytometry. The secretion of IFN-γ and TNF-α in CARTmeso-CXCL9 cells was higher than that in traditional CAR T cells (Fig. 3g), suggesting that CARTmeso-CXCL9 cells may have stronger antitumor activity. Therefore, we demonstrated in vivo that CARTmeso-CXCL9 cells drive T cells to tumor site and enhance the antitumor potential of CAR T cells.
Fig. 3.
CARTmeso-CXCL9 showed enhanced cytokine secretion and the potential of T cell recruiting in vivo. a Experimental design for b–g 1 day after the inoculation of 3 × 105 H322 cells, and 5 × 105 activated T cells were injected intravenously into NOD/SCID mice. UTD or CAR T cells were infused intravenously the next day. Before infusion, CAR T cells have been purified. After 7 days, samples of mice were gathered for analysis. b Frequencies of infiltrating T cells in tumor, spleen, and peripheral blood were measured by flow cytometry 7 days after the infusion of CAR T cells. c, d Representative images and score of stained immunohistochemistry for human CD3 in tumor tissue 7 days after T cell infusion. e The percentage of CD4+IFN-γ+ cells in tumor-infiltrating T or CAR T cells. f Flow cytometric analysis of CD69, PD-1, and Tim-3 in T or CAR T cells of the tumor site. g T or CAR T cells in tumor were analyzed for the expression of functional molecules (IFN-γ, TNF-α, and Ki67). N = 5 mice/group, *p < 0.05, **p < 0.01, ***p < 0.001 (repeated-measures one-way ANOVA or Student t test)
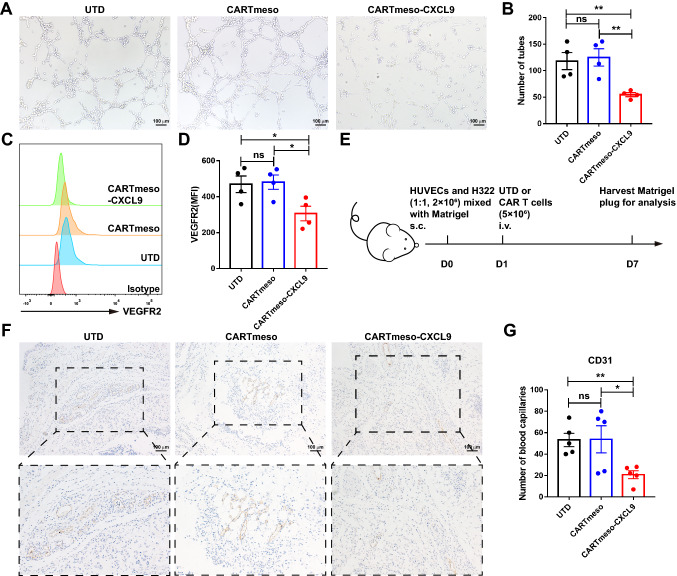
CXCL9 conferred antiangiogenic effect on CAR T cells
Previous studies have demonstrated that CXCL9 represses angiogenesis and tumor progression [10]. To elucidate the antiangiogenic function of CXCL9, tube formation assay and flow cytometry were conducted in vitro. The data showed that the addition of recombinant human CXCL9 protein not only inhibited tube formation in HUVECs, but also reduced VEGFR2 expression (Fig. S6). To explore whether CARTmeso-CXCL9 also has antiangiogenic ability, HUVECs were processed with the culture supernatants of UTD, CARTmeso, and CARTmeso-CXCL9 to evaluate their effects on HUVECs. As anticipated, the cell culture supernatant of CARTmeso-CXCL9 greatly decreased tubal structures and diminished VEGFR2 expression in HUVECs (Fig. 4a–d). Furthermore, a humanized tumor vascular model was constructed using a Matrigel plug assay in NOD/SCID mice to evaluate angiogenesis characteristics (Fig. 4e) [28]. Immunohistochemical staining for CD31 was performed to accurately quantify vascularization (Fig. 4f, g). There were fewer CD31 stained microvessels in the CARTmeso-CXCL9 group, which was consistent with the above results. The results demonstrated that the blood vessels of the Matrigel plug were lower in the CARTmeso-CXCL9 cell group than in the other control groups. To summarize, the data suggest that CARTmeso-CXCL9 cells have strong antiangiogenic properties.
Fig. 4.
CARTmeso-CXCL9 cells inhibited angiogenesis. a–d HUVECs were incubated with UTD or CAR T cell culture supernatant in vitro. a, b Representative images and number statistics of tubes formed by HUVECs were assessed. c, d Flow cytometric analysis of VEGFR2 expression in HUVECs. e–g Effect of CARTmeso-CXCL9 on angiogenesis using Matrigel plug assay in vivo. e Experiment setup of the Matrigel plug assay. f, g Representative images and number statistics of stained immunohistochemistry for human CD31 in Matrigel plugs 7 days after implantation. N = 5 mice/group, *p < 0.05, **p < 0.01 (repeated-measures one-way ANOVA)
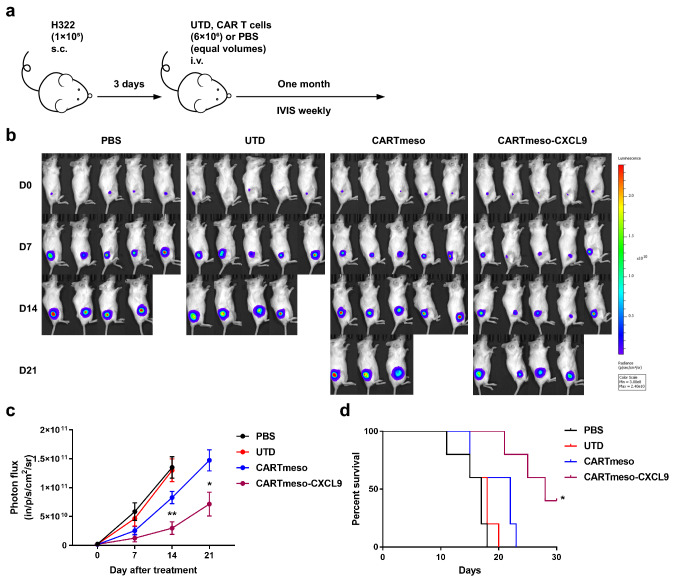
CAR T cells expressing CXCL9 exhibited superior antitumor efficacy in vivo
To study the antitumor effect of CARTmeso-CXCL9 cells further, we established a xenograft mouse model. NOD/SCID mice were subcutaneously injected with 1 × 105 luciferase-expressing H322 cells. PBS, UTD, CARTmeso, and CARTmeso-CXCL9 cells were intravenously injected into tumor-bearing mice (Fig. 5a). The CARTmeso-CXCL9 treatment group inhibited tumor growth and prolonged mouse survival than conventional CAR T cells or the control group, as assessed by bioluminescence imaging and survival curves (Fig. 5b–d). This prolongation may be modest, but it also illustrated the need for further optimization of the method. These results indicated that CARTmeso-CXCL9 cells have potent antitumor potential and improve the survival rate in the tumor-bearing mouse model.
Fig. 5.
CARTmeso-CXCL9 cells enhanced tumor eradication in a xenograft model. a Schematic diagram of the experimental process. H322 cells expressing luciferase were inoculated into NOD/SCID mice subcutaneously. On day 3, mice were treated with PBS, UTD, CARTmeso and CARTmeso-CXCL9 cells. b, c The bioluminescence image was recorded weekly to monitor tumor growth. d The survival curves of mice were analyzed. N = 5 mice/group, *p < 0.05 (the log-rank test)
Discussion
Despite promising results in B-cell malignancies, CAR T cells are still less effective in solid tumors [29, 30]. Improving the homing and accumulation of T cells in tumors is a prerequisite for their therapeutic activity. The increased lymphocyte infiltration in the tumor microenvironment is closely related to the good prognosis of various tumors [31, 32]. Chemokines and chemokine receptors are important regulators that mediate the migration of immune cells. At present, numerous studies have been devoted to modifying CAR T cells to overexpress chemokine receptors to augment T cell traffic to the tumor site [6, 33, 34]. CAR T cells overexpressing CXCR1 or CXCR2 can more effectively migrate to the tumor site generating IL-8, and significantly reducing the tumor burden [33]. Similarly, CAR T cells with CCR2b transduction enhance the migration and infiltration of tumor tissues [34]. Modifying CAR T cells for local delivery of chemokines is another viable strategy, which can recruit more immune cells to reach tumor site [35]. The forced expression of CCL19 in CAR T cells significantly inhibits tumor growth by inducing the infiltration of T cells and DC cells into tumor site [35]. Effector T cells, including CAR T cells, express high levels of the chemokine receptor CXCR3 and are attracted to tumors that express CXCR3 ligands (CXCL9, CXCL10, or CXCL11). There have been several studies focusing on increasing the concentration of CXCL9, CXCL10, or CXCL11 in the tumor microenvironment to recruit CXCR3+ effector T cells homing [22, 36–38]. In addition to the recruiting ability, CXCL9 also has the function of regulating Th1 cell differentiation and inhibiting angiogenesis, while CXCL11 polarizes CD4+ T cells into T regulatory type 1 cells and thus inhibits immune cell function [39]. And Dangaj D et al. found that among the three chemokines of CXCL9, CXCL10, or CXCL11, only the expression of CXCL9 was positively correlated with CD8A in various cancer types [8]. Moreover, various studies have shown that the expression of CXCL9 is highly correlated with tumor-infiltrating T cells [40, 41]. And CXCL9 can recruit immunocytes expressing its receptor CXCR3, which is mainly expressed in T cells, NK cells, DC cells and monocytes [42]. Compared with CCL19, CXCL9 not only increases the accumulation of various immune cells in the tumor microenvironment, but also exerts antitumor effects through other functions. CXCL9 regulates Th1 polarization to activate immune response [9]. Inhibition of tumor angiogenesis endows CXCL9 superior antitumor properties [15]. Various literature studies have increased the expression of CXCL9 in the TME by mesenchymal stem cell delivery, intratumoral injection, induced expression, and other methods, but have not applied CXCL9 to CAR T cells for tumor immunotherapy [22, 23]. Therefore, we constructed CART-CXCL9 cells that overexpress CXCL9. In addition, the high transcription level of CXCL9 can act as a positive prognostic factor in patients with multiple solid tumors [43–45], suggesting that CART-CXCL9 cells we modified can be used in various solid tumor models. Among malignant tumors, lung cancer is the primary cause of both high incidence and mortality rates in China [46]. Mesothelin is highly expressed in lung cancer patients, while its expression in normal tissues is limited to mesothelial cells [47]. Consequently, we designed CART-CXCL9 cells based on mesothelin (CARTmeso-CXCL9) in this study and selected to explore the function and efficacy of CARTmeso-CXCL9 in the lung cancer model.
T cells can directly or indirectly exert antitumor effects by producing effector factors IFN-γ and TNF-α. CD4+ T cells play a central role in coordinating adaptive immune responses. The effector subsets Th1 secrete cytokines (such as IFN-γ, TNF-α, and IL-2) to promote the tumor immune response. CXCL9 promotes the polarization of Th1 cells [14, 48], and our study verified these results. Similarly, our data indicated that CXCL9 could promote the production of CD3+ T cell cytokines and endow CAR T cells with enhanced effector function and cytotoxicity. Activated T cells highly expressed the CXCL9 receptor CXCR3. The in vivo and in vitro results showed that CAR T cells overexpressing CXCL9 could recruit and accumulate T cells at the tumor site. T cell migrating to tumor depends on a match between chemokine receptors on effector T cells and chemokines expressed at the tumor site [49]. The literature indicates that effector T cells, including CAR T cells, express high-level chemokine receptor CXCR3 [37, 50]. Thus, effector T cells are attracted to tumors that express CXCR3 ligand (i.e., CXCL9). Moreover, we examined CXCR3 expression on CAR T cells prior to infusion into mice, and both CARTmeso-CXCL9 and CARTmeso cells expressed more than 30% of CXCR3 (data were not shown), suggesting that CXCL9-modified CAR T cells have the potential to infiltrate into tumor sites. The antiangiogenic ability of CXCL9 has been reported to be the main factor inhibiting tumor growth [10, 15, 51], and CARTmeso-CXCL9 has the same characteristics. In the Matrigel plug assay, compared with conventional CARTmeso cells, CARTmeso-CXCL9 cells significantly reduced angiogenesis in the tumor Matrigel. Moreover, the CXCL9-CXCR3 axis enhances the anti-PD-1 efficacy in tumors and plays a vital function in immunotherapy [8, 16], suggesting that our modified CARTmeso-CXCL9 cells can be combined with anti-PD-1 therapy to exert a superior curative effect. However, there is a lack of mouse models to evaluate the long-term safety, and the introduction of suicide genes or synNotch system to control CXCL9 expression in CAR T cells may be necessary for future.
Our investigation demonstrates that a novel CAR structure can overexpress CXCL9, improve CAR T cell immune functions, and delay tumor growth. To the best of our knowledge, no previous studies have investigated the association of CAR T cells with the chemokine CXCL9. We also consider that CAR T cells overexpressing CXCL9 could enhance the effector function of CAR T cells, increase the infiltration of immune cells (such as T cells and NK cells) in the solid tumor microenvironment, and inhibit tumor angiogenesis to induce tumor regression in patients. However, these still need more research to prove. Our study also constructs humanized CAR T cells that kill human tumor cells, and the results provide a theoretical basis for solid tumor treatment with CARTmeso-CXCL9 cells. Its enhanced antitumor efficacy provides preclinical evidence for the expansion into the human system, which has clinical translational significance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage for English language editing and the staff and students of the Biotherapy Center at the First Affiliated Hospital of Zhengzhou University for their valuable help in this study.
Author contribution
YZ and SY designed and supervised the study. YT, CW, ZZ and YL performed the experiments. FL, QZ, CY, and KN helped to complete the experiment. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81771781), National Science and Technology Major Project of China (2020ZX09201-009), Program of the Major Research Plan of the National Natural Science Foundation of China (91942314), and Major public welfare projects in Henan Province (201300310400), and National Natural Science Foundation of China General Program (81872333).
Data availability
The datasets used in this study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All authors agree with submission to Cancer Immunology, Immunotherapy.
Ethical approval and consent to participate
Our study was reviewed and approved by the Ethics Committee of First Hospital of Zhengzhou University.
Human or animal rights
All animal experiments were approved by the Animal Care and Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Informed consent
Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yonggui Tian, Chunli Wen, Zhen Zhang and Yanfen Liu contributed equally to this work.
Contributor Information
Shengli Yang, Email: slyang@sibs.ac.cn.
Yi Zhang, Email: yizhang@zzu.edu.cn.
References
- 1.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Liu S, Zhang B, Qiao L, Zhang Y, Zhang Y. T cell dysfunction and exhaustion in cancer. Front Cell Dev Biol. 2020 doi: 10.3389/fcell.2020.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu J, Mei Q, Chen L, Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother. 2021;70(3):619–631. doi: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Y, Li Y, Shao Y, Zhang Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J Hematol Oncol. 2020 doi: 10.1186/s13045-020-00890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, Rui W, Zheng H, Huang D, Yu F, Zhang Y, Dong J, Zhao X, Lin X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur J Immunol. 2020;50(5):712–724. doi: 10.1002/eji.201948457. [DOI] [PubMed] [Google Scholar]
- 7.Jena B, Dotti G, Cooper LJN. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, Lanitis E, Duraiswamy J, Tanyi JL, Benencia F, Conejo-Garcia J, Ramay HR, Montone KT, Powell DJ, Gimotty PA, Facciabene A, Jackson DG, Weber JS, Rodig SJ, et al. Cooperation between constitutive and inducible chemokines enables T Cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885–900. doi: 10.1016/j.ccell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H, Lenz H-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation – a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47. doi: 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazari A, Ahmadi Z, Hassanshahi G, Abbasifard M, Taghipour Z, Falahati-pour SK, Khorramdelazad H. Effective treatments for bladder cancer affecting CXCL9/CXCL10/CXCL11/CXCR3 axis: a review. Oman Med J. 2020;35(2):e103–e103. doi: 10.5001/omj.2020.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauldin IS, Wages NA, Stowman AM, Wang E, Smolkin ME, Olson WC, Deacon DH, Smith KT, Galeassi NV, Chianese-Bullock KA, Dengel LT, Marincola FM, Petroni GR, Mullins DW, Slingluff CL., Jr Intratumoral interferon-gamma increases chemokine production but fails to increase T cell infiltration of human melanoma metastases. Cancer Immunol Immunother. 2016;65(10):1189–1199. doi: 10.1007/s00262-016-1881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouedraogo DE, Makinson A, Kuster N, Nagot N, Rubbo PA, Bollore K, Foulongne V, Cartron G, Olive D, Reynes J, Vendrell JP, Tuaillon E. Increased T-cell activation and Th1 cytokine concentrations prior to the diagnosis of B-cell lymphoma in HIV infected patients. J Clin Immunol. 2013;33(1):22–29. doi: 10.1007/s10875-012-9766-0. [DOI] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, Barsheshet Y, Karp CL, Karin N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Investig. 2014;124(5):2009–2022. doi: 10.1172/jci71951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune–vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 2018;18(3):195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, Luster AD. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity. 2019;50(6):1498–1512. doi: 10.1016/j.immuni.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Huang H, Wang Z, Zhang G. The inflammatory CXC chemokines, GROαhigh, IP-10low, and MIGlow, in tumor microenvironment can be used as new indicators for non-small cell lung cancer progression. Immunol Invest. 2017;46(4):361–374. doi: 10.1080/08820139.2017.1280052. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Huang X, Han X, Li Z, Zhu Q, Yan J, Yu S, Jin Z, Wang Z, Zheng Q, Wang Y. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed Pharmacother. 2016;78:8–13. doi: 10.1016/j.biopha.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Lu P, Xia Y, Ding S, Fan Y, Li X, Han P, Liu J, Tian D, Liu M. CXCL9: evidence and contradictions for its role in tumor progression. Cancer Med. 2016;5(11):3246–3259. doi: 10.1002/cam4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine mig (CXCL9) in a murine model. J Immunother. 2007;30(5):490–498. doi: 10.1097/CJI.0b013e318031b551. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Su S, Li H, Shao J, Meng F, Yang M, Qian H, Zou Z, Qian X, Liu B. Low dose irradiation increases adoptive cytotoxic T lymphocyte migration in gastric cancer. Exp Ther Med. 2017 doi: 10.3892/etm.2017.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, Wang Y, Sun J, Tan T, Cai X, Lin P, Tan Y, Zheng B, Wang B, Wang J, Xu L, Yu Z, Xu Q, Wu X, Gu Y. Role of CXCR3 signaling in response to anti-PD-1 therapy. EBioMedicine. 2019;48:169–177. doi: 10.1016/j.ebiom.2019.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin P, Gui L, Wang C, Yan J, Liu M, Ji L, Wang Y, Ma B, Gao WQ. Targeted delivery of CXCL9 and OX40L by mesenchymal stem cells elicits potent antitumor immunity. Mol Ther. 2020;28(12):2553–2563. doi: 10.1016/j.ymthe.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Y, Weng J, Wei X, Qin L, Lai P, Zhao R, Jiang Z, Li B, Lin S, Wang S, Wu Q, Tang Z, Liu P, Pei D, Yao Y, Du X, Li P. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia. 2017;32(3):801–808. doi: 10.1038/leu.2017.249. [DOI] [PubMed] [Google Scholar]
- 25.Mei Z, Zhang K, Lam AKY, Huang J, Qiu F, Qiao B, Zhang Y. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2019;9(2):640–652. doi: 10.1002/cam4.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JY, Li F, Wang LP, Chen XF, Wang D, Cao L, Ping Y, Zhao S, Li B, Thorne SH, Zhang B, Kalinski P, Zhang Y. CTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2015;113(5):747–755. doi: 10.1038/bjc.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karin N, Wildbaum G, Thelen M. Biased signaling pathways via CXCR3 control the development and function of CD4 + T cell subsets. J Leukoc Biol. 2016;99(6):857–862. doi: 10.1189/jlb.2MR0915-441R. [DOI] [PubMed] [Google Scholar]
- 28.Nör JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, Polverini PJ. Up-regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61(5):2183–2188. [PubMed] [Google Scholar]
- 29.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2019;17(3):147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discovery. 2020;19(3):185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 31.Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117(4):451–460. doi: 10.1038/bjc.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Yu W, Lian J, Wu Q, Liu S, Yang L, Li F, Huang L, Chen X, Zhang Z, Li A, Liu J, Sun Z, Wang J, Yuan W, Zhang Y. Th17 cells inhibit CD8 + T cell migration by systematically downregulating CXCR3 expression via IL-17A/STAT3 in advanced-stage colorectal cancer patients. J Hematol Oncol. 2020 doi: 10.1186/s13045-020-00897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, Dyson KA, Grippin AJ, Deleyrolle LP, Zhang W, Rajon DA, Wang QJ, Yang JC, Kresak JL, Sayour EJ, Rahman M, Bova FJ, Lin Z, Mitchell DA, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019 doi: 10.1038/s41467-019-11869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y, Yin H, An H, Zhou C, Zhou L, Chen S, McGowan E. Chemokine receptor CCR2b expressing anti-Tn-MUC1 CAR-T cells enhanced anti-breast cancer activity. Ann Oncol. 2019;30:xi12. doi: 10.1093/annonc/mdz448.002. [DOI] [Google Scholar]
- 35.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 36.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing bat f3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711–723. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon EK, Wang LS, Bekdache K, Lynn RC, Lo A, Thorne SH, Albelda SM. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology. 2018;7(3):e1395997. doi: 10.1080/2162402X.2017.1395997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia M, Chen J, Meng G, Shen H, Dong J. CXCL10 encoding synNotch T cells enhance anti-tumor immune responses without systemic side effect. Biochem Biophys Res Commun. 2021;534:765–772. doi: 10.1016/j.bbrc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Karin N, Wildbaum G, Thelen M. Biased signaling pathways via CXCR3 control the development and function of CD4 + T cell subsets. J Leukoc Biol. 2016;99(6):857–862. doi: 10.1189/jlb.2MR0915-441R. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Xu J, Wu X, Yao H, Yan Z, Guo T, Wang W, Wang P, Li Y, Yang X, Li H, Bian H, Chen Z-N. CD147 regulates antitumor CD8 + T-cell responses to facilitate tumor-immune escape. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-00570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng Z, Ren D, Zhang K, Zhao J, Jin X, Wu H. Using estimate algorithm to establish an 8-mRNA signature prognosis prediction system and identify immunocyte infiltration-related genes in pancreatic adenocarcinoma. Aging. 2020;12(6):5048–5070. doi: 10.18632/aging.102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H, Lenz HJ. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47. doi: 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu L, Yang X, Xu C, Sun J, Fang Z, Pan H, Han W. Comprehensive analysis of the expression and prognostic value of CXC chemokines in colorectal cancer. Int Immunopharmacol. 2020;89:107077. doi: 10.1016/j.intimp.2020.107077. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K, Zhang L, Mi Y, Tang Y, Ren F, Liu B, Zhang Y, Zheng P. A ceRNA network and a potential regulatory axis in gastric cancer with different degrees of immune cell infiltration. Cancer Sci. 2020;111(11):4041–4050. doi: 10.1111/cas.14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang B, Han W, Sheng Z-F, Shen G-L. Identification of immune-related biomarkers associated with tumorigenesis and prognosis in cutaneous melanoma patients. Cancer Cell Int. 2020 doi: 10.1186/s12935-020-01271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA A Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 47.Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2015;6(2):133–146. doi: 10.1158/2159-8290.cd-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groom Joanna R, Richmond J, Murooka Thomas T, Sorensen Elizabeth W, Sung Jung H, Bankert K, von Andrian UH, Moon James J, Mempel Thorsten R, Luster AD. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4 + T helper 1 cell differentiation. Immunity. 2012;37(6):1091–1103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ager A, Watson HA, Wehenkel SC, Mohammed RN. Homing to solid cancers: a vascular checkpoint in adoptive cell therapy using CAR T-cells. Biochem Soc Trans. 2016;44(2):377–385. doi: 10.1042/bst20150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong M, Puaux A-L, Huang C, Loumagne L, Tow C, Mackay C, Kato M, Prévost-Blondel A, Avril M-F, Nardin A, Abastado J-P. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71(22):6997–7009. doi: 10.1158/0008-5472.Can-11-1466. [DOI] [PubMed] [Google Scholar]
- 51.Gudowska-Sawczuk M, Kudelski J, Mroczko B. The role of chemokine receptor CXCR3 and its ligands in renal cell carcinoma. Int J Mol Sci. 2020;21(22):8582. doi: 10.3390/ijms21228582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study are available from the corresponding author on reasonable request.