Abstract
Cholangiocarcinoma, the second most common liver malignancy, after hepatocarcinoma is highly aggressive and usually diagnosed in advanced cases. In the era of personalized medicine, targeted therapy protocols are limited for cholangiocarcinoma and the only potential curative treatment, surgical resection, is seldom applicable.
This retrospective study included all cases with pathology-confirmed intrahepatic cholangiocarcinoma admitted in a tertiary healthcare facility during a 10-year timeframe. Clinical information, laboratory values, imaging studies, and survival data were retrieved, and PD-L1 immunostaining was performed on representative pathology slides, for each case.
From the total of 136 included cases (49 surgical resections and 87 liver biopsies), 38.97% showed PD-L1 positivity on tumoral cells, 34.8% on tumor infiltrating immune cells, 10.11% on epithelial cells within the peritumoral area and 15.95% on immune cells from the peritumoral area. Overall survival was significantly higher in the first two scenarios. However, after adjusting for age, tumor number, tumor size, and tumor differentiation in a multivariate analysis, only PD-L1 positivity on tumor infiltrating immune cells remained a favorable prognostic for survival. High immune cell counts also correlated with increased overall survival.
Our study demonstrated that PD-1/PD-L1 checkpoint pathway in the microenvironment of intrahepatic cholangiocarcinoma bears prognostic significance. PD-L1 expression on immune cells, in both resection and biopsy specimens, might be a strong independent predictor for a favorable outcome.
Keywords: Cholangiocarcinoma, PD-L1, Tumor cells, Immune cells, Overall survival
Introduction
Cholangiocarcinoma (CCA) is a rare, aggressive malignancy of the hepato-biliary epithelial cells. The latest WHO Classification of Digestive System Tumors emphasizes the importance of case stratification as two different subtypes—intrahepatic cholangiocarcinoma (iCCA), which represents only 10% of CCA cases, and extrahepatic cholangiocarcinoma (eCCA)—including perihilar and distal CCA, as the two entities with different clinical and molecular features [1–3].
CCA is typically diagnosed in advanced clinical stages. Consequently, patients are left with limited therapeutic options and poor outcomes. Surgical resection (partial hepatectomy) remains the only potentially curative treatment but is rarely applicable due to tumoral extent, vascular and/or lymphatic invasion, or distant metastasis [2]. Despite all the recent advances in precision medicine for cancer patients, the five-year survival rate of CCA remains at a persistently discouraging rate of 10–40% [2, 3]. Furthermore, palliation is suboptimal, as standard chemotherapy regimens (gemcitabine + cisplatin) have only achieved a median overall survival (OS) of 6–8 months [4]. Hence, diagnosis and therapeutic approaches are in dire need of progress, as there is a stark demand for novel improved strategies.
Immunotherapy has arguably revolutionized cancer treatment with the addition of immune checkpoint inhibitors in the therapeutic armamentarium since 2012 in various malignancies like melanoma, non-small lung cancer, renal cell cancer, and others. These types of drugs have provided a breakthrough in personalized cancer treatment. There are two main classes of immune checkpoint molecules, namely the programmed cell death protein-1 (PD-1), its ligand (PD-L1), and cytotoxic T-lymphocyte associated protein-4 [5, 6]. In 2017, the FDA approved pembrolizumab, a PD-1 inhibitor, for treating cholangiocarcinoma patients with particular genetic characteristics (high microsatellite instability of defective mismatch repair) [7–9].
Cancer cells can initiate different immune pathways that harbor immunosuppressive functions. One of the processes by which tumor cells can evade immune surveillance is by triggering immune checkpoint pathways. PD-L1 resides typically at the surface of activated T cells, having a protective role in a healthy body: when PD-1 binds to it, the axis triggers the programmed death of T cells, eluding overactivation of the immune system; in the neoplastic tissue, the overexpression of PD-L1 on the surface of tumor cells or in the tumor microenvironment (TME) stimulates the process of immune escape [7, 10]. The aberrant expression of PD-L1 thus interferes with antitumor immunity, and the PD-1/PD-L1 axis has been frequently identified as a key pathway utilized by tumors to escape the immune system.
To this point, understanding the applicability of immune therapy in CCA and its effects within TME is relatively limited [11, 12]. The exact mechanism used by CCA to surpass immune checkpoints remains to be elucidated [13]. Controversy overshadows the prognostic signification of PD-1/PD-L1 in CCA [14]. Immune checkpoint therapy correlative studies have identified PD-L1 as a potentially useful biomarker for predicting outcome or therapy response in CCA. Reports declare an incidence of PD-L1 positivity between 49 and 98% in biliary tract cancers [15]. PD-L1 overexpression is correlated with decreased survival in several studies [16–18], while other authors contradicted this association [19, 20]. Additionally, PD-L1 expression on stroma cells has been incriminated in having a clinical impact in CCA patients: it seems that various types of cells from the TME like tumor-associated neutrophils, tumor-associated macrophages, or myeloid suppressor cells can overexpress PD-L1, possibly leading to an immunosuppressive microenvironment, although there is still a need for further molecular studies in order to fully comprehend the relationship between PD-L1 expression in stromal cells, cancer cells and their interaction with the tumor microenvironment [18].
The discrepancy in the results is influenced by several factors, including various staining techniques, different interpretation criteria, and inconsistent cut-off values for PD-L1 levels. Only a few studies [11, 12] evaluated PD-L1 in a complex manner, assessing its expression in the TME, the tumor invasion front (TIF) and in healthy tissue. Additionally, to our knowledge, most studies on PD-L1 expression were developed on tissue samples from patients who underwent surgical resection. However, since the majority of CCA patients are unsuitable for surgery [4], the question of whether small sample biopsies provide sufficient material for PD-L1 staining is still unanswered.
In the context of a lethal malignancy (with one curative option, poor systemic treatments alternatives, and no prognostic biomarkers), but in the era of immunotherapy, more studies to evaluate PD-L1 expression in CCA are mandatory for several reasons: (a) Inhibiting the PD-1/PD-L1 axis already proved to be a promising direction to pursue [21]; (b) PD-L1 might represent a prognostic biomarker helping clinicians to better select patients for adjuvant treatment after surgery or for immunotherapy in case of a nonresectable patient, and (c) the fact that anti-PD-L1 treatment in various tumor types is directly correlated with the immunohistochemical expression of PD-L1 on tumoral tissue has been already proven [5, 22].
Since the overexpression of PD-L1 in cancer and stroma cells has been linked to the outcome of CCA patients, we proceeded to investigate whether PD-L1 expression in the tumor mass (TME) and tumor invasion front (TIF) was correlated with overall survival in both large resection specimens and small-sized liver biopsies in patients with iCCA. Secondly, we analyzed the associations between PD-L1 expression and other negative clinical and histopathologic prognostic factors. We also compared the PD-L1 expression in large resection cases versus small-sized liver biopsies to establish whether liver biopsy offers enough material for future PD-L1 immunohistochemistry studies in patients with CCA.
Materials and methods
Case selection
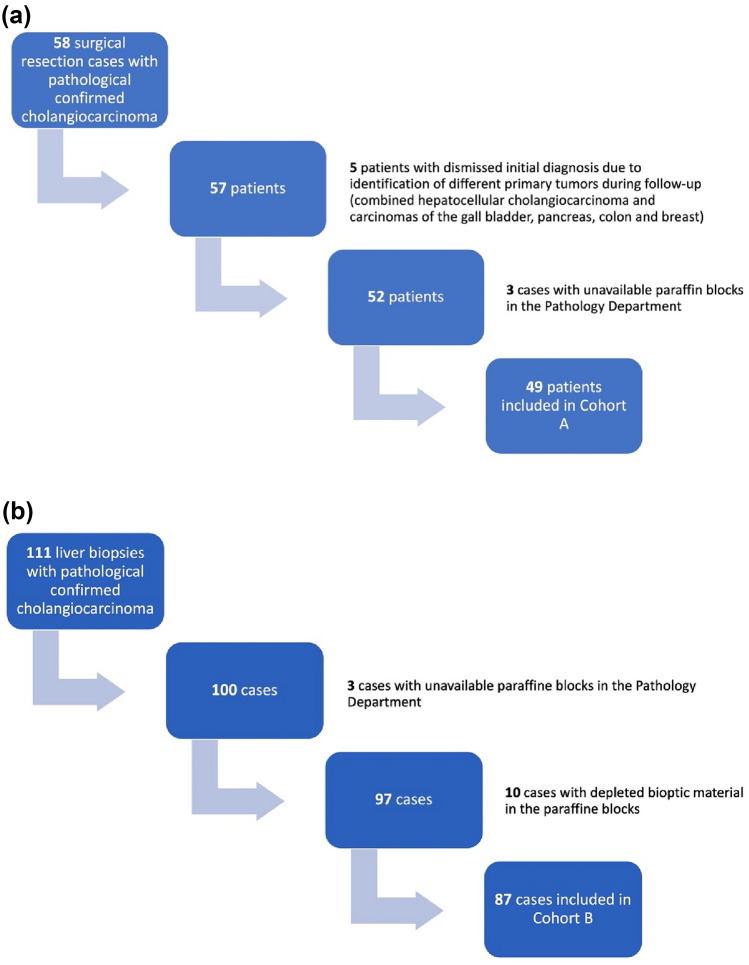
This was a retrospective study conducted in a high-volume tertiary-care facility (The "Octavian Fodor" Regional Institute of Gastroenterology and Hepatology) from Cluj-Napoca, Romania. The study was conducted per the Declaration of Helsinki and approved by both the Institutional Ethics Committee (165/09.12.2021) and the Ethics Committee of “Iuliu Hatieganu” University of Medicine and Pharmacy Cluj-Napoca, Romania (34/13.12.2021). The medical records of all patients hospitalized in our center during a ten-year timeframe (2011–2021) with a pathology-confirmed diagnosis of iCCA were reviewed for possible inclusion in the study. We included two cohorts of patients: patients with iCCA who underwent surgical resection with a curative intent (Cohort A) and patients with iCCA judged to be inoperable and in whom a liver biopsy offered the definitive proof of malignancy (Cohort B). The exclusion criteria were as follows: unavailable paraffin blocks, depleted tumoral tissue in the paraffin blocks, cases of combined hepatocellular-cholangiocarcinoma, cases that were reclassified after further analysis of clinical and imagistic data and patients with other known malignancies. The flowchart of the study population is depicted in Fig. 1.
Fig. 1.
Flowchart of the study population after applying the inclusion and exclusion criteria: a Cohort A – 49 surgical resection cases, b Cohort B – 87 liver biopsies
Patient demographics, risk factors, laboratory findings, and imagistic work-up were retrieved from the medical records. Pathologic data in cohort A, including tumor size, number, differentiation, perineural infiltration, vascular invasion, inflammatory infiltrate, lymph node involvement, distant metastasis, and resection margins, were recorded from the pathology department. In cohort B, tumor size, number, vascular invasion, lymph node involvement, and distant metastasis were recorded based on CT and/or MRI exams. The liver biopsy in cohort B was performed under ultrasound guidance (Logiq 7, Logiq 8, and Logiq 10 system from General Electric, Milwaukee, WI, USA) using 1.2 mm diameter (18 gauge) cutting needles with a 1.4 cm long sampling notch coupled to a Biopsy Gun (Bard Medical, Covington, GA, USA). If a patient had two or more potential target lesions, the gastroenterologist chose one of the lesions as the biopsy target, taking into consideration the lesion visibility and technical difficulty of the biopsy.
Pathologic assessment
Two experienced, independent pathologists evaluated all histologic slides. They reclassified them according to the 5th edition of the WHO Classification of Digestive System Tumors [1] and the 8th edition of the AJCC Cancer Staging Manual [23]. Representative formalin fixed and paraffin-embedded blocks from matched tumoral and peritumoral tissue with a high density of inflammatory infiltrate were chosen for all hepatic resection cases. All paraffin blocks of liver biopsies were checked to ensure the availability of proper tissue for further immunostaining.
PD-L1 immunostaining and interpretation
PD-L1 immunohistochemistry was performed on 3 μm tissue sections of the chosen blocks of resection cases and on all liver biopsy blocks. The slides were processed by completely automated systems (Leica Bond-Max Immunostainer; Leica Biosystems, Nussloch, Germany) in conformity with the manufacturer’s instructions, using a murine monoclonal anti-PD-L1 antibody (MAB 1561, 1/100; R&D Systems, Minneapolis, MA). Positive and negative controls were included during the staining procedure.
PD-L1 immunohistochemical expression was appraised using light microscopy by two pathologists without knowledge of the clinical characteristics. Only membranous staining was considered positive. The percentage of cells exhibiting cell surface staining for PD-L1 was estimated in two distinct tumor areas: the tumor mass and at the intersection between tumor and healthy tissue (TIF). PD-L1 was assessed in tumor cells, tumor-infiltrating immune cells, non-tumoral cholangiocytes, and immune cells from the TIF. Similar to previously published studies, we used a scoring system for the intensity of the staining (0 for negative staining, 1 for weak staining, 2 for moderate staining, and 3 for intense staining), while the extent rate of positive cells was appreciated in percentage (0 to 100%) [16, 18, 19]. The intensity grade was further multiplied by the percentage of positive cells, resulting in a final score with a range from 0 to 300. If the final score was ≥ 3, PD-L1 was considered positive. Next, we also checked for immune cell infiltration in both peri-tumoral and intratumoral areas. We classified four groups: absent, mild, moderate, and intense immune cell infiltrates.
Statistical analysis
Categorical data were presented as counts and percentages. Comparisons of categorical data were performed with the Chi-square test or Fisher’s exact test in case of low expected frequencies. Continuous normally distributed data were presented as means and standard deviations, while skewed data were presented as medians and quartiles. Comparisons of continuous skewed data were performed with the Wilcoxon rank sum test. Correlations between continuous skewed data were done with Spearman’s correlation coefficient and its associated statistical test.
The overall survival was defined as the time from treatment (in cohort A) or time of diagnosis (in cohort B) until death or the study ending date (May 2022). Survival data were presented graphically using the Kaplan–Meier method. The optimal cut-off value for serum PD-L1 regarding overall survival was identified using previous data from the literature [16, 19, 24, 25]. The relation between tissue PD-L, among other predictors, and survival was verified using univariate proportional Cox regressions. To evaluate whether these relations were not spurious, we further added known predictors for survival as adjusting variables in multivariate Cox regression models. The proportional hazard assumption was checked with a formal statistical test for all these models, while the linear functional form for continuous variables was checked using model residuals plots inspection. For multivariate models, multicollinearity was assessed with variance inflation factors. For all statistical tests, the two-tailed p value was computed, and the results were considered statistically significant for values below 0.05. All analyses were computed using the R environment for statistical computing and graphics (R Foundation for Statistical Computing, Vienna, Austria), version 3.6.3 [R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria; 2019.
Results
Patient and disease characteristics
The study population included 136 tumor samples from patients undergoing surgery for the management of CCA (Cohort A; n = 49) or patients undergoing liver biopsy for definite proof of malignancy (Cohort B; n = 87). The median age of all patients was 62 years, and 75 out of 136 were male (55.14%). The mean tumor size was 7.82 cm, and 52.94% patients presented with multiple lesions. The histologic evaluation revealed: good differentiation in 22 cases (16.17%), moderate differentiation in 55 cases (40.44%), poor differentiation in 42 cases (30.88%); tumor differentiation was not available in 17 patients (12.5%). Other patient, laboratory, and tumor characteristics are depicted in Table 1.
Table 1.
Clinical and pathological characteristics of the study population
| Population characteristics | Cohort A ( n = 49) | Cohort B ( n = 87) | P value |
|---|---|---|---|
| Age | |||
| Mean ± SD | 60.48 ± 9.6 | 62.67 ± 9.78 | 0.218 |
| Range | 39–77 | 43–84 | |
| Gender | |||
| Male, n(%) | 30 (61.22) | 45 (51.72) | 0.369 |
| Female, n(%) | 19 (38.77) | 42 (48.27) | |
| Environment | |||
| Urban, n(%) | 34 (69.38) | 50 (57.47) | 0.200 |
| Rural, n(%) | 15 (30.61) | 37 (42.52) | |
| Underlying disease, n(%) | |||
| Hepatitis B virus | 4 (8.16) | 8 (9.19) | 0.273 |
| Hepatitis C virus | 1 (2.04) | 8 (9.19) | |
| Liver cirrhosis | 2 (4,08) | 13 (14.94) | |
| Liver steatosis | 9 (18.36) | 10 (11.49) | |
| Obesity | 3 (6.12) | 8 (9.19) | |
| Type 2 diabetes mellitus | 9 (18.36) | 18 (20.69) | |
| Tumor characteristics | |||
| Tumor size | |||
| Mean ± SD (cm) | 6.73 ± 34.5 | 8.69 ± 3.98 | 0.004 |
| Range (cm) | 1—14 | 0.6—19 | |
| Tumor number | |||
| Solitary | 30 (61.22) | 34 (39.08) | 0.012 |
| Multiple | 19 (38.77) | 53 (60.92) | |
| Pathologic differentiation, n(%) | |||
| Good | 8 (16.32) | 14 (16.09) | 0.016 |
| Medium | 26 (53.06) | 29 (33.33) | |
| Poor | 15 (30.61) | 27 (31.03) | |
| Not available | 17 (19.54) | ||
| Resection margin, n(%) | |||
| R0 | 25 (51.02) | * | |
| R1, R2 | 24 (48.97) | * | |
| Perineural infiltration, n(%) | 29 (59.18) | † | |
| Vessel infiltration | |||
| Lymphatic, n(%) | 26 (53.06) | † | |
| Venal, n(%) | 21 (42.85) | † | |
| Hepatic capsule invasion, n(%) | 19 (38.77) | * | |
| AJCC stage | |||
| I, II | 22 (44.89) | 0 (0) | < 0.001 |
| III, IV | 27 (55.1) | 87 (100) | |
*Gross and/or microscopic aspects not applicable in cohort B due to lack of surgical specimens data not available in cohort B due to small sizes of the biopsies
Further on, we analyzed for the presence of intratumoral and peri-tumoral immune cell infiltrates. An abundant intratumoral immune cell infiltrate was found in 13 out of 136 cases (9.55%), moderate immune cell infiltrate in 43 cases(31.61%), mild immune cell infiltrate in 76 cases (55.88%) while no intratumoral immune infiltrates were reported in 4 patients from cohort B.
PD-L1 expression in tumor and tumor front
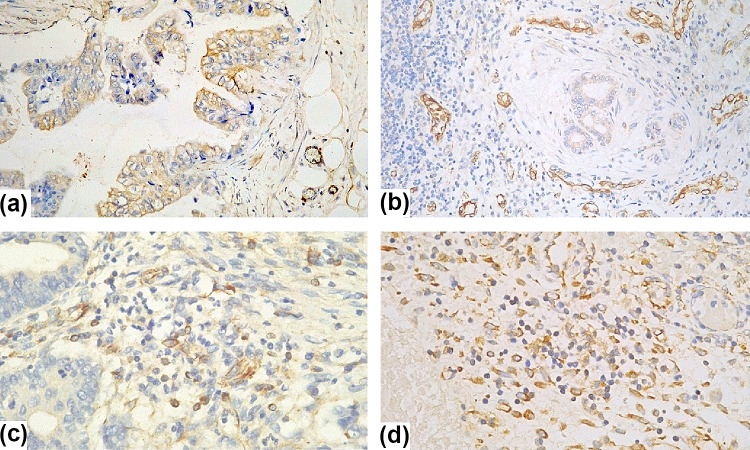
PD-L1 expression was evaluated in two different compartments: inside the tumor (on both cancer cells and tumor-infiltrating immune cells) and at the TIF (on both healthy cells and peri-tumoral immune cells)—Fig. 2.
Fig. 2.
Membranous positivity of PD-L1 on immunostained pathology slides: a positive cancer cells within tumoral tissue, 20 × magnification; b positive epithelial cells in the tumor invading front, 20 × magnification; c positive immune cells scattered among unstained cells within tumoral tissue, 40 × magnification and d positive immune cells in the tumor invading front, 40 × magnification
PD-L1 was positive on cancer cells in 38.97% cases and on tumor infiltrating immune cells in 34.8% cases. In the TIF area, PD-L1 was positive in "healthy" epithelial cells in 10.11% cases, while in peri-tumoral immune cells, it was scored positive in 15.95% cases. A parallel between PD-L1 expression in cohort A versus cohort B is delineated in Table 2.
Table 2.
PD-L1 expression in both large resection specimens (Cohort A) and small-sized liver biopsies (Cohort B) in patients with iCCA, after applying the scoring system for interpretation of the staining
| PD-L1 immunostaining | Cohort A | Cohort B | P value |
|---|---|---|---|
| Tumoral cells, n (%) | |||
| Negative | 10 (20.4) | 73 (83.9) | |
| Positive, mild | 5 (10.2) | 3 (3.44) | |
| Positive, moderate | 18 (36.73) | 9 (10.34) | < 0.001 |
| Positive, intense | 16 (32.65) | 2 (2.29) | |
| Tumor infiltrating immune cells | |||
| Negative | 19 (38.77) | 67 (77.01) | |
| Positive, mild | 2 (4.08) | 4 (4.59) | |
| Positive, moderate | 18 (36.73) | 10 (11.49) | |
| Positive, intense | 10 (20.4) | 2 (2.29) | < 0.001 |
| Not available* | 0 | 4 (4.59) | |
| Epithelial cells in the tumor invasion front | |||
| Negative | 40 (81.63) | 40 (45.97) | |
| Positive, mild | 5 (10.2) | 0 | |
| Positive, moderate | 2 (4.08) | 0 | |
| Positive, intense | 2 (4.08) | 0 | 0.009 |
| Not available* | 0 | 47 (54.02) | |
| Immune cells from the tumor invasion front | |||
| Negative | 40 (81.63) | 39 (44.82) | |
| Positive, mild | 2 (4.08) | 0 | |
| Positive, moderate | 1 (2.04) | 6 (6.89) | 0.632 |
| Positive, intense | 6 (12.24) | 0 | |
| Not available* | 0 | 42 (48.27) | |
*Data not available due to lack of benign tissue in some cases from Cohort B Negative = intensity grade *percentage of positive cells < 3 Positive = intensity grade * percentage of positive cells ≥ 3
The relation between PD-L1 expression and overall survival
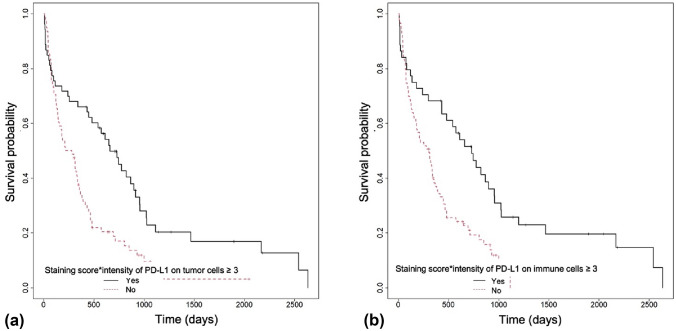
Until data gathering (May 2022), 116 patients out of 136 died (85.29%). Median OS was 20.96 months in cohort A and 9.45 months in cohort B. There was no significant discrepancy in OS based on the expression of PD-L1 in the TIF (p = 0.691 for "healthy" epithelial cells and p = 0.848 for peri-tumoral immune cells. Survival was, however, significantly higher in patients with positive PD-L1 expression in cancer cells (p < 0.001, Fig. 3a) and positive staining for PD-L1 in tumor infiltrating immune cells (p < 0.001, Fig. 3b).
Fig. 3.
Kaplan–Meier survival analysis for a cases that displayed PD-L1 positivity on tumor cells (p < 0.001) and b PD-L1 positivity on tumor infiltrating immune cells (p < 0.001)
Next, we tested whether a high intratumoral immune infiltrate could influence OS compared to a moderate or a low immune infiltrate. We found that patients with high intratumoral immune infiltrate had better OS compared to patients with moderate or low immune infiltrates (p = 0.008). Further on, we performed a multivariate analysis to check whether PD-L1 expression on cancer cells or tumor-infiltrating immune cells remains a favorable prognostic factor for OS after adjusting for age, tumor number, tumor size, and tumor differentiation. The multivariate analysis found that only PD-L1 expressed on tumor infiltrating immune cells is a favorable prognostic factor (in contrast to PD-L1 expressed on cancer cells). Both univariate and multivariate analyses of OS are summarized in Table 3. A positive resection margin was the only negative prognostic factor found in our studied population.
Table 3.
Univariate and multivariate analysis of overall survival in intrahepatic cholangiocarcinoma patients
| OS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Cohort A | ||||
| Age (years) | 0.9571–1.0292 | 0.684 | ||
| Tumor stage | 0.482–2.1016 | 0.98 | ||
| Positive resection margin | 1.7562–9.6872 | 0.001 | 1.7397–9.6244 | 0.001 |
| Perineural invasion | 0.7184–4.1668 | 0.22 | ||
| Venous vascular invasion | 0.5084–2.2804 | 0.84 | ||
| Lymphatic vascular invasion | 1.2549–8.1498 | 0.015 | ||
| Liver capsule invasion | 0.3008–1.3396 | 0.23 | ||
| PD-L1 positive on tumor immune cells | 0.2713–1.2826 | 0.183 | ||
| Cohort A and B | ||||
| Age (years) | 0.9829–1.0218 | 0.83 | ||
| Multiple tumors | 0.893–1.9025 | 0.17 | ||
| Tumor size (cm) | 0.9869–1.1059 | 0.13 | ||
| Tumor differentiation | 0.5168–1.5617 | 0.70 | ||
| PD-L1 positive on cancer cells | 0.3333–0.7497 | < 0.001 | ||
| PD-L1 positive on tumor immune cells | 0.3001–0.721 | < 0.001 | 0.2751–0.892 | 0.019 |
| High intratumoral immune infiltrates | 0.1849–0.7981 | 0.01 | 0.1496–0.8436 | 0.019 |
PD-L1 = programmed death ligand 1; HR = hazard ratios; p = level of significance; CI = confidence interval, cm = centimeters; in Cohort A the multivariate analysis was adjusted for age, tumor stage, tumor resection margin, and PD-L1 positive on tumor infiltrating immune cells; in Cohort A and B, the multivariate analysis was adjusted for age, tumor number, tumor size, and tumor differentiation
Relationship of PD-L1 expression on tumor infiltrating immune cells with other pathological data
Last, we evaluated whether there was any relationship between PD-L1 expressed on tumor infiltrating immune cells and several other pathological data from the resection specimen including: liver capsule invasion, tumor differentiation, perineural invasion, lymphatic invasion, venous invasion, resection margin status, tumor stage, and tumor number. As shown in Table 4, only one parameter—multiple tumors, was correlated with PD-L1 expression on tumor infiltrating immune cells (p = 0.005). Regarding PD-L1 and other laboratory markers, we found no significant correlation (including the evaluation of CA-19–9 levels, p = 0.91).
Table 4.
Relationship of PD-L1 expression on tumor infiltrating immune cells and several pathological variables in the resection specimens (Cohort A)
| PD-L1 positive in tumor infiltrating immune cells | Yes ( n = 29) | No ( n = 18) | p |
|---|---|---|---|
| Liver capsule invasion (yes), n (%) | 17 (58.62) | 11 (61.11) | 0.866 |
| Tumor differentiation | 0.856 | ||
| Good | 5 (17.24) | 3 (15.79) | |
| Moderate | 16 (55.17) | 9 (47.37) | |
| Poor | 8 (27.59) | 7 (36.84) | |
| Perineural invasion (yes), n (%) | 18 (60) | 11 (61.11) | 0.939 |
| Lymphatic invasion (yes), n (%) | 15 (50) | 11 (57.89) | 0.59 |
| Venous vascular invasion (yes), n (%) | 14 (46.67) | 7 (36.84) | 0.498 |
| Positive resection margin (yes), n (%) | 13 (43.33) | 11 (61.11) | 0.233 |
| Tumor stage (I and II) n (%) | 14 (48.28) | 8 (42.11) | 0.675 |
| Multiple tumors | 7 (23.33) | 12 (63.16) | 0.005 |
PD-L1 = programmed death ligand 1; n = number; % = percent; p = level of significance
Discussion
Based on the analysis of one of the largest cohorts of Caucasian patients with iCCA, our data suggest that the PD-1/PD-L1 checkpoint pathway in the TME bears prognostic significance, regardless of disease staging and therapeutic option (curative/palliation). While not of particular clinical consequence when expressed in the tumor invasion front (“healthy” peritumoral cholangiocytes and immune cells), PD-L1 expression on the malignant cells and, most importantly, on the tumor infiltrating immune cells appears to be a critical outcome predictor, as these patients had a significantly higher overall survival. Moreover, patients with PD-L1 expression on the TME appear to be diagnosed in an earlier stage, amendable with curative intent surgery, as suggested by the disproportionate rates of PD-L1 expression between the two groups.
While these results appear straightforward, the pathophysiology behind these findings might be more intricate, as the precise interaction within the TME still resembles a black box. Therefore, a cautious approach is needed to avoid premature conclusions. CCA is a highly desmoplastic tumor, in which the per se malignant cells are far outnumbered by the peritumoral stromal cells, which include a wide array of fibroblasts, endothelial and immune cells [12]. As an oversimplification, the gross understanding is that the abundant peritumoral stroma resembles a protective coating for the active malignant core(s), providing a frontline against the host antitumoral response and thus, generating an autonomous, self-sufficient, and protected CCA enclave. Most of the elements of the TME are proven pro-tumoral agents, such as cancer-associated fibroblasts [12, 26, 27], tumor-associated macrophages, and neutrophils [28–30]. On the other hand, the role of lymphocytes in the TME appears to be equivocal. Most of the data on the role of natural killer cells is extrapolated from hepatocellular carcinoma, as only few studies suggest their antitumoral role in CCA [31]. However, tumor infiltrating lymphocytes (TILs) are of particular interest in CCA, as they appear to be a double-edged sword in the disease progression. TILs encompass a large heterogeneity of cells comprising B lymphocytes, CD4 + , CD8 + , and regulatory T cell lymphocytes. The consensus is that a high TIL count correlates with improved outcomes in terms of disease staging, invasiveness, and OS [12]. However, a more thorough analysis reveals a rather heterogeneous data pool and conflicting nuances, as most of the studies include largely eCCAs and have evaluated different lymphocyte population subtypes [32, 33].
Our data suggest that an abundant immune infiltrate is an independent predictor of a better OS, which is consistent with previous reports [28, 32, 34]. However, while the rest of these studies focus either on the entire spectrum of biliary tract cancers or solely on eCCA, our design has included only iCCA, which is by far the rarest subtype, accounting for 10% of CCAs [35]. The directionality of the rich immune infiltrate—better outcome relationship is, however, a matter of debate, as the hypothesis that during the CCA natural history, the tumor develops mechanisms of immune escape, thus leading to lower TIL count in advanced disease appears epistemologically reasonable, despite lacking solid evidence [12].
The role of the PD-1/PD-L1 checkpoint pathway has been extensively studied in multiple cancers and, along with a better understanding of other checkpoint molecules, has arguably led to the advent of a novel approach in cancer therapy in the past decade. In brief, malignant cells manipulate these pathways to evade immune surveillance, typically by overexpression which leads to immune tolerance. Thus, inhibiting these pathways with specific molecules cancels their cancer-promoting effect and enhances tumor control [12]. However, how each tumor accomplishes immune tolerance may vary widely, as is the case for iCCA. Our analysis revealed that patients eligible for surgery (cohort A) had a significantly higher proportion of positive malignant cell and tumor infiltrating immune cells PD-L1 staining, regardless of positivity intensity. Furthermore, on univariate analysis, both PD-L1 positive malignant cell and tumor infiltrating immune cells were significant predictors for an improved outcome, while PD-L1 positive immune cells were independent predictors on multivariate analysis, thus negating a potential staging bias. Upon first impression, these results may appear counter-intuitive, as the consensus states that high PD-L1 expression is leading to a worse outcome. On the one hand, there are studies, mostly on eCCAs (both hilar and distal), that support conventional wisdom, reporting that a high PD-L1 expression within the TME (either malignant cell or tumor infiltrating immune cells) is associated with a worse outcome [16, 19, 36]. However, such conclusive evidence has yet to be found regarding iCCA, with a potential explanation being subtle differences in tumor biology. The study by Lim Y et al. suggests significant discrepancies in PD-L/PD-L1 pathway expression between hilar and distal CCA. Nevertheless, no comparisons with iCCA are available [36].
On the other hand, data regarding iCCA are scarce and inconclusive, which might support a different role of the PD-1 axis. Moreover, our study is among the few to evaluate the prognostic role of PD-L1 expression on in vivo iCCA and provides the most extensive available dataset. Another small-scale study that included 31 surgically resected iCCA reported a high PD-1 expression within the TME, which correlated with a more advanced stage, raising the hypothesis of immune evasion. However, the study reported no outcome data [37]. One in vitro study proposed a reversed role of PD-L1 in CCA cancer stem cells, as highly positive PD-L1 cell lines had significantly lower tumorigenicity and exhibited less aggressive traits [38]. Albeit not yet validated in the case of iCCA, the apparent role reversal of this immune checkpoint axis has been observed in studies on non-small cell [39] and small cell lung cancer [40], colorectal [41], breast [42], and head and neck malignancies [43]. Therefore, it appears that in some clinical scenarios, the overexpression of PD-L1 bears the significance of a battle scar, rather than military draft evasion, being the direct result of antitumor immune pressure and TIL interferon γ production within the TME, as suggested by Kurt Schalper from the Yale School of Medicine [42].
While our study revealed significant correlations between OS and PD-L1 expression on both malignant cells and tumor infiltrating immune cells, only tumor infiltrating immune cells PD-L1 positivity was an independent predictor for a better outcome on multivariate analysis. A similar pattern was reported in a large-scale study on head and neck squamous cell cancer, in which tumor cell PD-L1 positivity did not alter prognosis significantly. In contrast, tumor infiltrating immune cells positivity did [43]. The authors raised the hypothesis of a potential role discrepancy of the PD-1/PD-L1 axis when expressed in tumor cells vs. TILs. The current understanding of the TME might not be sufficient to support this claim. Furthermore, our study did not reveal significant discrepancies regarding PD-L1 expression in the normal cholangiocytes or peri-tumoral immune cells. This suggests that whatever interactions occur on the checkpoint inhibitor level, they occur solely within the TME and might not be systemic.
Our design was also anchored on the clinical needs and thus is, to our knowledge, the largest study which included PD-L1 expression on pre-therapeutic biopsy specimens. Our data revealed significant discrepancies between resection and biopsy specimens regarding PD-L1 expression, which might have two potential explanations. The first follows the rationale discussed above, which correlates overexpression with a favorable prognosis, and, in extension, to less advanced disease at the time of diagnosis. Therefore, an acknowledged selection bias arose: biopsy specimens belonged to a clinical scenario in which patients required pathology for chemotherapy commencement. In contrast, resection specimens belonged to patients who were in theory eligible for curative intent surgery. A second hypothesis might suggest that the discrepancy between the groups might be explained by iCCA heterogeneity. Thus, the small tissue sample size on the biopsy specimen might lead to false-negative results. As the role of precision medicine expands, a future research direction might be to evaluate the utility of percutaneous CCA biopsy in providing specific data on TME using designs that compare either entire resection specimen analysis vs. random biopsy or pre-operative biopsy.
Another valuable characteristic of our study is the inclusion of one of the largest Western cohorts, given that most of the available studies on CCA (either intrahepatic, extrahepatic or in general) come from Asia, a geographic area with a significantly higher incidence of CCA, which exhibits different risk factors and, potentially differences in tumor biology [35].
Our results warrant a cautious approach. The study design has important limitations, some of which are inherent, some amendable by future research. Significant caveats might be generated by the retrospective monocentric design. There is a lack of data regarding the type of treatment administered to both Cohort A and B, which could influence the outcome of the patients and therefore, create a bias. Standard chemotherapy regimens provide median of a 6–8 months of OS [4]. We acknowledge that the lack of intermittent follow-up data (including disease-free survival, disease progression, systemic therapy, and therapeutic response) might generate concerns regarding the interpretation of our results. However, all the patients were further referred to oncology departments and benefited from standard therapy. Given the low palette of therapeutic options for iCCA, which at the time of enrollment consisted only of the gemcitabine/cisplatin combination [44], there is little room for bias regarding therapy selection. Furthermore, patients not eligible for potential therapy were not biopsied in the first place, as the risk/cost/benefit analysis was unfavorable. Therefore, treatment heterogeneity within cohort B is unlikely, regardless of the actual collection of the data. Regarding cohort A, the therapeutic approach is even more straightforward. Patients eligible for curative resection had no indication for adjuvant/neoadjuvant treatment, and any potential disease recurrence was treated according to the same protocol. None of the patients in our study have benefited from loco-regional therapies such as radiofrequency ablation or trans-arterial chemoembolization, as we do not use these methods for CCA treatment in our center. The correlation between PD-L1 levels and OS thus needs to be tested in large-scale prospective studies.
There might be concerns regarding the apparent reversal of the bench-to-bedside paradigm, given the lack of basic scientific evidence regarding the role of the PD-L1 axis in CCA. However, the past decade has brought to spotlight the translational gap phenomena, highlighting the low conversion rate from animal experimental-to-human-to-clinical models. Therefore, a potential solution to circumvent this caveat is to expand laterally what was previously a vertical research trajectory (cell-animal-human-clinical practice). Thus, whenever technically feasible and with a negligible risk profile, extracting as much basic science data from clinical scenarios appears reasonable, if not promising. The strength of our findings should be tested in further clinical trials [45, 46].
An additional shortcoming is the insufficient characterization of the TME, especially regarding the nature of the tumor infiltrating immune cells. However, both pathologist that analyzed the data had no difficulties in saying that tumor infiltrating lymphocytes were the ones positive for PD-L1 and responsible for a better OS. Given the complex nature and heterogeneity of TILs [47], an in-depth analysis might better explain both the results and their clinical significance.
Conclusion
While the iCCA TME Pandora's box has yet to be opened, our study is the first to bring evidence that tumor infiltrating immune cells PD-L1 expression on both resection and biopsy specimens might be a strong independent predictor for a favorable outcome in iCCA. Further large-scale, multicentric studies are undeniably needed before any solid conclusion can be made.
Abbreviations
- CCA
Cholangiocarcinoma
- iCCA
Intrahepatic cholangiocarcinoma
- OS
Overall survival
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- TIF
Tumor invasion front
- TILs
Tumor infiltrating lymphocytes
- TME
Tumor microenvironment
Author contributions
L.P.M., T.M., M.I., C.M.M. developed the concept of the study. L.P.M., Z.S., N.H., I.R. gathered the database. L.P.M., C.S.M, I.R. performed pathologic assessments. D.L., T.M., M.S., M.I, performed the statistical analysis, interpreted and sketched the results. L.P.M., R.C., C.G., T.M. drafted the manuscript. T.M, Z.S., N.H., C.M.M. supervised the findings and provided feedback for the article. All authors discussed the results and reviewed the manuscript.
Funding
Funding was provided by Universitatea de Medicină şi Farmacie Iuliu Haţieganu Cluj-Napoca (Grant No. 1530/58/18.01.2019).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Classification of Tumours Editorial Board . Digestive System Tumours, WHO Classification of Tumours. 5. World Health Organization: IARC Publications; 2019. [Google Scholar]
- 2.Endo I, Gonen M, Yopp AC, et al. Intrahepatic Cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 3.Banales JM, Cardinale V, Carpino G, et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 4.Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819–828. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Pang Z, Ding N, Dong W, Ma W, Li Y, Du J, Liu Q. The efficacy and potential predictive factors of PD-1/PD-L1 blockades in epithelial carcinoma patients: a systematic review and meta analysis. Oncotarget. 2016;7(45):74350. doi: 10.18632/oncotarget.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng F, Chen J. Application of immune checkpoint inhibitors in the treatment of Cholangiocarcinoma. Technol Cancer Res Treat. 2021;20:153303382110399. doi: 10.1177/15330338211039952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mou H, Yu L, Liao Q, et al. Successful response to the combination of immunotherapy and chemotherapy in cholangiocarcinoma with high tumour mutational burden and PD-L1 expression: a case report. BMC Cancer. 2018;18:1105. doi: 10.1186/s12885-018-5021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Yao J, Song L, Zhang S, Huang T, Li Y. Local and abscopal responses in advanced intrahepatic cholangiocarcinoma with low TMB, MSS, pMMR and negative PD-L1 expression following combined therapy of SBRT with PD-1 blockade. J Immunother Cancer. 2019;7(1):1–9. doi: 10.1186/s40425-019-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Tian L, Ma J, Ma L, et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J Surg Onc. 2020;18:303. doi: 10.1186/s12957-020-02082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabris L, Perugorria MJ, Mertens J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39:63–78. doi: 10.1111/liv.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu G, Sun L, Li Y, et al. The Clinicopathological and prognostic value of PD-L1 expression in Cholangiocarcinoma: a meta-analysis. Front Oncol. 2019;9:897. doi: 10.3389/fonc.2019.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monge C, Pehrsson EC, Xie C, et al. A phase II study of Pembrolizumab in combination with capecitabine and oxaliplatin with molecular profiling in patients with advanced biliary tract carcinoma. Oncologist. 2022;27:e273–e285. doi: 10.1093/oncolo/oyab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma K, Wei X, Dong D, et al. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol Lett. 2017;14:250–256. doi: 10.3892/ol.2017.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Wang X-Y, Zhang Y, et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses. CMAR. 2018;10:4113–4123. doi: 10.2147/CMAR.S172719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitano Y, Yamashita Y, Nakao Y, et al. Clinical significance of PD-L1 expression in both cancer and stroma cells of cholangiocarcinoma patients. Ann Surg Oncol. 2020;27:599–607. doi: 10.1245/s10434-019-07701-4. [DOI] [PubMed] [Google Scholar]
- 19.Walter D, Herrmann E, Schnitzbauer AA, et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71:383–392. doi: 10.1111/his.13238. [DOI] [PubMed] [Google Scholar]
- 20.Fontugne J, Augustin J, Pujals A, Compagnon P, Rousseau B, Luciani A, Tournigand C, Cherqui D, Azoulay D, Pawlotsky JM, Calderaro J. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(15):24644. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Squires MH, Woelfel I, Cloyd JM, Pawlik TM. Emerging treatment options for cholangiocarcinoma. Expert Opin Orphan Drugs. 2018;6:527–536. doi: 10.1080/21678707.2018.1476235. [DOI] [Google Scholar]
- 22.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti–PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. (Eds.) (2017) AJCC Cancer Staging Manual (8th edition), 8th ed. Springer Cham
- 24.Kriegsmann M, Roessler S, Kriegsmann K, et al. Programmed cell death ligand 1 (PD-L1, CD274) in cholangiocarcinoma – correlation with clinicopathological data and comparison of antibodies. BMC Cancer. 2019;19:72. doi: 10.1186/s12885-018-5254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng M, Li SH, Fu X, Yan XP, Chen J, Qiu YD, Guo RP. Relationship Between PD-L1 Expression, CD8+ T-Cell Infiltration and Prognosis in Intrahepatic Cholangiocarcinoma Patients. Cancer Cell Int. 2021;21(1):1. doi: 10.1186/s12935-021-02081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thuwajit P. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009 doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 27.Sirica AE, Campbell DJ, Dumur CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2011;27:276–284. doi: 10.1097/MOG.0b013e32834405c3. [DOI] [PubMed] [Google Scholar]
- 28.Kitano Y, Okabe H, Yamashita Y, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118:171–180. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Z-Y. Prognostic value of neutrophil distribution in cholangiocarcinoma. WJG. 2015;21:4961. doi: 10.3748/wjg.v21.i16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wongkham S. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010 doi: 10.3892/mmr_00000303. [DOI] [PubMed] [Google Scholar]
- 31.Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in Cholangiocarcinoma progression. Hepatology. 2021;73:75–85. doi: 10.1002/hep.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109:2665–2674. doi: 10.1038/bjc.2013.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura T, Yoshizawa T, Hirai H, Seino H, Morohashi S, Wu Y, Wakiya T, Kimura N, Kudo D, Ishido K, Toyoki Y. Prognostic impact of CD163+ macrophages in tumor stroma and CD8+ T-cells in cancer cell nests in invasive extrahepatic bile duct cancer. Anticancer Res. 2017;37(1):183–190. doi: 10.21873/anticanres.11304. [DOI] [PubMed] [Google Scholar]
- 34.Goeppert B, Frauenschuh L, Zucknick M, et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br J Cancer. 2015;113:1343–1349. doi: 10.1038/bjc.2015.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39:19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 36.Lim YJ, Koh J, Kim K, et al. High ratio of programmed cell death protein 1 (PD-1)+/CD8+ tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother Oncol. 2015;117:165–170. doi: 10.1016/j.radonc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Ye Y, Zhou L, Xie X, et al. Interaction of B7–H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–504. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- 38.Tamai K, Nakamura M, Mizuma M, et al. Suppressive expression of CD 274 increases tumorigenesis and cancer stem cell phenotypes in cholangiocarcinoma. Cancer Sci. 2014;105:667–674. doi: 10.1111/cas.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun C, Zhang L, Zhang W, et al. Expression of PD-1 and PD-L1 on tumor-infiltrating lymphocytes predicts prognosis in patients with small-cell lung cancer. OTT. 2020;13:6475–6483. doi: 10.2147/OTT.S252031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. OncoImmunology. 2014;3:e29288. doi: 10.4161/onci.29288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HR, Ha S-J, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Leenaars CHC, Kouwenaar C, Stafleu FR, et al. Animal to human translation: a systematic scoping review of reported concordance rates. J Transl Med. 2019;17:223. doi: 10.1186/s12967-019-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pound P, Ritskes-Hoitinga M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J Transl Med. 2018;16:304. doi: 10.1186/s12967-018-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41:127. doi: 10.1186/s13046-022-02340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]