Abstract
Background
Anti-PD-(L)1 blocking agents can induce immune-related adverse events (irAEs), which can compromise treatment continuation. Since circulating leukocyte–platelet (PLT) complexes contribute to inflammatory and autoimmune diseases, we aimed to analyze the role of these complexes as predictors of irAEs in non-small cell lung cancer (NSCLC) patients receiving anti-PD-(L)1.
Materials and methods
Twenty-six healthy donors (HD) and 87 consecutive advanced NSCLC patients treated with anti-PD-(L)1 were prospectively included. Percentages of circulating leukocyte–PLT complexes were analyzed by flow cytometry and compared between HD and NSCLC patients. The association of leukocyte–PLT complexes with the presence and severity of irAEs was analyzed.
Results
NSCLC patients had higher percentages of circulating leukocyte–PLT complexes. Higher percentages of monocytes with bound PLT (CD14 + PLT +) were observed in patients who received prior therapies while CD4 + T lymphocytes with bound PLT (CD4 + PLT +) correlated with platelets counts. The CD4 + PLT + high percentage group presented a higher rate of dermatological irAEs while the CD4 + PLT + low percentage group showed a higher rate of non-dermatological irAEs (p < 0.001). A lower frequency of grade ≥ 2 irAEs was observed in the CD4 + PLT + high percentage group (p < 0.05). Patients with CD4 + PLT + low and CD14 + PLT + high percentages presented a higher rate of grade ≥ 3 irAEs and predominantly developed non-dermatological irAEs (p < 0.01).
Conclusions
Our results suggest that circulating leukocyte–PLT complexes and the combination of CD4 + PLT + and CD14 + PLT + percentages can be used as a predictive biomarker of the development and severity of irAEs in advanced NSCLC patients receiving anti-PD-(L)1 agents.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02793-4) contains supplementary material, which is available to authorized users.
Keywords: Leukocyte–platelet complexes, irAEs, Immunotherapy, NSCLC
Introduction
Immune checkpoints are regulatory molecules of the immune system that play an important role in maintaining immune homeostasis and self-tolerance. The programmed cell death-1 receptor (PD-1) is an inhibitory co-receptor primarily expressed on the surface of activated T lymphocytes which, upon binding to the programmed cell death ligand-1 (PD-L1), modulates the T lymphocyte effector function, including proliferation, cytokine production and survival. In the tumor microenvironment, the PD-L1 expressed by tumor cells interacts with PD-1 to inhibit the effector function of T lymphocytes. The interruption of PD-1/PD-L1 signaling by anti-PD(L)-1 blockade agents can regenerate T-cell-mediated antitumor immunity, resulting in an increased response against tumor cells [1, 2]. Anti-PD(L)-1 blockade agents have improved the treatment outcome of several types of solid tumors, including advanced non-small cell lung cancer (NSCLC), either as monotherapy [3, 4] or in combination with chemotherapy and/or other immune-checkpoint blockade agents [5–7]. However, T-cell activation can induce inflammatory side effects, so-called immune-related adverse events (irAEs), which can appear in up to 80% of patients receiving anti-PD(L)-1 blockade agents. The most commonly reported irAEs are skin rash, pruritus, diarrhea, and thyroid disorders. Any organ or system can be involved and although generally mild, up to 25% of cases can be severe and may require immunosuppressors or treatment discontinuation [8]. An early detection of patients who will develop irAEs is crucial for their prompt management, especially for the most severe forms of irAEs [9–11].
The physiopathology of irAEs seems to differ between cutaneous and noncutaneous subtypes. While the cutaneous subtype is a reminiscent of a dermal hypersensitivity reaction (allergic-like), with a perivascular lymphocytic and eosinophilic infiltrates, nondermatological irAEs display more complex patterns of acute and chronic inflammation, which are similar to those found in autoimmune disorders with neutrophilic infiltrates and granulomas [12–15].
Inflammatory cytokines may be involved in the development of irAEs, since patients experiencing irAEs have shown elevated levels of inflammatory cytokines, and several irAEs have improved with anti-tumor necrosis factor (TNF) therapy. Also, low baseline IL-6 levels and increase in IL-6 after anti-PD-(L)1 therapy, as well as higher levels of IL-17, have been associated with a greater incidence of severe immune-related colitis [16–18].
Platelets (PLT) are essential components of hemostasis. However, PLT function is not restricted to the process of coagulation. Recently, it has been shown that PLT also play a role in cancer metastasis and have been recognized as an immunoregulatory cellular component. Upon activation, PLT release cytokines, chemokines, growth factors and platelet-derived microparticles which, in addition to the expression of activation molecules on their surface, cause the platelet to bind to the leukocyte [19–22]. Several molecules are involved in PLT–leukocyte binding, such as the interaction between P-selectin with PSGL-1, which appears to be essential for this union, as well as CD40–CD40L, GPIb–CD11b, and GPIIb/IIIa–CD11/CD18 [23]. The binding of PLT to leukocytes and the presence of certain platelet-derived soluble factors (such as TGFβ, PF4, sCD40L) decrease T cell proliferation and inflammatory cytokine production and increase IL-10 production by T lymphocytes and monocytes [21, 22, 24–26]. However, the interaction of PLT with monocytes does not always have an anti-inflammatory function. Patients with systemic inflammation or autoimmunity disorders present higher levels of monocyte–PLT complexes [27, 28]. Moreover, activated PLT have been shown to induce the expression of TNFα, IL-1β, IL-6, IL-8, IL-12, and MIP-1β on resting monocytes in vitro [29, 30].
The levels of circulating leukocyte–PLT complexes in cancer patients have not yet been studied. Taking into account their participation in inflammatory processes and the importance of predicting irAEs early, the aim of our study was to analyze the possible role of circulating leukocyte–PLT complexes levels as a predictor of irAEs development in advanced NSCLC patients receiving anti-PD-(L)1 blockade agents.
Materials and methods
Patients
Twenty-six healthy donors (HD) and 87 consecutive patients with advanced NSCLC treated at our institution with anti-PD-(L)1 blockade agents alone or in combination with chemotherapy, irrespective of treatment line, were prospectively included in this study from May 2015 to January 2019. The end of follow-up was September 2019. Written informed consent was obtained from each patient and ethical approval for the study was granted by the Institutional Ethics Committee. Patients’ data were collected from electronical medical records.
Immune-related adverse events
IrAEs were defined as any adverse event with a potential immunologic basis that required close monitoring and/or potential intervention with immunosuppressives or hormone replacement. Patients’ symptoms, physical exploration and laboratory data were assessed at every cycle. Thyroid function was evaluated at baseline and every six weeks thereafter. IrAEs severity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [31]. Clinical and analytical alterations were also prospectively recorded in all the visits.
For patients experiencing more than one irAE, the type and severity of the first irAE was used for the analysis of the 12-month grade ≥ 2 or grade ≥ 3 irAEs free survival and to calculate irAE severity. For the rest of analyses, all irAEs developed by each patient were considered.
Sample collection and whole blood staining
Whole blood samples from HD and NSCLC patients were collected in heparinized BD Vacutainer tubes (BD, Franklin Lakes, NJ) before starting anti-PD-(L)1 therapy. One-hundred microliters (µL) of whole blood were incubated with anti-CD3–PECy7 (clone HIT3a), anti-CD8–PercP (clone SK1), anti-CD19–PercP (clone SJ25C1) (Biolegend, San Diego, USA), anti-CD41a–FITC (clone HIP8), anti-CD62P–APC (clone HI62P) (Immunotools, Friesoythe, Germany) and anti-CD14–PECy7 (clone M5E2) (BD) monoclonal antibodies and the corresponding isotype controls. Then, red blood cells were lysed and white cells fixed using BD FACS lysing solution (BD Bioscience), washed with 2 mL of PBS 1X and resuspended in 400 µL of PBS 1X to be analyzed by flow cytometry.
Flow cytometry analysis
Lymphocytes were gated according to Forward scatter (FSC) and Side scatter (SSC). CD4 + and CD8 + T lymphocytes with bound PLT were identified on gated lymphocytes as CD3 + CD8–CD41a + (CD4 + PLT +) and CD3 + CD8 + CD41a + (CD8 + PLT +), respectively. B lymphocytes and NK cells with bound PLT were identified on gated lymphocytes as CD19 + CD41a + (CD19 + PLT +) and CD3–CD8 + (NK PLT +), respectively. Monocytes were gated according to CD14 expression (CD14 +) and SSC. Neutrophils were gated as SSC high CD14 − cells. Monocytes and neutrophils with bound PLT were identified as CD14 + CD41a + (CD14 + PLT +) and SSC high CD14 + CD41a + (Neutrophils PLT +). To analyze PLT and activated PLT in blood, samples were acquired using logarithmic FSC and SSC. Blood PLT were identified as CD41a + events and activated PLT as CD41a + CD62P + (PLTCD62P +) events. To determine the patients with high and low levels of CD4 + PLT + or CD14 + PLT + complexes, we calculated the cutoffs of 95% of confidence interval of HD values. These cutoffs exclude the 95% of HD values in a normal distribution. Statistically, the 95% of confidence interval is equal to the mean plus 1.96 standard deviations. Samples were acquired with the MACSQuant Analyzer 10 flow cytometer (Miltenyi Biotec, Bergisch Galdbach, Germany). The percentage of positive cells (% cells) and event/µL for each population was obtained using FlowJo version X (FlowJo LLC, Ashland, USA).
Determination of IFNɣ, IL-10, and IL-17 in plasma
Plasma concentrations of IFNɣ (Mabtech, Nacka Strand, Sweden), IL-10 (Immunotools) and IL-17 (Peprotech, London, UK) were determined using specific ELISA kits according to the manufacturers’ instructions and using the specific standard curves of recombinant molecules. The limits of detection were: 4 pg/mL for IFNɣ, 16 pg/mL for IL-10, and 10 pg/mL for IL-17.
Statistical analysis
The Kolmogorov–Smirnov test was used to analyze data with normal distribution. To describe our population, numbers and percentages were used for qualitative variables, while the median (interquartile ranges, IQR) was calculated for ordinal and quantitative variables with an asymmetric distribution. Comparisons between groups were tested with the Student’s t or the Mann–Whitney test, according to a Gaussian distribution. ANOVA and Kruskal–Wallis tests were used for comparisons between more than two groups. Fisher and Chi-square tests were used for the comparison of frequencies. Correlation analyses were carried out with Pearson’s or Spearman correlations. The long-rank Mantel Cox test was used to analyze differences in irAEs grade during the follow-up period.
All p values were based on a two-sided hypothesis, and those under 0.05 were considered statistically significant. All the analyses were performed using Graph Pad Prism 7 software.
Results
Patient characteristics
The median age of advanced NSCLC patients was 66.29 years [IQR 60.28–73.38], 75.86% were males and 24.14% were female. The majority of patients were current or former smokers (94.25%) and baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0–1 in 82.75% of patients. Non-squamous was the most common histology (70.12%).
Twenty-three patients (26.43%) received anti-PD-(L)1 blockade agents in first line therapy and 64 (73.57%) in second line or beyond. Anti-PD-(L)1 blockade agents were given alone in 80 patients (92%), and in combination with chemotherapy in 7 patients (8%). No differences in patient characteristics were observed according to the presence of irAEs.
Table 1 shows the characteristics of the patients included in this study. Median follow-up was 13.56 months [IQR: 7–18.46].
Table 1.
Patient characteristics and comparison according to the presence of irAEs
| Category |
Total n = 87 (%) |
irAEs n = 64 (%) |
No irAEs n = 23 (%) |
p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 66 (75.86) | 48 (75) | 18 (78.26) | 0.49 |
| Female | 21 (24.14) | 16 (25) | 5 (21.74) | |
| Age, median [range] | 66.29 [36.98–85.34] | 66.36 [53.56–83.73] | 66.27 [36.98–66.27] | 0.97 |
| Smoking status | ||||
| Non- or light smoker | 5 (5.75) | 4 (6.25) | 1 (4.35) | 0.23 |
| Current or former smoker | 82 (94.25) | 60 (93.75) | 22 (95.65) | |
| ECOG PS | ||||
| 0–1 | 72 (82.75) | 55 (85.93) | 17 (73.91) | 0.12 |
| 2 | 15 (17.25) | 9 (14.07) | 6 (26.09) | |
| PD-L1 expressiona | ||||
| Low (1–49%) | 22 (31.88) | 18 (28.13) | 4 (17.39) | 0.58 |
| High (≥ 50%) | 24 (34.79) | 19 (29.69) | 5 (21.75) | |
| Negative (< 1%) | 23 (33.33) | 16 (25) | 7 (30.43) | |
| Histologic subtype | 0.19 | |||
| Squamous | 26 (29.88) | 17 (26.57) | 9 (39.14) | |
| Non-squamous | 61 (70.12) | 47 (73.43) | 14 (60.86) | |
| Treatment line | 0.38 | |||
| 1st line | 23 (26.43) | 21 (32.82) | 5 (21.74) | |
| ≥ 2nd line | 64 (73.57) | 43 (67.18) | 18 (78.26) | |
| Anti-PD-(L)1 blockade agents | 0.38 | |||
| Nivolumab | 32 (36.78) | 21 (32.81) | 11 (47.83) | |
| Pembrolizumab | 35 (40.22) | 27 (42.19) | 8 (34.78) | |
| Atezollizumab | 15 (17.25) | 11 (17.19) | 4 (17.39) | |
| Avelumab | 5 (5.76) | 5 (7.81) | 0 | |
| ICB schedule | 0.26 | |||
| Monotheraphy | 80 (92) | 60 (93.75) | 20 (86.95) | |
| Combination with chemotherapy | 7 (8) | 4 (6.25) | 3 (13.05) | |
| Prednisone equivalent ≥ 10 mg/daily use | 0.39 | |||
| No | 49 (56.32) | 37 (57.8) | 12 (52.2) | |
| Yes | 38 (43.68) | 27 (42.2) | 11 (47.8) | |
| Pre-ICB initiation | 9 (23.68) | |||
| Post-ICB initiation | 29 (76.32) |
irAEs immune-related adverse events, ECOG PS Eastern Cooperative Oncology Group performance status, ICB Immune-checkpoint blockade
aPD-L1 expression analysis in tumor samples was available from 69 patients (79.1%), and missing in 11 patients (17.18%) in the irAEs group and 7 patients (30.43%) in the non-irAEs group
IrAE characteristics
Sixty-four patients (73.56%) experienced a total of 121 irAEs. The median number of irAEs per patient was 1 [IQR 1–3], and 29 patients (33.33%) experienced two or more irAEs. The most common irAE subtypes were pruritus (34.49%), rash (26.43%), diarrhea (24.14%) and hypothyroidism (11.49%).
According to CTCAE version 4.0, 86 irAEs (71.08%) were grade 1, 24 (19.83%) were grade 2, 7 (5.79%) were grade 3, 2 (1.65%) were grade 4 and 2 (1.65%) were grade 5. The characteristics of irAEs are described in Table 2.
Table 2.
Description of immune-related adverse events
| Types of irAEs | All patients, n = 87 (%) | Median onset time [range], weeks | ||
|---|---|---|---|---|
| All grades n = 121a |
Grade 3–5 n = 11 |
irAEs requiring prednisone equivalent ≥ 10 mg/d n = 18d |
||
| Cutaneous | ||||
| Rash | 23 (26.43) | 0 | 1 (1.15) | 6.35 [0.28–87] |
| Pruritus | 30 (34.49) | 0 | 2 (2.29) | 6 [0.28–145] |
| Diarrhea | 21 (24.14) | 2 (2.29) | 3 (3.44) | 7 [0.28–145] |
| Endocrine dysfunction | ||||
| Hypothyroidism | 10 (11.49) | 0 | 0 | 11.14 [3.50–106] |
| Hyperthyroidism | 4 (4.59) | 0 | 1 (1.15) | 8.07 [4–22] |
| Adrenal insufficiency | 1 (1.15) | 1 (1.15) | 1 (1.15) | 23 |
| Pneumonitis | 5 (5.75) | 4b (4.59) | 4 (4.59) | 28 [3.14–36] |
| Hepatic toxicity | 6 (6.89) | 3c (3.44) | 3 (3.44) | 8.7 [2–28] |
| Mucositis | 2 (2.29) | 1 (1.15) | 0 | 2.14 [1.57–2.71] |
| Arthritis | 9 (10.34) | 0 | 2 (2.29) | 12 [2.71–119] |
| Other | 9 (10.34) | 0 | 1 (1.15) | |
| Flu-like | 3 | 2.85 [0.14–15.57] | ||
| Nephritis | 1 | 5.85 | ||
| Vitiligo | 1 | 10 | ||
| Myositis | 2 | 2.57 [2.42–2.71] | ||
| Infusion reaction | 2 | 0 | ||
irAEs immune-related adverse events. Percentages may add up to more than 100 because some patients experienced more than one event
aTotal number of irAEs
bTwo cases of pneumonitis were grade 5
cOne case of hepatic toxicity was grade 4
dTotal number of irAEs requiring ≥ 10 mg of prednisone equivalent daily is greater than total number of patients who required them because some patients experienced more than one event
Advanced NSCLC patients showed higher percentages of circulating leukocyte–PLT complexes
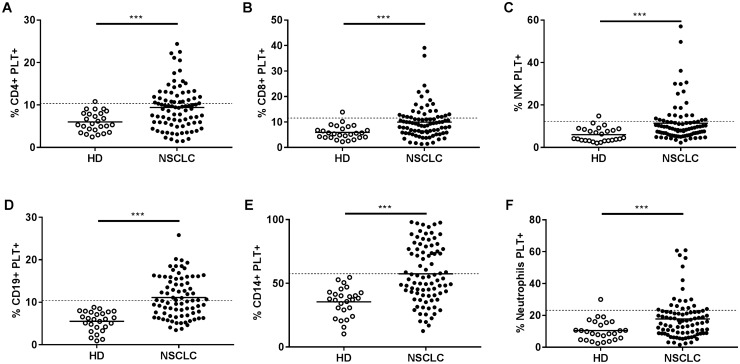
There were higher percentages of circulating subpopulations of leukocytes (neutrophils, monocytes, NK cells, CD4 + , and CD8 + T lymphocytes and CD19 + B lymphocytes) with bound PLT in advanced NSCLC patients compared with HD (Fig. 1). The percentages of circulating CD19 + B cells, NK cells, CD4 + , and CD8 + T lymphocytes with bound PLT correlated strongly to each other (Spearman correlations: r = 0.88, p < 0.0001 for % CD4 + PLT + vs % CD8 + PLT + ; r = 0.75, p < 0.0001 for % CD4 + PLT + vs % CD19 + PLT + ; r = 0.64, p < 0.0001 for % CD4 + PLT + vs % NK PLT + ; r = 0.64, p < 0.0001 for % CD8 + PLT + vs % NK PLT + ; r = 0.72, p < 0.0001 for % CD8 + PLT + vs % CD19 + PLT + ; r = 0.65, p < 0.0001 for % NK PLT + vs % CD19 + PLT +). We also observed a correlation between the percentage of CD14 + PLT + and neutrophil PLT + complexes (Spearman correlation: r = 0.54, p < 0.0001). Since the percentage of circulating CD4 + PLT + complexes correlated strongly with the other lymphocyte subpopulations with bound PLT, and the percentage of circulating CD14 + PLT + complexes correlated with neutrophils PLT + complexes, we limited the following analysis to the association of circulating CD4 + PLT + and CD14 + PLT + complexes, with clinical parameters and irAEs as the representative lymphoid and myeloid lineage cells, respectively.
Fig. 1.
Percentage of circulating leukocyte–PLT complexes in advanced NSCLC patients and HD. Dot plots show the percentage of circulating a CD4 + , b CD8 + , c NK, d CD19 + , e CD14 + , and f neutrophils with bound platelets (PLT +) obtained by HD flow cytometry (n = 26) and NSCLC (n = 87). Values of CD19 + PLT + from eight patients were missing. The data are presented as means. The dotted line in each graph represents the mean plus two standard deviation of HD values. The statistical analysis was performed using the t test (CD4 + PLT + , CD19 + PLT + , CD14 + PLT +) and the Mann–Whitney test (CD8 + PLT + , NK PLT + , Neutrophils PLT +). ***p < 0.001
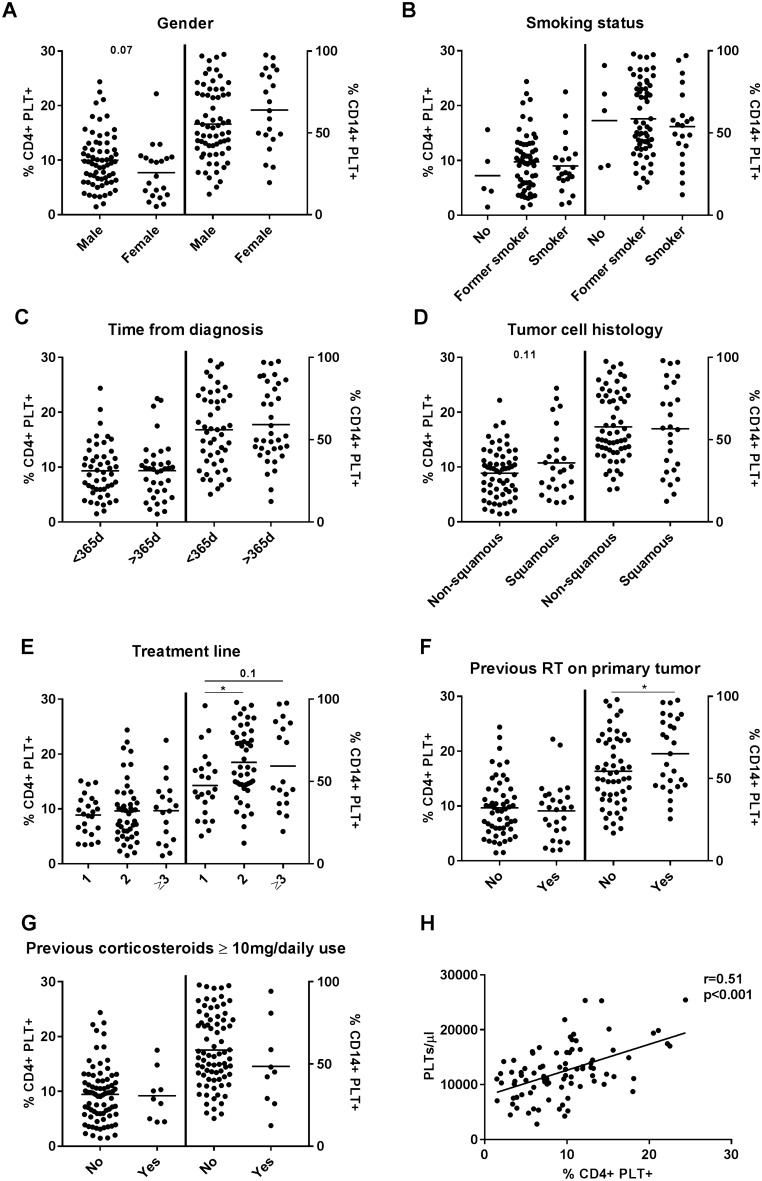
No differences in the percentages of baseline circulating CD4 + PLT + complexes were observed according to gender, smoking status, time from diagnosis, tumor cell histology and previous treatment with corticoesteroids (Fig. 2). However, increased percentages of circulating CD14 + PLT + complexes were observed in NSCLC patients previously treated with chemotherapy or radiotherapy (Fig. 2e, f). The percentage of circulating CD4 + PLT + complexes correlated with PLT blood levels in advanced NSCLC patients (Fig. 2h).
Fig. 2.
Percentage of circulating CD4 + PLT + and CD14 + PLT + according to clinical and demographic characteristics of advanced NSCLC patients. Dot plots show the percentage of CD4 + (left axes) and CD14 + (right axes) PLT complexes (PLT +) according to a gender, b smoking status, c time from diagnosis, d tumor cell histology, e treatment line, f previous radiotherapy on primary tumor, and g corticosteroids previous to anti PD-(L)1 treatment. h Correlation of percentage of CD4 + PLT + and PLT/µl in NSCLC patients. The statistical analysis was performed using the t test. Pearson’s correlation was performed for the correlation analysis. *p < 0.05
Percentages of circulating CD4 + PLT + complexes differed according to irAE type and severity
We analyzed the association between circulating CD4 + PLT + and CD14 + PLT + complexes and the development of irAEs. Percentages of circulating CD4 + PLT + and CD14 + PLT + complexes were comparable regarding the presence of irAEs, severity or number of irAEs per patient (Supplementary Figs. 1a–f).
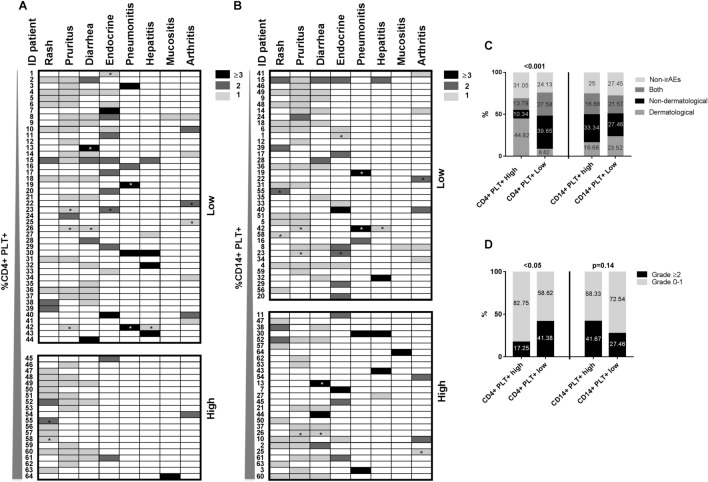
To assess the association between the percentages of circulating CD4 + PLT + or CD14 + PLT + complexes and the type of irAEs and their grade, we represented NSCLC patients who developed any irAE (n = 64) in a heat map in which patients were segregated in CD4 + PLT + high (n = 20) and CD4 + PLT + low (n = 44), or in CD14 + PLT + high (n = 27) and CD14 + PLT + low (n = 37), taking 10.6% as a cutoff for CD4 + PLT + complexes and 58.07% as a cutoff for CD14 + PLT + complexes (after computing the 95% confidence interval of samples from HD as indicated in Materials and Methods). We observed that CD4 + PLT + high patients presented a greater incidence of dermatological irAEs (CD4 + PLT + high: 17 out of 20; and CD4 + PLT + low: 22 out of 44 p = 0.01). However, CD4 + PLT + low patients presented a higher incidence of nondermatological irAEs that were grade ≥ 2 (CD4 + PLT + high: 4 out of 20 and CD4 + PLT + low: 21 out of 44; p = 0.05) (Fig. 3a). No differences were observed between CD14 + PLT + high and low patients (Fig. 3b).
Fig. 3.
The percentage of circulating CD4 + PLT + was associated with the type and grade of irAEs development. Heat maps show the type and grade of irAEs in advanced NSCLC patients previously ordered and represented from lower to higher percentages of a CD4 + PLT + and b CD14 + PLT + . Each patient was identified in both heat maps with the same ID. Stacked bars show the percentage of c the type of irAEs grouped as dermatological, non-dermatological or both and d the grade of irAEs in CD4 + PLT + high (n = 29), CD4 + PLT + low (n = 58), CD14 + PLT + high (n = 36) and CD14 + PLT + low (n = 51) groups of patients. Patients marked with an asterisk had less than 6 months of follow-up. The Chi-square test was used to analyze differences in frequencies of irAEs types and the Fisher test was used to analyze differences in frequencies of irAE grades between different groups of NSCLC patients. NSCLC patients were classified as CD4 + PLT + high and CD4 + PLT + low, or in CD14 + PLT + high and CD14 + PLT + low according to the cut-offs of 95% of confidence interval of HD values (10.6% for CD4 + PLT + complexes and 58.07% for CD14 + PLT + complexes)
The frequency distribution of patients without irAEs, with only dermatological irAEs, with only non-dermatological irAEs or with both types of irAEs was different between patients with CD4 + PLT + high and low percentages but not between patients with CD14 + PLT + high and low percentages (Fig. 3c). The proportion of patients with grade of irAEs ≥ 2 was higher in the CD4 + PLT + low group than in the CD4 + PLT + high group (Fig. 3d).
Advanced NSCLC patients with circulating CD4 + PLT + high and CD14 + PLT + low percentages only developed grade ≤ 1 dermatological irAEs
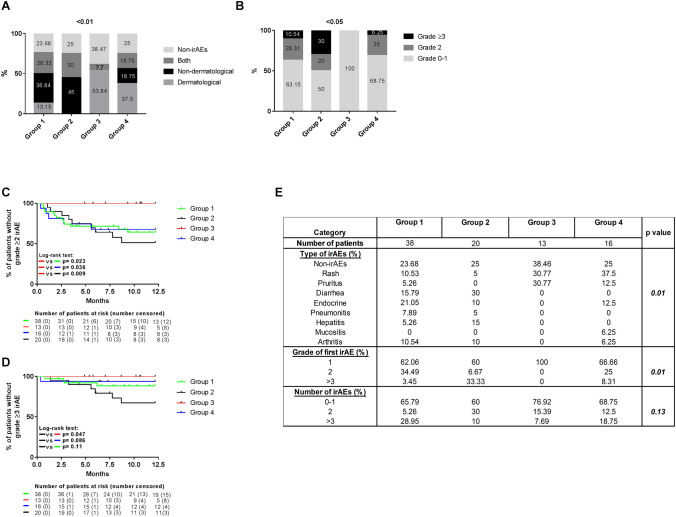
We then grouped patients into four groups according to the percentage of both CD4 + PLT + and CD14 + PLT + complexes: Group 1) CD4 + PLT + low and CD14 + PLT + low percentages (n = 38), Group 2) CD4 + PLT + low and CD14 + PLT + high percentages (n = 20), Group 3) CD4 + PLT + high and CD14 PLT + low percentages (n = 13) and Group 4) CD4 + PLT + high and CD14 + PLT + high percentages (n = 16). No clinical or demographic differences were observed between these four groups (Supplementary Table 1). There were differences in the frequency distribution of patients without irAEs, with only dermatological irAEs, with only non-dermatological irAEs or with both types of irAEs between the four groups (Fig. 4a). Patients in Group 3 had the lowest rate of irAEs and the highest frequency of dermatological irAEs. In contrast, patients in Group 2 developed more non-dermatological irAEs (Fig. 4a). With regard to the severity of irAEs, Group 2 had the highest rate of grade ≥ 3 irAEs, whereas all irAEs were grade 0–1 in Group 3 (Fig. 4b).
Fig. 4.
Differences in type and grade of irAEs in advanced NSCLC patients grouped according to circulating CD4 + PLT + and CD14 + PLT + complexes. The stacked bar shows a the type of irAEs grouped as dermatological, non-dermatological, or both and b the maximum grade of irAEs developed by each patient in the four groups of advanced NSCLC patients: Group 1 with CD4 + PLT + low and CD14 + PLT + low percentages (n = 38), Group 2 with CD4 + PLT + low and CD14 + PLT + high percentages (n = 20), Group 3 with CD4 + PLT + high and CD14 + PLT + low percentages (n = 13) and Group 4 with CD4 + PLT + high and CD14 + PLT + low percentages (n = 16). The staircase graph shows the percentage of patients c free of grade ≥ 2 or d grade ≥ 3 in the four groups. e The table shows the percentages of irAE types, the grade of the first irAE and number of irAEs during the 12 months of follow-up. The Chi-square test was used for the statistical analysis of frequencies and the log-rank Mantel–Cox test was used for the analysis of irAEs types during the 12 months of follow-up
We also analyzed the presence of grade ≥ 2 irAEs in the first 12 months of follow-up. No patient in Group 3 had grade ≥ 2 irAEs (Group 1: HR = 3.96, p = 0.023; Group 2: HR = 5.81, p = 0.009 and Group 4: HR = 6.56, p = 0.036, when compared with Group 3) (Fig. 4c). Grade ≥ 3 irAEs were observed in 32.81% of patients in Group 2, 11.58% in Group 1 and in 6.25% in Group 4 in the first 12 months of follow-up (Group 1: HR = 2.92, p = 0.11; Group 3: HR = 5.26, p = 0.047 and Group 4: HR = 5.19, p = 0.086, when compared with Group 2) (Fig. 4d).
Significant differences in irAE subtypes were found between these four groups (p = 0.012): patients in Group 3 only developed skin rash and pruritus, skin rash predominated in Group 4, endocrine alterations and diarrhea in Group 1, and diarrhea and hepatitis in Group 2 (Fig. 4e).
CD4 + PLT + percentages correlated with PLT levels and ratio of PLT/CD4 + T lymphocytes
To better understand the relationship between circulating leukocyte–PLT complexes, PLT characteristics and leukocyte levels, we compared the count/µL of CD4 + T lymphocytes, CD14 + monocytes and PLT, the percentage and the count/µL of activated PLT (PLT CD62P +) and the ratio of PLT to CD4 + T lymphocytes or CD14 + monocytes between patients with and without irAEs and between the four groups of advanced NSCLC patients. PLT/µL, the percentage and counts/µL of activated PLT and the ratios of PLT/CD4 + and PLT/CD14 + were similar according to the development of irAEs (Supplementary Figs. 2a–e).
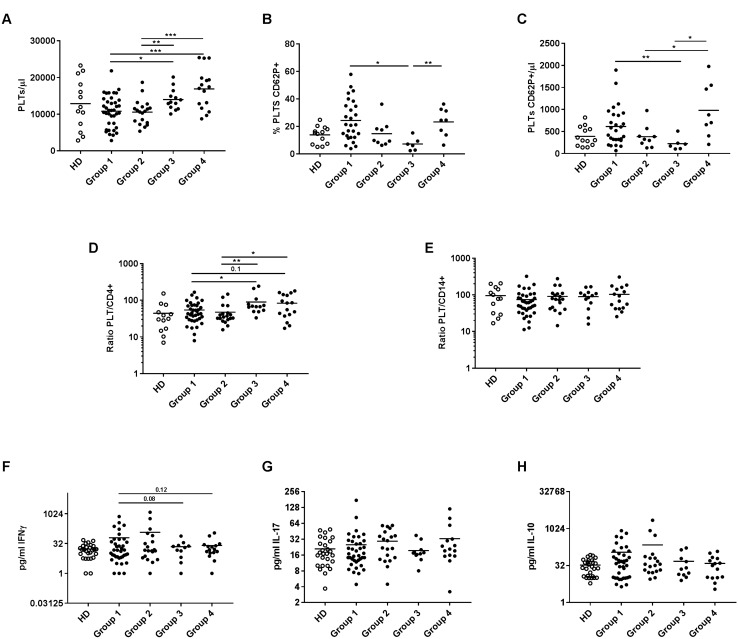
The counts/µL of CD14 + monocytes and CD4 + T lymphocytes were comparable in the four groups (data not shown). We observed a superior PLT/µL and a higher ratio of PLT/CD4 + T in Groups 3 and 4 (Fig. 5a, d). However, Group 3 had the lowest percentage and counts/µL of activated PLT (Fig. 5b, c). No differences in the ratio of PLT/CD14 + were observed between groups (Fig. 5e).
Fig. 5.
PLT blood levels, activated PLT, ratio of PLT/CD4 + and PLT/CD14 + and plasmatic cytokines in four groups of NSCLC patients grouped according to both CD4 + PLT + and CD14 + PLT + prior to anti-PD-(L)1 therapy. The dot plots show a PLT/µL, b percentage of activated PLT (PLT CD62P +) and c PLT CD62P + /µL in the four groups of advanced NSCLC patients: Group 1 with CD4 + PLT + low and CD14 + PLT + low percentages (n = 38), Group 2 with CD4 + PLT + low and CD14 + PLT + high percentages (n = 20), Group 3 with CD4 + PLT + high and CD14 + PLT + low percentages (n = 13) and Group 4 with CD4 + PLT + high and CD14 + PLT + low percentages (n = 16). Values of percentages of PLT CD62P + and PLT CD62P + /µL from 30 NSCLC patients were missing. Ratio of d PLT/CD4 + T lymphocytes and e PLT/CD14 + monocytes of four groups of NSCLC patients are shown. Levels (pg/mL) of f IFNɣ g IL-17 and h IL-10 in the four groups of NSCLC patients are shown. An unpaired t test was used for the analysis of PLT/µL, % PLT CD62P + and PLT CD62P + /µL; and the Mann–Whitney test for the analysis of the PLT/CD4 + , PLT/CD14 + , IFNɣ, IL-17 and IL-10 ratio. *p < 0.05 and **p < 0.01
To analyze the association between the pro- and anti-inflammatory cytokine profile of patients with and without irAEs and between the four groups of advanced NSCLC patients, we determined plasmatic IFNɣ, IL-17, and IL-10 concentrations. IFNɣ, IL-17, and IL-10 levels were comparable according to the development of irAEs (Supplementary Figs. 2f–h). No significant differences were observed in IL-17 or IL-10 levels between the four groups (Fig. 5g, h). However, a trend to higher IFNγ levels were observed in Groups 1 and 2 compared with the other two groups (Fig. 5f).
Discussion
This is the first time that circulating leukocyte–PLT complexes have been identified and quantified in patients with cancer. Here, we have shown that advanced NSCLC patients have a higher percentage of circulating leukocyte–PLT complexes. Our findings suggest that these complexes may play a significant role in the development of irAEs in advanced NSCLC patients receiving anti-PD-(L)1 blockade agents. Moreover, our results suggest that the combination of circulating CD4 + PLT + and CD14 + PLT + percentages can also predict the development and severity of irAEs. We focused our analysis on circulating leukocyte–PLT complexes because there is a close relation between PLT and cancer. On the one hand, it has been reported that tumor cells can activate PLT by both secreting soluble factors and by direct contact [32]. On the other hand, PLT have been shown to participate in the development of cancer and metastasis by different mechanisms; first, the binding of PLT to tumor cells favors the induction of tumor cells into the epithelial–mesenchymal transition invasive phenotype; second, PLT facilitate tumor spreading by confering protection against NK cell attack [33–35].
We found that higher blood PLT counts were associated with higher percentages of lymphocyte–PLT complexes in NSCLC patients. This may be explained by the fact that PLT circulate in an activated state expressing higher levels of the molecules essential for them to bind to leukocytes [23]. One interesting finding in our cohort was that pretreated NSCLC patients had an increased percentage of CD14 + PLT + . In line with this, it has been reported that cisplatin triggers PLT activation and chemotherapy may therefore increase the percentage of circulating leukocyte–PLT complexes [36].
A significant increase in circulating CD4 + PLT + and CD14 + PLT + complexes was found in advanced NSCLC patients, even though there was no correlation between the percentage of both types of complexes. This finding can be explained by the fact that the binding of PLT to lymphocytes or to monocytes can involve different receptors and, moreover, binding could be different in patients with malignant tumors and HD. The binding of PLT to activated CD4 + T lymphocytes involves the following receptor–ligand pairs: P-selectin–PSGL1, P-selectin–ALCAM, GPVIIb/IIIa–Mac1, and CD40L–CD40 ligation [37, 38]. However, the binding of PLT to monocytes involves P-selectin–PSGL1, P-selectin–CD15, GPIIbβ3–MAC1, GPIb–Mac1, JAM-C–Mac1, CD40L–CD40, TREM1L–TREM1, and CD36–trombospondin–CD36 ligations, in addition to the CD147 pathway [39, 40]. Variations in PSGL-1 glycosylation can also explain some differences in the percentages of monocyte and lymphocyte–PLT complexes. The reason is that unlike monocytes, which have the capacity to bind to P-selectin [41], only lymphocytes that express alpha3 fucosyl transferase, and which therefore have fucosylated PSGL-1, can bind to P-selectin [42].
We observed differences in the type and severity of irAEs according to the percentages of circulating CD4 + PLT + complexes. When this percentage was low, the predominant irAEs were severe non-dermatological irAEs. A possible explanation to this finding is that the binding of PLT downregulates the proliferation and production of IFNγ by T cells, which is in agreement with our previous results in chronic inflammatory patients. Those inflammatory patients with a low percentage of CD4 + PLT + complexes had the highest levels of pro-inflammatory IFNγ and IL-17 and the lowest levels of anti-inflammatory IL-10 in plasma [21], suggesting a predominant Th1 response. Considering that the development of irAEs is associated with the immune checkpoint blockade response [43] and that a Th1 response with IFNγ is crucial to achieving an effective antitumor response [44], the Th1 shift originated by less binding of PLT to CD4 lymphocytes would lead to an increased development of irAEs.
It is important to underline that we found that circulating CD4 + PLT + complexes, but not CD14 + PLT + complexes, were independently associated to the type and severity of irAEs. Differences between these two complexes could explain this finding. The binding of PLT to lymphocytes or monocytes involves molecules with different intracellular signaling and gene expression. However, not only are the receptors ligands different for PLT and lymphocytes and monocytes, but each type of complex also has a distinctive effect, and even have opposite immunological effects on lymphocytes and monocytes. We have previously reported that the binding of PLT to lymphocyte decreases T lymphocyte proliferation and pro-inflammatory cytokine production while increasing IL-10 production in HD and in patients with chronic inflammatory diseases [21, 22]. With regard to CD14 + PLT + complexes, a dual role of PLT was found depending on monocyte status. In HD, we found that the binding of PLT to toll-like receptor (TLR)-activated monocytes decreased TNFα and increased IL-10 production [24]. However, it has been also reported that the binding of activated PLT in resting monocytes in vitro induces the expression of TNFα, IL-1β, IL-6, IL-8, IL-12, and MIP-1β [29, 30]. A possible explanation is that PLT also secrete soluble factors that could have different effects on T lymphocytes and monocytes [21, 22, 26]. Therefore, the binding of PLT to lymphocytes but not to monocytes, would indicate an anti-inflammatory context. Our results are in line with this hypothesis since patients with CD4 + PLT + low and CD14 + PLT + high percentages were the group with higher levels of plasmatic IFNɣ and IL-17, in addition to the lowest PLT/CD4 + ratio. Morover, this group had more nondermatological irAEs, and a higher percentage of grade ≥ 3 irAEs. These findings suggest that those patients would require strict monitoring. Other studies in melanoma have already reported an upregulation of IL-17 and other cytokines at baseline in patients with severe irAEs [16, 45]. In contrast, patients with high percentages of CD4 + PLT + and low percentages of CD14 + PLT + made up the group with the lowest rate and severity of toxicity, which may be explained by a greater modulation of the inflammatory response. Taking all these considerations into account, circulating leukocyte–PLT complexes could serve as a predictive biomarker of the irAEs subtype depending on the underlying pathophysiological mechanism (allergic-like or nonautoimmune, and autoimmune), as previously mentioned. In addition, we have with a quick, non-invasive method to identify at baseline a group of patients that is more likely to present severe toxicity in order to provide them with a closer follow-up, which gives an extrapotential value to our study.
Nervertheless, there are some limitations to our study. First of all, the classification of irAEs is fundamentally clinical, and its translation into immunological terms is complex, which makes it difficult to fully assess the correlation between circulating leukocyte–PLT complexes and the development of immune-related toxicity. Another limitation is that the heterogeneity of irAEs subtypes, the broad range of their severity and the fact that many patients developed more than one type of irAE, may also hinder the analysis of these results. Also, the percentages of circulating leukocyte–PLT complexes were only analyzed at baseline,and may change during anti-PD-(L)1 therapy. Finally, an extended cohort of patients with a longer follow-up which may help to detect those irAEs that can appear later, would be required to validate our findings in a prospective study, overcome the afore-mentioned limitations and adequately assess the real impact of the percentage of circulating leukocyte–PLT complexes by accounting for potential confounding factors.
In conclusion, the combination of the percentage of circulating CD4 + PLT + and CD14 + PLT + complexes at baseline may serve as a predictive biomarker of irAEs subtypes and severity in advanced NSCLC patients receiving anti-PD-(L)1 blockade agents, which may prove useful for detecting the patients most likely to present severe toxicity and who may require closer monitoring.
Conclusions
We found that the percentages of circulating leukocyte–PLT complexes were higher in advanced NSCLC patients before starting anti-PD-(L)1 therapy. Increased percentages of circulating CD14 + PLT + complexes were observed in pretreated NSCLC while percentages of circulating CD4 + PLT + correlated with PLT blood levels. Patients with low percentages of circulating CD4 + PLT + complexes showed a higher frequency of non-dermatological and grade ≥ 2 irAEs. Patients with high percentages of CD4 + PLT + and low percentages of CD14 + PLT + had the lowest rate of irAEs, showing ≤ grade 1 dermatological irAEs. In contrast, patients with low levels of circulating CD4 + PLT + and high levels of CD14 + PLT + made up the group of patients with the highest frequency and severity of nondermatological irAEs. Our results suggest that the binding of PLT to leukocytes may play a role in advanced NSCLC and that the combination of the percentage of circulating CD4 + PLT + and CD14 + PLT + complexes can be used as a predictive biomarker for irAEs subtypes and severity in advanced NSCLC patients receiving anti-PD-(L)1 agents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file2 Supplementary Fig.1 Association of percentage of circulating CD4+PLT+ and CD14+PLT+ with the presence of irAEs. Percentage of CD4+PLT+ and CD14+PLT+ complexes in (A-B) non-irAEs vs irAEs groups, (C-D) grade of irAEs (0-≥3) and (E-F) number of irAEs per patient. The statistical analysis was performed using the t-test. *p<0.05 (TIF 484 kb)
Supplementary file3 Supplementary Fig. 2 The association of platelet blood levels, the percentage and levels of activated platelets, the ratio of PLT/CD4+ and PLT/CD14+ and plasmatic levels of IFNɣ, IL-17 and IL-10 with irAE manifestation. The dot plots show (A) Platelet blood levels (PLT/µL), (B) the percentage of activated platelets, (C) the levels of activated platelets (PLT CD62P+/µL), (D) the ratio of PLT/CD4+, (E) the ratio of PLT/CD14+, pg/mL of (F) IFNɣ, (G) IL-17 and (H) IL-10 according to non-irAE and irAE groups. An unpaired t-test was used for the analysis of PLT/µL, % PLT CD62P+ and PLT CD62P+/µL, and the Mann-Whitney test for the analysis of the PLT/CD4+, PLT/CD14+, IFNɣ, IL-17 and IL-10 ratios (TIF 604 kb)
Acknowledgements
SV was supported by “Fondo Investigaciones Sanitarias” and a participant in the Program for Stabilization of Investigators the “Direcció i d’Estrategia i Coordinació del Departament Salut de la Generalitat de Catalunya.”
Abbreviations
- ECOG
Eastern Cooperative Oncology Group
- FSC
Forward scatter
- HD
Healthy donors
- irAEs
Immune-related adverse events
- IQR
Interquartile ranges
- NSCLC
Non-small cell lung cancer
- PD-1
Programmed cell death-1 receptor
- PD-L1
Programmed cell death ligand-1
- PLT
Platelets
- PS
Performance status
- SSC
Side scatter
Author contribution
All authors were involved in revising intellectual content, and all authors approved the final version to be published. CZ, LPS, LAL and MAO performed cellular staining and flow cytometry analysis and ELISAs. CZ and LPS and MAO analysed results of flow cytometry and ELISAS; MR, GAP, IS, ABJ, JSL, OG, JGD, ABM, and MMT collected samples and clinical data; CZ and SVA performed statistical analysis. CZ, MR, MMT, and SVA wrote the manuscript. MMT and SVA design the study.
Funding
This work was supported by the Bristol Myers Squibb.
Compliance with ethical standards
Conflict of interest
The authors declare that they have not conflict of interest.
Ethical approval
The study was conducted in accordance with the Helsinki Declaration and approved by the Researchs Ethics Board of Hospital de la Santa Creu i Sant Pau, Barcelona (IIBSP-PDL-2017-82).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carlos Zamora, Mariona Riudavets, Margarita Majem Tarruella and Silvia Vidal Alcorisa contributed equally to this work.
References
- 1.Naidoo J, Page DB, Wolchok JD. Immune checkpoint blockade. Hematol Oncol Clin North Am. 2014;28:585–600. doi: 10.1016/j.hoc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 6.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 9.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 10.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toi Y, Sugawara S, Sugisaka J, et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019;5:376–383. doi: 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158–176. doi: 10.1111/cup.12858. [DOI] [PubMed] [Google Scholar]
- 13.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Sbeih H, Ali FS, Luo W, et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018 doi: 10.1186/s40425-018-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–1375. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 16.Callahan MK, Yang A, Tandon S, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol. 2011;29:2505–2505. doi: 10.1200/jco.2011.29.15_suppl.2505. [DOI] [Google Scholar]
- 17.Stucci S, Palmirotta R, Passarelli A, et al. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol Lett. 2017;14:5671–5680. doi: 10.3892/ol.2017.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaehler KC, Piel S, Livingstone E, et al. Update on immunologic therapy with antiCTLA-4 antibodies in melanoma: Identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37:485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Dinkla S, van Cranenbroek B, van der Heijden WA, et al. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood. 2016;127:1976–1986. doi: 10.1182/blood-2015-04-640300. [DOI] [PubMed] [Google Scholar]
- 20.McNicol A, Israels S. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Targets. 2008;8:99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
- 21.Zamora C, Canto E, Nieto JC, et al. Functional consequences of platelet binding to T lymphocytes in inflammation. J Leukoc Biol. 2013;94:521–529. doi: 10.1189/jlb.0213074. [DOI] [PubMed] [Google Scholar]
- 22.Zamora C, Canto E, Nieto JC, et al. Binding of platelets to lymphocytes: a potential anti-inflammatory therapy in rheumatoid arthritis. J Immunol. 2017;198:3099–3108. doi: 10.4049/jimmunol.1601708. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Ji Q, Hjemdahl P. Platelet-lymphocyte conjugation differs between lymphocyte subpopulations. J Thromb Haemost. 2006;4:874–881. doi: 10.1111/j.1538-7836.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 24.Zamora C, Canto E, Nieto JC, et al. Inverse association between circulating monocyte-platelet complexes and inflammation in ulcerative colitis patients. Inflamm Bowel Dis. 2018;24:818–828. doi: 10.1093/ibd/izx106. [DOI] [PubMed] [Google Scholar]
- 25.Liu CY, Battaglia M, Lee SH, et al. Platelet factor 4 differentially modulates CD4 + CD25 + (Regulatory) versus CD4 + CD25 − (Nonregulatory) T cells. J Immunol. 2005;174:2680–2686. doi: 10.4049/jimmunol.174.5.2680. [DOI] [PubMed] [Google Scholar]
- 26.Rachidi S, Metelli A, Riesenberg B, et al. Platelets subvert T cell immunity against cancer via GARP-TGF axis. Sci Immunol. 2017 doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elalamy I, Chakroun T, Gerotziafas GT, et al. Circulating platelet-leukocyte aggregates: a marker of microvascular injury in diabetic patients. Thromb Res. 2008;121:843–848. doi: 10.1016/j.thromres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Marques P, Collado A, Martinez-Hervás S, et al. Systemic inflammation in metabolic syndrome: increased platelet and leukocyte activation, and key role of CX3CL1/CX3CR1 and CCL2/CCR2 axes in arterial platelet-proinflammatory monocyte adhesion. J Clin Med. 2019;8:708. doi: 10.3390/jcm8050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki J, Hamada E, Shodai T, et al. Cytokine secretion from human monocytes potentiated by P-selectin-mediated cell adhesion. Int Arch Allergy Immunol. 2013;160:152–160. doi: 10.1159/000339857. [DOI] [PubMed] [Google Scholar]
- 30.Weyrich AS, Elstad MR, McEver RP, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(2009) National Cancer Institute. Common Terminology Criteria for Adverse Events. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- 32.Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer. 2016;138:2078–2087. doi: 10.1002/ijc.29847. [DOI] [PubMed] [Google Scholar]
- 33.Miyashita T, Tajima H, Makino I, et al. Metastasis-promoting role of extravasated platelet activation in tumor. J Surg Res. 2015;193:289–294. doi: 10.1016/j.jss.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 34.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Placke T, Kopp HG, Salih HR. Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun. 2011;3:374–382. doi: 10.1159/000323936. [DOI] [PubMed] [Google Scholar]
- 36.Togna GI, Togna AR, Franconi M, Caprino L. Cisplatin triggers platelet activation. Thromb Res. 2000;99:503–509. doi: 10.1016/s0049-3848(00)00294-2. [DOI] [PubMed] [Google Scholar]
- 37.Starossom SC, Veremeyko T, Yung AWY, et al. Platelets play differential role during the initiation and progression of autoimmune neuroinflammation. Circ Res. 2015;117:779–792. doi: 10.1161/CIRCRESAHA.115.306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 39.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 40.Rong M, Wang C, Wu Z, et al. Platelets induce a proinflammatory phenotype in monocytes via the CD147 pathway in rheumatoid arthritis. Arthritis Res Ther. 2014;16:478. doi: 10.1186/s13075-014-0478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yago T, Tsukuda M, Minami M. P-selectin binding promotes the adhesion of monocytes to VCAM-1 under flow conditions. J Immunol. 1999;163:367–373. doi: 10.4049/jimmunol.163.1.367. [DOI] [PubMed] [Google Scholar]
- 42.Van Wely CA, Blanchard AD, Britten CJ. Differential expression of α3 fucosyltransferases in Th1 and Th2 cells correlates with their ability to bind P-selectin. Biochem Biophys Res Commun. 1998;247:307–311. doi: 10.1006/bbrc.1998.8786. [DOI] [PubMed] [Google Scholar]
- 43.Shafqat H, Gourdin T, Sion A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. Semin Oncol. 2018;45:156–163. doi: 10.1053/j.seminoncol.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1–based immunotherapy. Clin Cancer Res. 2019;25:1557–1563. doi: 10.1158/1078-0432.CCR-18-2795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file2 Supplementary Fig.1 Association of percentage of circulating CD4+PLT+ and CD14+PLT+ with the presence of irAEs. Percentage of CD4+PLT+ and CD14+PLT+ complexes in (A-B) non-irAEs vs irAEs groups, (C-D) grade of irAEs (0-≥3) and (E-F) number of irAEs per patient. The statistical analysis was performed using the t-test. *p<0.05 (TIF 484 kb)
Supplementary file3 Supplementary Fig. 2 The association of platelet blood levels, the percentage and levels of activated platelets, the ratio of PLT/CD4+ and PLT/CD14+ and plasmatic levels of IFNɣ, IL-17 and IL-10 with irAE manifestation. The dot plots show (A) Platelet blood levels (PLT/µL), (B) the percentage of activated platelets, (C) the levels of activated platelets (PLT CD62P+/µL), (D) the ratio of PLT/CD4+, (E) the ratio of PLT/CD14+, pg/mL of (F) IFNɣ, (G) IL-17 and (H) IL-10 according to non-irAE and irAE groups. An unpaired t-test was used for the analysis of PLT/µL, % PLT CD62P+ and PLT CD62P+/µL, and the Mann-Whitney test for the analysis of the PLT/CD4+, PLT/CD14+, IFNɣ, IL-17 and IL-10 ratios (TIF 604 kb)