Abstract
Background
Anti-PD-1 antibodies plus lenvatinib therapeutic regimens have demonstrated a relatively high antitumor response in many solid cancers; however, the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer (GBC) has not been reported.
Methods
Advanced GBC patients who received anti-PD-1 antibodies plus lenvatinib were retrospectively screened. Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR), PD-L1 expression and safety were evaluated to identify efficacy biomarkers.
Results
A total of 31 GBC patients were included in this study. After a median follow-up of 8 months and 23 deaths were observed. The median PFS was 5.0 months (95% CI: 4.1–8.0 months), and the median OS was 11.3 months (95% CI: 7.5–20.9 months). Overall, the ORR was 32.3%, the DCR was 83.9%, and the CBR was 41.9%. Moreover, after treatment, 3 patients received conventional surgery, in which 1 patient achieved a pathological complete response. All patients (100%) experienced adverse events (AEs), and 58.1% of the patients experienced grade 3 AEs. The most commonly observed grade 3 AEs included fatigue (5/31, 16.1%), decreased appetite (5/31, 16.1%), hypertension (4/31, 12.9%) and bilirubin elevation (4/31, 12.9%). Subgroup analysis revealed that positive PD-L1 expression maybe associate with a longer PFS.
Conclusion
Anti-PD-1 antibodies plus lenvatinib represent an effective and tolerable therapy for patients with advanced gallbladder cancer.
Keywords: Gallbladder cancer, PD-1, Lenvatinib, PD-L1 expression
Introduction
Gallbladder cancer (GBC) is a rare malignant tumor with a high mortality rate, and 219,420 new cases and 1,65,087 deaths were reported globally in 2018 [1–3]. Approximately half of gallbladder cancers were detected as a result of emergency surgery due to accidents, and this incidental gallbladder cancer is always associated with better survival than nonincidental gallbladder cancer. Unfortunately, for nonincidental gallbladder cancer patients, less than 20% of these patients can receive surgical treatment [4]. For the remaining patients, chemotherapy, targeted therapy and immunotherapy represent alternative therapies, of which chemotherapy is most commonly used.
Cisplatin plus gemcitabine is widely used as the first-line treatment in patients with unresectable gallbladder cancer [5, 6]. However, the objective response rate (ORR) of cisplatin plus gemcitabine regimens in ABC-002 research is approximately 21 to 37%, which is not quite satisfactory [7]. In addition, for patients with metastatic gallbladder cancer who experience progression even after chemotherapy, efficacious treatment options are lacking [8]. The shortage of available antitumor solutions for patients with advanced gallbladder cancer has troubled doctors and patients for a long time. Immunotherapy using anti-programmed cell death protein 1 (PD-1) blockade has shown promising effects in patients with advanced biliary tract cancer (BTC) in early clinical trials [9–11].
In our report, we focused on real-world advanced gallbladder cancer patients who received anti-PD-1 antibodies plus lenvatinib. Previous prospective clinical trials have shown that greater than one-third of patients with advanced or metastatic gallbladder cancer can obtain an objective response with tolerable adverse effects. These studies have motivated increasing clinical investigations of this regimen in patients with refractory cancers. Importantly, either lenvatinib or pembrolizumab has exhibited a satisfactory safety index in the treatment of patients with unresectable BTC [12, 13]. Thus, we believe that anti-PD-1 antibodies plus lenvatinib may become an encouraging therapeutic regimen in patients with advanced gallbladder cancer.
Materials and methods
Study population
From May 2018 to May 2021, advanced gallbladder cancer patients who received anti-PD-1 antibodies plus lenvatinib therapy at Peking Union Medical College Hospital (PUMCH) were enrolled in this study. The baseline characteristics of all 31 patients are summarized in Table 1. The primary eligibility criteria included histologically confirmed GBC and at least one measurable tumor lesion according to the RECIST v1.1 criteria [14]. Demographic, histological, pathological, disease stage, systemic treatment and the types of anti-PD-1 antibodies information were compiled and recorded. The study was registered at ClinicalTrials.gov (identifier: NCT03892577). And the study was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) of PUMCH (approval number: JS-1391).
Table 1.
Baseline characteristics of the study population
| Parameters | Total (N = 31) |
|---|---|
| Age, years (median, IQR) | 62 (58–69) |
| < 60 | 10 [32.3%] |
| ≥ 60 | 21 [67.7%] |
| Sex, n [%] | |
| Female | 17 [54.8] |
| Male | 14 [45.2] |
| ECOG performance, n [%] | |
| 0 | 15 [48.4] |
| 1 | 13 [41.9] |
| 2 | 3 [9.7] |
| Child–Pugh score, n [%] | |
| A | 26 [83.9] |
| B | 5 [16.1] |
| CA19-9, U/mL (median, IQR) | 324 (78.6–2338.0) |
| < 200 | 12 [38.7] |
| ≥ 200 | 13 [41.9] |
| NA | 6 [19.4] |
| Histology, n [%] | |
| Adenocarcinoma | 31 [100] |
| Differentiated histology, n [%] | |
| Poor | 14 [45.2] |
| Moderate | 12 [38.7] |
| Well | 1 [3.2] |
| Undifferentiated | 1 [3.2] |
| NA | 3 [9.7] |
| Bile stone, n [%] | 13 [41.9] |
| Site of metastases, n [%] | |
| Intrahepatic | 20 [64.5] |
| Lymph nodes | 26 [83.9] |
| Lung | 4 [12.9] |
| TNM stage, n [%] | |
| III | 6 [19.4] |
| IV | 25 [80.6] |
| PD-L1 expression, n [%] | |
| Positive | 13 [41.9] |
| Negative | 18 [58.1] |
| Previous antitumor therapy, n [%] | |
| Radical surgery resection | 9 [29.0] |
| Systemic chemotherapy | 11 [35.5] |
| Radiotherapy | 4 [12.9] |
| Type of anti-PD-1 antibodies, n [%] | |
| Pembrolizumab | 12 [38.7] |
| Nivolumab | 3 [9.7] |
| Sintilimab | 6 [19.4] |
| Toripalimab | 9 [29.0] |
| Camrelizumab | 1 [3.2] |
ECOG Eastern Cooperative Oncology Group, CA19-9 cancer antigen 19–9, TNM tumor node metastasis classification
Treatment
Information regarding the dates of initiation and completion of treatment, initial dose, dose modifications, radiological evaluation, laboratory data, and adverse events (AEs) during treatment were systematically collected. Lenvatinib was administered at a dosage of 12 mg (for patients with a body weight ≥ 60 kg) or 8 mg (for patients with a body weight < 60 kg) orally once a day. The PD-1 dose included a fixed dosage of 200 mg (240 mg for toripalimab) every 3 weeks or a fixed dosage of 3 mg/kg body weight every 3 weeks.
Outcome assessment
The clinical objective response was measured by the RECIST v1.1 criteria [15]. and was evaluated by professional radiologists at the center of PUMCH. To assess the tumor growth rate and treatment response, computed tomography/magnetic resonance imaging (CT/MRI) or positron emission tomography (PET) CT images were regularly evaluated. Progression‑free survival (PFS), overall survival (OS), ORR, disease control rate (DCR) and clinical benefit rate (CBR) were used to assess the efficacy of treatments. CBR is defined as the proportion of patients with a radiologically confirmed objective response (CR or PR) or SD for more than 6 months [16].
Safety assessment and grading were recorded in the electronic medical records or collected by the investigators using the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) as a reference.
PD-L1 expression evaluation
Whole sections of formalin fixed and paraffin embedded tumor specimens were subjected to immunohistochemistry. For each tissue section, consecutive 5 μm thick sections were selected and placed on a glass slide. The primary antibody used is anti-PD-L1, secondary antibodies were added to all sections. And the evaluation of PD-L1 expression was performed by independent pathologists who were blinded to the clinicopathological data.
Statistical analysis
The data from the cutoff date (May 5, 2021) of the analysis in this report were used to generate summaries of the baseline characteristics, therapeutic efficacies and AEs. The molecular marker analyses included treated patients with available data as of May 5, 2021. The PFS, OS, 6-month PFS and 12-month OS were all estimated using the Kaplan–Meier method, and the comparisons were analyzed using the log-rank test. Hazard ratios of each clinicopathological feature for PFS and OS were estimated by Cox proportional hazard modeling. To compare the individual variables, the t-test, Mann–Whitney U test, χ2 test and Fisher’s exact test were performed as appropriate. To identify the predictive factors associated with the treatment response to nivolumab, univariate and multivariable logistic regression models were applied. A two-tailed P-value < 0.05 was considered significant. Statistical analyses were performed using R-4.0.2 software.
Results
Baseline characteristics of the 31 advanced GBC patients
A total of 31 patients with gallbladder cancer who received PD-1 plus lenvatinib were included in the study. The demographics and baseline characteristics of all 31 patients are summarized in Table 1. At the time of initial treatment, the median age was 62 years, and 54.8% of the total patients were female. Twenty-eight (90.3%) patients had an ECOG (Eastern Cooperative Oncology Group) performance status of 0–1, and 26 (83.9%) patients had Child–Pugh stage A. All histopathological subtypes were adenocarcinomas. At baseline, the median CA19-9 level was 324 U/mL. In total, 14 (45.2%) patients had poorly differentiated gallbladder cancer, and 13 (41.9%) patients had biliary stones.
At baseline before treatment, most patients had metastatic cancer in the liver (20/31, 64.5%), lymph nodes (26/31, 83.9%) and lungs (4/31, 12.9%). In total, 25 (80.6%) patients had TNM stage IV. Sixteen (51.6%) patients received PD-1 plus lenvatinib as first-line therapy. Regarding prior treatments, 9 (29%) patients underwent radical surgical resection, 11 (35.5%) patients received systemic chemotherapy, and 4 (12.9%) received radiotherapy (Table 1).
Treatment and efficacy
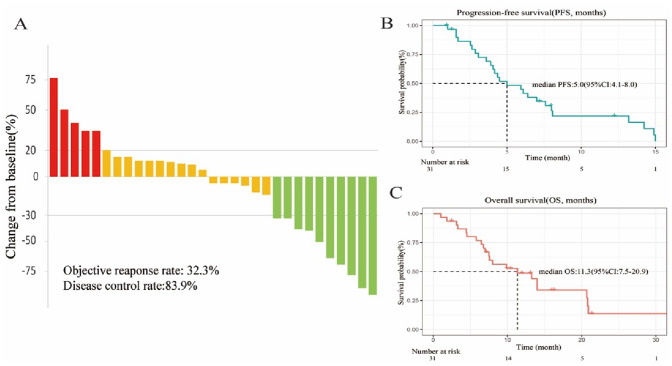
For all participants in our cohort, the median duration of follow-up was 9.9 (IQR, 6.1–14.0) months. All patients underwent complete radiological evaluations. Overall, 15 patients (48.4%) exhibited a decrease in tumor size from baseline (Fig. 1a). Ten (32.3%) patients achieved an objective response. Of these patients, all showed a PR, and no CR was observed. In total, 16 (51.6%) patients exhibited SD. Therefore, the overall radiologically confirmed ORR was 32.3%, and the DCR was 83.9% (Table 2).
Fig. 1.
Therapeutic efficacy of lenvatinib combined with PD-1 in patients with gallbladder cancer. A Maximum percentage change in the sum of the diameters of the target lesions from baseline. B Kaplan–Meier estimation of progression-free survival of the entire cohort. C Kaplan–Meier estimation of overall survival of the entire cohort
Table 2.
Therapeutic efficacy of response and survival outcomes
| Therapeutic response assessment | Evaluable patients (N = 31) |
|---|---|
| Confirmed objective response rate (ORR, n, %) | 10 (32.3%) |
| Complete response (CR, n, %) | 0 |
| Partial response (PR, n, %) | 10 (32.3%) |
| Stable disease (SD, n, %) | 16 (51.6%) |
| Disease control rate (DCR, n, %) | 26 (83.9%) |
| Clinical benefit rate (CBR n, %) | 13 (41.9%) |
| Progressive disease (PD, n, %) | 5 (16.1%) |
CR complete response, PR partial response SD stable disease PD progressive disease, ORR objective response rate, DCR disease control rate, CBR clinical benefit rate
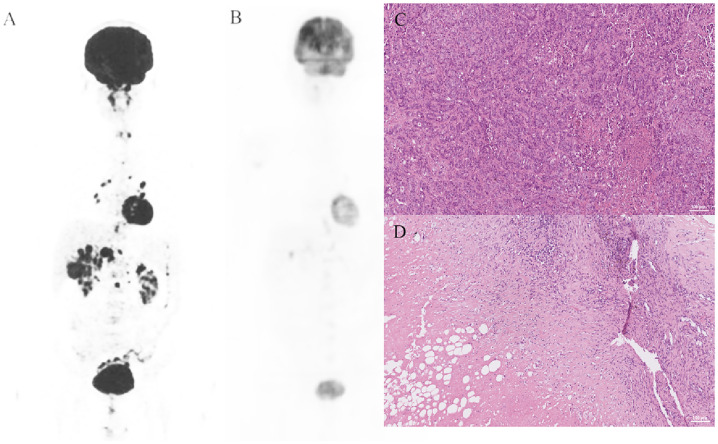
The survival outcomes of the enrolled patients were investigated in the entire cohort, and eight patients were still alive. For the entire cohort, the median PFS was 5.0 (95% CI: 4.1–8.0) months (Fig. 1b), and the 6-month PFS rate was 44.9% (95% CI: 30–67.2%). The median OS was 11.3 (95% CI: 9.6–12.3) months (Fig. 1c), and the 1-year OS rate was 48.7% (95% CI: 33.4–71%). We further determined the CBR in all assessment-available patients. Impressively, the CBR in all 31 patients was 41.9%. Another encouraging aspect of the results is that 3 patients were successfully converted to surgery, and one of them achieved pathological complete response (pCR), which has not been previously reported in gallbladder cancer. The details are shown in Fig. 2.
Fig. 2.
A representative case. The patient underwent laparoscopic biopsy in December 2019. PET-CT and H&E staining are shown in 2A and 2C, and pathology revealed a median differentiated gallbladder carcinoma. The patient regularly received 8 mg/day lenvatinib and 200 mg camrelizumab every 3 weeks. Then, in May 2020, PET-CT (2B) showed that the lesions had obviously shrank, and a conventional operation was performed. H&E staining of the surgically resected specimen showed pCR. PET-CT, positron emission tomography computed tomography; H&E, hematoxylin and eosin; pCR, pathological complete response
Subgroup analyses and PD-L1 expression
Among these patients, 41.9% (13/31) were determined to have positive PD-L1 expression. When patients were stratified by PD-L1 expression, Kaplan–Meier survival curve and log-rank test analysis demonstrated that patients with positive PD-L1 expression had a longer median PFS (7.1 vs.4.2 months, P = 0.015, Fig. 3c), however, no significantly (P = 0.31, Fig. 3d) longer of overall survival in the group with positive PD-L1 expression.
Fig. 3.
Association of PD-L1 expression detected by immunohistochemistry staining with survival outcomes. A Subgroup analyses of progression-free survival (PFS) in the entire population; B subgroup analyses of overall survival (OS) in the entire population; C PFS was longer in the PD-L1 positive group (n = 13) than in the PD-L1 negative group (n = 18) (P = 0.015); D Significantly longer overall survival was not observed in the group with positive PD-L1 expression (P = 0.31). ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; TNM, tumor node metastasis classification
Safety
The median duration of treatment of lenvatinib and PD-1 antibodies was 9.8 (IQR: 6.0–13.9) months. In total, AEs encountered during anti-PD-1 antibodies plus lenvatinib therapy were reported in all 31 (100%) patients. No grade 5 AE occurred, and only 2 patients experienced grade 4 SAEs (gastrointestinal hemorrhage). For severe AEs (SAEs), 19 (51.6%) patients had grade 3 AEs (Table 3). The most common AEs (any grade) were fatigue (18/31, 58.1%), elevated aminotransferase (ALT or AST) levels (15/31, 48.4%) and decreased appetite (13/31, 41.9%). Most AEs occurring during combination immunotherapy were safe, well tolerated and controlled. Regarding SAEs, the most common grade 3 SAEs were fatigue (5/31, 16.1%), decreased appetite (5/31, 16.1%), hypertension (4/31, 12.9%) and bilirubin elevation (4/31, 12.9%). After careful treatment, these AEs could be controlled.
Table 3.
Commonly observed adverse events
| Adverse events (AEs) | Any grade, n (%) | Grade 3, n (%) | Grade 4–5, n (%) |
|---|---|---|---|
| Fatigue | 18 (58.1) | 5 (16.1) | 0 |
| Hypertension | 12 (38.7) | 4 (12.9) | 0 |
| ALT or AST elevation | 15 (48.4) | 0 | 0 |
| Hypothyroidism | 12 (38.7) | 0 | 0 |
| Proteinuria | 8 (25.8) | 3 (9.7) | 0 |
| Decreased appetite | 13 (41.9) | 5 (16.1) | 0 |
| Abdominal pain | 6 (19.4) | 0 | 0 |
| Vomiting | 6 (19.4) | 0 | 0 |
| Diarrhea | 4 (12.9) | 0 | 0 |
| Bilirubin elevation | 10 (32.3) | 4 (12.9) | 0 |
| Decreased weight | 6 (19.4) | 0 | 0 |
| Skin rash | 11 (35.5) | 1 (3.2) | 0 |
| Myelosuppression | 3 (9.7) | 0 | 0 |
| Gastrointestinal hemorrhage | 3 (9.7) | 1 (3.2) | 2 (6.5) |
| Nasal hemorrhage | 2 (6.5) | 0 | 0 |
| Mouth ulcer | 3 (9.7) | 0 | 0 |
| Pneumonia | 2 (6.5) | 1(3.2) | 0 |
ALT alanine aminotransferase, AST aspartate aminotransferase
Discussion
To our knowledge, this is the largest study to analyze the treatment results of patients receiving lenvatinib combined with anti-PD-1 antibodies in patients with advanced gallbladder cancer. In this present retrospective study, we evaluated the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer. The treatment options for advanced gallbladder cancer patients are limited, and the overall survival is poor. Thus, new therapeutic strategies are needed. We found that these patients achieved an ORR of 32.3%, a DCR of 83.9% and a CBR of 41.9% with a median PFS of 5.0 (95% CI: 4.1–8.0) months and a median OS of 11.3 (95% CI: 7.5–20.9) months. One of the most encouraging aspects of the results was that 3 (9.7%) out of 31 patients converted to surgery, and 1 patient achieved pCR, which has not yet been reported worldwide [17]. This finding supported the feasibility and efficacy of anti-PD-1 antibodies plus lenvatinib for advanced gallbladder cancer patients.
This study revealed an ORR similar to that noted for the cisplatin plus gemcitabine regimen in the ABC-002 study. However, a limitation of the chemotherapy regimen is that its use cycle was approximately 6–8 months, whereas our regimen can be used for a long time. Previous reports on immunotherapy were mainly focused on pembrolizumab use in patients with advanced BTC, yielding an ORR of 13–17% (KEYNOTE-028 and KEYNOTE-158) [9, 13, 18]. Another study on immunotherapy in gallbladder cancer involved nivolumab and ipilimumab, yielding an ORR of 23% and DCR of 44% [19]. We hypothesized that the reason why the ORR of gallbladder cancer was higher than that of other BTCs may be related to the increased PD-L1 expression [20]. There have been relatively few studies on targeted drugs in gallbladder cancer; however, preclinical studies and clinical evidence have shown that targeted drugs can enhance the antitumor efficacy of PD1/PD-L1 immunotherapy by targeting VEGF/VEGFR to inhibit angiogenesis [21–23]. Simultaneously, we found that targeted drugs combined with anti-PD-1 antibodies can achieve better therapeutic effects in clinical practice. We looked forward to seeing more prospective trials for immunotherapy combined with TKIs as well as immunotherapy combined with chemotherapy.
Relevant studies on predicting tumor prognosis based on biomarkers have been performed. In our study, through univariate analysis of multiple indicators, we found that positive PD-L1 expression in pretreated tumor tissues was significantly associated with an improved PFS. No significant difference in OS was noted between the two groups, but some trends are evident from the curves. As the sample size increased, significant differences might be obtained. However, the role of PD-L1 expression as a biomarker for targeted therapy combined with immunotherapy remains controversial [24, 25]. Further research is needed to demonstrate the role of PD-L1 expression.
Regarding therapeutic safety, although all patients experienced AEs, no grade 5 AE were reported, and 2 patients experienced grade 4 SAEs. Approximately 61.3% of patients experienced grade 3 SAEs, but all these SAEs were reversible. These results are similar to the outcomes reported for hepatocellular carcinoma. The results indicate that the combination of anti-PD-1 antibodies and lenvatinib is a potential effective and safe therapy for patients with advanced gallbladder cancer. Compared with traditional chemotherapy, our treatment regimen was milder and had relatively fewer adverse effects. Compared with the low tolerability of chemotherapy, our treatment was more suitable for Asians. However, randomized controlled prospective studies are needed to further support this hypothesis.
The limitations of our exploratory analysis must be acknowledged. Our study is a preliminary single-arm exploratory analysis, which has limitations and requires a well-designed clinical trial with a controlled arm. Second, different anti-PD-1 antibodies were used in this study. Third, there is evidence that endogenous PD-1 may induce the growth of hepatocellular carcinoma [26]. We did not measure the expression of PD-1, and there is a bias in whether it has an effect on patients with gallbladder cancer. Although the mechanisms and signaling pathways associated with these antibodies are reportedly similar, bias could occur in a strict sense [27].
Conclusion
We reported the outcomes of 31 patients with advanced gallbladder cancer who received anti-PD-1 antibody plus lenvatinib therapy regimens. Our research found that anti-PD-1 antibodies plus lenvatinib could be safely and effectively used to treat advanced gallbladder cancer patients. PD-L1expression were positively correlated with a good therapeutic effect. Further research on prospective larger cohort is needed.
Acknowledgements
We thank the patients participating in this study and all staff at the hospital for their contributions to this study.
Funding
This work was supported by International Science and Technology Cooperation Projects (2016YFE0107100), CAMS Clinical and Translational Medicine Research Funds (2019XK320006), CAMS Innovation Fund for Medical Science (CIFMS) (2017-I2M-4–003 and 2018-I2M-3–001), Beijing Natural Science Foundation (L172055 and 7192158), the Fundamental Research Funds for the Central Universities (3332018032), CSCO-hengrui Cancer Research Fund (Y-HR2019-0239) and National Ten-thousand Talent Program. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of ethics
The study protocol complied with the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) of Peking Union Medical College Hospital (PUMCH). (Approval Number: JS-1391).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bangyou Zuo, Xiaobo Yang, Xu Yang have contributed equally to this work.
Contributor Information
Xinting Sang, Email: sangxt@pumch.cn.
Haitao Zhao, Email: ZhaoHT@pumch.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Valle JW, Kelley RK, Nervi B, et al. Biliary tract cancer. Lancet. 2021;397:428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 3.Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132–1138. doi: 10.1093/jnci/89.15.1132. [DOI] [PubMed] [Google Scholar]
- 4.Ethun CG, Le N, Lopez-Aguiar AG, et al. Pathologic and Prognostic Implications of Incidental versus Nonincidental Gallbladder Cancer: A 10-Institution Study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg. 2017;83:679–686. doi: 10.1177/000313481708300721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1015–1027. doi: 10.1200/JCO.18.02178. [DOI] [PubMed] [Google Scholar]
- 6.Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Zhao H. Systemic management for patients with hepatobiliary tumors in a multi-dimensional view. Hepatobiliary Surg Nutr. 2019;8:626–628. doi: 10.21037/hbsn.2019.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piha-Paul SA, Oh DY, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 10.Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Yang X, Long J, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. 2020;9:414–424. doi: 10.21037/hbsn-20-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vienot A, Neuzillet C (2018) Cholangiocarcinoma: the quest for a second-line systemic treatment. Trans Cancer Res S275–S288 [DOI] [PMC free article] [PubMed]
- 13.Bang Y-J, Ueno M, Malka D, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J Clin Oncol. 2019;37:4079–4079. doi: 10.1200/JCO.2019.37.15_suppl.4079. [DOI] [Google Scholar]
- 14.Schwartz LH, Seymour L, Litière S, et al (2016) RECIST 1.1—standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur J Cancer 62:138–45 [DOI] [PMC free article] [PubMed]
- 15.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Hu Y, Yang K, et al (2021) Cell-free DNA copy number variations predict efficacy of immune checkpoint inhibitor-based therapy in hepatobiliary cancers. J Immunother Cancer 9 [DOI] [PMC free article] [PubMed]
- 17.Zhao L, Zhao H. Conversion surgery for hepatocellular carcinoma in the new era of targeted and immune checkpoint inhibitor therapies. Hepatobiliary Surg Nutr. 2020;9:809–811. doi: 10.21037/hbsn-20-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno M, Chung HC, Nagrial A, et al (2018) Pembrolizumab for advanced biliary adenocarcinoma: results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol 29:viii210
- 19.Klein O, Kee D, Nagrial A, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6:1405–1409. doi: 10.1001/jamaoncol.2020.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody K, Starr J, Saul M, et al. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J Gastrointest Oncol. 2019;10:1099–1109. doi: 10.21037/jgo.2019.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shigeta K, Datta M, Hato T, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71:1247–1261. doi: 10.1002/hep.30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Patel A, Normolle D, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer. 2019;125:902–909. doi: 10.1002/cncr.31872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Li X, Liu S, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology. 2017;66:1920–1933. doi: 10.1002/hep.29360. [DOI] [PubMed] [Google Scholar]
- 27.Patsoukis N, Wang Q, Strauss L, et al (2020) Revisiting the PD-1 pathway. Sci Adv 6 [DOI] [PMC free article] [PubMed]