Abstract
Metabolic reprogramming of cancer cells generates a tumour microenvironment (TME) characterised by nutrient restriction, hypoxia, acidity and oxidative stress. While these conditions are unfavourable for infiltrating effector T cells, accumulating evidence suggests that regulatory T cells (Tregs) continue to exert their immune-suppressive functions within the TME. The advantages of Tregs within the TME stem from their metabolic profile. Tregs rely on oxidative phosphorylation for their functions, which can be fuelled by a variety of substrates. Even though Tregs are an attractive target to augment anti-tumour immune responses, it remains a challenge to specifically target intra-tumoral Tregs. We provide a comprehensive review of distinct mechanistic links and pathways involved in regulation of Treg metabolism under the prevailing conditions within the tumour. We also describe how these Tregs differ from the ones in the periphery, and from conventional T cells in the tumour. Targeting pathways responsible for adaptation of Tregs in the tumour microenvironment improves anti-tumour immunity in preclinical models. This may provide alternative therapies aiming at reducing immune suppression in the tumour.
Keywords: Treg, Metabolism, Nutrient depletion, Hypoxia, Acidity, Oxidative stress
Introduction
Tumour cells frequently exhibit increased metabolism compared to normal cells to fulfil their metabolic demands to sustain proliferation [1]. They perform aerobic glycolysis, also known as the “Warburg effect”, wherein glucose-derived pyruvate is shunted to lactate instead of being metabolized in the mitochondria despite the presence of oxygen [2]. In addition, increased mitochondrial respiration in tumour cells is essential for the biosynthesis of macromolecules and for tumorigenesis [3]. On the other hand, tumour hypoxia, mainly caused by defective vasculature in fast-growing tumours, forces cancer cells to rely on aerobic glycolysis, consequently increasing lactic acid production [4, 5]. While lactic acid is a major contributing factor to tumour acidity, also carbonic acid formed during oxidative phosphorylation can acidify the tumor microenvironment (TME) [6, 7]. Tumour cells produce elevated level of reactive oxygen species (ROS), which in turn can lead to increased basal metabolic activity and mitochondrial dysfunction [8–11].
Substrates for the tri-carboxylic acid (TCA) cycle can vary from glucose-derived pyruvate, oxidation of fatty acids, glutamine-derived α-ketoglutarate (via glutaminolysis), or through the conversion of branched-chain amino acids (BCAA) [11]. Increased uptake of amino acids by tumours is also essential for nucleic acid synthesis, to maintain redox balance and to mediate epigenetic regulation [12]. The tumour microenvironment is thus devoid of nutrients while being enriched in metabolic intermediates such as lactic acid, glutamate, kynurenine and ROS [7, 13–16]. Nutrient restriction, hypoxia, acidity and oxidative stress are all described to impair the function of tumour-infiltrating effector T cells [17, 18].
Regulatory T cells (Tregs), characterised by the expression of the transcription factor forkhead box P3 (Foxp3), are a heterogeneous population of immunosuppressive cells, which are critical for establishing peripheral tolerance [19, 20]. They originate either from the thymus after positive selection (termed tTregs or nTregs) or can be induced in the periphery (pTregs) from naïve CD4+ T cells after T cell receptor stimulation in the presence of transforming growth factor beta (TGF-β) and interleukin-2 (IL-2) [21–23]. Both subsets are present in the tumour and independently contribute to immune suppression [24–26]. The presence of immune-suppressive cytokines in the tumour can also lead to conversion of conventional CD4+ T cells to Tregs [27, 28]. In the review, we will refer in general to tumour-infiltrating Tregs (TI-Tregs), comprising both subsets.
Tregs can suppress immune responses via secretion of granzymes and perforins, immune-suppressive cytokines (IL-10, TGF-β, IL-35), or by expressing co-inhibitory molecules such as CTLA-4, PD-1, LAG-3, TIM-3 and TIGIT [29–31]. In addition, they can degrade extracellular adenosine triphosphate (ATP) or adenosine diphosphate (ADP) to immune-suppressive adenosine by combined activities of the ecto-enzymes CD39 and CD73 [30]. High Foxp3+ Treg infiltration is associated with tumour progression and poor recurrence-free and overall survival across melanomas, cervical, renal and breast cancers [32]. Transient systemic Treg depletion in metastasized melanoma patients, achieved by administering IL-2 conjugated to diphtheria toxin fragments A and B, causes significant regression of the tumours [33]. This supports previous mouse studies in which transient Treg depletion correlated with increased effector T cell activation, delayed tumour growth and enhanced survival in a range of solid tumours [34–36]. In addition, Treg depletion by the use of the anti-CD25 antibody Daclizumab resulted in increased immune response in combination with dendritic cell vaccination in breast cancer patients [37]. This was, however, not observed in another trial with melanoma patients [38]. The limited success of such Treg depleting strategies could be attributed to their lack of specificity to TI-Tregs [36]. Of note, long-term systemic depletion of Tregs results in fatal autoimmunity in adult mice, which also raises concerns regarding autoimmune side effects in human trials with long-term systemic Treg depletion [39].
Selective inhibition of Tregs in the tumour is therefore critical for developing safe and effective therapies to overcome immune suppression. Increasing evidence points out to the metabolic differences between peripheral and TI-Tregs and between TI-Tregs and effector T cells. Therefore, we aim to outline the current knowledge about metabolic profiles of Tregs in the TME and highlight several pathways of metabolic adaptation engaged by TI-Tregs that support their survival and suppressive activity.
Metabolic profile of Tregs: balance between glycolysis and mitochondrial respiration
The metabolic differences between conventional T cells (Tconv) and Tregs in normal physiology and during inflammation are being increasingly highlighted over the past years [40–46]. When Tconv cells are activated, they shift their metabolism from oxidative phosphorylation (OXPHOS) to aerobic glycolysis, to support cell growth and proliferation, much like tumour cells [47, 48]. Mammalian target of rapamycin (mTOR), consisting of complexes I and II, modulates glycolysis in T cells [49]. Upregulation of glycolysis is also observed during activation of Tregs. Stimulating mouse tTregs with TLR1 and TLR2 agonist Pam3CSK4 induces mTORC1 signalling, increases glycolysis and results in highly proliferative Tregs [42]. However, such glycolytic Tregs lost their suppressive capabilities in vitro [42]. Likewise, increase in glycolysis in Tregs induced by overexpression of glucose transporter (GLUT)-1 also caused a loss of suppressive function in vitro and in vivo in an inflammatory bowel disease model [42]. The migratory capacity of Tregs to the secondary lymphoid organs is dependent on mTORC2-mediated upregulation of glycolytic enzymes suggesting that Tregs utilize glycolysis for migration [50]. It was, however, not determined if the glycolytic Tregs were able to suppress Tconv cells in this model [50].
While glycolysis seems to be imperative for the proliferation and migration of Tregs, their functionality is dependent on alternative metabolic routes involving mitochondrial metabolism. For instance, impaired OXPHOS by Treg-specific deletion of mitochondrial complex III in adult mice results in loss of Treg suppressive functions, while there is no effect on the expression of FoxP3 or the number of FoxP3+ Tregs [51]. Mice lacking the metabolic sensor Lkb1 in Tregs develops lethal autoimmunity due to disrupted mitochondrial metabolism, further highlighting the essentiality of OXPHOS for Treg functionality [52, 53]. Moreover, Tregs take up exogenous fatty acids rather than relying on de novo fatty acid synthesis for their survival and functionality [44]. Inhibiting lipid uptake by knockdown of fatty acid-binding protein (FABP) 5, or acute treatment with the FABP inhibitor BMS309403, impaired OXPHOS, lipid metabolism finally disrupting mitochondrial structure specifically in Tregs (Fig. 1) [54]. Interestingly, such Tregs suppressed effector responses through increased IL-10 production [54]. It is not clear from the former studies if impaired OXPHOS in Tregs also caused alterations in mitochondrial structure or led to release of mitochondrial DNA. Moreover, it is conceivable that a specific metabolic pathway in Tregs dictates their mode of suppression.
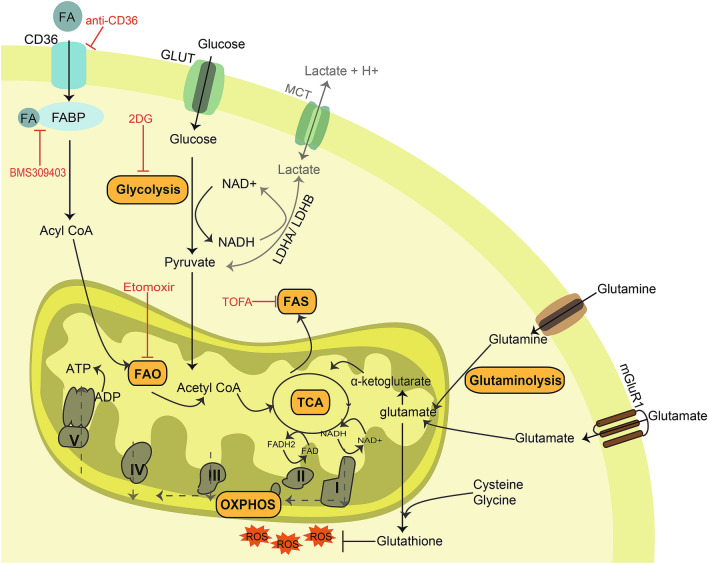
Fig. 1.
Tumour-infiltrating (TI) Tregs adapt to the metabolic stresses often experienced in the tumour microenvironment (TME). TI-Tregs increase their glucose uptake via glucose transporters (GLUT) converting it to pyruvate via glycolysis. The conversion of pyruvate to lactate seldom occurs in TI-Tregs. Pyruvate is instead converted to acetyl-CoA and metabolized in the mitochondria to fuel the tri-carboxylic acid (TCA) cycle. The intermediates of TCA cycle can also be utilized for fatty acid synthesis (FAS) by TI-Tregs. Alternatively, TI-Tregs can take up free fatty acids (FA) from the TME via fatty acid transporters (e.g. CD36). This process is aided by the presence of fatty acid-binding proteins (FABP). FA is then converted to fatty acyl CoA to further be oxidized in the mitochondria, termed fatty acid oxidation (FAO). In addition, the TCA cycle can also be fuelled by the derivatives of amino acid metabolism. For example, glutamate produced from glutamine is converted to α-ketoglutarate which enters the TCA cycle. Furthermore, TI-Tregs have increased expression of metabotropic glutamate receptor 1 (mGluR1), which allows the uptake of glutamate from the TME. The reducing equivalents NADH and FADH2 are synthesized during the TCA cycle which in turn transfers electrons to the electron transport chain (ETC). The direction of electron transfer across the ETC complexes is depicted by dashed lines. The membrane potential created by the ETC drives phosphorylation of ADP to ATP in the presence of oxygen (OXPHOS). The reactive oxygen species (ROS) produced due to increased OXPHOS is scavenged by glutathione thereby protecting TI-Tregs from oxidative stress. Inhibitors that are shown to alter TI-Treg metabolism and their targets are depicted in red
The ability of Tregs to switch between different metabolic routes has been attributed to the expression of FoxP3. Upon activating murine naïve CD4+ T cells in vitro in the presence of TGF-β, induced FoxP3+ Tregs had increased mitochondrial respiration compared to their FoxP3− counter parts [55]. In addition, exogenous expression of FoxP3 in activated CD4+ T cells causes a shift towards OXPHOS [42]. Expression of FoxP3 suppresses Myc signalling, by binding to its promoter, which in turn affects glycolysis and glutaminolysis [55, 56]. The importance of metabolic regulation for Treg functionality was reiterated using in the Foxp3∆EGFPiCre mouse model, wherein Tregs harbour loss of FoxP3 while expressing humanized Cre recombinase fused with enhanced green fluorescent protein, enabling the tracing of FoxP3− Tregs [57]. Such FoxP3− Tregs have increased glycolysis and OXPHOS. Treg-specific deletion of mTORC2 reduces glycolysis and restores the phenotype and functionality of FoxP3− Tregs and reduces inflammation in vivo. Interestingly, mTORC2 deficiency does not upregulate mitochondrial metabolism suggesting that alternative pathways are responsible [57]. In summary, current results indicate that Tregs utilize glycolysis for proliferation and migration, while their suppressive function is dependent on mitochondrial respiration [42, 43, 50, 51]. Tregs might switch their metabolic profile in response to inflammatory cues, which in turn affects their functionality, thereby allowing immune resolution only after pathogen clearance. While these studies have deciphered the metabolic profile of Tregs in the context of normal physiology or by using models of autoimmune disorders, the unique metabolic feature of Tregs could prove advantageous in the metabolically restrictive tumour microenvironment as well.
Tregs in the tumour microenvironment
Nutrient depletion
Glucose and free fatty acids
Reliance on OXPHOS for functionality might prove beneficial to Tregs in the nutrient-depleted TME, since they can utilize substrates either derived from glycolysis or from fatty acid oxidation (FAO) (Fig. 1) [43, 44, 58]. Accordingly, Tregs increase glycolytic as well as oxidative capacity within the tumour compared with Tconv cells [59]. TI-Tregs from MC-38 tumour-bearing mice have higher intracellular lipid content compared with TI-Tconv cells and with splenic Tregs. However, there was no increased uptake of fatty acids by these cells, suggesting that fatty acid synthesis (FAS) contributed to the increased lipid pool in TI-Tregs. The tri-carboxylic acid (TCA) cycle intermediates for FAS mostly originated from glycolysis, since inhibiting this with 2-deoxy-D-glucose (2DG) caused a reduction in lipid accumulation in TI-Tregs (Fig. 1) [59].
On the contrary, studies utilizing similar tumour models conclude that fatty acid uptake contributes to the increased lipid pool of TI-Tregs (Fig. 1) [60, 61]. In low glucose conditions, human Tregs utilize fatty acids efficiently for their expansion, survival and for suppressive functions in vitro [62]. In mice bearing GL-261 glioblastoma tumours, Tregs have higher lipid uptake compared with Tconv cells within the tumour [60]. Surface expression of the fatty acid transporters CD36 and SLC27A1 is significantly increased in Tregs in the brain compared to Tregs in the periphery. Of note, increased levels of fatty acid were found within the tumour compared with surrounding healthy brain tissue, suggesting that TME with high level of fatty acid might allow for accumulation of Tregs [60]. This was confirmed in a recent study, wherein the proliferation and suppressive function of effector Tregs were increased with higher doses of fatty acid palmitate in vitro [62]. Furthermore, gastric tumours harbouring Ras homolog family member A (RHOA) mutation produce more fatty acid compared with wild-type (WT) tumours, which in turn allows expansion and increased suppression by TI-Tregs [62]. Accordingly, CD36 is selectively upregulated in TI-Tregs, and this is accompanied by higher fatty acid uptake and a higher lipid content compared with peripheral Tregs [61]. In line with this, Treg-specific genetic ablation of CD36 severely decreased lipid uptake and content in TI-Tregs, which then impaired OXPHOS and skewed their metabolic preference towards aerobic glycolysis. As a result, CD36-deficient TI-Tregs displayed reduced suppressive capacity ex vivo, while their splenic counterparts displayed comparable suppressive capacity. This suggests that CD36-mediated fatty acid uptake is specific to TI-Tregs [61].
While most studies imply that Tregs employ OXPHOS for their functionality in the TME, TI-Treg cell lines generated from primary melanoma or breast cancer tumours were highly glycolytic and upregulated glucose transporters [63]. The competition for glucose in turn caused senescence of effector T cells, which did not occur in high glucose conditions [63]. However, the extent of glucose limitation in the TME and the potential effect of this on TI-Tregs were not described. The reliance of TI-Tregs on glycolysis for their suppressive functions is not shown by additional studies. Hence, TI-Tregs presumably rely on mitochondrial metabolism for their functionality which endows them with a broader choice of substrates obtained either from glucose or from fatty acids in the TME.
Amino acids
Amino acid metabolism also plays an important role in Treg development, thereby allowing their adaptation within the TME. During stimulation of naïve T cells, depriving glutamine in the media or addition of the glutaminase inhibitor 6-diazo-5-oxo-l-norleucine (DON) results in increased FoxP3 expression in vitro in a TGF-β dependent manner [64, 65]. Such Tregs induced under glutamine limitation maintain suppressive function and can persist in vivo [64]. In addition, FoxP3+ Tregs obtained from healthy human donors are resilient to glutamine restriction in vitro [66]. Moreover, high glutamate concentration altered cytokine production by dendritic cells which in turn fostered Tregs in an experimental autoimmune encephalomyelitis model [67]. Increased glutamate can also directly alter Tregs and enhance their proliferation and suppressive function [68]. Tumour cells have increased consumption of glutamine, converting it to glutamate, which can be exported in exchange for cystine, rendering the TME low in glutamine and high in glutamate levels, potentially sustaining TI-Tregs [13, 69, 70]. In line with this, vascular endothelial growth factor (VEGF) blockade increased glutamate production in murine glioblastoma tumours, thereby favouring Treg accumulation [68]. Depleting Tregs prior to VEGF blockade in this model led to tumour growth control and increased survival [68].
Moreover, tumour cells express indoleamine 2,3-dioxygenase (IDO) which mediates the conversion of tryptophan to kynurenine [71]. IDO-mediated tryptophan depletion and the resulting tryptophan metabolites facilitate induction of FoxP3+ Tregs and activate suppressive function of Tregs in a dendritic cell-dependent manner [72–75]. Kynurenine is also known to activate and signal through the aryl hydrocarbon receptor (AHR), which is essential for TGF-β dependent Treg induction [76, 77]. Furthermore, overexpression of IDO in mouse B16 melanoma tumour resulted in increased expression of AHR on TI-Treg and also enhanced their suppressive functions [78]. Tissue resident Tregs and activated Tregs from peripheral blood can deplete arginine due to their high expression of arginase and also utilize this pathway to suppress proliferation of effector T cells [79]. Such Tregs with high expression of arginase were also seen in tumours from melanoma patients, suggesting additional mode of TI-Treg mediated suppression [79]. Taken together, these studies imply that TI-Tregs can utilize the amino acid profiles within the TME for induction, survival and function.
Feeding mice a diet low in isoleucine or a Treg-specific deletion of amino acid transporter SLC3A2 resulted in low Treg numbers in vivo due to reduced proliferation suggesting that isoleucine is essential to sustain Treg proliferation [80]. Isoleucine, valine and leucine, collectively termed as the branched-chain amino acids (BCAA), are taken up by the tumour to sustain increased metabolic demands [11, 81, 82]. However, the level of BCAA in the TME and their effect on tumour growth is different across tumour types [81, 82]. It would thus be interesting to further explore the effect of altered BCAA levels in the TME on metabolic rewiring of TI-Tregs.
Hypoxia
The hypoxic TME actively recruits Tregs due to enhanced intra-tumoral expression of chemokine CCL-28 [83, 84]. Moreover, hypoxia induces upregulation of TGF-β1 through hypoxia-inducible factor (HIF)-1α binding to its promoter, thereby inducing Foxp3 expression in CD4+ T cells both in vitro and in vivo [85, 86]. Some studies also show an increased Treg suppressive function in hypoxic conditions [87, 88]. HIF-1α stimulates glycolysis by inducing glucose transporters and glycolytic enzymes, while inhibiting mitochondrial respiration [5]. Genetic deletion of HIF-1α in Tregs increased mitochondrial metabolism ex vivo compared with Tregs from WT mice, thereby enhancing their suppressive functions [60]. Interestingly, enhanced suppression by HIF-1α knockout (KO) Tregs was limited to hypoxic conditions, indicating that Tregs utilize a HIF-1α driven metabolic switch only under hypoxia, such as in the tumour [60]. However, it was not evaluated in this model if Tregs can cope with increased mitochondrial activity despite possibly low oxygen levels in the tumour for prolonged duration. HIF-1α stabilization under hypoxia is essential to reduce oxygen consumption thereby assisting cell survival, whereas HIF-1α KO cells continue to consume oxygen eventually disrupting intracellular oxygen tension [89]. It is therefore possible that 1% oxygen can sustain mitochondrial activity in HIF-1α KO conditions albeit for a short duration. In line with the earlier observations, loss of glycolytic capacity of Tregs lacking HIF-1α reduced their migratory capability into the TME, contributing to the increased survival of mice bearing GL-261 brain tumour [50, 60]. Thus, targeting HIF-1α could prevent migration into the tumour, but this might improve inhibitory function for Tregs already present in the tumour.
Acidity
Increased extracellular lactic acid due to high aerobic glycolysis by tumour cells is a major source for acidification of the TME [14]. The conversion of glucose-derived pyruvate to lactate regenerates NAD+, which is essential to sustain glycolysis. This process also occurs in activated Tconv cells. As a result, the presence of extracellular lactic acid or the pH neutral form- sodium lactate causes loss of proliferation of Tconv cells since they are unable to excrete lactate produced due to loss of gradient [55, 90]. On the contrary, Tregs prefer to oxidize pyruvate in the mitochondria, instead of converting it to lactic acid, which is supported by their increased ratio of NAD/NADH (Fig. 1) [55]. Consequently, Tregs are able to proliferate and suppress Tconv cells in the presence of pH neutral extracellular lactate. In addition, the presence of extracellular lactate increases the frequency of iTregs in a TGF-β dependent manner [55]. Furthermore, the increased frequency of iTregs (CD4+FoxP3+) in the presence of extracellular lactate in low glucose condition is due to a relative reduction in proliferation of non iTregs (CD4+FoxP3−) [55]. While these results indicate that the survival and functional advantage of FoxP3+ Tregs in high lactate environments mainly stems from their preference to oxidize pyruvate in the mitochondria, Tregs could potentially utilize lactate as an alternative fuel source for OXPHOS.
However, it is described that loss of proliferation and cytokine production by human effector T cells in the presence of lactic acid is a consequence of acidification which can be reversed by buffering the pH of the medium [90]. Furthermore, sodium lactate at physiological pH did not inhibit Tconv function [90, 91]. It is thus interesting to determine if Tregs also have an advantage in low pH conditions. This was recently addressed using Tregs induced in the presence of tumour conditioned medium [61]. Such Tregs had a survival advantage in the presence of extracellular lactic acid in vitro due to the upregulation of the fatty acid transporter CD36 [61]. Knock out of CD36 in Tregs reversed this advantage due to reduced mitochondrial respiration which depleted their NAD pool [61]. It is, however, not completely deciphered if the ability to survive in the presence of lactic acid is limited to TI-Tregs due to their increased CD36 expression or if Tregs in general are more resistant to extracellular acidification owing to their reduced glycolytic rates.
Oxidative stress
Tregs have elevated intracellular ROS levels compared with effector T cells combined with low level of antioxidant regulator NRF2 and its associated gene transcripts, thus being relatively sensitive to oxidative stress in the TME [55, 92]. TI-Tregs sorted and expanded from human ovarian tumours were highly apoptotic. Culturing mouse Tregs with human ovarian cancer ascites also induced apoptosis in Tregs, due to their increased ROS production [92]. Surprisingly, apoptotic Tregs retain their capability to suppress anti-tumour immune response in vivo in MC38 and B16-F10 tumour models. Suppressive function of such apoptotic Tregs was dependent on the ecto-enzymes CD39 and CD73. These apoptotic Tregs released high levels of ATP via pannexin-1-dependent channels, and in turn metabolized it to the immune-suppressive adenosine by the combined activity of CD39 and CD73 [92].
On the contrary, it was shown earlier that human nTregs show greater resilience towards ROS mediated cell death compared with Tconv cells due to increased levels of antioxidant- thioredoxin-1 [93]. Moreover, iTregs have lower intracellular ROS compared with activated Tconv cells despite their increased mitochondrial metabolism [94]. This enhanced capability of scavenging intracellular ROS is due to increased levels of the antioxidant glutathione (Fig. 1) [94]. Glutathione is synthesized by the enzyme glutamate cysteine ligase (Gclc) utilizing glutamine, glycine and cysteine [95]. Genetic deletion of Gclc specifically in Tregs caused severe autoimmunity in mice. Gclc deficiency in Tregs triggered an intracellular accumulation of serine due to feedback regulation of serine by glutathione. This in turn resulted in increased mTOR activation and dysregulated Treg metabolism causing reduced FoxP3 expression and loss of Treg functionality in both mice and human Tregs [94]. The requirement of glutathione by Tregs is also noteworthy and points out to a potential dependence of Tregs on cysteine availability [95]. It is also interesting to evaluate if cysteine limitation in the tumour microenvironment might in turn engage trans-sulfuration pathway in TI-Tregs [96, 97]. Furthermore, Treg Gclc deletion led to slower growth of tumours in B16-F10 melanoma model. It was not evaluated if Tregs underwent apoptosis or if apoptotic Tregs also contributed to immune suppression in this model [94]. It is thus imperative to determine how ROS levels vary across tumour models and if this in turn leads to differences in Treg metabolic profiles determining maintenance and suppressive functions.
Targeting intra-tumoral Treg metabolism
The differences in metabolic profile of TI-Tregs compared with peripheral Tregs and Tconv cells provide opportunities to tackle the persistent problem of selective TI-Treg targeting. Since glycolysis is essential for the migration of Tregs, inhibiting glycolysis in Tregs could prevent recruitment into the TME [50, 60]. It is, however, possible that glycolysis inhibition might result in improved suppressive function of Tregs already present in the TME [42]. This could be circumvented by preceding glycolysis inhibition with transient Treg depletion. Additional studies are, however, essential to find targets that can modulate glycolysis specifically in Tregs, sparing other immune subtypes. Expression of FoxP3 is known to downregulate mTOR activation in Tregs which further reduces glycolysis [42]. Chronic activation of mTOR in Tregs could therefore destabilize their metabolism, reducing their functionality in tumour models.
Since inhibition of OXPHOS reduces suppressive function of Tregs in vitro and in vivo, this strategy can also be used to target suppressive function of intra-tumoral Tregs [51, 57]. 5-tetradecyloxy-2-furoic acid (TOFA) is known to inhibit acetyl-CoA carboxylase, thereby inhibiting fatty acid synthesis (Fig. 1). Abolishing fatty acid accumulation in Tregs by treatment with TOFA, significantly inhibits the proliferation of Tregs [59]. However, the effect on tumour growth control in vivo was due to a direct toxicity on tumour cells rather than immune-mediated events [59]. TI-Tregs are enriched in the expression of the fatty acid transporter CD36 in melanoma models (Fig. 1) [61]. Treatment with a monoclonal antibody blocking CD36 reduced accumulation of TI-Tregs without systemic loss of Treg numbers or functions thereby leading to a significant increase in infiltration and effector functions of CD8+ T cells in the tumour. Subsequently, reduced tumour growth was observed [61]. CD36 blockade also reduced TI-Tregs and increased the ratio of CD8+ T cells to Tregs in the RHOA mutant MC38 colon cancer model, characterised by high production of free fatty acids [62]. VT1021, a peptide that induces thrombospondin-1 (Tsp-1), in turn targeting CD36, is currently being evaluated in a phase 1/2 clinical trial [98]. Determining its effect on TI-Treg functionality could provide additional insights into the potential of targeting Treg metabolism.
Treatment with Etomoxir, known to inhibit FAO by inhibiting carnitine palmitoyl-transferase 1a, severely affects Treg proliferation and suppressive capability by the inhibition of lipid metabolism (Fig. 1) [59, 60]. Intracranial administration of Etomoxir into mice bearing GL-261 brain tumour led to an increased ratio of effector T cell/ Tregs resulting in an immune-mediated improvement in survival, suggesting that blocking FAO might specifically target Treg functionality [60]. This must be further verified with additional specific inhibitors for FAO, since Etomoxir was shown to have off-target effects such as reduced electron transport chain (ETC) activity and increased induction of oxidative stress [99–101]. In addition, dysregulated mitochondrial metabolism of effector CD8+ T cells in the TME results in reduced effector functions thereby increasing tumour growth [102–104]. It is thus probable that broad inhibitors of fatty acid metabolism, while reducing Treg functionality might also hamper effector responses.
Studies that evaluate the potential of modulating TI-Treg metabolism to enhance the efficacy of checkpoint blockade are recently surfacing. Encouragingly, anti-PD-1 monoclonal antibody effectively limited tumour progression and prolonged survival in mice lacking CD36 in Tregs, indicating that CD36 and PD-1 targeting could synergize to control tumour growth [61]. In line with this observation, combination of CD36 blockade and anti-PD1 antibody significantly reduced growth of RHOA mutated MC38 tumours, while either antibody alone did not lead to tumour growth control [62]. Injecting apoptotic Tregs along with tumour diminished the beneficial effect of PD-L1 antibody on anti-tumour T cell response and control of MC38 tumour, indicating that modulating TI-Treg metabolism likely augments response to immune therapy [92]. Targeting Tregs by the use of CTLA-4 blocking antibody in combination with PD-1 blockade caused regression of RHOA mutant MC38 tumours that are otherwise resistant to either treatment alone [62]. Signalling via CTLA-4 is known to reduce glycolysis in Tregs [50]. It is therefore interesting to evaluate the potential of CTLA-4 blockade on modulating TI-Treg metabolism and if this in part contributes to the efficacy of the antibody. Preliminary results showed that CTLA-4 blockade in low glycolytic tumours destabilized Treg functions and led to increased survival in mice [105].
Decreased ratios of Teff/Tregs in the tumour are often associated with poor prognosis in cancer and impaired outcome upon checkpoint inhibition [32, 106, 107]. Favourable ratios can also be achieved by empowering Tconv to overcome the metabolic stresses in the TME. To do so, one could make use of the lessons learnt from TI-Treg metabolic profiles. In contrast to Tregs, Tconv produces lactic acid owing to increased aerobic glycolysis, as a result of which they lose their functionality in TME with high level of extracellular lactic acid [55, 90]. Treatment with the LDH inhibitor GSK 2837808A in vitro rescued the Tconv from L-lactate inhibition [55]. In line with this observation, blocking LDH (with NCI-737) and IL-21 synergistically rewired the metabolic profile of CD8+ T cells and led to reduced tumour growth and longer survival in B16 melanoma bearing mice [108]. Alternatively, promoting mitochondrial biogenesis and function in CD8+ T cells also improved anti-tumour activity in vivo [102, 103, 109, 110].
Concluding remarks
Despite the plethora of metabolic challenges such as nutrient restriction, hypoxia, acidity and oxidative stress, imposed by TME on infiltrating immune cells, Tregs can still survive and function. In this review, we provided a comprehensive understanding of how Tregs deal with these metabolic stresses. While most studies indicate that Tregs rely on OXPHOS for their suppressive functions in normal physiology and during disease [42, 43, 51, 60], the substrate for this in the tumour could either be derived from glucose or from fatty acids [54, 59, 61]. Though it seems plausible that the preference for a particular fuel source can be specific to a certain microenvironment, there are discrepancies across studies that utilize similar tumour models [59, 61]. It is evident that the metabolic reprogramming of TI-Tregs is not dependent on a single pathway, but it is an amalgamation of responses of Treg-intrinsic regulators to the environmental cues. Further characterisation of the regulators of Treg metabolic responses to TME is required. The competitive advantage of Tregs due to their metabolism, however, could also become their Achilles heel, which provides new avenues for specific therapeutic targeting, which already shows promising early results [60, 61]. One can envision that such approaches may synergize with current effective anti-tumour immune therapies, like T cell therapies or checkpoint inhibition, thereby providing an additional and independent approach in cancer immunotherapy [61, 62]
Abbreviations
- AHR
Aryl hydrocarbon receptor
- BCAA
Branched-chain amino acids
- ETC
Electron transport chain
- FAO
Fatty acid oxidation
- FAS
Fatty acid synthesis
- HIF
Hypoxia-inducible factor
- IDO
Indoleamine 2, 3-dioxygenase
- iTreg
Induced Tregs
- mTOR
Mammalian target of rapamycin
- nTregs
Natural Tregs
- OXPHOS
Oxidative phosphorylation
- RHOA
Ras homolog family member A
- ROS
Reactive oxygen species
- TCA
Tri-carboxylic acid
- Tconv
Conventional T cells
- TGF-β
Transforming growth factor beta
- TI-Tregs
Tumour-infiltrating Tregs
- TME
Tumour microenvironment
- Treg
Regulatory T cells
- WT
Wild-type
Author contributions
D.R, R.L and C.U.B conceptualised the idea for the manuscript. All authors contributed to the literature search. The first draft of the manuscript was written by D.R, F.V and R.L. All authors were involved in critical revision of the manuscript and approval of the final manuscript.
Funding
None.
Compliance with ethical standards
Conflicts of interest
C.U.B declares the following potential COI: advisory roles for BMS, MSD, Roche, Novartis, GSK, AZ, Pfizer, Lilly, GenMab, Pierre Fabre, Third Rock Ventures, research funding from BMS, Novartis, NanoString, co-founder of Immagene B.V. D.S.P received research support from MSD and BMS and is co-founder, shareholder and advisor of Immagene B.V.
Footnotes
Ruben Lacroix and Christian U Blank: shared last authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang LV. Tumor microenvironment and metabolism. Int J Mol Sci. 2017;18:278. doi: 10.3390/ijms18122729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber V, Camisaschi C, Berzi A, et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017;43:74–89. doi: 10.1016/j.semcancer.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Longo DL, Bartoli A, Consolino L, et al. In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res. 2016;76:6463–6470. doi: 10.1158/0008-5472.CAN-16-0825. [DOI] [PubMed] [Google Scholar]
- 8.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 9.Yao CH, Wang R, Wang Y, et al. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife. 2019 doi: 10.7554/eLife.41351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, McMillan-Ward E, Kong J, et al. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 11.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200–e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacroix R, Rozeman EA, Kreutz M, et al. Targeting tumor-associated acidity in cancer immunotherapy. Cancer Immunol Immunother. 2018;67:1331–1348. doi: 10.1007/s00262-018-2195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho P-C, Bihuniak JD, Macintyre AN, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim AR, Rathmell WK, Rathmell JC. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife. 2020;9:e55185. doi: 10.7554/eLife.55185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, Saha J. Tumour, oxidative stress and host T cell response: cementing the dominance. Scand J Immunol. 2015;82:477–488. doi: 10.1111/sji.12350. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103–114. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 21.Vaeth M, Wang YH, Eckstein M, et al. Tissue resident and follicular Treg cell differentiation is regulated by CRAC channels. Nat Commun. 2019;10:1–16. doi: 10.1038/s41467-019-08959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson J, Tran DQ, Pesu M, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G, Levitsky HI. Natural regulatory T cells and De novo-induced regulatory T Cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 25.Waight JD, Takai S, Marelli B, et al. Cutting edge: epigenetic regulation of Foxp3 defines a stable population of CD4 + regulatory T cells in tumors from mice and humans. J Immunol. 2015;194:878–882. doi: 10.4049/jimmunol.1402725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadzadeh M, Pasetto A, Jia L, et al. Tumor-infiltrating human CD4(+) regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci Immunol. 2019 doi: 10.1126/sciimmunol.aao4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y, Josefowicz S, Chaudhry A, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moo-Young TA, Larson JW, Belt BA, et al. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother. 2009;32:12–21. doi: 10.1097/CJI.0b013e318189f13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker LSK, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms oftreg-mediatedt cell suppression. Front Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99. doi: 10.1016/j.jaut.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep. 2015 doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasku MA, Clem AL, Telang S, et al. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008 doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher SA, Aston WJ, Chee J, et al. Transient Treg depletion enhances therapeutic anti-cancer vaccination: immunity. Inflamm Dis. 2017;5:16–28. doi: 10.1002/iid3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teng MWL, Ngiow SF, von Scheidt B, et al. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 36.Arce Vargas F, Furness AJS, Solomon I, et al. Fc-Optimized Anti-CD25 depletes tumor-infiltrating regulatory T Cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rech AJ, Mick R, Martin S, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:13462–13462. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs JFM, Punt CJA, Lesterhuis WJ, et al. Dendritic cell vaccination in combination with Anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16:5067–5078. doi: 10.1158/1078-0432.CCR-10-1757. [DOI] [PubMed] [Google Scholar]
- 39.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 40.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev. 2013;252:52–77. doi: 10.1111/imr.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerriets VA, Kishton RJ, Nichols AG, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerriets VA, Kishton RJ, Johnson MO, et al. Foxp3 and toll-like receptor signaling balance T reg cell anabolic metabolism for suppression. Nat Immunol. 2016;17:1459–1466. doi: 10.1038/ni.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4 + T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berod L, Friedrich C, Nandan A, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 45.Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28:514–524. doi: 10.1016/j.smim.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 46.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang A, Luan HH, Medzhitov R. An evolutionary perspective on immunometabolism. Science. 2019;363(6423):eaar3932. doi: 10.1126/science.aar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmond RJ. mTOR regulation of glycolytic metabolism in T cells. Front Cell Dev Biol. 2018;6:122. doi: 10.3389/fcell.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kishore M, Cheung KCP, Fu H, et al. Regulatory T Cell migration is dependent on glucokinase-mediated glycolysis. Immunity. 2017;47:875–889.e10. doi: 10.1016/j.immuni.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberg SE, Singer BD, Steinert EM, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–499. doi: 10.1038/s41586-018-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He N, Fan W, Henriquez B, et al. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc Natl Acad Sci USA. 2017;114:12542–12547. doi: 10.1073/pnas.1715363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang K, Blanco DB, Neale G, et al. Homeostatic control of metabolic and functional fitness of T(reg) cells by LKB1 signalling. Nature. 2017;548:602–606. doi: 10.1038/nature23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Field CS, Baixauli F, Kyle RL, et al. Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for Treg suppressive function. Cell Metab. 2020;31:422–437.e5. doi: 10.1016/j.cmet.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R, Dillon CP, Shi LZ, et al. The transcription factor myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charbonnier L-M, Cui Y, Stephen-Victor E, et al. Functional reprogramming of regulatory T cells in the absence of Foxp3. Nat Immunol. 2019;20:1208–1219. doi: 10.1038/s41590-019-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kareva I. Metabolism and gut microbiota in cancer immunoediting, CD8/Treg ratios, immune cell homeostasis, and cancer (immuno)therapy: concise review. Stem Cells. 2019;37:1273–1280. doi: 10.1002/stem.3051. [DOI] [PubMed] [Google Scholar]
- 59.Pacella I, Procaccini C, Focaccetti C, et al. Fatty acid metabolism complements glycolysis in th selective regulatory t cell expansion during tumor growth. Proc Natl Acad Sci USA. 2018;115:E6546–E6555. doi: 10.1073/pnas.1720113115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miska J, Lee-Chang C, Rashidi A, et al. HIF-1α is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of Tregs in glioblastoma. Cell Rep. 2019;27:226–237. doi: 10.1016/j.celrep.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Franco F, Tsui YC, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21:298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumagai S, Togashi Y, Sakai C, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 2020;53:187–203.e8. doi: 10.1016/j.immuni.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Liu X, Sanders KL, et al. TLR8-mediated metabolic control of human Treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 2019;29:103–123. doi: 10.1016/j.cmet.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klysz D, Tai X, Robert PA, et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci Signal. 2015 doi: 10.1126/scisignal.aab2610. [DOI] [PubMed] [Google Scholar]
- 65.Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metzler B, Gfeller P, Guinet E. Restricting glutamine or glutamine-dependent purine and pyrimidine syntheses promotes human T cells with High FOXP3 expression and regulatory properties. J Immunol. 2016;196:3618–3630. doi: 10.4049/jimmunol.1501756. [DOI] [PubMed] [Google Scholar]
- 67.Fallarino F, Volpi C, Fazio F, et al. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat Med. 2010;16:897–902. doi: 10.1038/nm.2183. [DOI] [PubMed] [Google Scholar]
- 68.Long Y, Tao H, Karachi A, et al. Dysregulation of glutamate transport enhances Treg function that promotes vegf blockade resistance in glioblastoma. Cancer Res. 2020;80:499–509. doi: 10.1158/0008-5472.CAN-19-1577. [DOI] [PubMed] [Google Scholar]
- 69.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timmerman LA, Holton T, Yuneva M, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24:450–465. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 72.Sharma MD, Hou D-Y, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan Y, Zhang G-X, Gran B, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 77.Mezrich JD, Fechner JH, Zhang X, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campesato LF, Budhu S, Tchaicha J, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11:4011. doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lowe MM, Boothby I, Clancy S, et al. Regulatory T cells use arginase 2 to enhance their metabolic fitness in tissues. JCI Insight. 2019 doi: 10.1172/jci.insight.129756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikeda K, Kinoshita M, Kayama H, et al. Slc3a2 mediates branched-chain amino-acid-dependent maintenance of regulatory T cells. Cell Rep. 2017;21:1824–1838. doi: 10.1016/j.celrep.2017.10.082. [DOI] [PubMed] [Google Scholar]
- 81.Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care. 2018;21:64–70. doi: 10.1097/MCO.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sivanand S, Vander Heiden MG. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell. 2020;37:147–156. doi: 10.1016/j.ccell.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren L, Yu Y, Wang L, et al. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget. 2016;7:75763–75773. doi: 10.18632/oncotarget.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T reg cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 85.Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng B, Zhu J-M, Wang Y, et al. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-β1 in gastric cancer. PLoS ONE. 2013;8:e63777. doi: 10.1371/journal.pone.0063777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Shoshan J, Maysel-Auslender S, Mor A, et al. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1α. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 88.Westendorf A, Skibbe K, Adamczyk A, et al. Hypoxia enhances immunosuppression by Inhibiting CD4+ Effector T cell function and promoting Treg activity. Cell Physiol Biochem. 2017;41:1271–1284. doi: 10.1159/000464429. [DOI] [PubMed] [Google Scholar]
- 89.Papandreou I, Cairns RA, Fontana L, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 90.Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 91.Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses energy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 92.Maj T, Wang W, Crespo J, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mougiakakos D, Johansson CC, Jitschin R, et al. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 94.Kurniawan H, Franchina DG, Guerra L, et al. Glutathione restricts serine metabolism to preserve regulatory T Cell function. Cell Metab. 2020;31:920–936.e7. doi: 10.1016/j.cmet.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garg SK, Yan Z, Vitvitsky V, Banerjee R. Differential dependence on cysteine from transsulfuration versus transport during T cell activation. Antioxid Redox Signal. 2011;15:39–47. doi: 10.1089/ars.2010.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu J, Berisa M, Schwörer S, et al. Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metab. 2019;30:865–876.e5. doi: 10.1016/j.cmet.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cieslewicz M, Mahalingam D, Harb WA, et al. A phase I open label study evaluating VT1021 in patients with advanced solid tumors. J Clin Oncol. 2019;37:TPS3158. doi: 10.1200/JCO.2019.37.15_suppl.TPS3158. [DOI] [Google Scholar]
- 99.Raud B, Roy DG, Divakaruni AS, et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 2018;28:504–515.e7. doi: 10.1016/j.cmet.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Connor RS, Guo L, Ghassemi S, et al. The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci Rep. 2018;8:6289. doi: 10.1038/s41598-018-24676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao C-H, Liu G-Y, Wang R, et al. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. PLoS Biol. 2018;16:e2003782. doi: 10.1371/journal.pbio.2003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y-R, Imrichova H, Wang H, et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat Immunol. 2020 doi: 10.1038/s41590-020-0793-3. [DOI] [PubMed] [Google Scholar]
- 103.Scharping NE, Menk AV, Moreci RS, et al. The tumor microenvironment represses T Cell mitochondrial biogenesis to drive intratumoral T Cell metabolic insufficiency and dysfunction. Immunity. 2016;45:374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fischer M, Bantug GR, Dimeloe S, et al. Early effector maturation of naïve human CD8(+) T cells requires mitochondrial biogenesis. Eur J Immunol. 2018;48:1632–1643. doi: 10.1002/eji.201747443. [DOI] [PubMed] [Google Scholar]
- 105.Zappasodi R, Serganova I, Cohen I, et al. Abstract 3257: CTLA-4 blockade drives loss of regulatory T cell functional stability in glycolysis defective tumors. Cancer Res. 2020;80:3257–3257. doi: 10.1158/1538-7445.AM2020-3257. [DOI] [Google Scholar]
- 106.Ouwerkerk W, van den Berg M, van der Niet S, et al. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res. 2019;29:453–464. doi: 10.1097/CMR.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hermans D, Gautam S, García-Cañaveras JC, et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor immunity. Proc Natl Acad Sci. 2020;117:6047–6055. doi: 10.1073/pnas.1920413117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, Kurupati R, Liu L, et al. Enhancing CD8+ T Cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377–391.e9. doi: 10.1016/j.ccell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Menk AV, Scharping NE, Rivadeneira DB, et al. 4–1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med. 2018;215:1091–1100. doi: 10.1084/jem.20171068. [DOI] [PMC free article] [PubMed] [Google Scholar]