Abstract
Background
Foxp3+RORγt+ T cells possess both characteristics of regulatory T cells and T helper 17 cells and show significant immunoregulatory functions in autoimmune diseases. However, the role and clinical significance of Foxp3+RORγt+ T cells in gastric cancer remains unclear.
Methods
We enrolled 452 gastric cancer tissue microarray samples and 60 fresh tumor tissue samples from Zhongshan Hospital. The infiltration of Foxp3+RORγt+ T cells and immune contexture were examined by immunohistochemistry and flow cytometry. Survival analyses of patient subgroups were conducted by Kaplan–Meier curves, log-rank test and Cox proportional model.
Results
High infiltration of Foxp3+RORγt+ T cells predicted poor overall survival (P = 0.0222 and 0.0110) and inferior therapeutic response (P = 0.003 for interaction) in gastric cancer. Foxp3+RORγt+ T cells were associated with impaired effective function of CD8+ T cells featured by decreased interferon-γ, granzyme B and CD107a expression. Co-evaluation of Foxp3+RORγt+ T cells and CD8+ T cells could predict survival outcomes and chemotherapeutic responsiveness more precisely.
Conclusions
We found that Foxp3+RORγt+ T cells could potentially attenuate effective functions of CD8+ T cells and led to adverse survival outcomes and inferior chemotherapeutic responsiveness. Moreover, the novel co-evaluation system might be useful for prognosis prediction for appropriate treatment in gastric cancer.
Novelty and impact statements
Clinical significance of Foxp3+RORγts+ T cells has not been studied in gastric cancer. Herein, we investigated the prognostic value of Foxp3+RORγt+ T cells in 452 patients. We demonstrated that intratumoral Foxp3+RORγt+ T cell infiltration was a prognostic biomarker for overall survival and the identification of patients might benefit from post-gastrectomy 5-fluorouracil. These findings allow a more precise stratification upon the co-evaluation with CD8+ T cells to better clinical management for patients who would benefit from 5-fluorouracil.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02950-3.
Keywords: Gastric cancer, Foxp3+RORγt+ T cells, Prognosis, Adjuvant chemotherapy, Chemotherapeutic responsiveness
Introduction
Gastric cancer is the fifth most frequently diagnosed malignancy and the third leading cause of cancer-associated deaths worldwide. Every year, gastric cancer takes charge of over 1,000,000 new cases, half of which occur in China [1, 2]. Current management for gastric cancer patients is composed of endoscopic detection followed by gastrectomy, adjuvant chemotherapy (ACT) or neo-adjuvant chemotherapy (NACT). However, even though patients receive adjuvant therapy, the recurrence rate for advanced gastric cancer still ranges from 25 to 40% [3–5]. Consequently, further investigation of other potential adjuvant therapeutic strategies that can prolong survival and reduce disease recurrence for gastric cancer patients is of urgent need in clinical practice.
In recent years, immunotherapy has started a brand new era in the war against cancer. Immune checkpoint inhibitors (ICIs), which aim to reactivate antitumor immunity and reinvigorate effective function of T cells, have shown promising efficacy and manageable safety in a series of solid malignancies [6]. Unfortunately, the objective response rate of immunotherapy is merely less than 15% in gastric cancer [7, 8]. Previous studies have reported that the heterogeneity of tumor cells and tumor microenvironment might attenuate the efficacy of immunotherapy [9]. Consequently, it is of much clinical significance to identify distinct subtypes of gastric cancer with more precise and specific markers, so as to facilitate patient counseling, decision-making on individualized adjuvant therapy, and follow-up scheduling better [2].
As an inflammatory-associated malignancy, gastric cancer is featured by infiltration of heterogeneous polynuclear and mononuclear immune cells, including macrophages [10, 11], granulocytes [12], and various T lymphocytes [13, 14]. In inflammatory conditions, regulatory T (Treg) cells can express specific transcription factors and cytokines that are associated with CD4+ T helper cell lineages. Treg cells have been reported as able to protect the host from tumorigenicity by reducing the inflammation mediated by T cells [15]. Meanwhile, Treg cells have shown pro-tumorigenic effects by suppressing antitumor immunity [16]. Especially, inflammation combined with expansion of Treg cells in tumor tissue might lead to cytotoxic T lymphocyte dysfunction and indicate poor prognosis [17, 18]. This phenomenon may result from the presence of different Treg subsets with dissimilar functional phenotypes, due to the heterogeneity of tumor microenvironment [19]. Notably, a specific Treg cell population, which co-expresses transcription factor forkhead box P3 (Foxp3) and retinoic acid–related orphan receptor-γt (RORγt), has been identified. RORγt is a marker and key regulator of Th17 cells, and modulates the production of pro-inflammatory cytokine interleukin-17 (IL-17) [20]. Lines of evidence suggest that Foxp3+RORγt+ Treg cells might represent an intermediate stage of differentiation between suppressive Treg cells and pro-inflammatory Th17 cells. Also known as "ex-Treg cells," these Treg cells may show decreased expression of Foxp3, yet are endowed with fragility and ability to secret effective molecules such as IL-17 [21]. Our previous study has reported that IL-17 could reinvigorate antitumor immunity, thus leading to better survival and superior chemotherapeutic responsiveness in gastric cancer [22]. However, the role of Treg-specific RORγt expression in gastric cancer and whether such controversial Treg subset would impact clinical outcomes still remain unknown.
Here, we inspected the clinical significance of Foxp3+RORγt+ Treg cells. Detailed phenotypic analyses revealed that the Foxp3+RORγt+ Treg cells potentially suppressed antitumor immune responses in gastric cancer. Furthermore, stratification of gastric cancer patients based on Foxp3+RORγt+ T cells and CD8+ T cells infiltration predicts prognosis and therapeutic responsiveness to ACT. However, our study was a retrospective research in nature and the quantity of patients receiving ACT was relatively small. Therefore, we advocate further validation in a prospective, larger, and multi-centered randomized trial.
Materials and method
Patients and gastric tissue samples
Four hundred and ninety-six gastric cancer patients from Zhongshan Hospital, Fudan University (Shanghai, China) were enrolled in our current study. Given the data missing, metastatic diseases or dot loss, the remaining 452 patients were randomly divided into two independent patient datasets, testing set (n = 226, cohort 1) and validation set (n = 226, Cohort 2). The patients underwent radical gastrectomy and standard D2 lymphadenectomy between 2007 and 2008. All tissue samples were formalin-fixed and paraffin-embedded (FFPE). Patient clinicopathological characteristics, including age, gender, tumor size, location, grade, Lauren classification, TNM stage, ACRG classification and application of fluorouracil-based ACT, were collected retrospectively. TNM stage was assessed according to the 2010 International Union Against Cancer TNM staging system, and the ACRG classification were assessed on the basis of high-throughput protein and mRNA expression [23]. None of the patients accepted radiotherapy. Only the patients with stage II and III tumors received routine fluorouracil-based ACT after surgery. The endpoint of interest was overall survival. Survival time was recorded from the date of gastrectomy to the date of death or the last follow-up. An additional flow cytometry (FCM) cohort (Cohort 3) consisted of 60 patients from Zhongshan Hospital, Fudan University (Shanghai, China) was also recruited. These patients received gastrectomy between August 2018 and November 2018. Expression of effector molecules and immune checkpoints within CD8+ T cells was detected by FCM. Corresponding 60 FFPE tissue blocks of Cohort 3 were retrospectively obtained and established as an independent tissue microarray (TMA) to evaluate the intratumoral Foxp3+RORγt+ Treg cells infiltration in Cohort 3. Our research was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Every patient was informed of and signed the permission to the usage of resected gastric tissue samples.
Assay methods
Immunohistochemistry (IHC) was conducted as previously described [22]. The slides were dewaxed in dry-heat oven, xylene and graded alcohols, and then washed with phosphate-buffered saline for 3 times. After treated with 3% H2O2 for 30 min at 37 °C, the slides were boiled in sodium citrate buffer with a microwave oven for 14 min and then treated with 10% normal goat serum blocking solution for 120 min at 37 °C. Primary antibodies (Foxp3 and RORγt; diluted as preceding described) were applied in a moist chamber at 4 °C overnight. After washed off the primary antibody, an Envisionplus detection system was applied (AP/RA; HRP/Mo) with vector blue (VB) and 3,30-diaminobenzidine (DAB) to visualize the reaction marks, respectively. All slides were scanned with Nikon Ti and each section was evaluated (magnification: × 200). The quantity of intratumoral and peritumoral cells (CD4+ T cells, CD8+ T cells, Foxp3+ Treg cells, CD66b+ neutrophils, CD56+ Natural Killer [NK] cells, CD68+ macrophages, CD163+ macrophages and IFN-γ+ cells) was recorded as the average of positive cells from 3 randomized fields (× 200) counted by two independent pathologists (Dr. Peipei Zhang and Dr. Lingli Chen) who were blinded to the clinical data. The mean count of their evaluation was determined. Variations in the numeration exceeding 5 cells were re-evaluated separately by both pathologists to reach consensus. The intratumoral and peritumoral region referred to the tumor center area and the area 2 cm away from the tumor margin, respectively.
Flow cytometry
Fresh tumor tissues were collected as soon as the samples were resected during the surgery Fresh tissues were digested by collagenase IV and incubated with BD GolgiStop™ Protein Transport Inhibitor (Containing Monensin, BD Biosciences) for 2 h. Next, the single-cell suspensions were centrifuged and incubated in RBC lysis buffer on ice for 10 min and then incubated with Human TruStain FcX (Biolegend) for Fc receptor blocking for 15 min. Cells were stained for surface markers in cell staining buffer for 30 min at 4 °C in the dark. Washed with cell staining buffer, cells were fixed and permeabilized with Fixation/Permeabilization Solution Kit (BD Biosciences) for 20 min. Intracellular cytokine staining was established in intracellular staining permeabilization wash buffer at 4 °C in the dark for 30 min. Stained cells were washed, re-suspended in phosphate-buffered saline/0.1% bovine serum albumin plus azide. Flow cytometry was performed with use of FACSCelesta flow cytometer (BD Biosciences) and analyzed by FlowJo Software (Tree Star, Ashland, OR, USA). In all stained samples, dead cells were excluded with the use of Fixable Viability Stain 510 (BD Biosciences). The compensation was dependent on BD CompBeads set (BD Biosciences) adjusted by the machine automatically. Antibodies applied for FCM were listed (Sulppementary Table S1).
Statistical analysis
SPSS 25 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, including one-way ANOVA followed by Tukey’s multiple comparison test, Kaplan–Meier curves, log-rank test, and multivariate analysis based on the Cox proportional hazards method. The cutoff value of Foxp3+RORγt+ T cells was evaluated by X-Tile (Version 3.6.1, Yale University). GSEA (4.1.0) was used for gene set database from TCGA, GSE9650 and GO analysis. The genes included in Foxp3+RORγt+ T cells signature and CD8+ T cell gene set are listed in Supplementary Table S2. The signature scores, calculated by geometric mean, were used to indicate the relative abundance of Foxp3+RORγt+ T cells in TCGA samples. The outcomes were visualized by Graphpad Prism (Version 8.0, Graphpad Software, La Jolla, California, USA). All statistical analyses were two-sided, and P < 0.05 was identified as statistically significant.
Results
Foxp3+RORγt+ T cells expand in gastric cancer
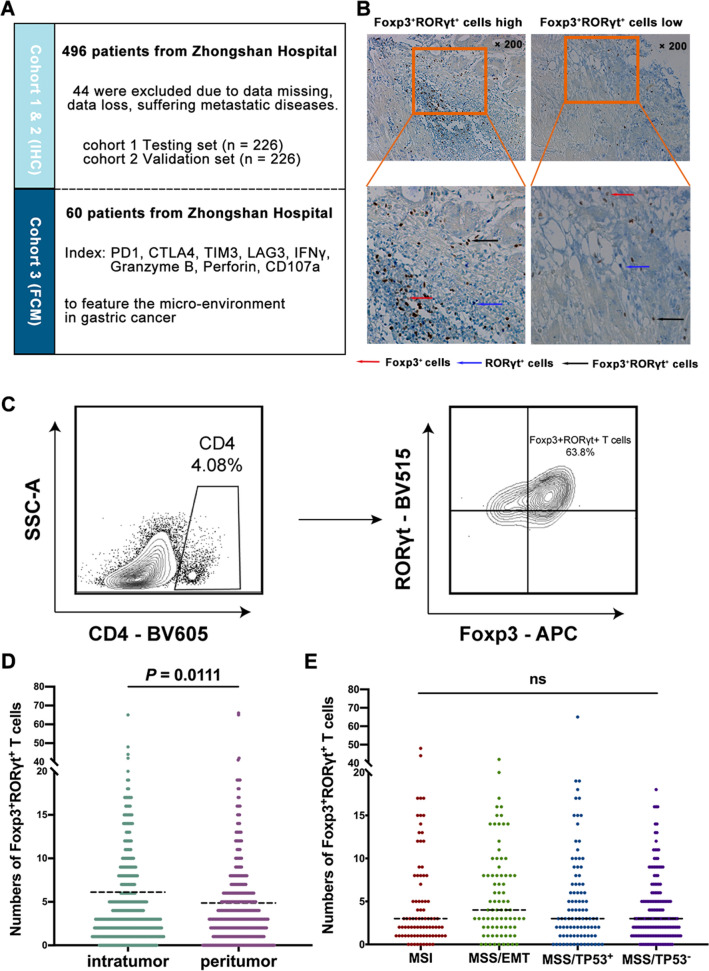
The current study was designed as listed (Fig. 1a). IHC staining and Flow Cytometry were performed for Foxp3+RORγt+ T cells (Fig. 1b, c). Compared with peritumor tissues, intratumor tissues showed significantly higher infiltration of Foxp3+RORγt+ T cells (Fig. 1d). Consequently, we concentrated on intratumoral Foxp3+RORγt+ T cells in our following study. Notably, the infiltration of Foxp3+RORγt+ T cells showed no significant differences among distinct ACRG classification subtypes (Fig. 1e), indicating Foxp3+RORγt+ T cells might potentially identify a novel subtype of gastric cancer. Detailed patient clinicopathological data were listed in Table 1. Conclusively, Foxp3+RORγt+ T cells showed expansion in gastric cancer and potentially indicated a novel subtype of gastric cancer.
Fig. 1.
Foxp3+RORγt+ T cells are accumulated in gastric cancer and independent of ACRG classification. a The brief summary of the current study. b Immunohistochemistry (IHC) staining of Foxp3+RORγt+ T cells in gastric cancer tissues (intratumor and peritumor). Magnification: × 200. Differenet colors represent corresponding cell subtypes as tagged. c The typical flow cytometry image of Foxp3+RORγt+ T cells was displayed. d IHC score of intratumoral and peritumoral Foxp3+RORγt+ T cells (n = 452). e Association between intratumoral Foxp3+RORγt+ T cells and ACRG classification. Dotted horizontal lines indicate the mean (± SD). NS refers to not significant (one-way ANOVA followed by Tukey’s multiple comparison test)
Table 1.
Relationship between Foxp3+RORγt+ T cells and baseline characteristics of patients
| Testing set (n = 226) | Validation set (n = 226) | |||||
|---|---|---|---|---|---|---|
| Factors | Foxp3+RORγt+ T low (n = 134) | Foxp3+RORγt+ T high (n = 92) | P | Foxp3+RORγt+ T low (n = 136) | Foxp3+RORγt+ T high (n = 90) | P |
| Age (years) | 0.635 | 0.303 | ||||
| Mean (± SD) | 60.7 (± 11.3) | 60.2 (± 11.8) | 59.4 (± 11.3) | 59.8 (± 12.5) | ||
| Gender | 0.6204 | 0.6906 | ||||
| Female | 42 | 26 | 36 | 26 | ||
| Male | 92 | 66 | 100 | 64 | ||
| Tumor size (cm) | 0.330 | 0.391 | ||||
| Mean (± SD) | 4.04 (± 2.12) | 3.64 (± 2.33) | 3.97 (± 2.17) | 3.59 (± 2.00) | ||
| Lauren’s classification | 0.1481 | 0.2403 | ||||
| Intestinal | 76 | 61 | 92 | 54 | ||
| Diffuse | 58 | 31 | 44 | 36 | ||
| Grade | 0.3049 | 0.2889 | ||||
| G1 | 5 | 1 | 3 | 5 | ||
| G2 | 31 | 17 | 25 | 20 | ||
| G3 | 98 | 74 | 108 | 65 | ||
| pTNM stage | 0.8350 | 0.1103 | ||||
| Stage I | 37 | 21 | 22 | 26 | ||
| Stage II | 25 | 20 | 41 | 20 | ||
| Stage III | 70 | 50 | 70 | 41 | ||
| Stage IV | 2 | 1 | 3 | 3 | ||
| Adjuvant chemotherapya | 0.8137 | 0.0550 | ||||
| Yes | 78 | 55 | 87 | 46 | ||
| No | 56 | 37 | 49 | 44 | ||
pTNM Pathologic tumor-node-metastasis
aPatients with adjuvant chemotherapy received at least one cycle of fluorouracil-based chemotherapy
High infiltration of Foxp3+RORγt+ T cells predicts poor prognosis in gastric cancer
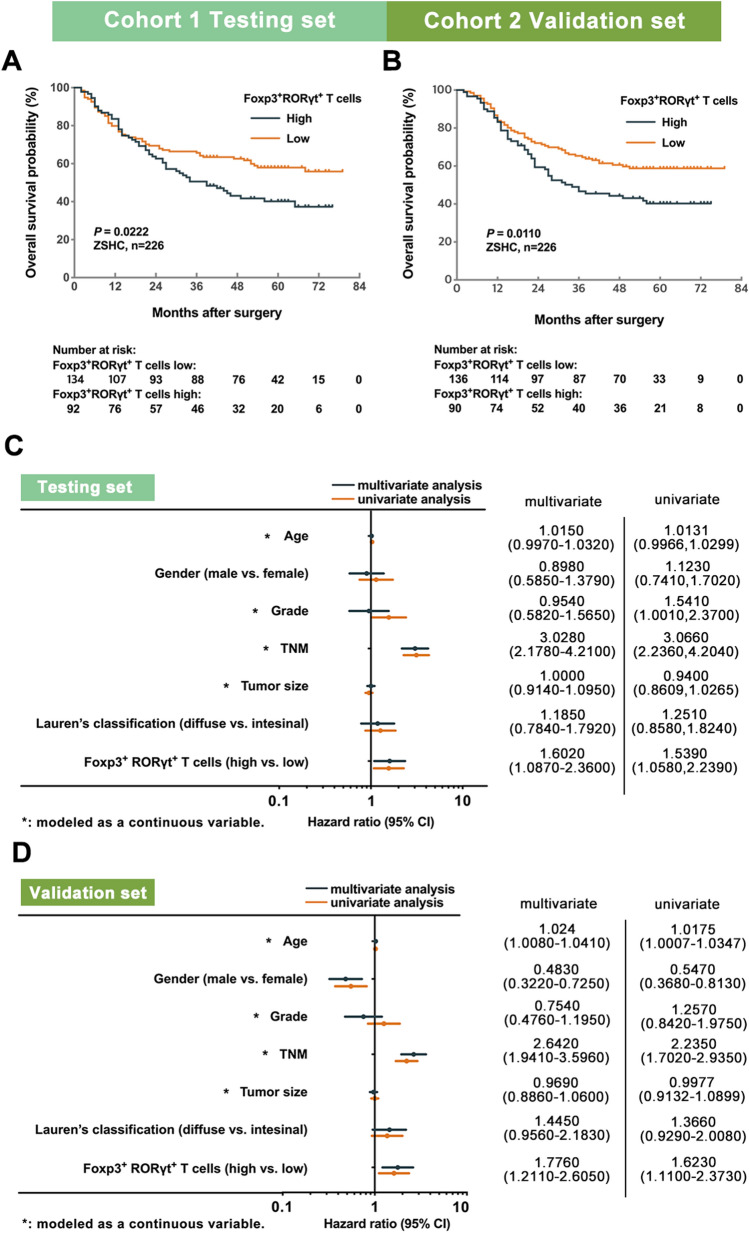
To investigate the clinical significance of Foxp3+RORγt+ T cells, we compared the overall survival (OS) between Foxp3+RORγt+ T cells high and low subgroups, with the use of Kaplan–Meier curves and log-rank test. In Testing set, high infiltration of Foxp3+RORγt+ T cells predicted significantly poorer OS (log-rank test, P = 0.0222; Fig. 2a). Furthermore, we applied univariate and multivariate analyses to discover whether Foxp3+RORγt+ T cells could serve as an independent prognosticator for survival outcomes. Clinicopathological parameters, including age, gender, tumor grade, TNM stage, tumor size, Lauren’s classification and infiltration of Foxp3+RORγt+ T cells were taken into consideration. Notably, we found that the intratumoral infiltration of Foxp3+RORγt+ T cells was an independent adverse prognosticator for OS (multivariate analysis, hazards ratio [HR]: 1.6020, 95% Confidence Interval [CI]: 1.0870–2.3600; Fig. 2c). These results were validated in Validation set (log-rank test, P = 0.0110 and multivariate analysis, HR: 1.7760, 95% CI: 1.2110–2.6050; Fig. 2b, d). Consequently, these results indicated that Foxp3+RORγt+ T cells could be an independent adverse prognosticator for survival outcomes in gastric cancer.
Fig. 2.
High infiltration of Foxp3+RORγt+ T cells Predicts poor prognosis in gastric cancer. a, b Patients with high infiltration of Foxp3+RORγt+ T cells had significantly worse survival outcomes (log-rank test, P = 0.0222 and P = 0.0110, respectively). c, d Univariate and multivariate analyses identified intratumoral infiltration of Foxp3+RORγt+ T cells were an independent adverse prognosticator for survival outcomes
Foxp3+RORγt+ T cells indicate inferior responsiveness to adjuvant chemotherapy in stage II and III gastric cancer
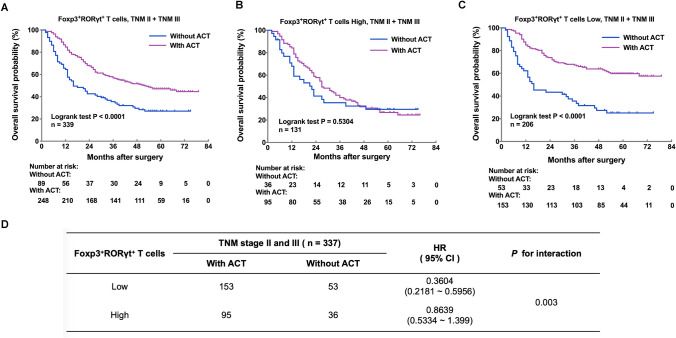
We inspected the association between intratumoral infiltration of Foxp3+RORγt+ T cells and therapeutic responsiveness to fluorouracil-based adjuvant chemotherapy (ACT) in a database of pooled TNM stage II and III gastric cancer patients. As to TNM stage II and III gastric cancer patients, receiving ACT could lead to significantly better OS (P < 0.0001; Fig. 3a). Next, we divided TNM stage II and III gastric cancer patients into Foxp3+RORγt+ T cells high and low subgroups. Notably, in Foxp3+RORγt+ T cells high subgroup, patients could not benefit from ACT (P = 0.5304; Fig. 3b). In Foxp3+RORγt+ T cells low subgroup, however, patients could significantly benefit from ACT (P < 0.0001; Fig. 3c). Furthermore, the interaction test indicated that patients with high infiltration of Foxp3+RORγt+ T cells might have inferior therapeutic responsiveness to ACT (P = 0.003 for interaction; Fig. 3d). Collectively, these results indicated that high infiltration of Foxp3+RORγt+ T cells might indicate inferior chemotherapeutic responsiveness in gastric cancer.
Fig. 3.
High infiltration of Foxp3+RORγt+ T cells indicates inferior responsiveness to adjuvant chemotherapy in gastric cancer. a For stage II/III gastric cancer patients, receiving ACT could lead to significantly better OS (P < 0.0001). b In Foxp3+RORγt+ T cells high subgroup, patients could not benefit from ACT (P = 0.5304). c In Foxp3+RORγt+ T cells low subgroup, however, patients could significantly benefit from ACT (P < 0.0001). d The interaction test between Foxp3+RORγt+ T cells infiltration and chemotherapeutic responsiveness indicated that patients with high infiltration of Foxp3+RORγt+ T cells might have inferior therapeutic responsiveness to ACT (P = 0.003)
Foxp3+RORγt+ T cells are associated with impaired CD8+ T cell effective function
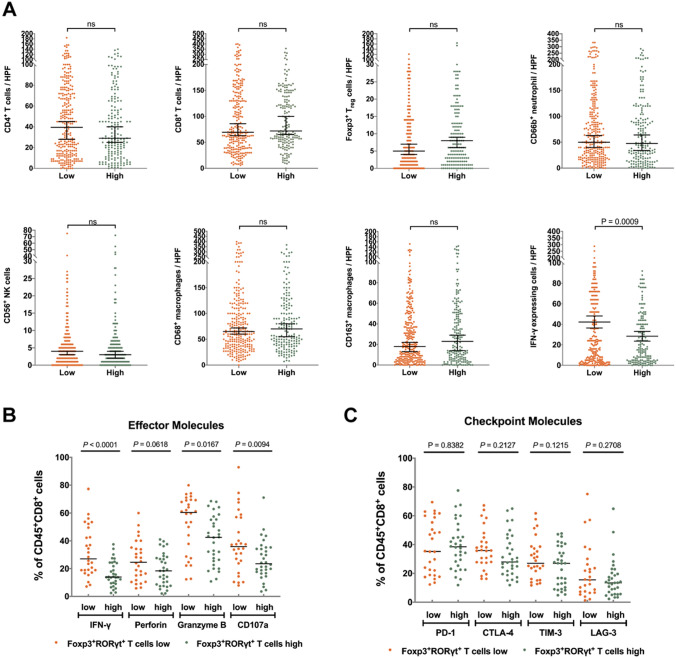
Since Foxp3+RORγt+ T cells might show both features of Treg cells and Th17 cells, we next evaluated the possible association between Foxp3+RORγt+ T cells and immune contexture, including CD4+ T cells, CD8+ T cells, Foxp3+ Tregs, CD66b+ neutrophils, CD56+ NK cells, CD68+ macrophages, CD163+ macrophages and IFN-γ+ cells in gastric cancer. Notably, we only observed decreased infiltration of IFN-γ+ cells in Foxp3+RORγt+ T cells high subgroup, compared with Foxp3+RORγt+ T cells low subgroup (P = 0.0009; Fig. 4a).
Fig. 4.
High infiltration of Foxp3+RORγt+ T cells is associated with reduced CD8+ T cell effective function. a Comparison of the infiltration of various immunocytes between Foxp3+RORγt+ T cells high/low subgroups. b Comparison of the expression of effector molecules within CD8+ T cells between Foxp3+RORγt+ T cells high/low subgroups. c Comparison of the expression of immune checkpoints within CD8+ T cells between Foxp3+RORγt+ T cells high/low subgroups
Although no association was observed between the infiltration of CD8+ T cells and Foxp3+RORγt+ T cells, considering that CD8+ T cells are the main source of IFN-γ, we further investigated the potential impact of Foxp3+RORγt+ T cells on the functional phenotype of CD8+ T cells. Notably, in Foxp3+RORγt+ T cells high subgroup, expression of effector molecules (IFN-γ, Granzyme B and CD107a) was significantly decreased within CD8+ T cells (P < 0.0001, P = 0.0617, P = 0.0094; Fig. 4b). Expression of perforin within CD8+ T cells also showed a trend towards reduction in Foxp3+RORγt+ T cells high subgroup (P = 0.0618; Fig. 4b). However, no association was observed between Foxp3+RORγt+ T cells infiltration and immune checkpoint expression within CD8+ T cells (Fig. 4c). Thus, we suggested that it was the functional and proliferative phenotype, but not the number of of CD8+ T cells that might influence the effect stage of tumor-reactive immune responses. Through the investigation from gene level, we found that the stage of CD8 + T cells was more inclined to exhaustion and the T cells proliferation were less activated in tumors with high infiltration of Foxp3 + RORγt + T cells (Supplemtary Figure S2).
Consequently, these results suggested that Foxp3+RORγt+ T cells were associated with impaired CD8+ T cell effective function in gastric cancer.
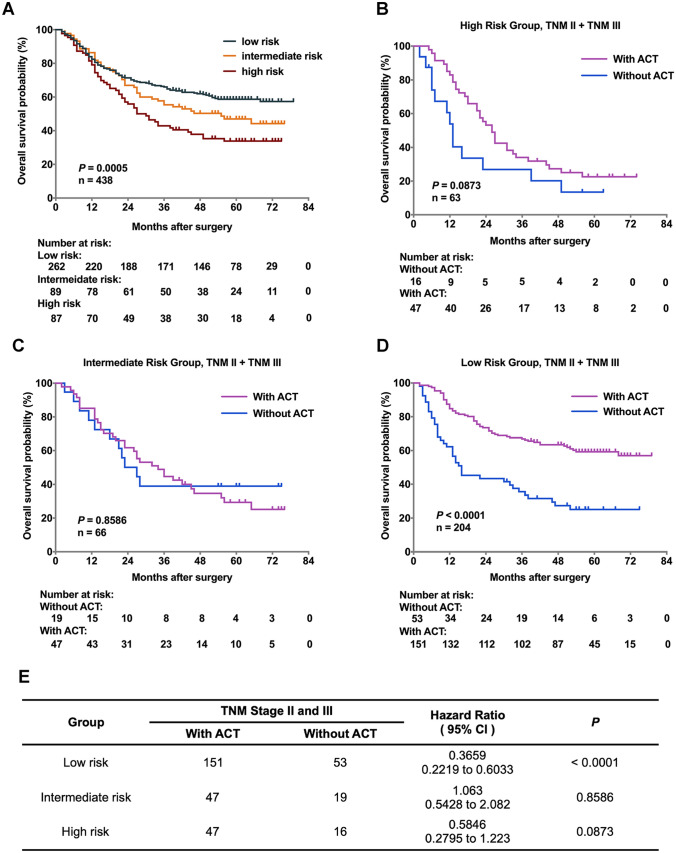
Stratification of patients based on Foxp3+RORγt+ T cells and CD8+ T cells infiltration predicts prognosis and therapeutic responsiveness to ACT in gastric cancer
We have proven that Foxp3+RORγt+ T cells indicated impaired effective function of CD8+ T cells (Fig. 4). In order to stratify gastric cancer patients more precisely, we combined and co-evaluated Foxp3+RORγt+ T cells and CD8+ T cells infiltration in gastric cancer. Based on the combined evaluation system which was established on four groups sketchily (Figure S1), we divided patients into three distinct risk groups, high risk group (Foxp3+RORγt+ T cells high and CD8+ T cells low), intermediate risk group (Foxp3+RORγt+ T cells high and CD8+ T cells high), and low risk group (Foxp3+RORγt+ T cells low, P = 0.0005; Fig. 5a). Furthermore, we sought to discover whether the three risk groups could indicate distinct therapeutic responsiveness to ACT in patients with stage II and III gastric cancer. Surprisingly, the patients in low risk group could significantly benefit from ACT, while either high risk group or intermediate risk group merely had a trend towards improved survival after receiving ACT (Fig. 5b–e). Conclusively, these results indicated that the infiltration of Foxp3+RORγt+ T cells together with CD8+ T cells could be used to identify gastric cancer patients into various risk subgroups, thus predicting diverse prognosis and therapeutic responsiveness to ACT.
Fig. 5.
Stratification of patients based on FOXP3+RORγt+ T cells and CD8+ T cells predicts prognosis and therapeutic responsiveness in gastric cancer. a In order to stratify gastric cancer patients more precisely, we combined and co-evaluated the infiltration of Foxp3+RORγt+ T cells and CD8+ T cells in gastric cancer. Based on the combined evaluation system, we divided patients into high risk group (Foxp3+RORγt+ T cells high and CD8+ T cells low), intermediate risk group (Foxp3+RORγt+ T cells high and CD8+ T cells high), and low risk group (Foxp3+RORγt+ T cells low). The three risk groups showed distinct survival outcomes (P = 0.0005). b–e The three risk groups also showed distinct therapeutic responsiveness to ACT. The patients of lower risk group could significantly benefit from ACT (HR: 0.3659, 95% CI: 0.2219–0.6033, P < 0.0001), while either high risk group or intermediate risk group merely had a trend towards improved survival after receiving ACT (HR: 0.5846, 95% CI: 0.2795–1.223, P = 0.0873 and 1.063, 95% CI: 0.5428–2.082, P = 0.8586)
Discussion
Foxp3+ Treg cells have diverse roles in the pathogenesis of human malignancies, and are associated with both favorable prognosis and progressive disease [24, 25]. The heterogeneity of Treg cell population could explain why Treg cells might have paradoxical effects on tumor progression. Phenotypic plasticity enables Treg cells to down-regulate Foxp3 expression and acquire Th cell properties [26]. Notably, the enhanced expression of RORγt in Treg cells could mark their potential plasticity toward Th17 cells. Interestingly, Foxp3+RORγt+ T cells share the features of both Th17 cells and Treg cells. This population might be particularly detrimental for prognosis by suppressing adaptive antitumor immunity, whereas the pro-inflammatory nature might promote tumorigenesis by supporting a tumor microenvironment enriched in tumor growth factors, pro-inflammatory cytokines, pro-angiogenic factors, and reactive oxygen species [27].
In the current study, we compared the infiltration of immune cells between Foxp3+RORγt+ T cells high and low subgroups and found that the quantity of IFN-γ expressing cells decreased most obviously in Foxp3+RORγt+ T cells high subgroup. Considering that CD8+ T cells are the main source of IFN-γ, we decided to focus on the potential impact of Foxp3+RORγt+ T cells on functional phenotype of CD8+ T cells [28]. Interestingly, we observed decreased expression of effective molecules (IFN-γ, Granzyme B and CD107a) of CD8+ T cells in Foxp3+RORγt+ T cells high subgroup, indicating Foxp3+RORγt+ T cells might orchestrate the impairment of T cell effective function and degranulation. Notably, previous studies have reported that T cell functional status might impact therapeutic responsiveness to immunotherapy [29]. Consequently, we assumed that Foxp3+RORγt+ T cells might also have a potential impact on immunotherapeutic responsiveness in gastric cancer. However, the effector molecules and immune checkpoints detected in the current study are limited, and the correlation between Foxp3+RORγt+ T cells and other novel T cell-associated effector molecules and immune checkpoints remains further investigation in our following studies.
Since CD8+ T cells might be regulated by Foxp3+RORγt+ T cells, we wondered whether the co-evaluation of Foxp3+RORγt+ T cells and CD8+ T cells would be of more clinical significance. We developed a new classification system based on Foxp3+RORγt+ T cells and CD8+ T cells infiltration. Interestingly, we found the novel stratification system could predict patients with distinct prognosis and responsiveness to ACT more precisely. This might be useful for prognosis prediction and decision-making for appropriate treatment in gastric cancer.
In conclusion, we revealed the clinical significance of Foxp3+RORγt+ T cells and the potential impact of Foxp3+RORγt+ T cells on tumor immune microenvironment. However, our study was a retrospective research in nature and the quantity of patients receiving ACT was relatively small. Therefore, we advocate further validation in a prospective, larger, and multi-centered randomized trial.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their contribution to the evaluation of immunohistochemistry results and excellent pathological technology help.
Author contributions
Y. Fei, Y. Cao, Y. Gu and H. Fang for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; Y. Chen, J. Wang, X. Liu, K. Lv, X. He, C. Lin, H. Liu, H. Li and H. He for technical and material support; R. Li, H. Zhang and W. Zhang for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding
This study was funded by grants from National Natural Science Foundation of China (81671628, 81871306, 81871930, 81902402, 81902901, 81972219, 82003019) and Shanghai Sailing Program (17YF1402200, 18YF1404600, 19YF1407500, 21YF1407600). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Data availability
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.
Declarations
Conflicts of interest
The authors declare no conflicts of interest.
Consent for publication
All authors provide their consent for publication of the manuscript.
Ethical approval
Written informed consent was obtained from each patient included and the protocol of all study cohorts were approved by the institutional review board and ethics committee of Zhongshan hospital, Fudan University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuchao Fei, Yifan Cao, Yun Gu and Hanji Fang have contributed equally to this work.
Contributor Information
Ruochen Li, Email: rcli12@fudan.edu.cn.
Heng Zhang, Email: zhang.heng@zs-hospital.sh.cn.
Weijuan Zhang, Email: weijuanzhang@fudan.edu.cn.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA: A Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 4.Bang Y-J, Kim Y-W, Yang H-K, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. JCO. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus Ipilimumab in patients with metastatic esophagogastric cancer. JCO. 2018;36:2836–2844. doi: 10.1200/JCO.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Hu N, Yang HH, et al. Comparison of global gene expression of gastric cardia and noncardia cancers from a high-risk population in China. PLoS ONE. 2013;8:e63826. doi: 10.1371/journal.pone.0063826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Wang X, Shen Z, et al. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Cao Y, Li R, et al. Poor clinical outcomes of intratumoral dendritic cell–specific intercellular adhesion molecule 3–grabbing non-integrin–positive macrophages associated with immune evasion in gastric cancer. Eur J Cancer. 2020;128:27–37. doi: 10.1016/j.ejca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Liu H, Shen Z, et al. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267:311–318. doi: 10.1097/SLA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Lin C, Li H, et al. Tumor-infiltrating γδT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. OncoImmunology. 2017;6:e1353858. doi: 10.1080/2162402X.2017.1353858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R, Liu H, Cao Y, et al. Identification and validation of an immunogenic subtype of gastric cancer with abundant intratumoural CD103+CD8+ T cells conferring favourable prognosis. Br J Cancer. 2020;122:1525–1534. doi: 10.1038/s41416-020-0813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuniyasu Y, Takahashi T, Itoh M, et al. Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol. 2000;12:1145–1155. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]
- 16.Chang L-Y, Lin Y-C, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF- -mediated killing of CD8+ T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Kim MK, Di Caro G, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–1063. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115–139. doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro G, Liu X, Ngo K, et al. RORγt and RORα signature genes in human Th17 cells. PLoS ONE. 2017;12:e0181868. doi: 10.1371/journal.pone.0181868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Wang JT, Li H, Zhang H, et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol. 2019;30:266–273. doi: 10.1093/annonc/mdy505. [DOI] [PubMed] [Google Scholar]
- 22.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 23.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 24.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chellappa S, Hugenschmidt H, Hagness M, et al. Regulatory T cells that co-express RORγt and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. OncoImmunology. 2016;5:e1102828. doi: 10.1080/2162402X.2015.1102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummel MF, Mahale JN, Uhl LFK, et al. Paracrine costimulation of IFN-γ signaling by integrins modulates CD8 T cell differentiation. Proc Natl Acad Sci USA. 2018;115:11585–11590. doi: 10.1073/pnas.1804556115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varn FS, Tafe LJ, Amos CI, Cheng C. Computational immune profiling in lung adenocarcinoma reveals reproducible prognostic associations with implications for immunotherapy. OncoImmunology. 2018;7:e1431084. doi: 10.1080/2162402X.2018.1431084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almanzar G, Klein M, Schmalzing M, et al. Disease manifestation and inflammatory activity as modulators of Th17/Treg balance and RORC/FoxP3 methylation in systemic sclerosis. Int Arch Allergy Immunol. 2016;171:141–154. doi: 10.1159/000450949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.