Abstract
Specific extracts of selected vegetables (SV) have been shown to benefit the survival of stage IIIb/IV non-small cell lung cancer patients in phase I/II studies and is currently in a phase III trial. However, the underlying mechanism of SV-mediated antitumor immune responses has not been elucidated. Our results indicate that SV modulated the NK and adoptive T cell immune responses in antitumor efficacy. Furthermore, antitumor effects of SV were also mediated by innate myeloid cell function, which requires both TLR and β-glucan signaling in a MyD88/TRIF and Dectin-1-dependent manner, respectively. Additionally, SV treatment reduced granulocytic myeloid-derived suppressor cell (MDSC) infiltration into the tumor and limited monocytic MDSC toward the M2-like functional phenotype. Importantly, SV treatment enhanced antigen-specific immune responses by augmenting the activation of antigen-specific TH1/TH17 cells in secondary lymphoid organs and proliferative response, as well as by reducing the Treg population in the tumor microenvironment, which was driven by SV-primed activated M-MDSC. Our results support the idea that SV can subvert immune-tolerance state in the tumor microenvironment and inhibit tumor growth. The present study suggests that features, such as easy accessibility, favorable clinical efficacy, no detectable side effects and satisfactory safety make SV a feasible, appealing and convincing adjuvant therapy for the treatment of cancer patients and prevent tumor recurrence and/or metastases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02927-2.
Keywords: MDSC, TH1, Immune modulating agents, Nutrient supplement, Cancer immunotherapy
Introduction
Lung cancer is one of the most challenging cancer types among both men and women, accounting for 27% of all cancer deaths in the USA [1]. Abundant treatment options for lung cancer have been developed, including surgery, radiotherapy, chemotherapy and targeted therapies based on specific genomic alterations carried by patients. However, the 5-year survival rate has not been significantly improved and long-term disease control remains a main challenge for lung cancer treatment [2]. Recent progress in immune checkpoint inhibition has been translated into major therapeutic advances in the treatment of lung cancer, including the CTLA-4 inhibitor, ipilimumab [3], and the PD-1 inhibitor, nivolumab [4]. Although the results with ipilimumab and nivolumab in small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) patients suggest a survival benefit when given with chemotherapy compared to chemotherapy alone, the typical autoimmune complications and endocrine toxicities were also reported [5,6]. Therefore, immunotherapies with efficacies equivalent to immune checkpoint inhibition that benefit disease control without significant toxicities are urgently needed.
Over the past several decades, accumulating evidence has shown that certain botanical products contain phytochemicals, such as inositol hexaphosphate (IP6) [7], daidzein [8,9], genistein [8], and coumestrol [10], with immune-modulatory or antitumor bio-activities. In previous studies [11], we designed a selected vegetable (SV) therapy using extract of selective vegetables and herbs based on two criteria: non-toxicity and the presence of antitumor or immune-enhancing activity [12]. These selected vegetables may contain cancer prevention-related components recommended by the American Cancer Society and the National Cancer Institute. Additionally, increased consumption of those components has been associated with reduced cancer-related deaths in recent years [13,14]. We initiated two clinical pilot studies of adjuvant SV therapy in Stage IIIb and IV NSCLC patients [11,12]. The results indicated that weight maintenance, Karnofsky performance status and the survival rates of the 18 patients enrolled were improved without any detectable toxicity. Interestingly, one lead case remained tumor free for 133 months; a second case showed complete regression of multiple brain lesions after SV administration and radiotherapy; another eight patients had no new metastases after SV administration [12]. We also observed that SV treatment inhibited tumor growth in the early phase in a murine lung tumor model (14). Currently SV is in phase 3 clinical trial for late stages NSCLC (FDA IND-63605). However, the mechanisms of SV-mediated antitumor effects are unclear.
Myeloid-derived suppressor cells (MDSC), a population of immature myeloid cells and myeloid progenitors, possess potent immune-suppressive activity through the generation of reactive oxygen species (ROS) and immunoregulatory cytokines to maintain an immune-tolerant network in the tumor microenvironment [15]. They can suppress both innate and adaptive immunity by attenuating host effector T-cell responses, thereby resulting in the failure of immune surveillance and the promotion of tumor progression and metastasis [16,17]. It is reported that the number of MDSC in the peripheral blood of lung cancer patients was significantly increased and positively correlated with disease stage [18]. This MDSC-targeted therapeutic strategy has been exploited to overcome the immuno-tolerant microenvironment in advanced lung cancer [19]. In our previous study [20], we have shown that MDSC can give rise to M1-type macrophages with potent antigen presentation function and type 1 immune response-enhancing ability through production of IL-12, TNFα and inducible nitric oxide synthase (iNOS) leading to tumor rejection and host survival [20]. It is reported that increased M1 macrophage density in tumor islets is positively correlated with prolonged survival in NSCLC patients [21]. Several phytochemicals extracted from botanical foods have been reported to promote the maturation and antigen presentation capabilities of phagocytic immune cells as well as other immune cells, including T cells, B cells and NK cells [22]. However, whether and how the antitumor effects of SV are mediated through modulating the host immune system remains to be elucidated.
In this study, we reported that SV food can modulate the antitumor immune responses of NK, adoptive T cells as well as innate myeloid cells, which is dependent on MyD88/TRIF and Dectin-1 signaling. Most importantly, SV can limit the infiltration of granulocytic MDSC into tumor tissue and limit monocytic MDSC toward the M2-like functional phenotype, thereby enhancing CD8 and TH1 cell activation and reducing Treg development in the tumor microenvironment. Along with previous pilot studies [11,12], our results suggest that SV can potentially be developed as an immune adjuvant therapy for use in conjunction with conventional anti-cancer therapies to prevent recurrence and/or metastases of lung cancer by converting the immuno-suppressive tumor microenvironment into an immune-conducive one.
Materials and methods
SV preparation
SV is a freeze-dried commercial vegetable soup prepared by Sun Farm (Milford, CT). SV was prepared following “good manufacturing practices” to minimize contamination with heavy metals, herbisides and bacteria as described in a previous study [11]. Briefly, SV includes soybeans, mushrooms, mung beans, red dates, scallions, garlic, lentils, leeks, Hawthorn fruit, onions, ginsengs, angelica root, licorice, dandelion root, senegal root, ginger, olive, sesame seeds, and parsley. The mix was blended, boiled, freeze-dried, pulverized and stored at − 20 °C until use. The extraction from raw materials and subsequent processing of SV was under stringent standard operation procedure (SOP) approved by U.S. FDA to assure the batch wise biochemical and biological consistency, as well as to fulfill the FDA approved safety measures with respect to heavy metal, pesticides and microbial contamination for the use in phase 3 clinical trial for non-small cell lung cancer (NSCLC, IND63605). Quality control for consistency of SV preparation with respective to composition and efficacy was performed for each batch. Random samples of SV were sent to Northeast Laboratory (Berlin, CT) for analysis of nutritional value, heavy metals, and bacteria as described previously [11]. A 40-g daily serving of SV (dry weight) for patients contains 28 g (70%) carbohydrate and dietary fiber, 10 g (25%) protein, and 2 g fat (5%). For mouse experiments, 20% SV and 80% regular mouse chow (wt/wt) mix was prepared.
Experimental animals
Congenic CD45.1+, ovalbumin (OVA)–specific MHC class II restricted TCR-transgenic (OT-II) C57BL/6, SCID mice (NOD.CB17-Prkdcscid/J), RAG1 knockout (KO) mice (B6.129S7-Rag1tm1Mom/J), MyD88 KO mice (B6.129P2 (SJL)-Myd88tm1.1Defr/J), TRIF KO mice and NOD2 KO mice were purchased from National Cancer Institute and The Jackson Laboratory. Bone marrow of Dectin-1 KO C57BL/6 mice was a generous gift from Dr. Jun Yan (University of Louisville). All animal experiments were conducted in accordance with the IACUC guidelines of Icahn School of Medicine at Mount Sinai.
Peptide and antibodies
OT-II OVA peptide and OVA protein were purchased from AnaSpec (Fremont, CA). Antibodies against CD4, CD8, CD25, CD115, F4/80, Foxp3, RORgt, Tbet, Ly6C, Ly6G, CD45.1, CD45.2, CD206 and Thy1.2 were purchased from eBioscience (SanDiego, CA). Anti-iNOS (inducible nitric oxide synthase) FITC was purchased from BD Pharmingen, anti-Arginase 1 (SanDiego, CA), and isotype-matched monoclonal antibodies were purchased from eBioscience.
Mouse lung tumor model and NK cell depletion
To establish the tumor model of lung cancer, C57BL/6-derived Lewis lung carcinoma tumor cells (LLC) and Balb/c-derived line 1 lung tumor cells (L1) [12] were subcutaneously injected in the right thigh. Test mice were fed with Lab Chow powder mixed with water as control (CT) food or hot water extracts of SV powder (20% wt/wt) starting 10 days before tumor inoculation and then continued through sacrifice. Tumor size was measured twice per week. A stable ovalbumin (OVA)-expressing LLC tumor cell clone was used to track tumor antigen specific T cell responses in vivo. To establish the tumor model of liver cancer, mice were implanted with 1 × 105 OVA-LLC tumor cells intrahepatically. For NK cell depletion, anti-CD49b antibody (Biolegend) was initially injected (i.p., 200 μg/mouse at 2 days before tumor inoculation then given every 4 days for a total of 4 injections.
Mouse model of lung and liver metastases and adoptive transfer experiments
In the intrahepatical OVA-LLC/C57BL/6 tumor model, when tumors reached a size of 5 × 5 mm2, purified tumor-specific (OT-II) CD45.1 T cells (5 × 106 per mouse) were injected via tail vein. Ten days after adoptive transfer, mice were sacrificed. The tumor weight was measured. The presence of tumor-specific (OT-II) Tregs in the tumor infiltrated leukocytes, bone marrow and spleen was assessed by flow cytometry. The proliferation of purified splenic tumor-specific (OT-II) CD45.1 T cells in response to OVA protein (5ug/mL) was assessed in the presence of irradiated naïve splenocytes.
Preparation of MDSCs
Mice with tumors greater than 10 × 10 mm2 were sacrificed and the spleens, tibias, and femurs were harvested. After lysis of the red blood cells (RBCs), bone marrow cells and splenocytes were cultured overnight. On the second day, loosely attached cells were harvested and fractionated on a Percoll (GE Healthcare, Piscataway, NJ) density gradient as previously described [20]. MDSCs were purified from the cells banding at 50–60% by anti-CD115-PE and anti-PE microbeads (MiltenyiBiotec, Auburn, CA). The purity of the sorted cell populations was > 95%, as determined by flow cytometry.
T cell proliferation assay
The sorted splenic Thy1.2+ T cells (2 × 105]) were co-cultured with irradiated (2000 rad) naïve splenic cells (1 × 104) as APC in the presence or absence of OT-II OVA peptide (5 µg/mL) in 96-well microplates. [3H] thymidine was added during the last 8 h of 72-h culture.
Cytokine detection by ELISA
Cytokine concentrations in culture supernatants were determined using mouse TNFα, IL-10, Interferonγ (IFN-γ), IL-17 ELISA kits (R&D Systems) according to manufacturer’s instructions.
Cytotoxic T Lymphocyte assay and macrophage-dependent cytotoxic assay
Mice were inoculated with 1 × 104 L1 cells in the right thigh. When tumors reached a size of 5 × 5 mm2, tumor-bearing mice were fed with CT or SV food. After 11 days of CT or SV treatment, splenic Thy1.2+ T cells or hepatic CD49b+ NK cells were purified and co-incubated with L1 tumor cells (target cells) at ratios of 12.5:1, 25:1, 50:1 and 100:1 for 4 h. Supernatants were collected to measure lactate dehydrogenase release (CytoTox96 Non-Radioactive Cytotoxicity Assay Kit, Promega). Specific killing (expressed as a percentage) was calculated as experimental LDH release/maximum LDH release as follows: % cytotoxicity = 100 (experimental release − effector spontaneous release − target spontaneous release)/(total target release − target spontaneous release).
Statistical analysis
Statistical analysis of survival rates was performed using the log-rank test. Student's t test was used for all other analyses. For samples with equal variance, the paired Student’s t test for equal variance was used. For samples with unequal variance, Wilcoxon signed-rank test was used for statistical analysis. p < 0.05 was considered to be statistically significant.
Results
SV effectively retards tumor growth and prolongs the survival of tumor-bearing mice in different tumor models
Since the ingredients in Selected Vegetable (SV) have been repeatedly proven to enhance antitumor responses in the past decades, we mixed SV food with normal mouse chow, and fed test mice from day 10 prior to tumor inoculation through termination. A number of different models were then performed to evaluate the antitumor effects of SV food. As shown in Fig. 1a, the tumor growth rate was significantly retarded and overall survival was significantly increased in the SV-fed test mice in L1 lung cancer model with BALB/c background. We further tested whether similar antitumor efficacy would be observed in a less immunogenic, Lewis lung cancer (LLC) tumor in a different mouse genetic background, the C57BL/6 mouse strain (Fig. 1b). Similar results were observed with SV-fed mice exhibiting significantly smaller tumors than the control food (CT)-fed mice. These results indicate that pretreatment with SV food led to an approximately 50% reduction in tumor size compared to the CT food group in both tumor models. The results suggest SV food induced potent antitumor responses against different lung tumor cells.
Fig. 1.
SV exerts antitumor effects, which are mediated by T cells as well as NK cells. a Mice were fed control or SV food from day 10 prior to tumor inoculation through termination. Overall survival of L1 lung tumor-bearing mice. *p < 0.05, SV food versus control food (CT) (log-rank test). b Tumor progression of LLC bearing mice. Tumor size was assessed 2 times per week and is reported as average tumor area (mm3) ± standard error, n = 8 mice per group. Error bars indicate SEM *p < 0.05, (Analysis of variance (ANOVA)). c) Tumor progression of LLC bearing WT or RAG−/− mice (C57BL/6 background). Tumor growth was determined at 3–4-day intervals. 7–10 mice per group. Error bars indicate SEM. *p < 0.05, ns, not significant. d The effect of CD49b depletion on tumor growth in SV versus CT food treated BALB/c mice. Left panel: Tumor growth was followed in L1 lung tumor-bearing mice receiving control food, SV food, or SV food with CD49b-depletion. *p < 0.05. Right panel: Overall survival in L1 lung tumor-bearing mice (n = 10). ***p < 0.001, SV food group or SV food with CD49b-depletion versus control food (log-rank test). e, f Cytotoxicity of T cells and NK cells from SV or control food treated tumor-bearing mice. 1 × 104 L1 cells were inoculated into test mice and started to fed with SV or CT food when tumor reach 5 mm × 5 mm. After 11 days of SV or CT food treatment, the effector cells, liver NK e and splenic T cells f, were purified from CT/SV-fed mice for use in direct cytolytic activity assays against target cells (L1 lung tumor cells) at various effector-to-target (E:T) ratios. Three mice per group were used. Data are presented as mean ± SEM *p < 0.05 (Student’s t test)
Natural killer cells (NK) and T cells were involved in SV food-mediated antitumor responses
It is well documented that the bio-activity of the active ingredients in SV may be mediated by regulation of the host immune systems. Therefore, we further determined which immune cell types were involved in SV food-mediated antitumor immunity. First, we employed the same treatment scheme and evaluated the potential T or B cell involvement in SV-mediated antitumor responses in the RAG1−/− mouse model. As shown in Fig. 1c, we observed a significantly increased tumor growth rate in SV-fed RAG−/− mice as compared to the corresponding syngeneic WT mice. Again, the tumor growth rate was significantly retarded in SV-fed WT mice when compared to CT-fed mice (p = 0.0285). Furthermore, the SV diet inhibited tumor growth slightly throughout the observation period compared with the CT diet in RAG−/− immune-deficient mice, suggesting that other immune cells, like natural killer (NK) cells, may play a role in SV-mediated antitumor effects. To elucidate the role of NK cells in SV-triggered antitumor immune responses, we depleted the pan-NK cell population using anti-CD49b antibody. The inhibitory effect of SV food on tumor growth was moderately attenuated after NK cell depletion (Fig. 1d, left, p = 0.03874); however, there were no significant differences between the survival rates of SV-fed groups with and without NK cell depletion (Fig. 1d, right, p < 0.0001). These results suggest that NK cells may be more effective in inhibiting the early stage tumor growth, but less so when a tumor progresses beyond certain size due to change of immune landscape in the tumor microenvironment. Liver is the first, and most important, organ for sorting the components from absorbed nutrients before entering the systemic circulation. Therefore, we isolated NK cells from liver and T cells from spleen of test mice after fed with CT- or SV-food for 11 days to assess their direct cytotoxic activity against tumor cells. The results showed that liver NK cells from SV-fed mice had a significant level of cytotoxic activity against target L1 tumor cells at all four E:T ratios from 100:1 to 12.5:1 (Fig. 1e). Furthermore, cytotoxic activity was also detected in splenic T cells from SV-fed mice at E:T ratios of 100:1 and 50:1 whereas those from CT-fed mice exhibited little cytotoxicity (Fig. 1f). These in vivo and in vitro results together may indicate that effector NK and T cells may play an important role in SV-mediated antitumor responses.
TLR and Dectin-1 downstream signaling is required for the SV mediated antitumor effect
Several key components identified in SV have been reported to potentially exhibit pro-inflammatory or anti-inflammatory effects by regulating the activation of Toll like receptor (TLR)-related signals, including β-glucans [23] and lectins. In order to elucidate the role of TLRs in SV-mediated signals, we evaluated the antitumor effects of SV in tumor-bearing mice, specifically with gene knockout of MyD88 or TRIF, two vital adapter proteins in TLR family signaling. In Fig. 2a, with the same experimental scheme, but in MyD88 KO or TRIF KO mice strains, we observed that MyD88 deficiency promoted tumor progression in CT-fed mice. Furthermore, the tumor growth rates of LLC-tumor-bearing MyD88 KO mice fed with a CT or SV diet showed no differences, while these in LLC-tumor-bearing WT KO mice, again, showed significant differences (p = 0.0017), indicating that MyD88 KO tumor-bearing mice significantly lost their responsiveness to SV. Likewise, we observed similar results in the TRIF KO tumor-bearing mice (Fig. 2b). The above results suggest that TLR signaling is required for the SV-mediated antitumor responses.
Fig. 2.
TLR- and β-glucan downstream signaling is required for the SV mediated antitumor effect. a MyD88 KO and wild type (WT) mice were fed either CT or SV diet starting 10 days prior to tumor inoculation. Mice received subcutaneous flank injections of 3 × 105 LLC cells in 0.1 ml of PBS on day 10 and remained on their corresponding diets. Tumors were measured every 3–4 days. Error bars indicate SEM. (n = 7). *p < 0.05, n.s., not significant. b Tumor growth of LLC tumor-bearing TRIF KO mice was shown (n = 6). Error bars indicate SEM. n.s., not significant. c Tumor growth in LLC tumor-bearing WT mice. Error bars indicate SEM. (n = 9). *p = 0.0231. Dectin-1 KO chimeric mice were generated to evaluate the effect of Dectin-1 deficiency in myeloid cells on SV-mediated antitumor responses. Tumor growth in LLC-tumor-bearing Dectin-1 KO chimeric mice is shown. Error bars indicate SEM. (n = 7). n.s., not significant. Data are representative of two experiments with n = 6–9 mice per experiment
It has been shown that antigen-presenting cells play a dominant role in directing immune responses and priming T effector lymphocytes. Among the aforementioned components in SV, β-glucans, polysaccharides of D-glucose monomers, have been reported as immune-stimulators that act by regulating the development and activation of myeloid cells through Dectin-1 receptor [24]. Dectin-1 receptor is dominantly expressed in hematopoietic cells and has been reported to activate antigen-presenting cells, such as dendritic cells and macrophages, after ligand engagement [25,26]. However, whether SV food can trigger the activation of antigen-presenting cells through engagement of the Dectin-1 receptor needs to be further validated experimentally. To address this question, we generated Dectin-1 KO chimeric mice by transferring bone marrow cells of Dectin-1KO (CD45.2+) mice into lethally irradiated wild-type (CD45.1+) mice. In contrast to what we observed in wild type mice, there were no significant differences in tumor growth rate between CT or SV-fed Dectin-1 KO chimeric mice (Fig. 2c). These findings suggest that Dectin-1 signaling is also required for SV augmentation of antitumor immune responses.
SV enhances antitumor immune responses by targeting two different subsets of myeloid-derived suppressor cells (MDSC) in tumor-bearing mice
A recent study reported that β-glucan, a ligand of Dectin-1, promoted MDSC differentiation [27]. Our previous study and other findings highly suggest that SV food component β-glucans have great potential for activating Dectin-1 on myeloid cells [11,12,27]. Therefore, we evaluated the potential effect of SV food on two subsets of MDSCs, granulocytic MDSC (G-MDSC) and monocytic MDSC (M-MDSC) in the tumor and secondary lymphoid organs.
SV treatment led to substantially decreased CD11b+Ly6G+Ly6C+ G-MDSC levels in both the tumor infiltrating leukocyte (TIL) population (~ 35% reduction, Fig. 3a, b) and in the spleen (~ 50% reduction, Fig. 3a, c). In contrast, the CD11b+Ly6G−Ly6C+ M-MDSC population was little affected by SV treatment in TIL and spleen. Interestingly, we found that CD11b+Ly6G−Ly6C+ M-MDSCs from SV-fed mice had significantly increased MHCII+ (Fig. 3d, e) and decreased arginase I+MHCIIlow cell populations (Fig. 3d, f) in tumor tissue, as well as in the spleen, indicating that SV may potentially attenuate immune-suppressive activity and promote maturation of CD11b+Ly6G−Ly6C+ M-MDSC populations. Furthermore, neither subset of MDSC was affected in bone marrow (data not shown).
Fig. 3.
Granulocytic, but not monocytic, myeloid-derived suppressor cells (MDSC) were decreased in tumor tissues of SV-fed LLC tumor-bearing mice. a Mice were fed control or SV food starting 10 days prior to tumor inoculation. C57BL/6 mice were subcutaneously challenged with 3 × 105 LLC cells. Mice were sacrificed on day 21. Tumor infiltrated leukocytes (TIL), splenocytes (SP) and bone marrow cells (BM) were isolated from tumor-bearing mice. G-MDSC (Ly6G+Ly6C+) and M-MDSC (Ly6G−Ly6C+) in TIL, SP and BM were analyzed by flow cytometry and presented as dot plots. b, c Statistical analysis of MDSC percentages in TIL (b) and Spleen (c) obtained from (a) is presented. *p < 0.05, ***p < 0.001, n = 5 mice per group. d The expression of MHC class II and Arginase type I of M-MDSC was analyzed by flow cytometry and presented as dot plots. e, f Statistical analysis of the percentage of MHCII + (e) and Arginase I + (f) in M-MDSC in TIL and spleen obtained from (d) is presented. n = 3 mice per group. Error bars indicate SEM. *p < 0.05, ANOVA
SV food promotes differentiation/maturation of M-MDSC to the M1 functional phenotype in different mouse backgrounds
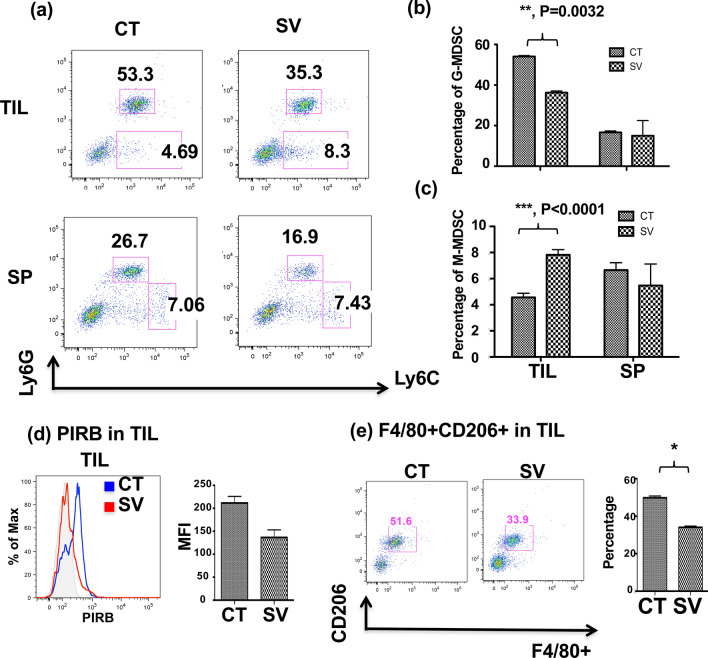
We further determined whether SV food had similar effects in different mouse backgrounds e.g. BABL/c. As shown in Fig. 4a, b, although the distribution of G-/M-MDSC varied in different mouse backgrounds, SV food significantly decreased accumulation of the G-MDSC population in the tumor in both backgrounds. The M-MDSC population in TIL was moderately increased by SV (Fig. 4a, c). No significant changes in the G-/M-MDSC population were found in the spleen.
Fig. 4.
SV treatment promotes the acquisition of an M1 phenotype by M-MDSC. a Mice were fed control or SV food starting 10 days prior to tumor inoculation. BALB/c mice were subcutaneously inoculated with 1 × 104 L1 lung cells in 0.1 ml of PBS on day 10 and remained on their corresponding diets. Mice were sacrificed on day 28. Tumor infiltrated leukocytes and splenocytes (SP) were isolated from tumor-bearing mice. G-MDSC (Ly6G+Ly6C+) and M-MDSC (Ly6G−Ly6C+) in TIL and SP were analyzed by flow cytometry and presented as dot plots. b, c Statistical analysis of percentages of G-MDSC (b) and M-MDSC (c) obtained from (a) is shown. **p < 0.01, ***p < 0.001, ANOVA. (d) Phenotypic analysis of PIRB expression in CD11b+ TIL is presented in histogram (left) and as statistical analysis (right). *p < 0.05, ANOVA. (e) The percentage of F4/80+CD206+ M2-type macrophages in TIL is shown in dot plot (left) and the statistical analysis is presented (right). *p < 0.05, ANOVA. Data were presented as the average ± SEM. n = 3 mice per group
Several studies have shown that M-MDSC can differentiate into an M1 phenotype, which may convert its intrinsic immune-suppressive to immuno-promoting activity and facilitate the tumor regression [20,28]. We further evaluated the potential immune-modulatory effect of SV food on the promotion of M-MDSC to an M1/M2 functional phenotype in the BALB/c background. Interestingly, we found that SV-fed mice exhibited decreased PIRB expression, which has been demonstrated to play an important role in M2-type macrophage differentiation among the CD11b+ cell population in the tumor (Fig. 4d), but not in the spleen (data not shown). Furthermore, we also observed that F4/80+CD206+ cells, M2-type macrophages, were decreased in tumors of SV-fed mice (Fig. 4e).
SV can significantly decrease Treg and increase TH1 development in the tumor infiltrating T cells of SV food-fed mice.
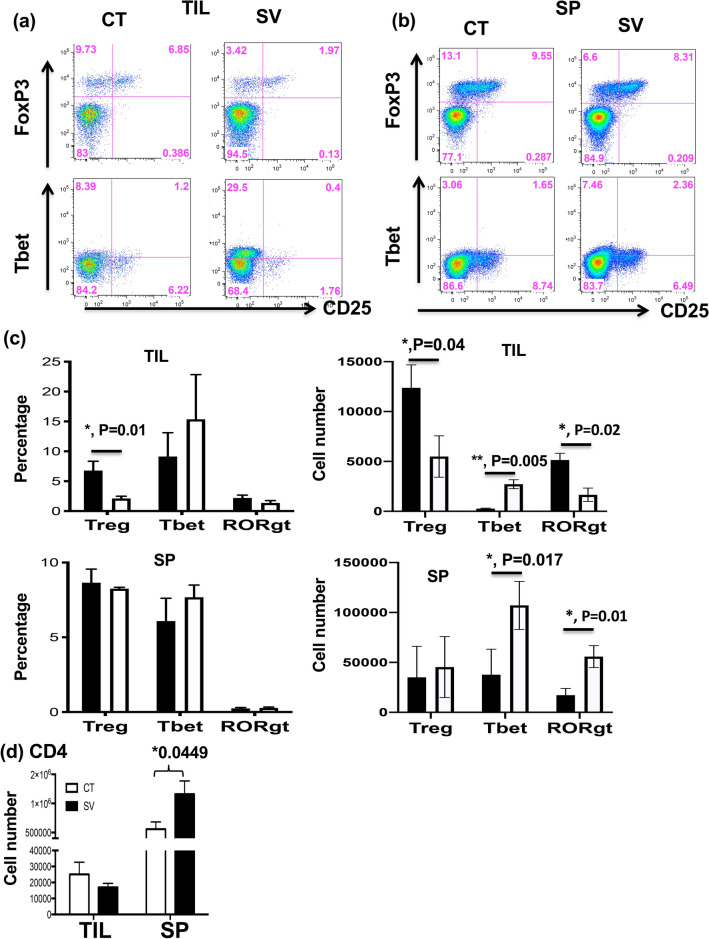
We further evaluated the effect of SV on T regulatory and helper cells in tumor and spleen from treated tumor-bearing mice. The results showed that the percentage (Fig. 5c left panels) and absolute cell number (Fig. 5c right panels) of CD4+CD25+Foxp3+ regulatory T cells were both significantly decreased in the TIL of SV-fed mice (Fig. 5c upper panels), but not in spleen (Fig. 5c lower panels). There were no significant changes in the percentages of CD4+Tbet+ or CD4+RORgt+ T cells in TIL or spleen (Fig. 5c). Worth noting is that the percentages of CD4+Tbet+ T cells in TIL varied between individual experiments (Supplementary Fig. 1), however, the absolute numbers of CD4+Tbet+ T cells in TIL and spleen of SV-fed mice were significantly increased (Fig. 5c). Furthermore, the absolute numbers of CD4+RORgt+ T cells were increased in the spleens of SV treated mice as well (Fig. 5c). In contrast to our observations in spleens, CD4+RORgt+ T cells were significantly reduced in TIL after SV food feeding. Moreover, the number of CD4 T cells was significantly increased in the spleens of SV-fed mice (Fig. 5d). These results indicate that the antitumor effects of the SV diet can also be mediated by regulating the Treg population as well as by development of effector TH1/TH17 in tumor and spleen tissues.
Fig. 5.
SV treatment results in a significant decrease in Treg and a significant increase in Tbet expression in Tumor infiltrating leukocytes (TIL) and spleen (SP) from tumor-bearing mice. a, b TIL and SP were isolated from tumor-bearing mice fed CT or SV food. The phenotype of tumor-infiltrating and splenic T cells was analyzed by flow cytometry. Cells were gated on the CD4+ T cell population. CD25+FoxP3+ (Treg) and Tbet+ T cell populations in TIL (a) and spleen (b) are presented as dot plots. c Statistical analysis of the percentage (left) and number (right) of Treg, Tbet+ and RORgt+ in TIL (upper, n = 3–8) and spleen (lower, n = 3) obtained from (a, b) is shown. *p < 0.05, ANOVA. d The number of CD4 in TIL and spleen is presented as the average ± the standard error (SEM). *p < 0.05, **p < 0.01 ANOVA
SV can reduce Treg and enhance tumor-specific TH1 and TH17 responses
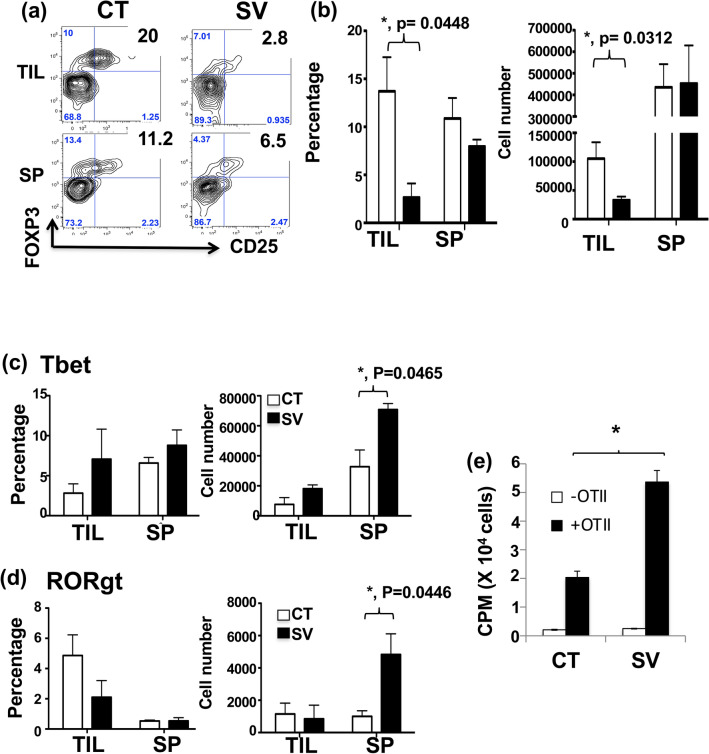
Our in vivo results from Dectin-1 KO mice showed that Dectin-1-related signaling may be involved in the SV food-mediated antitumor immune responses (Fig. 2c). Dectin-1 receptor plays a critical role in promoting the maturation and antigen-presenting capacities of phagocytic cells. Therefore, we determined whether part of the antitumor responses of SV food was mediated by augmentation of antigen-specific effector T cell responses. We analyzed the phenotype of adoptively-transferred CD45.1 + OT-II T cells in chicken ovalbumin (OVA)-overexpressed LLC-tumor-bearing mice after feeding with SV or CT food. In Fig. 6a, b, the percentage and cell numbers of Treg were significantly decreased in TIL. The cell numbers of CD4+Tbet+ (Fig. 6c) and CD4+RORgt+ (Fig. 6d) were both significantly increased in the spleen. Most importantly, adoptively-transferred CD45.1 + OT-II T cells from SV-fed mice exhibited significantly higher proliferative activity in response to OT-II antigens compared to those from control food-fed mice (Fig. 6e).
Fig. 6.
SV treatment reduces the Treg population and enhances tumor-specific T cell responses. a OVA-LLC tumor cells were intrahepatically inoculated into mice fed CT or SV food. After tumors reached 5 × 5 mm3, CD45.1+ OT-II T cells were adoptively transferred into tumor-bearing mice. On day 10 after adoptive transfer, the mice were sacrificed. The phenotype of tumor-specific (OT-II) T cells was analyzed by flow cytometry and presented as dot plot. b–c Statistical analysis of the percentage (left) and number (right) of Treg (b), Tbet+ (c) and RORgt+ (d) cells among CD4+CD45.1+ OT-II T cells in TIL and spleen obtained from (a) is shown. *p < 0.05, ANOVA. e The proliferative activity of CD45.1+ OT-II T cells purified from spleen in response to OVA peptides was determined. Data are presented as the average ± the standard error, n = 3 mice per group. Error bars indicate SD. *p < 0.05, ANOVA
Discussion
Whether a complementary treatment is beneficial for anti-cancer regimen has been a heated debate for decades. However, it is a matter of a balancing act between pros and cons. The current study showed the immune enhancing property of a selected vegetable therapy, specifically chosen for their anticancer attributes, supporting our previous clinical studies in patients with advanced lung cancer who benefited from SV administration and suffered no toxic side effects [11,12]. In previous studies [12], we found that the extracts of mushrooms and mung beans, two major components in SV, had striking antitumor effects individually or in combination in lung tumor-bearing mice. Furthermore, we have identified or quantitatively measured several critical components of SV that are reported to be beneficial for cancer prevention by the National Cancer Institute and the American Cancer Society, including β-glucans, saponins, isoflavones (daidzein and genistein), biochanin A and protease inhibitors Inositol hexaphosphate (IP6). Most of them have been shown to enhance host immunity by targeting different immune cell types, which supports our results in this study. Among those targeted immune cell types, we found that the antigen-presenting cells with highly phagocytotic activity might be the primary arm for initiation and persistence of long-term antitumor immunity.
Myeloid-derived suppressor cells (MDSCs) are broadly defined as immature myeloid cells and myeloid progenitors, CD11b+Ly6G−Ly6C+ monocytic MDSC (M-MDSCs) and CD11b+Ly6G+Ly6C+ granulocytic MDSC (G-MDSC), which can be identified based on Ly6C and Ly6G markers [29]. There are two major strategies to dampen the tumor-promoting activities of MDSCs, i.e. by depletion or by maturation. It has been reported that β-glucans are potential targets for antitumor immune-modulatory therapies by inducing the differentiation, and inhibiting the immuno-suppressive activities, of monocytic MDSCs [27], and inducing apoptosis of G-MDSC [26]. We found that SV indeed significantly decreased the G-MDSC population in TIL and spleen, which could be due to reduced recruitment or increased apoptosis in G-MDSC. M-MDSCs from tumor-bearing mice have been shown to resemble Ly6Chi inflammatory monocytes [30] or tumor-infiltrating monocytes [30,31]. In addition, Ly6Chi monocytes have been demonstrated to give rise to multiple subsets of tumor-associated macrophages (Ly6Cint, MHCIIhi and MHCIIlow), which possess limited antigen-presenting capacity but strong suppressive activity on allogenic T cell proliferation [30]. Furthermore, SV decreased the MHCloArghi, while increasing the MHChiArglo, population in M-MDSC in TIL and spleen (Fig. 3d–e). These findings suggest that the bio-components of SV can potentially increase the number of M-MDSCs present in the tumor microenvironment, and these tumor-infiltrating M-MDSCs have a more differentiated and less immune-suppressive phenotype.
Dectin-1, the receptor for β-glucans, can activate Syk, Raf1, and NFκB signaling pathways through an immunoreceptor tyrosine-based activation motif (ITAM) [32], which not only subverts the suppression of MDSC [33], but also triggers the potent antitumor immunity of dendritic cells through the induction of Th9 cells [34]. Dectin-1 induced production of IL-1β and TNFα in different subsets of murine macrophages and dendritic cells [35,36]. In addition to Dectin-1, β-glucans can act on complement receptors and TLR-2/6 and modulate both innate and adaptive responses by activating macrophages, neutrophils, monocytes, NK cells, and dendritic cells [23]. Furthermore, Dectin-1 has been shown to collaborate with TLRs (TLR2, 4, 5, 7, and 9) to enhance and sustain TLR-mediated I-κB degradation and NFκB nuclear translocation and cytokine production [35,37], which is Syk- and CARD9-dependent [35]. Additionally, Dectin-1 signaling was reported to induce innate memory in epigenetical reprogramming of monocytes, providing a preferential advantage for survival of memory subsets with long-term efficacy and persistence [38,39]. In the present study, we observed that SV food therapy lost its tumor suppressive activity when TLR-mediated signaling was absent due to deficiency in MyD88 and TRIF (Fig. 2a, b). It has been demonstrated that Dectin-1 plays an important role in MDSC expansion [27] and the limiting of MDSC toward M2-like differentiation [40,41]. In contrast, Dectin-1 is considered to be an M2-like marker and is increased by IL-13-mediated PPARγ activation [41,42]. Interestingly, M-MDSCs populations from TIL and spleen decreased arginase expression (Fig. 3f) and CD11b + myeloid cells from TIL of SV-fed mice exhibited decreased M2-like macrophages (F4/80+CD206+, Fig. 4e), and these observations, together with findings in previous studies suggest that SV may not only promote the further differentiation of M-MDSC, but also restrain the suppressive activity of M-MDSC or its polarization toward M2-like macrophages.
The aforementioned pattern recognition receptors, including dectin-1 and TLR, and mannose receptors, expressed on adaptive and innate immune cells can be directly bound by exotic substances/microbial motifs and elicit protective immune responses against invading microbes [43]. In addition to the impact of SV on myeloid cells as described above, we also found that the inhibitory effect of SV on tumor growth was decreased in T cell/B cell/NK cell-deficient mouse models (Fig. 1). Additionally, SV substantially decreased Treg in TIL, and increased Tbet and RORgt cell numbers in the spleens and/or tumors of treated mice (Figs. 5, 6), suggesting that SV treatment can enhance effector T cell development, especially TH1 development. It has been reported that β-glucan can directly activate NK cells by binding to Dectin-1 and mediate the antitumor effects of NK cells [26]. Interestingly, the cytolytic activity of liver NK cells was moderately increased in SV-fed mice (Fig. 1e), suggesting that SV can boost the cytotoxic effect of NK cells against tumor cells. However, whether SV can also activate immature NK cells, which favor tumor progression [44], remains to be studied. The above findings indicate that SV can potentially exert direct or indirect effects to activate the innate immune system to retard tumor growth. Most importantly, antigen-specific TH1/TH17 cells were significantly augmented in secondary lymphoid organs (Fig. 6c, d), which was accompanied by down-regulation of the antigen-specific Treg population in the tumor microenvironment (Fig. 6a, b). It is reported that MyD88 and TRIF-deficient immune cells have impaired responses to TLR ligands, such as reduced IL-6, TNFα and IL-12p40 production, which are key cytokines for TH1/TH17 development [45,46]. Our results showed that antitumor effect of SV food was mediated by TLR-associated MyD88 and TRIF signalings, suggesting that SV food might increase IL-6 and IL-12p40 production and promote TH1 (by IL-12p40) and TH17 (by IL-6 and TGFβ) in the tumor microenvironment. Dectin-1 is reported to activate dendritic cells through phospholipase (PLC)γ2 signaling and induce TH1 and TH17 differentiation [47]. Besides, Dectin-1 is also reported to convert Treg to TH17 development [48]. On the other hand, SV food might decrease Treg development through imbalance of Treg and effector TH1/TH17 in immune system. These studies supports that SV food might increase IL-6 and IL-12p40 production and promote TH1 and TH17 but decrease Treg development. In addition, SV treatment resulted in a significant increase in the proliferation of antigen-specific effector T cells (Fig. 6e). These effects can be driven by an increase in antigen-presenting capacity of SV-primed activated M-MDSC. In addition to β-glucans, a number of key components identified in SV[12], such as Isoflavones (daidzein, genistein,) and soy saponins have been shown to suppress the proliferation of Caco-2 cells in vitro [49]. Besides, protease inhibitors, inositol hexaphosphate, and other phytoestrogens have been reported to possess antitumor and immune-enhancing activities [50]. These studies and the above findings indicate that key components of SV can potentially induce M1-like phenotypes or direct antitumor responses.
In our previous study, we had shown that a specific dietary supplement, selected vegetables (SV), is associated with objective responses, prolonged survival and attenuation of the normal pattern of progression of unresectable stage IIIb and IV non-small cell lung cancer (NSCLC). Noticeably, the mean survival time of the SV group is significantly longer than the control group (15 months vs. 4.8 months, respectively) [12]. Moreover, rapid progress in the understanding of how lung cancer cells evade immunosurveillance and maintain immune-tolerance in the tumor microenvironment drive immunotherapy as an emerging major strategy in NSCLC treatment, e.g. the successful development of immune checkpoint inhibitors (PD-1 and PD-L1). These encouraging achievements led us to determine whether the anticancer efficacy of SV was due to escalating the host immune system or evoking specific immune responses against tumor by targeting immune-suppressive regulators, rather than just as nutritional supplements.
The mechanisms by which SV food retards tumor growth are proposed in Fig. 7. β-glucans may possess the most potent and unique biological properties among the active ingredients (saponins, isoflavone and etc.) of SV by triggering ITAM-, MyD88- and TRIF-signaling in Dectin-1 and TLR-expressing immune, mostly myeloid, cells. β-glucans can significantly inhibit immune-suppressive cells, such as G-MDSC, M2-like myeloid cells and the Treg population, while limiting toward an M2-like phenotype in myeloid cells and enhancing activation of TH1, TH17 and NK cells. In addition, SV can decrease the immune-suppressive activity of M-MDSC and enhance antigen-specific immune responses. Therefore, optimization of SV administration for cancer patients may enhance their innate and adaptive immunity against tumors, resulting in retarded tumor progression, ameliorating malnutrition and enhancing the therapeutic efficacy of conventional therapies.
Fig. 7.
The proposed mechanisms by which SV food retards tumor growth. SV containing β-glucans and other active ingredients (saponins, isoflavone and etc.) can exhibit anti-tumor activity by triggering ITAM-, MyD88- and TRIF-signaling through Dectin-1 and TLR on immune cells, mostly myeloid cells. The above effects can suppress immune-suppressive cells, such as G-MDSC, M2-like myeloid cells and the Treg population, while skewing toward an M1-like phenotype in myeloid cells and enhancing activation of TH1, TH17 and NK cells. Therefore, SV can not only be used as nutritional supplements, but also reinforce their innate and adaptive immunities and the antigen-specific immune responses, which hopefully can be applied as adjuvants for conventional antitumor therapy for late stage of cancer patients
Besides, SV can be potentially used by healthy individuals to prevent the development of lung cancer and lung cancer patients prior potential recurrence, which is partially supported by anti-tumor effects of SV food in preventive tumor models in this study. Although the immune system in patients prior recurrence can not be fully reflected by that in health individuals, the immune-enhancing effects of SV food is appreciated to potentially apply and benefit for cancer patients to prevent or limit the recurrence progression. The present study suggests that easy accessibility, favorable clinical efficacy, acceptable side effects and satisfactory safety make SV a feasible, appealing and convincing adjuvant therapy for lung cancer, and likely other types of cancer with involvement of immune modulation in tumor growth as well.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Ms. Marcia Meseck for editing the manuscript. The authors thanks Dr. Jun Yan (University of Louisville) for providing the bone marrow of Dectin-1 KO C57BL/6 mice for this study.
Authors' contributions
Dr. Hui-Ming Chen performed most of the research, analyzed data and wrote the manuscript; Dr. Linus Sun and Dr. Ping-Ying Pan made contributions to interpretation of data and intellectual content. Dr. Lu-Hai Wang and Dr. Shu-Hsia Chen made substantial contribution to the conception or design of the research, important intellectual content, project supervision and wrote the manuscript with valuable contribution from all other authors.
Availability of data and material
The data analyzed and shown in presented study is available from the corresponding authors upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing financial interests.
Ethics approval
All animal experiments were done in accordance with approved guidelines for animal experimentation of the Mount Sinai School of Medicine.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lu-Hai Wang, Email: luhaiwang@mail.cmu.edu.tw.
Shu-Hsia Chen, Email: shuhsia.chen@houstonmethodist.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol. 2009;471:469–486. doi: 10.1007/978-1-59745-416-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rijavec E, et al. Ipilimumab in non-small cell lung cancer and small-cell lung cancer: new knowledge on a new therapeutic strategy. Expert Opin Biol Ther. 2014;14:1007–1017. doi: 10.1517/14712598.2014.907786. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM. Nivolumab: a review in advanced squamous non-small cell lung cancer. Drugs. 2015;75:1925–1934. doi: 10.1007/s40265-015-0492-9. [DOI] [PubMed] [Google Scholar]
- 5.Cappelli LC, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Rodriguez E, Rodriguez-Abreu D, Spanish Group for Cancer, I.-B. Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist. 2016;21:804–816. doi: 10.1634/theoncologist.2015-0509(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vucenik I, Sakamoto K, Bansal M, Shamsuddin AM. Inhibition of rat mammary carcinogenesis by inositol hexaphosphate (phytic acid). A pilot study. Cancer Lett. 1993;75:95–102. doi: 10.1016/0304-3835(93)90193-D. [DOI] [PubMed] [Google Scholar]
- 8.Wang HZ, Zhang Y, Xie LP, Yu XY, Zhang RQ. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells(1) J Nutr Biochem. 2002;13:421–426. doi: 10.1016/S0955-2863(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z, et al. S-equol, a secondary metabolite of natural anticancer isoflavone daidzein, inhibits prostate cancer growth in vitro and in vivo, though activating the Akt/FOXO3a pathway. Curr Cancer Drug Targets. 2016;16:455–465. doi: 10.2174/1568009616666151207105720. [DOI] [PubMed] [Google Scholar]
- 10.Lim W, Jeong M, Bazer FW, Song G. Coumestrol inhibits proliferation and migration of prostate cancer cells by regulating AKT, ERK1/2, and JNK MAPK cell signaling cascades. J Cell Physiol. 2016 doi: 10.1002/jcp.25494. [DOI] [PubMed] [Google Scholar]
- 11.Sun AS, et al. Phase I/II study of stage III and IV non-small cell lung cancer patients taking a specific dietary supplement. Nutr Cancer. 1999;34:62–69. doi: 10.1207/S15327914NC340109. [DOI] [PubMed] [Google Scholar]
- 12.Sun AS, et al. Pilot study of a specific dietary supplement in tumor-bearing mice and in stage IIIB and IV non-small cell lung cancer patients. Nutr Cancer. 2001;39:85–95. doi: 10.1207/S15327914nc391_12. [DOI] [PubMed] [Google Scholar]
- 13.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailar JC, 3rd, Gornik HL. Cancer undefeated. N Engl J Med. 1997;336:1569–1574. doi: 10.1056/NEJM199705293362206. [DOI] [PubMed] [Google Scholar]
- 15.Bronte V, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang WC, Ma G, Chen SH, Pan PY. Polarization and reprogramming of myeloid-derived suppressor cells. J Mol Cell Biol. 2013;5:207–209. doi: 10.1093/jmcb/mjt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao J, et al. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol. 2011;77:12–19. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HM, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res. 2015;21:4073–4085. doi: 10.1158/1078-0432.CCR-14-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan PY, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma G, et al. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong L, et al. Regulating antigen presentation–anti-tumor immuno-therapy by integrative Chinese and Western medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:847–851. [PubMed] [Google Scholar]
- 23.Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batbayar S, Lee DH, Kim HW. Immunomodulation of fungal beta-glucan in host defense signaling by Dectin-1. Biomol Ther (Seoul) 2012;20:433–445. doi: 10.4062/biomolther.2012.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Becker C, Lowell CA, Underhill DM. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J Biol Chem. 2012;287:34149–34156. doi: 10.1074/jbc.M112.382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albeituni SH, Yan J. The effects of beta-glucans on dendritic cells and implications for cancer therapy. Anticancer Agents Med Chem. 2013;13:689–698. doi: 10.2174/1871520611313050003. [DOI] [PubMed] [Google Scholar]
- 27.Tian J, et al. beta-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol. 2013;43:1220–1230. doi: 10.1002/eji.201242841. [DOI] [PubMed] [Google Scholar]
- 28.Eisenstein S, et al. Myeloid-derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res. 2013;73:5003–5015. doi: 10.1158/0008-5472.CAN-12-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozao-Choy J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 31.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 32.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albeituni SH, et al. Yeast-derived particulate beta-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol. 2016;196:2167–2180. doi: 10.4049/jimmunol.1501853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun. 2016;7:12368. doi: 10.1038/ncomms12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodridge HS, et al. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J Immunol. 2009;182:1146–1154. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 37.Dennehy KM, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeed S, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintin J, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loures FV, et al. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary paracoccidioidomycosis. J Infect Dis. 2014;210:762–773. doi: 10.1093/infdis/jiu136. [DOI] [PubMed] [Google Scholar]
- 41.Lefevre L, et al. PPARgamma ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS ONE. 2010;5:e12828. doi: 10.1371/journal.pone.0012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coste A, et al. PPARgamma promotes mannose receptor gene expression in murine macrophages and contributes to the induction of this receptor by IL-13. Immunity. 2003;19:329–339. doi: 10.1016/S1074-7613(03)00229-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee DH, Kim HW. Innate immunity induced by fungal beta-glucans via Dectin-1 signaling pathway. Int J Med Mushrooms. 2014;16:1–16. doi: 10.1615/IntJMedMushr.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- 44.Rosinsky C, Antony PA. A role for pre-mNK cells in tumor progression. J Immunother Cancer. 2016;4:16. doi: 10.1186/s40425-016-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orr MT, et al. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. Eur J Immunol. 2013;43:2398–2408. doi: 10.1002/eji.201243124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar P, John V, Marathe S, Das G, Bhaskar S. Mycobacterium indicus pranii induces dendritic cell activation, survival, and Th1/Th17 polarization potential in a TLR-dependent manner. J Leukoc Biol. 2015;97:511–520. doi: 10.1189/jlb.1A0714-361R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tassi I, et al. Requirement of phospholipase C-gamma2 (PLCgamma2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol. 2009;39:1369–1378. doi: 10.1002/eji.200839313. [DOI] [PubMed] [Google Scholar]
- 48.Osorio F, et al. DC activated via Dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald RS, et al. Environmental influences on isoflavones and saponins in soybeans and their role in colon cancer. J Nutr. 2005;135:1239–1242. doi: 10.1093/jn/135.5.1239. [DOI] [PubMed] [Google Scholar]
- 50.Poonyachoti S, Deachapunya C. Modulatory effects of phytoestrogens on the expression of Fas ligand and the release of cytochrome C in normal and cancerous endometrial cells. J Med Assoc Thai. 2012;95(Suppl 12):S105–112. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed and shown in presented study is available from the corresponding authors upon reasonable request.