Abstract
STING is a pivotal mediator of effective innate and adaptive anti-tumor immunity; however, intratumoral administration of STING agonists have shown limited therapeutic benefit in clinical trials. The systemic effect of the intravenous delivery of STING agonists in cancer is not well-defined. Here, we demonstrated that systemic administration of STING agonist inhibited melanoma growth, improved inflammatory effector cell infiltration, and induced bone marrow mobilization and extramedullary hematopoiesis, causing widespread changes in immune components in the peripheral blood. The systemically administered STING agonist promoted HSC expansion and influenced lineage fate commitment, which was manifested as the differentiation of HSPCs was skewed toward myeloid cells at the expense of B-cell lymphopoiesis and erythropoiesis. Transcriptome analysis revealed upregulation of myeloid lineage differentiation-related and type I interferon-related genes. This myeloid-biased differentiation promoted the production and maturation of myeloid cells toward an activated phenotype. Furthermore, depletion of Gr-1+ myeloid cells attenuated the anti-tumor immunity of STING agonist. Our findings reveal the anti-tumor mechanism of systemic administration of STING agonist that involves modulating HSPC differentiation and promoting myeloid cells maturation. Our study may help explain the limited clinical activity of STING agonists administered intratumorally.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03502-7.
Keywords: Anti-tumor immunity, STING agonist, Hematopoietic stem and progenitor cell, Myeloid cell differentiation, Melanoma
Introduction
Immune checkpoint inhibitors (ICIs) have been used clinically to treat patients with various types of cancers [1]. Although most immunotherapies for cancer target the adaptive immune system, innate immune activation has been increasingly recognized for its role in promoting anti-tumor immune responses [2]. Adaptive anti-tumor immunity is also highly dependent on innate immunity, as innate immune cells facilitate signaling for effector T cell trafficking and enhance anti-tumor immunity [3].
Drugs that activate innate immunity have shown remarkable preclinical activity and promising results in early clinical trials, including Toll-like receptor (TLR)-9 and retinoic acid inducible gene-1 (RIG-I) agonists [4, 5]. Aberrant DNA species in the cytoplasm are recognized by cyclic GMP-AMP synthase (cGAS), which synthesizes cyclic GMP-AMP (cGAMP), and subsequently activate the downstream stimulator of interferon genes (STING, also known as TMEM173), leading to activation of TANK-binding kinase 1 (TBK1) and phosphorylation and nucleus translocation of interferon regulatory factor 3 (IRF3), resulting in the transcription of genes encoding type I interferon (IFN). The cGAS-STING pathway is an important TLR-independent mediator of the host innate defense and is essential for effective innate immune signaling [6–10]. Preclinical studies have provided evidence that activation of cGAS-STING signaling enhances anti-tumor immunity by stimulating various immune cells within the tumor microenvironment (TME), including CD8+ T cells, natural killer (NK) cells, dendritic cells (DCs), monocytes, and neutrophils. These findings have been observed in diverse cancer types [11–17]. However, most studies adopted an intratumoral administration method, which showed frustrating results in solid tumor. Therefore, more and more intravenous administered small molecule STING agonists are being developed, but the systemic effect of intravenous delivery of STING agonists remains unclear.
The outgrowth of malignant tumors such as melanoma involves global immune landscape changes beyond the TME [18, 19] and is associated with hematopoietic stem and progenitor cell (HSPC) dysfunction [19–22], which may predict adverse outcomes [23]. Hematopoietic stem cells (HSCs) have self-renewal abilities and multiple differentiation capacities [24]. HSCs remain quiescent at a steady state, but when exposed to various stimuli, they rapidly re-enter the cell cycle to replenish the entire hematopoietic system [25]; and terminally differentiated myeloid cells are key innate immune cells essential for activating adaptive immunity. During cancer development and progression, HSCs re-enter the cell cycle and differentiate into common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs); however, HSPCs gradually lose their full differentiation capacity under the pressure of chronic low-level activation signals from cancer cells, resulting in the accumulation of immature myeloid cells [26, 27]. Besides myeloid cells, melanoma and breast cancer growth can affect the hematopoietic system in the bone marrow (BM) and spleen, leading to a decrease in lymphoid lineage cells, including T cells [19, 28]. Cytosolic DNA-sensing pathways are highly and widely expressed in primitive HSPCs, including in HSCs and their early progenies. Pathogen-associated molecular patterns (PAMPs) can directly affect HSCs through specific pathogen recognition receptors such as cGAS [29]. The STING agonist can promote the myeloid-biased differentiation of HSPCs and strengthen the anti-infection capacity of the immune system [29, 30]. However, the role of STING agonists in regulating hematopoiesis in cancer is not well-understood.
In this study, we evaluated the anti-tumor effects and global changes on the immune system of systemically administered STING agonist, and determined the function of STING agonist exposure on HSPCs fate decision in a B16-F10 mouse model.
Materials and methods
Cell line
B16-F10 mouse melanoma cells were obtained from the China Infrastructure of Cell Line Resource (Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium containing nonessential amino acids, 1% penicillin–streptomycin, 1% l-glutamine, and 10% fetal bovine serum. All cell culture reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Mice and melanoma model
Six- to eight-week-old C57BL/6 mice were purchased from the Vital River (Beijing, China). The mice were housed under standard specific pathogen-free conditions. All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, with protocols approved by the Animal Care and Use Committee at the Peking University Cancer Hospital & Institute. Tumors were generated by injecting 2 × 105 B16-F10 cells subcutaneously into the flanks of C57BL/6 mice. The tumor diameter was measured every three days, and tumor volumes were calculated as 0.5 × (length × width2). The mice were treated intravenously or intratumorally with 100 μL of dimeric amidobenzimidazole (diABZI, MedChemExpress, Monmouth Junction, NJ, USA) or vehicle. To deplete immune cells, 200 mg of anti-CD8 antibody (BioXcell, Lebanon, NH, USA) or anti-Gr-1 antibody (BioXcell) was injected twice before treatment. Mice were euthanized when the tumor volume reached 2000 mm3. Blood was collected into ethylenediaminetetraacetic acid-coated tubes, and hematological and blood biochemical parameters were analyzed.
Flow cytometry analysis
Single-cell suspensions were prepared from the tumor tissues, peripheral blood, spleen, and BM. Red blood cells were lysed using ACK lysis buffer. The cells were stained with viability dye (TONBO, San Diego, CA, USA) to exclude dead cells and then resuspended in phosphate-buffered saline containing 2% fetal calf serum, followed by Fc blocking and antibody incubation. Intracellular staining was performed after surface staining, lysis, and fixation using Fixation buffer and Intracellular Staining Perm Wash Buffer (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions. The antibodies are listed in Supplementary Table S1, and the staining panel are listed in Supplementary Table S2. The gating strategy of the tumor tissues and spleen are illustrated in Supplementary Fig. S1A and S1B, respectively. Flow cytometry data were analyzed using CytoFLEX (Beckman Coulter, Brea, CA, USA) and CytExpert (Beckman Coulter) and FlowJo (TreeStar, Ashland, OR, USA) software.
In vitro analysis of HSCs
BM cells were harvest from the tibia and femur of B16-F10 melanoma-bearing C57BL/6 mice, and Lin− Sca-1+ c-kit+ (LSKs) single cells were sorted using fluorescence-activated cell sorting. The LSKs were cultured in StemSpan™ SFEM medium (serum-free medium for culture and expansion of hematopoietic cells, STEMCELL Technologies, Vancouver, Canada) containing human IL-11 (100 ng/mL, STEMCELL), human Flt3 ligand (100 ng/mL, STEMCELL), and murine SCF (50 ng/mL, STEMCELL). LSKs were treated with 2.5 uM diABZI or vehicle for one hour in vitro, and then the activation of STING pathway was analyzed by western blotting. Colony-forming units (CFUs) were assessed after 14 days of culture with or without diABZI treatment in MethoCult™ GF M3434 medium (STEMCELL Technologies). The CFUs were classified into small (< 500 μm) and large (≥ 500 μm) colonies.
Immunohistochemistry and immunofluorescence staining
Formalin-fixed and paraffin-embedded tissue sections were stained with hematoxylin and eosin for histological confirmation. Immunohistochemistry (IHC) was performed on the embedded samples using antibodies against CD8 (Cell Signaling Technology, Danvers, MA, USA), granzyme B (Proteintech, Rosemont, IL, USA) followed by a standard avidin–biotin detection protocol using 3-amino-9 ethylcarbazole. For immunofluorescence staining, the tissue slides were incubated with specific primary antibodies against CD3, CD8, CD4, B220, CD11b, and arginase I (Arg-1) (Servicebio, Wuhan, China), followed by incubation with anti-mouse/rabbit Alexa Fluor secondary antibodies (Cell Signaling Technology). DAPI was used for nuclear staining. Images were captured using a FluoView1000 laser scanning confocal microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
For isolated LSKs, western blotting analysis was performed using STING (Cell Signaling Technology), phosphorylated STING (Cell Signaling Technology), TBK1 (Cell Signaling Technology), phosphorylated TBK1 (Cell Signaling Technology), IRF3 (Proteintech), phosphorylated IRF3 (Cell Signaling Technology). CD11b+ Gr-1+ myeloid cells were isolated from the tumors of tumor-bearing mice using a mouse CD11b+ Gr-1+ isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) with an isolation efficiency above 90%, and western blotting analysis was performed using anti-Arg-1, anti-inducible nitric oxide (NO) synthase (iNOS), and anti-GAPDH antibodies (Cell Signaling Technology). The integrated density of the positive bands was quantified using ImageJ software (Bethesda, MD, USA).
T cell proliferation assay
T cells were obtained from murine spleens using the MojoSort™ Mouse CD8+ T Cell Isolation Kit (BioLegend, San Diego, CA, USA). T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, 1 mol/L) for 15 min at 37 °C and then co-cultured with tumor CD11b+ Gr-1+ cells (1:1) for 72 h in 12-well plates with/without Dynabeads™ Mouse T-Activator CD3/CD28 (Thermo Fisher Scientific) stimulation. The fluorescence intensity of CFSE-labeled T cells was detected using flow cytometry.
Smart-seq2 transcriptome profiling analysis
Single-cell suspensions of LSKs and Lin− Sca-1− c-kit+ CD16/32+ CD34+ (GMPs) were sorted from the BM of diABZI-treated and control mice and collected into tubes containing lysis and ribonuclease inhibitors. Amplification was performed using the Smart-Seq2 method as previously reported. The synthesized cDNA was assessed using a Qubit® 3.0 Fluorometer (Thermo Fisher Scientific) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and then randomly sheared using ultrasonic waves for library preparation. After library preparation, a PerkinElmer LabChip® GX Touch and Step OnePlus™ Real-Time PCR System (Waltham, MA, USA) was used to inspect the library quality. The libraries were then loaded onto an Illumina HiSeq platform (San Diego, CA, USA) for PE150 sequencing. DEGseq was used for differentially expressed gene analysis, and the P-value was adjusted using Benjamini and Hochberg’s approach. Genes showing a fold-change ≥ 2 and adjusted P-value < 0.05 were considered as differentially expressed. The data were deposited in the Gene Expression Omnibus database (accession number: GSE192927).
Statistical analysis
Data were analyzed using GraphPad Prism version 8 software (San Diego, CA, USA). The results are presented as the mean ± standard deviation (SD). Survival analyses were performed using the Kaplan–Meier method, and Log-rank tests were used to compare survival curves. Comparisons between two groups were performed using Student’s t-test, and values of P < 0.05 (two-tailed test) were considered to indicate statistically significant results.
Results
Systemic administration of STING agonist treatment inhibits melanoma growth and improves immune cell infiltration
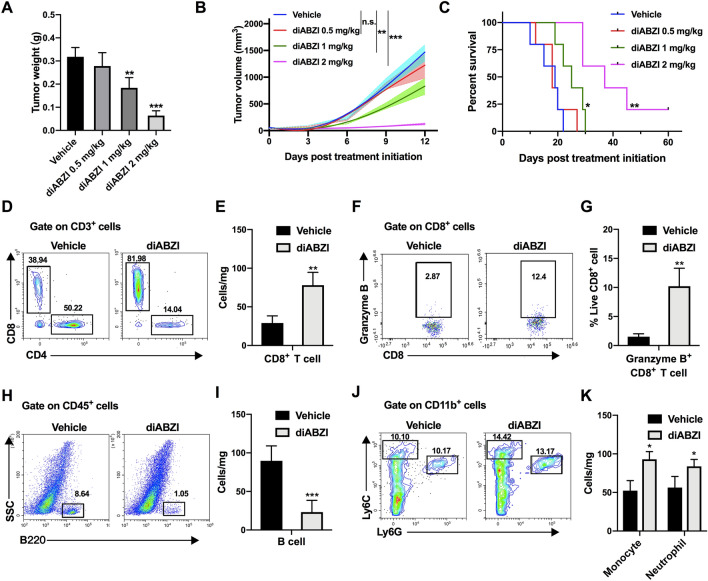
We first evaluated the therapeutic effects of systemically administration of diABZI, which is a synthetic small-molecule STING agonist [31], on a poorly immunogenic B16-F10 melanoma model. Tumor-bearing mice were intravenously treated with different doses of diABZI three times and continually monitored until the study endpoint (Supplementary Fig. S2A). The anti-tumor effect and survival benefit of diABZI were positively correlated with the dose level (Fig. 1A–C), with a dose of 2 mg/kg showing the most significant effect on tumor growth inhibition (P < 0.001). This dosage did not cause weight loss (Supplementary Fig. S2B) or behavioral abnormalities. Moreover, the significantly deteriorated biochemical parameters in tumor-bearing mice tended to be normal (Supplementary Fig. S2C). Based on these data, a dose of 2 mg/kg was used in subsequent experiments.
Fig. 1.
Systemic administration of STING agonist inhibits melanoma growth and stimulates an immunogenic tumor microenvironment. A–C B16-F10-bearing mice were intravenously injected with different concentrations of diABZI or vehicle (n = 5 mice in each group). A Tumor weights, B tumor growth curves and C overall survival curves are shown. Tumor-infiltrating D–G T cells, H, I B cells, J, K monocytes (CD11b+ Ly6Chi), and neutrophils (CD11b+ Ly6G+) were analyzed using flow cytometry at 48 h after last administration. Results displayed are representative of at least two independent experiments and are presented as the mean ± SD (n = 3–5 biologically independent samples). n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001 compared with the vehicle group
We next investigated changes in tumor-infiltrating leukocyte populations at 48 h after the third diABZI treatment using flow cytometry, as the drug exerted strong anti-tumor effects at this time. Tumor-bearing mice treated with diABZI showed significantly higher infiltration of CD3+ (Supplementary Fig. S3A and S3B) and CD8+ T cells within B16-F10 tumors (Fig. 1D and E), with a concomitant increase in the CD8+/CD4+ T cell ratio (Supplementary Fig. S3C). CD8+ T cells in the diABZI group exhibited higher granzyme B expression (Fig. 1F and G), and the percentage of Treg cells was decreased in the diABZI group (Supplementary Fig. S3D and S3E). Notably, we also detected significantly decreased B cell numbers (Fig. 1H and I) but an elevated number of monocytes and neutrophils in the tumors of diABZI-treated mice compared to that in control mice (Supplementary Fig. S3F and S3G, Fig. 1J and K). There were also slight increases in NK cells, macrophages, and DCs in diABZI-treated tumors, but the increase were not significant (Supplementary Fig. S3H). Consistent with these observations, immunofluorescence analysis showed increased expression of CD3, CD8 and CD11b and decreased expression of CD4 and B220 expression in diABZI-treated tumors (Supplementary Fig. S3I).
Systemic administration of STING agonist induces widespread immune component changes in peripheral blood, spleen, and bone marrow
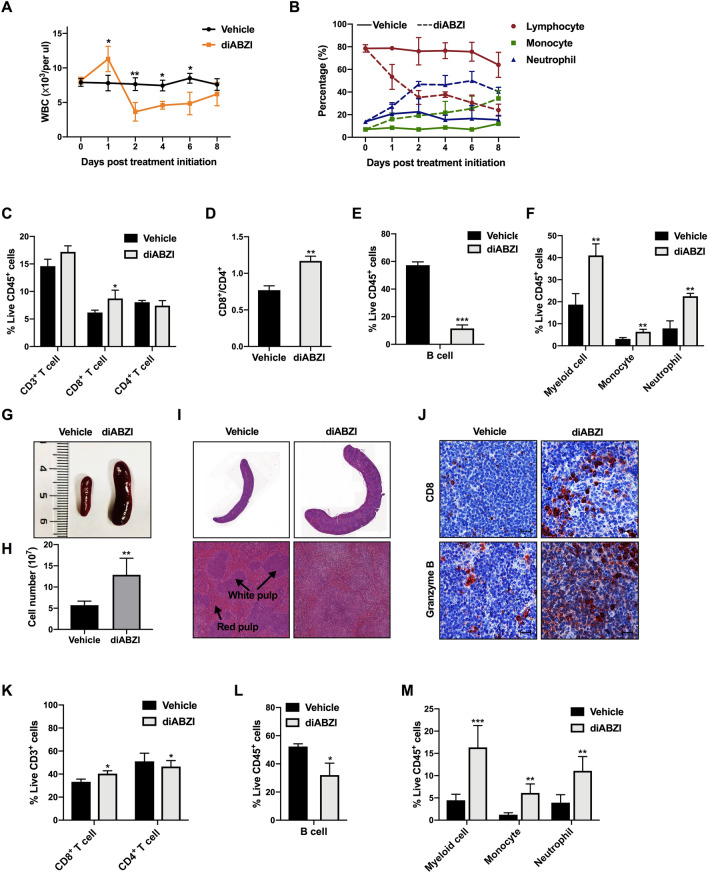
Cancer is a systemic disease in which cell components in the peripheral blood are associated with clinical outcomes in patients with melanoma receiving immunotherapy [32]. We found that the changes in immune compartments upon diABZI treatment were not restricted to the tumor environment but also occurred systemically in the peripheral blood, spleen, and BM. Kinetic analysis of the hematological parameters was performed in the two groups of mice. The absolute number of white blood cells (WBC) increased immediately after diABZI injection and then declined significantly after 24 h when the tumors started to shrink rapidly (Fig. 2A). Specifically, the numbers of monocytes and neutrophils were significantly increased, whereas lymphocytes exhibited the opposite trend (Fig. 2B). Although the total number of lymphocytes decreased after diABZI treatment, the flow cytometry analysis showed that the percentage of CD8+ T cells and the CD8+/CD4+ T cell ratio significantly increased (Fig. 2C and D, Supplementary Fig. S4A and S4B). Notably, the percentage of B cells in CD45+ cells dramatically dropped 4.8-fold (Fig. 2E, Supplementary Fig. S4C). This may be the reason for the drastic decrease in the lymphocyte proportion. Therefore, the reduction in the total number of leukocytes in peripheral blood might be due to a large number of effector T lymphocytes recruited to the TME coupled with the decrease in B cells. A 2.1-fold increase in CD11b+ myeloid cells was observed, as well as an increase in monocytes and neutrophils (Fig. 2F, Supplementary Fig. S4D). An increase in NK cells and DCs was also observed in peripheral blood (Supplementary Fig. S4E). The mice showed severe anemia and thrombocytopenia after the diABZI treatment, as indicated by a 2.5-fold decrease in the red blood cell (RBC) number along with a 2.0-fold reduction in hemoglobin (HGB) content, and a 3.3-fold reduction in blood platelets (PLTs) (Supplementary Fig. S4F).
Fig. 2.
Systemic administration of STING agonist induces immune cellular composition change in peripheral blood and spleen. A Mice were intravenously injected with diABZI or vehicle, and WBC counts were analyzed on the indicated days after injection. B Percentages of lymphocytes, monocytes, and neutrophils in peripheral blood after treatment. C, D Percentage of T cells, E B cells, F monocytes, and neutrophils in peripheral blood CD45+ cells. G Representative spleen size and morphology images of control and diABZI-treated mice. H Number of splenocytes post-RBC lysis. I Representative images of hematoxylin and eosin staining and J IHC staining of CD8 and granzyme B in spleen sections. Scale bar, 100 μm. K Percentage of T cells, L B cells, M monocytes, and neutrophils at 48 h after the third administration in the spleens. Results displayed are representative of two independent experiments. Data are presented as the mean ± SD (n = 3–5 per group). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the vehicle group
In addition to perturbation in the peripheral blood, splenomegaly was observed when the mice were euthanized (Fig. 2G). The total number of splenocytes post-red blood cell lysis was increased by 2.3-fold compared to that in control mice (Fig. 2H). Histological investigation of control mice revealed a well-demarcated separation between the red pulp and white pulp, whereas no distinct separation of splenic red and white pulp was observed in diABZI-treated mice, indicating systemic diABZI treatment had an impact on the architecture of spleen (Fig. 2I). The spleen is a reservoir of peripheral immune cells with key roles in mediating immune system effects. Therefore, immune compartment alterations in the spleen after diABZI treatment were examined. IHC staining showed that the expression of CD8 and granzyme B in the spleens of the diABZI group was increased significantly (Fig. 2J). Flow cytometry revealed a significant increase in CD8+ T cells and slight decrease in the proportion of CD4+ T cells in the spleen (Fig. 2K, Supplementary Fig. S5A). Consistent with the observations in tumors and blood, the percentage of B cells was significantly reduced (Fig. 2L, Supplementary Fig. S5B) and percentage of monocytes and neutrophils was strikingly increased (Fig. 2M, Supplementary Fig. S5C) in splenic CD45+ cells. DCs were also significantly increased in the spleens of diABZI-treated mice (Supplementary Fig. S5D). These widespread changes in the peripheral blood and spleen indicated that the variation of tumor-infiltrating leukocytes after systemic infusion of the STING agonist is associated with hematopoiesis. Therefore, BM-resident immune cells were further examined. In the BM of diABZI-treated mice, the CD8+/CD4+ T cell ratio was significantly increased (Supplementary Fig. S6A–C); the BM showed similar variation tendencies as the spleen for other immune cells (Supplementary Fig. S6D–H). The peripheral blood WBC count increased immediately after diABZI treatment, and the consistent changes in the spleen and BM indicated that systemic administration of the STING agonist rapidly mobilizes HSPCs in steady state and affects BM hematopoiesis and extramedullary hematopoiesis.
Systemic administration of STING agonist promotes HSC expansion and myeloid-biased differentiation
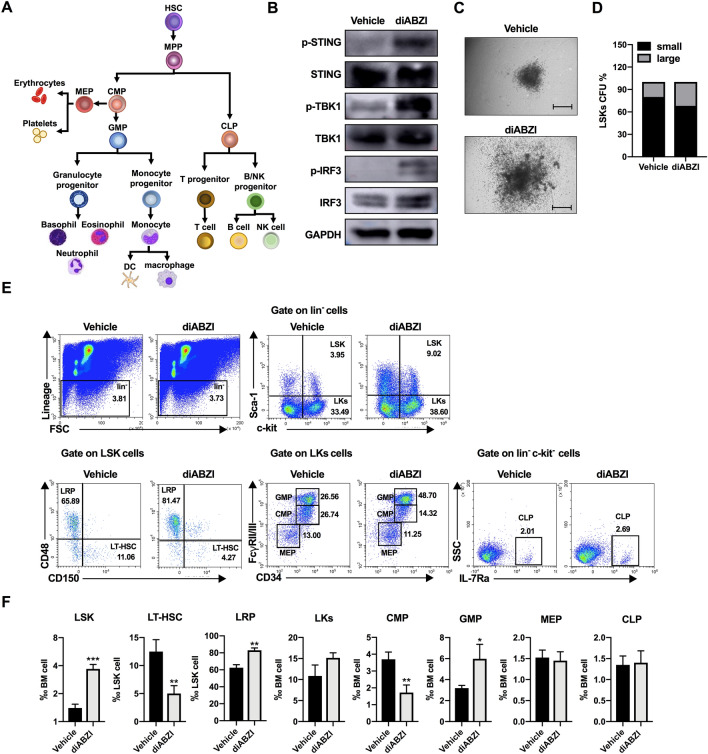
To determine whether the systemic STING agonist affected the hematopoietic system, we investigated HSPC compartments (Fig. 3A) in melanoma-bearing mice. First, we tested the effects of diABZI on HSCs in vitro and found that diABZI activated STING signaling by phosphorylating STING and its downstream proteins, TBK1 and IRF3 (Fig. 3B). A colony-forming unit assay indicated that after 14 days of culture with or without diABZI, the diABZI group showed larger CFUs (Fig. 3C and D). In vivo, the changes in HSPCs in the BM (Fig. 3E and F) and spleen (Supplementary Fig. S7) were analyzed using flow cytometry. Mouse HSCs were referred to as LSKs and were found to be increased in both the BM and spleens of diABZI-treated mice. The LSK pool contains LT-HSCs (CD150+ CD48−) with long-term hematopoietic reconstituting activity and lineage-restricted progenitors (LRPs; CD150− CD48+) that have no self-renewal capacity but maintain downstream lineage differentiation potential [33]. The diABZI-treated mice showed a significant decrease in LT-HSCs and an increase in LRPs in both the BM and spleen. The downstream progenitor Lin− Sca-1− c-Kit+ (LKs) exhibited a modest increase in the BM but a 1.7-fold decrease in the spleen. The LK pool contains CMPs, GMPs, and megakaryocyte-erythrocyte progenitors (MEPs). After diABZI treatment, GMPs rapidly expanded and CMPs were reduced in both the BM and spleen. There was no obvious change in the frequency of common lymphoid progenitors (CLPs) in the BM or spleen after diABZI treatment. These results indicated that the STING agonist disrupted the dormant state of HSCs and promoted HSC expansion and myeloid-biased differentiation.
Fig. 3.
Systemic administration of STING agonist promotes bone marrow HSPC mobilization and myeloid-biased differentiation. A Schematic illustration of HSPC differentiation. HSC hematopoietic stem cell, MPP multipotent progenitor, CMP common myeloid progenitors, MEP megakaryocyte-erythrocyte progenitor, GMP granulocyte-monocyte progenitor, CLP common lymphoid progenitor. (B) LSKs were isolated and stimulated with 2.5 μM diABZI for 1 h. STING, TBK1, and IRF3 expression levels and their phosphorylation states were determined by western blotting. C, D The colony-forming units (CFUs) were detected after 14 days of culture with or without diABZI (2.5 μM). C Representative pictures of colonies. Scale bar, 500 μm. D Percentage of small (< 500 μm) and large (≥ 500 μm) colonies of LSKs treated with vehicle or diABZI. E Representative flow cytometry images and F relative ratio of LSKs (Lin− Sca-1+ c-kit+), LT-HSCs (long-term HSCs, Lin− Sca-1+ c-kit+ CD48− CD150+), LRP (Lin− Sca-1+ c-kit+ CD48+ CD150−), LKs (Lin− Sca-1− c-kit+), CMPs, GMPs, MEPs, and CLPs obtained from the bone marrow of control and diABZI-treated mice. Results displayed are representative of two independent experiments. Data are presented as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the vehicle group
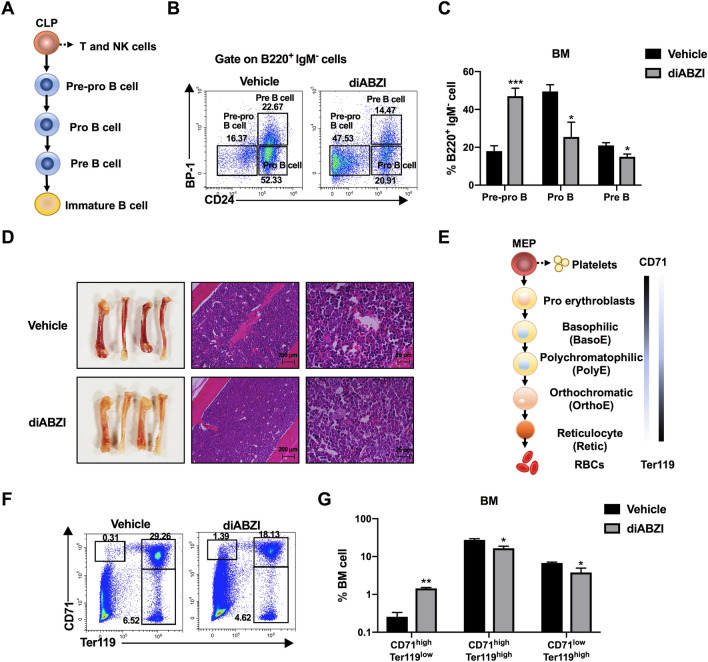
Although CLPs did not change after STING agonist treatment, T cells and NK cells were increased and B cells were decreased. Therefore, B cells at different stages of differentiation (Fig. 4A) were examined to clarify why B cells were decreased. As shown in Fig. 4B and C, pre-pro-B cells increased by 2.7-fold in diABZI-treated mice compared to in control mice. The proportions of pro-B cells, pre-B cells, and mature B cells were reduced after diABZI treatment, demonstrating that B cell development was arrested at the pre-pro-B cell to pro-B cell stage. Furthermore, we investigated the direct cytotoxic effect of diABZI on B cells. Our data revealed that diABZI treatment significantly induced apoptosis in B cells (Supplementary Fig. S8), which also helps to explain this finding. Given that diABZI-treated mice showed anemia (Fig. 4D), erythroid differentiation was analyzed in the BM of these mice. CD71 and Ter119 are the most commonly used cell-surface antigens to identify different stages of erythroid differentiation [34]. As shown in Fig. 4E, the expression of CD71 decreased gradually during maturation of the erythroid lineage, whereas the expression of Ter119 increased. Flow cytometry analyses revealed a reduction in CD71high Ter119high and CD71low Ter119high cells in the BM and an increase in CD71high Ter119low cells, indicating that the reduction in mature erythroid cells occurred because of blocking of the differentiation of pro-erythrocytes (CD71high Ter119low) to early erythrocytes (CD71high Ter119high) (Fig. 4F and G).
Fig. 4.
Systemic administration of STING agonist blocks B lineage and erythroid lineage development. A Schematic illustration of CLP differentiation to terminal B cells. B, C Different stages of B cells in the BM were identified using CD24 and BP-1 markers within the B220+ and IgM− cells, and detected by flow cytometry. D Representative images of femurs and H&E staining from vehicle- and diABZI-treated mice. E Schematic illustration of MEP differentiation to terminal red blood cells. F, G Erythroid lineages in the BM were analyzed by flow cytometry to detect CD71 and Ter119. Results displayed are representative of two independent experiments. Data are presented as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the vehicle group
Furthermore, we also conducted an in vivo experiment to assess the effects on the hematopoietic system following intratumoral injections with diABZI. We collected the spleens and BM samples and found no histopathological changes compared to the control group (Supplementary Fig. S9A). Flow cytometry analysis revealed no significant alterations in the frequency of myeloid cells and B cells in the BM following intratumoral diABZI treatment (Supplementary Fig. S9B). These findings indicate that, unlike intratumoral injection, intravenous treatment of diABZI showed a unique effect on the hematopoietic system.
Systemic administration of STING agonist shifts the transcriptional profiles of HSPCs toward myeloid cell differentiation
To better understand the effects of diABZI on HSPCs, LSKs and GMPs were sorted from the BM and subjected to Smart-seq2 sequencing to explore transcriptome alterations after diABZI treatment. Gene Ontology (GO) analysis demonstrated that many biological processes are influenced by LSKs. As shown in Fig. 5A, the regulation of myeloid cell differentiation, hematopoiesis, innate immune response, response to ROS, positive regulation of lymphocyte-mediated immunity, negative regulation of B cell activation, and type I IFN signaling pathway were enriched in LSKs from diABZI-treated mice. Type I IFN signaling in myeloid cells was previously considered to promote anti-tumor immunity and is a prerequisite for efficient melanoma T cell therapy [35], and type I IFN has been reported to activate dormant HSCs [36]. Gene set enrichment analysis (GSEA) further revealed significant enrichment of the gene response to type I IFN in LSKs from diABZI-treated mice (Fig. 5B).
Fig. 5.
Systemic administration of STING agonist alters the transcriptional profiles in LSKs and GMPs. A GO enrichment analysis of differentially expressed genes and B GSEA analysis of type I IFN response pathway in LSKs of vehicle- and diABZI-treated mice (n = 3 mice in each group). C GO enrichment analysis of differentially expressed genes and D GSEA analysis of type I IFN response pathway in GMPs. E Volcano plot showing the distribution of the differential gene expression in GMPs. F, G Heat maps of F genes related to myeloid cell differentiation and G genes involved in type I IFNs in GMPs from diABZI-treated mice as compared to that in the control group
GO analysis of the GMPs showed that the innate immune response, regulation of myeloid cell differentiation, regulation of ROS metabolic process, and response to IFN-β were among the top differentially enriched pathways between GMPs from the two groups (Fig. 5C). GSEA showed that GMPs from diABZI-treated mice were enriched in the type I IFN pathway (Fig. 5D). The volcano plot shows the differentially expressed genes in GMPs from diABZI-treated mice and control mice (Fig. 5E). Heatmaps of individual genes related to myeloid cell differentiation and the type I IFN pathway are shown in Fig. 5F and G, respectively. In GMPs from diABZI-treated mice, we observed upregulated expression of signal transducer and activator of transcription 1 (STAT1), which induces splenic extramedullary hematopoiesis and myeloid cell output [37], and increased expression of positive regulators that participate in monocyte differentiation (BATF2, ZFP36L1) [38, 39]. The expressions of several genes that participate in immunosuppressive myeloid cell differentiation and recruitment were downregulated in the GMPs of diABZI-treated mice, such as colony-stimulating factor 1 receptor (CSF1R) and EP300 [40–42]. These transcriptional data verified that HSPCs were also targets of the STING agonist, leading to upregulation of type I IFN-related genes and differentiation toward myeloid cells. Additionally, the differentiation of immature myeloid cells which exert immunosuppressive function may be repressed by diABZI.
The myeloid cells induced by systemically administered STING agonist exert anti-tumor effect
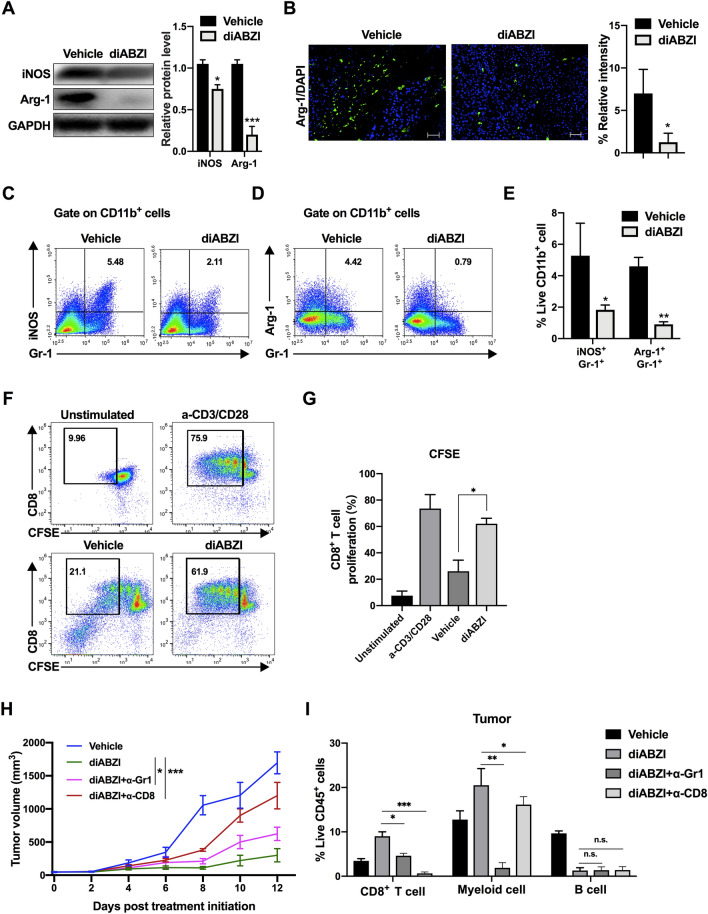
Cancer causes HSPC dysfunction and result in the accumulation of immature myeloid cells [43]. These immature myeloid cells share their surface markers with other mature myeloid cells (including monocytes and neutrophils) but exert immunosuppressive effects by releasing iNOS, which generates NO, Arg-1, and ROS to suppress T cell proliferation [43, 44]. Given that the systemic STING agonist induced HSPCs toward a myeloid-biased differentiation and promoted the accumulation of CD11b+ Gr-1+ (Ly6C+/Ly6G+) myeloid cells, we examined the functional characteristics of these cells. Western blotting and immunofluorescence staining showed that the expression of Arg-1 and iNOS was reduced in the CD11b+ Gr-1+ myeloid cells isolated from the tumors of diABZI-treated mice (Fig. 6A and B), and the flow cytometry analysis showed similar results (Fig. 6C–E). To detect the effect of CD11b+ Gr-1+ myeloid cells on T cells, splenic CD8+ T cells were isolated and co-incubated with tumor-derived CD11b+ Gr-1+ cells, and T cell proliferation was assessed using CFSE labeling. As shown in Fig. 6F and G, the proliferation of T cells was significantly higher after co-incubated with diABZI-induced CD11b+ Gr-1+ cells compared with that of co-incubated with vehicle-treated CD11b+ Gr-1+ cells. The function of myeloid cells was further examined in vivo through depletion with a Gr-1 monoclonal antibody. The results showed that diABZI-induced tumor regression was partially restrained by anti-Gr-1 (Fig. 6H), verifying that myeloid cells induced by systemically administered STING agonist exert anti-tumor effect. As shown in Fig. 6I and Supplementary Fig. S10, the depletion of Gr-1+ myeloid cells resulted a reduction in the density of CD8+ T cells in tumor and spleen. Although there was a tendency of decrease in blood and BM, the changes were not statistically significant. Following diABZI treatment, the proportion of Gr-1+ myeloid cells was reciprocally reduced in mice depleted of CD8+ cells in tumors, but no significant changes were observed in blood, spleen, or BM. Furthermore, the depletion of myeloid cells or CD8+ T cell did not impact the proportion of B cells, which exhibited a rapid reduction after diABZI treatment. Our data indicated that the diABZI-induced mature myeloid cells were necessary for the systemic expansion of CD8+ T cells. The therapeutic effect observed in our study dependents on the synergistic communication between HSPCs-derived myeloid cells and CD8+ T cells.
Fig. 6.
The myeloid cells induced by systemically administered STING agonist exhibit anti-tumor phenotype. A–E Myeloid cells were separated from the tumors of mice treated with vehicle or diABZI. A iNOS and Arg-1 expression levels were determined by western blotting. Histogram on the right showed quantification of western blotting data from 3 independent experiments. B Representative images of immunofluorescence staining of Arg-1 in sections. Scale bar, 100 μm. The histogram showed the density of positivity cells for each section. C–E Flow cytometry dot plot and quantification of iNOS+ and Arg-1+ myeloid cells in tumors of mice treated with vehicle or diABZI. F, G Effect of tumor-derived myeloid cells on CD8+ T cell proliferation was detected using CFSE-labeled T cells. H Tumor growth curves of B16-F10-bearing mice injected with the vehicle or diABZI after Gr-1+ cell or CD8+ T cell depletion using anti-Gr-1 or anti-CD8 antibody (n = 5). I Quantification of CD8+ T cells, myeloid cells and B cells following the administration in the tumor. Results displayed are representative of at least two independent experiments and are presented as the mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the vehicle group
Discussion
The cGAS-STING signaling pathway is a promising treatment target in cancer [45]. However, clinical trials of STING agonists have shown discouraging results. In 2018, it was reported that single-agent treatment with MK-1454 did not achieve a partial or complete response in patients with advanced solid tumors or lymphomas, and the objective response rate of MK-1454 plus anti-PD-1 antibody Keytruda was less than 30% [46]. A phase I study has shown that the efficacy of single-agent ADU-S100 (MIW815) is limited in the patients with advanced solid tumors and lymphomas [47]. According to a clinical trial of ADU-S100 combined with the anti-PD-1 antibody spartalizumab for treating advanced solid tumors and lymphomas, which was reported at the 2019 ASCO annual meeting, although anti-tumor activity was observed in PD-1-naïve triple-negative breast cancer and PD-1-relapsed/refractory melanoma, most patients showed no benefit from the treatment [48]. Notably, these clinical trials utilized synthetic cyclic dinucleotides (CDNs) as STING agonists, which were administered through intratumoral injection. Consequently, the therapeutic potential of these STING agonists is limited to patients with accessible tumors. In order to address this challenge for patients with multiple heterogenous and distal tumors, Ramanjulu et al. introduced a systemically administered STING agonist called diABZI, which showed a significantly lower half-maximal effective concentration compared to traditional CDNs in CT-26 colorectal tumors [31]. We confirmed the dramatic anti-tumor effect of systemic diABZI treatment in a B16-F10 melanoma mouse model. The survival of tumor-bearing mice was prolonged, and B16-F10 tumors changed from cold to hot, with elevated infiltration of CD8+ T cells and various other immune cells; these results are consistent with those obtained following intratumoral injection of CDNs.
Persistent stimulation signals from tumors disturbed the differentiation of HSPCs and generated immature myeloid cells that have immune-suppressive activity [49]. Therefore, restoring the ability of HSCs to differentiate into mature cells with normal immune activity would be beneficial for patients with cancer. We found that systemic diABZI administration comprehensively induced HSPC mobilization and extramedullary hematopoiesis in melanoma, resulting in the expansion of LSKs, LRPs, and GMPs in both the BM and spleen, indicating that STING agonists send a strong signal to HSPCs and induce myeloid-biased differentiation in cancer. The regulatory function of the STING agonist in HSPC homeostasis was also observed for other pattern recognition receptor agonists: systemic administration of a TLR2 agonist induced HSPC expansion in the BM and spleen of mice [50], and exposure to the systemic TLR7/8 agonist R848 led to HSPC expansion and mobilization in the BM [51]. Furthermore, the systemic administration of the STING agonist expanded GMPs and changed the fate of myeloid cells into mature cells with decreased the production of iNOS, Arg-1, and promoted the proliferation of T cells in melanoma. In addition, Gr-1+ cell depletion confirmed that these myeloid cells were responsible for the anti-tumor efficacy of the STING agonist. These findings demonstrated that the depleted Gr-1+ cells within the di-ABZI treated mice were not immature suppressive myeloid cells but were mature myeloid cells that can exert anti-tumor effect. Weiss et al. reported that systemic injection of the murine STING agonist DMXAA resulted in cooperation of myeloid cell subsets and T cells to exert the most efficient anti-tumor effect in a breast tumor model [52], which was in accordance with our findings. Thus, STING agonist administration activated both innate and adaptive anti-tumor immunity, and STING stimulation of myeloid-biased differentiation may be an effective tool for rapidly replenishing the innate immune system during tumor progression.
Rather than simply replenishing downstream immune cell subsets, the expansion of myeloid cells and differentiation of HSPCs prompted by the STING agonist were unbalanced, similar to the effects of TLR agonists. Differentiation of the B lineage and erythroid lineage in the BM of melanoma-bearing mice administered the STING agonist was blocked, perhaps because they share the same developmental niche with myeloid progenitors, and thus liberate space to allow for myeloid cell maturation. Luca et al. also found that a TLR1/2 agonist decreased B cells in the BM and correspondingly increased macrophages and granulocytes [53]. The STING agonist may repress the production of some cytokines that influence B cell differentiation patterns, such as Cxcl12 [54], which was downregulated in LSKs from diABZI-treated mice according to our transcriptome analysis (data not shown). B cells in tumor perform various functions, such as antigen presenting that contribute to T cell activation, and regulatory effects that exert immunosuppressive functions. The related mechanism of the disturbance in B cell development requires further investigation.
In addition to BM mobilization, splenic extramedullary hematopoiesis is commonly regarded as a compensatory response to fulfill the increased demand for myeloid cells to alleviate pathological conditions [55]. In cancer, the spleen serves as a major site for priming HSPCs that differentiate into potent myeloid suppressor cells, and targeted abrogation of splenic extramedullary hematopoiesis can synergistically enhance the anti-tumor effect of ICIs [56]. We observed erythropoiesis blockade and anemia in diABZI-treated mice. Therefore, HSPC mobilization to the spleen may correspond to loss of the erythropoietic potential in the BM, which contributes to supplementation of red cells. It should be noticed that systemic inflammation can adversely affect the later stages of erythropoiesis [57–59], and chronic inflammation-mediated anemia has been acknowledged as a form of anemia that can develop in patients with various disorders, including cancer [60]. Therefore, clinicians need to be attentive to the possibility of such anemia when administering STING agonists to cancer patients. The systemic administration of STING agonists may exacerbate anemia and affect have an impact on patients’ quality of life.
Immune cells pre-existing within TME also play a crucial role in the anti-tumor response during systemic administration of STING agonists. Multiple studies have demonstrated that STING agonists can reprogram the immune cells present within the TME towards a more immunogenic, anti-tumor phenotype [13–15]. Cancer is a systemic disease; however, current cancer immune therapies mainly focus on the local TME, whereas the fact that anti-tumor immunity is regulated by continuous interactions between various cells in peripheral and diverse tissues, including the BM and spleen, is not widely considered [18]. Here, we determined the global immunological consequences of systemic STING agonists, providing insight into the essential contribution of HSPCs to efficacious anti-tumor immune responses in cancer. Based on these findings, we predicted why intratumoral STING agonists have shown limited efficacy in clinical treatment. Firstly, we observed that intratumoral injection of diABZI did not induce splenic extramedullary hematopoiesis or histopathological changes in the BM. This suggests that intratumoral injection may not effectively target HSPCs or correct abnormal hematopoiesis. Dysregulated HSPCs in patients with cancer induce sustained expansion of immature myeloid cells that inhibit anti-tumor immunity. In addition, many cancers gradually decrease the number and self-renewal ability of LT-HSCs during tumor progression [19, 20]. All failed clinical trials of STING agonists involved patients who were in advanced stages of disease. Long-term tumor-bearing status and pre-treatment conditions may have resulted in HSC exhaustion and BM failure. Under such conditions, STING agonists cannot effectively mobilize HSCs and cannot reprogram the fate of myeloid cells. Therefore, the curative effect may be improved if STING agonists are intravenously administered to patients with cancer at an early stage.
In conclusion, we demonstrated that STING agonist could mobilize HSCs and favor HSPC differentiation into immunogenic myeloid cells in melanoma. Consequently, immature myeloid cells-mediated T cell suppression was abolished, and the anti-tumor activity of CD8+ T cells was further enhanced (Fig. 7). Our findings reveal the role of STING agonists in regulating HSC differentiation and reprogramming immature myeloid cells. Systemic administration of STING agonists is a promising treatment for melanoma.
Fig. 7.

Graphical Summary. Systemic STING agonist diABZI mobilize murine HSCs and remodel lineage fate commitment, favoring HSPC differentiation into immunogenic myeloid cells. This myeloid-biased differentiation promoted the production of potential anti-tumor myeloid cells, thereby enhancing systemic anti-tumor immunity
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Hui Xu and the Laboratory Animal Facility of Peking University Cancer Hospital and Institute for assistance with constructing the mouse model. We are grateful to Peking University Medical and Health Analysis Center for assistance with cell sorting. We also thank Tao Xu and Beijing Biokorad Biotechnology for assistance with FACS analysis, and Annoroad Gene Technology for assistance with transcriptome sequencing and analysis.
Author contributions
Conceptualization: TX, JD, LS, JG; data curation: TX, JD, LS, JG; formal analysis: TX, JD, LT, LS, LS, JG; methodology: TX, JD, LT, LS; investigation: TX, JD, LT, LS; supervision: JD, LS, JG; validation: TX, JD, LT, LS; original draft preparation: TX, JD; revision and editing: LT, LS, LS, JG.
Funding
This work was funded by the National Natural Science Foundation of China to Jie Dai (82073011) and to Lu Si (82272676) and the Beijing Municipal Administration of Hospitals’ Ascent Plan to Jie Dai (QML20231107) and to Lu Si (DFL20220901).
Data availability
The Smart-seq2 transcriptome data were deposited in the Gene Expression Omnibus database (accession number GSE192927). All data generated or analyzed in this study were included in this article or in the supplementary information files.
Declarations
Conflict of interest
The authors state no conflict of interest.
Ethical approval
The studies involving mice were reviewed and approved by the Ethics Committee of Peking University Cancer Hospital and Institute.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianxiao Xu and Jie Dai contributed equally to this work.
Contributor Information
Lu Si, Email: silu15_silu@126.com.
Jun Guo, Email: guoj307@126.com.
References
- 1.Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27:11–37. doi: 10.1038/cr.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berraondo P, Minute L, Ajona D, Corrales L, Melero I, Pio R. Innate immune mediators in cancer: between defense and resistance. Immunol Rev. 2016;274:290–306. doi: 10.1111/imr.12464. [DOI] [PubMed] [Google Scholar]
- 3.Sayour EJ, Mitchell DA. Manipulation of innate and adaptive immunity through cancer vaccines. J Immunol Res. 2017;2017:3145742. doi: 10.1155/2017/3145742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Medina T, Kummar S, et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a phase Ib. Multicenter Study Cancer Discov. 2018;8:1250–1257. doi: 10.1158/2159-8290.CD-18-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Such L, Zhao F, Liu D, et al. Targeting the innate immunoreceptor RIG-I overcomes melanoma-intrinsic resistance to T cell immunotherapy. J Clin Invest. 2020;130:4266–4281. doi: 10.1172/JCI131572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–U74. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12:35. doi: 10.1186/s13045-019-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 10.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–U111. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahal LN, Dou L, Hussain K, et al. STING activation reverses lymphoma-mediated resistance to antibody immunotherapy. Can Res. 2017;77:3619–31. doi: 10.1158/0008-5472.CAN-16-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao L, Qi J, Zhao Q, et al. Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics. 2020;10:498–515. doi: 10.7150/thno.37745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, Allen C. Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade. Cancer Immunol Res. 2016;4:1061–71. doi: 10.1158/2326-6066.Cir-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivick KE, Desbien AL, Glickman LH, et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep. 2018;25:3074–85 e5. doi: 10.1016/j.celrep.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, Johnson BD, Dwinell MB. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7:115. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shae D, Becker KW, Christov P, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–78. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolai CJ, Wolf N, Chang IC, Kirn G, Marcus A, Ndubaku CO, McWhirter SM, Raulet DH. NK cells mediate clearance of CD8(+) T cell-resistant tumors in response to STING agonists. Sci Immunol. 2020 doi: 10.1126/sciimmunol.aaz2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamran N, Li Y, Sierra M, Alghamri MS, Kadiyala P, Appelman HD, Edwards M, Lowenstein PR, Castro MG. Melanoma induced immunosuppression is mediated by hematopoietic dysregulation. Oncoimmunology. 2018;7:e1408750. doi: 10.1080/2162402X.2017.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sio A, Chehal MK, Tsai K, et al. Dysregulated hematopoiesis caused by mammary cancer is associated with epigenetic changes and hox gene expression in hematopoietic cells. Can Res. 2013;73:5892–904. doi: 10.1158/0008-5472.CAN-13-0842. [DOI] [PubMed] [Google Scholar]
- 21.Ubellacker JM, Haider MT, DeCristo MJ, Allocca G, Brown NJ, Silver DP, Holen I, McAllister SS. Zoledronic acid alters hematopoiesis and generates breast tumor-suppressive bone marrow cells. Breast Cancer Res. 2017;19:23. doi: 10.1186/s13058-017-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giles AJ, Chien CD, Reid CM, Fry TJ, Park DM, Kaplan RN, Gilbert MR. The functional interplay between systemic cancer and the hematopoietic stem cell niche. Pharmacol Ther. 2016;168:53–60. doi: 10.1016/j.pharmthera.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, Wang Z, Zheng L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci USA. 2014;111:4221–6. doi: 10.1073/pnas.1320753111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- 26.Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020 doi: 10.1126/sciimmunol.aay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudd CE. A new perspective in cancer immunotherapy: PD-1 on myeloid cells takes center stage in orchestrating immune checkpoint blockade. Sci Immunol. 2020 doi: 10.1126/sciimmunol.aaz8128. [DOI] [PubMed] [Google Scholar]
- 28.Allen BM, Hiam KJ, Burnett CE, et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med. 2020;26:1125–34. doi: 10.1038/s41591-020-0892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao W, Du C, Wang J. The cGAS-STING pathway in hematopoiesis and its physiopathological significance. Front Immunol. 2020;11:573915. doi: 10.3389/fimmu.2020.573915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi H, Kobayashi CI, Nakamura-Ishizu A, et al. Bacterial c-di-GMP affects hematopoietic stem/progenitors and their niches through STING. Cell Rep. 2015;11:71–84. doi: 10.1016/j.celrep.2015.02.066. [DOI] [PubMed] [Google Scholar]
- 31.Ramanjulu JM, Pesiridis GS, Yang J, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–43. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 32.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22:2908–18. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 35.Ruotsalainen J, Lopez-Ramos D, Rogava M, Shridhar N, Glodde N, Gaffal E, Holzel M, Bald T, Tuting T. The myeloid cell type I IFN system promotes antitumor immunity over pro-tumoral inflammation in cancer T-cell therapy. Clin Transl Immunol. 2021;10:e1276. doi: 10.1002/cti2.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 37.Gawish R, Bulat T, Biaggio M, et al. Myeloid cells restrict MCMV and drive stress-induced extramedullary hematopoiesis through STAT1. Cell Rep. 2019;26:2394–406 e5. doi: 10.1016/j.celrep.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matatall KA, Jeong M, Chen S, Sun D, Chen F, Mo Q, Kimmel M, King KY. Chronic infection depletes hematopoietic stem cells through stress-induced terminal differentiation. Cell Rep. 2016;17:2584–95. doi: 10.1016/j.celrep.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen MT, Dong L, Zhang XH, et al. ZFP36L1 promotes monocyte/macrophage differentiation by repressing CDK6. Sci Rep. 2015;5:16229. doi: 10.1038/srep16229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Can Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–81. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruger AM, Dorhoi A, Esendagli G, et al. How to measure the immunosuppressive activity of MDSC: assays, problems and potential solutions. Cancer Immunol Immunother. 2019;68:631–44. doi: 10.1007/s00262-018-2170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng J, Mo J, Zhu T, et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19:133. doi: 10.1186/s12943-020-01250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrington KJ BJ, Ingham M, Strauss J, Cemerski S, Wang M, Tse A, Khilnani A, Marabelle A, Golan T. (2018) Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. ESMO. https://oncologypro.esmo.org/meeting-resources/esmo-2018-congress/preliminary-results-of-the-first-in-human-fih-study-of-mk-1454-an-agonist-of-stimulator-of-interferon-genes-sting-as-monotherapy-or-in-combin
- 47.Meric-Bernstam F, Sweis RF, Hodi FS, et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an Intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28:677–88. doi: 10.1158/1078-0432.CCR-21-1963. [DOI] [PubMed] [Google Scholar]
- 48.Funda Meric-Bernstam SKS, Hamid O, Spreafico A, Kasper S, Dummer R, Shimizu T, Steeghs N, Lewis N, Talluto CC, Dolan S, Bean A, Brown R, Trujillo D, Nair N, Luke NJ (2019) Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J Clin Oncol. https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.2507
- 49.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herman AC, Monlish DA, Romine MP, Bhatt ST, Zippel S, Schuettpelz LG. Systemic TLR2 agonist exposure regulates hematopoietic stem cells via cell-autonomous and cell-non-autonomous mechanisms. Blood Cancer J. 2016;6:e437. doi: 10.1038/bcj.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Yao JC, Li JT, Schmidt AP, Link DC. TLR7/8 agonist treatment induces an increase in bone marrow resident dendritic cells and hematopoietic progenitor expansion and mobilization. Exp Hematol. 2021;96:35–43 e7. doi: 10.1016/j.exphem.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss JM, Guerin MV, Regnier F, et al. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. Oncoimmunology. 2017;6:e1346765. doi: 10.1080/2162402X.2017.1346765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Luca K, Frances-Duvert V, Asensio MJ, et al. The TLR1/2 agonist PAM(3)CSK(4) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia. 2009;23:2063–74. doi: 10.1038/leu.2009.155. [DOI] [PubMed] [Google Scholar]
- 54.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Chen D, Long H, Zhu B. The mechanisms of pathological extramedullary hematopoiesis in diseases. Cell Mol Life Sci. 2020;77:2723–38. doi: 10.1007/s00018-020-03450-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C, Ning H, Liu M, et al. Spleen mediates a distinct hematopoietic progenitor response supporting tumor-promoting myelopoiesis. J Clin Invest. 2018;128:3425–38. doi: 10.1172/JCI97973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–8. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramos P, Casu C, Gardenghi S, et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med. 2013;19:437–45. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider RK, Schenone M, Ferreira MV, et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med. 2016;22:288–97. doi: 10.1038/nm.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ganz T. Anemia of inflammation. N Engl J Med. 2019;381:1148–57. doi: 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Smart-seq2 transcriptome data were deposited in the Gene Expression Omnibus database (accession number GSE192927). All data generated or analyzed in this study were included in this article or in the supplementary information files.