Abstract
Background
This phase II study evaluated camrelizumab in different PD-L1 expression cohorts of patients with previously treated advanced/metastatic non-small cell lung cancer (NSCLC; NCT03085069, registered March 21, 2017).
Methods
Patients who progressed during/after chemotherapy were enrolled and divided into four cohorts based on PD-L1 tumor proportion score (TPS). Patients with EGFR/ALK alterations and PD-L1 TPS ≥ 50% were also eligible. All enrolled patients received camrelizumab at 200 mg IV Q2W. The primary endpoint was objective response rate.
Results
A total of 146 patients were enrolled. As of data cutoff on Aug 20, 2020, the median follow-up was 29.5 months (95% CI 27.4–30.8). Objective response rate was 17.8% (95% CI 12.0–25.0) and improved with the increasing PD-L1 TPS (TPS < 1%, 12.2% [95% CI 5.7–21.8]; ≥ 1–< 25%, 19.4% [95% CI 7.5–37.5]; ≥ 25–< 50%, 36.4% [95% CI 10.9–69.2]; ≥ 50%, 23.3% [95% CI 9.9–42.3]). No response was observed in the five patients harboring EGFR mutations. Median progression-free survival was 3.2 months (95% CI 2.0–3.4), and patients with positive PD-L1 TPS had longer progression-free survival. Median overall survival was 14.8 months (95% CI 10.2–18.7). Treatment-related adverse events (TRAEs) of any grade occurred in 87.7% of patients, and 21.2% had grade ≥ 3 TRAEs.
Conclusion
Camrelizumab showed improved efficacy compared with historical data of the second-line chemotherapy in pre-treated advanced/metastatic NSCLC. Patients with positive PD-L1 expression derived greater benefit from camrelizumab. Camrelizumab has a manageable safety profile.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03091-3.
Keywords: Camrelizumab, PD-L1, Immunotherapy, NSCLC, Phase II study
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) as the predominant subtype [1, 2]. Before the availability of targeted therapy, chemotherapy was the standard treatment strategy for patients with NSCLC, with limited overall survival and poor adverse event profile [3]. Targeted therapy is effective in patients with oncogenic driver alterations, which accounts for only parts of NSCLCs [4–6]. Since blocking the binding of programmed cell death 1 (PD-1) to its ligands, programmed cell death ligand 1/2 (PD-L1/PD-L2) on tumor cells could stimulate T-cell response and hinder tumor cells to escape immune surveillance [7, 8], so for patients without amenable alterations, immunotherapy is becoming a new treatment paradigm with promising survival outcomes. Although immunotherapy is commonly used in the first-line treatment of NSCLCs, it also shows encouraging anti-tumor activity as second-line therapy [9–14]. Therefore, developing reliable and validated biomarkers that stratify patients with different probabilities of response to immunotherapy is of great importance for clinical practice [15–18].
The correlation between PD-L1 expression and immunotherapeutic efficacy in second-line and beyond settings of NSCLC had been interrogated in several studies, but remains controversial. Pembrolizumab is superior to docetaxel in terms of progression-free survival in patients with PD-L1 tumor proportion score (TPS) ≥ 50%, but not in the total population (TPS ≥ 1%), as shown in KEYNOTE-010 study [12]. The magnitude of benefits from nivolumab versus docetaxel improved with the increasing PD-L1 expression in advanced non-squamous NSCLC whose tumors expressed PD-L1 (TPS ≥ 1%, hazard ratio [HR] for overall survival 0.59, HR for progression-free survival 0.70; ≥ 5%, 0.43, 0.54; and ≥ 10%, 0.40, 0.52), as revealed in CheckMate 057 study [10]. But this result was inconsistent with that from CheckMate 017 study which showed that the expression of the PD-L1 was neither prognostic nor predictive of benefit in squamous cell NSCLC and that from CheckMate 078 study which showed that the survival benefit with nivolumab was observed regardless of PD-L1 expression level [9, 11]. Both POPLAR and OAK study showed that the improvement of overall survival by atezolizumab versus docetaxel was associated with increase of PD-L1 expression, but the improvement of survival in PD-L1 low or undetectable subgroup by atezolizumab was only reported in OAK study [13, 14]. Thus, no clear evidence supported the use of PD-L1 expression to guide patient selection for immunotherapy in the second-line treatment.
Currently, there were no clinical trials specifically designed to explore the value of PD-L1 expression in guiding second-line immunotherapy of NSCLC. Therefore, we conducted this phase II trial, which offers a new trial design aiming to test different types of patients or treatments in an innovative and effective way, to assess the efficacy and safety of camrelizumab (a humanized IgG4 anti-PD-1 monoclonal antibody which has shown promising activity in multiple malignances [19–23]) in different PD-L1 expression cohorts of patients with previously treated advanced or metastatic NSCLC.
Materials and methods
Study design and participants
This was an open-label, single-arm, multicenter phase II study conducted at 14 medical centers in China. This study assessed efficacy of camrelizumab in different PD-L1 expression cohorts simultaneously to evaluate the correlation of camrelizumab with PD-L1 expression. We enrolled patients aged 18–80 years; with a histologically and cytologically confirmed NSCLC; had stage IIIb or IV disease according to International Association for the Study of Lung Cancer (IASLC) Staging Manual in Thoracic Oncology (the seventh edition); relapsed or progressed during/after platinum-based chemotherapy; provision of fresh tissue or core needle biopsy; with at least one measurable tumor lesion detected by computed tomography or magnetic resonance imaging according to Response Evaluation for Criteria for Solid Tumors (RECIST) version 1.1; and with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients with EGFR mutations or ALK gene rearrangements were eligible provided they had disease progression with at least one approved tyrosine kinase inhibitor and had PD-L1 expression of 50% or greater in tumor. Exclusion criteria were patients with previous treatment of anti-PD-1/PD-L1/PD-L2/CTLA-4 antibody, antitumor vaccine, immune-stimulatory antitumor agent, systemic corticosteroids or immunosuppression (within 14 days before the initiation of study treatment), those with central type lung cancer, interstitial pneumonitis, active tuberculosis, carcinomatous meningitis or central nervous system metastases.
Study treatments and assessments
Eligible patients were assigned to four cohorts based on PD-L1 TPS: PD-L1 TPS < 1%, TPS ≥ 1% and < 25%, TPS ≥ 25% and < 50%, and TPS ≥ 50%. Patients were intravenously given camrelizumab 200 mg every 2 weeks in each 4-week cycle until disease progression, intolerable toxic effects, physician decision or patient withdrawal. Dose reduction was not permitted. Dose delay which was defined as a delay in delivering treatment by at least 3 days was allowed to manage toxicities. When treatment-related adverse events (TRAEs) recovered to grade ≤ 1 or baseline status, the treatment would be re-started. Response (complete response/partial response) had to be confirmed at least 4 weeks after first noted. Tumor response was assessed at week 8 from the initiation of treatment and every 6 weeks thereafter using radiographic imaging according to RECIST, version 1.1. Adverse events were monitored continuously throughout the study period and assessed every 30 days after treatment discontinuation until 90 days after the last dose of study treatment. Adverse events were graded as per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.03. The extent of PD-L1 expression in tumors was measured at a central laboratory with the PD-L1 immunohistochemistry 22C3 PharmDx assay (Dako, Carpinteria, CA) in accordance with the Manufacturer’s instructions. PD-L1 expression was quantified as TPS, which was defined as the percentage of viable tumor cells showing partial or complete membrane staining (≥ 1 +), relative to all viable tumor cells present in the sample [24]. The expression of PD-L1 was finally confirmed by pathologist group.
Outcomes
The primary endpoint was objective response rate, which was defined as the proportion of patients with a confirmed complete response or partial response as per RECIST, version 1.1. The secondary endpoints were duration of response (the time from first evidence of response to disease progression per RECIST 1.1 or death due to any cause), progression-free survival (the time from treatment initiation until disease progression per RECIST 1.1 or death due to any cause, which occurred first), 12-month overall survival rate (the probability of surviving during the first 12 months from treatment initiation), and safety.
Statistical analysis
On the basis of results of PD-L1 expression level, patients were assigned to one of the four PD-L1 expression study cohorts in a non-randomized manner. For each of the PD-L1 expression cohort, sample size was calculated using the exact method based on the primary endpoint of objective response rate. The desirable objective response rate (p1) and unacceptable objective response rate (p0) are 10% and 1% for TPS < 1% cohort, 20% and 4% for TPS ≥ 1–< 25% cohort, 25% and 5% for TPS ≥ 25–< 50% cohort, and 30% and 8% for TPS ≥ 50% cohort. With an alpha of 0.05 and power of 80%, the trial was conducted aiming to enroll a sample size of 42 in PD-L1 TPS < 1% cohort, 27 in TPS ≥ 1–< 25% cohort, 21 in TPS ≥ 25–< 50% cohort and 21 in TPS ≥ 50% cohort. To assess the preliminary anti-tumor activity of camrelizumab, each of the cohort was conducted using a two-stage study design. In the first stage, at least one response in the first 15 patients of each cohort was required for further cohort expansion; otherwise, the cohort would be closed for futility.
Patients administrated with one dose of study medication constituted the intent-to-treat population. We assessed both efficacy and safety in intent-to-treat population. Median progression-free survival, overall survival, and duration of response were estimated using the Kaplan–Meier method, with their 95% confidence intervals (CIs) calculated using Brookmeyer–Crowley method. The 12-month survival rate was also calculated using the Kaplan–Meier method, with its corresponding 95% CI calculated using the log–log transformation according to normal distribution approximation with back transformation to CI on the untransformed scale. The estimate of objective response rate was presented with 95% CI using the Clopper–Pearson method. All statistical analyses were conducted using SAS, version 9.4.
Results
Patients
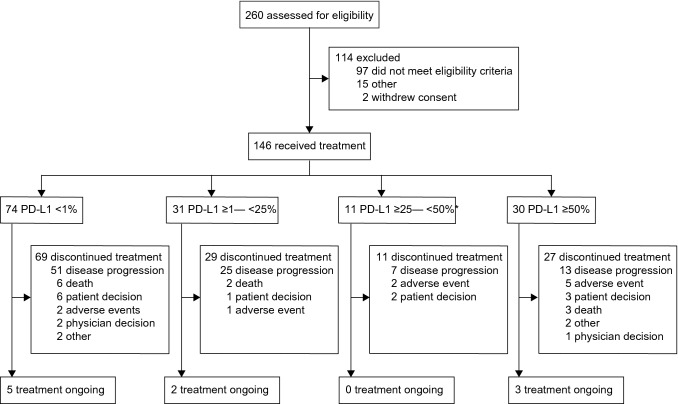
Between May 24, 2017, and Aug 1, 2018, 260 participants were assessed for eligibility. A total of 146 patients were enrolled and received study treatment. Of them, 74 (50.7%) had a PD-L1 TPS of < 1%, 31 (21.2%) of ≥ 1–< 25%, 11 (7.5%) of ≥ 25–< 50%, and 30 (20.5%) of ≥ 50% (Fig. 1). In patients with TPS ≥ 50%, five participants harbored EGFR mutations and 25 did not.
Fig. 1.
Study profile. *Among the screened patients, proportion of patients with PD-L1 ≥ 25–< 50% was relatively lower. Number of patients in this cohort did not reach the planned sample size, when all other cohorts had already reached. But the pre-set objective response had been achieved with 11 patients. After comprehensive consideration, enrollment of this cohort was stopped
Patient demographics and disease characteristics were well-balanced across different PD-L1 TPS populations at baseline (Table 1). Among them, the median age was 62 years (range 35–76); 78.8% (115/146) were males; 54.8% (80/146) had non-squamous cell carcinomas; and 45.2% (66/146) had squamous cell carcinomas; and 89.7% (131/146) had stage IV NSCLC.
Table 1.
Baseline demographics and disease characteristics
| Cohorts by PD-L1 expression | All patients (n = 146) | ||||||
|---|---|---|---|---|---|---|---|
| < 1% (n = 74) | ≥ 1–< 25% (n = 31) | ≥ 25–< 50% (n = 11) | ≥ 50% | ||||
| Total (n = 30) | EGFR − (n = 25) | EGFR + (n = 5) | |||||
| Age, median years (range) | 62 (35–74) | 61 (38–74) | 64 (46–76) | 59 (40–74) | 59 (40–74) | 57 (41–66) | 62 (35–76) |
| Male | 58 (78.4) | 24 (77.4) | 10 (90.9) | 23 (76.7) | 20 (80.0) | 3 (60.0) | 115 (78.8) |
| ECOG performance status | |||||||
| 0 | 5 (6.8) | 1 (3.2) | 0 | 2 (6.7) | 2 (8.0) | 0 | 8 (5.5) |
| 1 | 69 (93.2) | 30 (96.8) | 11 (100) | 28 (93.3) | 23 (92.0) | 5 (100) | 138 (94.5) |
| Tumor histology | |||||||
| Adenocarcinoma | 43 (58.1) | 18 (58.1) | 3 (27.3) | 14 (46.7) | 9 (36.0) | 5 (100) | 78 (53.4) |
| Squamous cell carcinoma | 30 (40.5) | 13 (41.9) | 7 (63.6) | 16 (53.3) | 16 (64.0) | 0 | 66 (45.2) |
| Others | 1 (1.4) | 0 | 1 (9.1) | 0 | 0 | 0 | 2 (1.4) |
| Disease stage | |||||||
| IIIa | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 1 (0.7) |
| IIIb | 8 (10.8) | 4 (12.9) | 1 (9.1) | 1 (3.3) | 0 | 1 (20.0) | 14 (9.6) |
| IV | 65 (87.8) | 27 (87.1) | 10 (90.9) | 29 (96.7) | 25 (100) | 4 (80.0) | 131 (89.7) |
| Metastases | 74 (100) | 31 (100) | 11 (100) | 30 (100) | 25 (100) | 5 (100) | 146 (100) |
| Number of metastatic organs | |||||||
| ≤ 2 | 53 (71.6) | 22 (71.0) | 8 (72.7) | 23 (76.7) | 20 (80.0) | 3 (60.0) | 106 (72.6) |
| > 2 | 21 (28.4) | 9 (29.0) | 3 (27.3) | 7 (23.3) | 5 (20.0) | 2 (40.0) | 40 (27.4) |
| Previous therapies | |||||||
| Surgery | 28 (37.8) | 15 (48.4) | 4 (36.4) | 10 (33.3) | 8 (32.0) | 2 (40.0) | 57 (39.0) |
| Chemotherapy | 71 (95.9) | 30 (96.8) | 11 (100) | 30 (100) | 25 (100) | 5 (100) | 142 (97.3) |
| Radiotherapy | 26 (35.1) | 12 (38.7) | 4 (36.4) | 6 (20.0) | 5 (20.0) | 1 (20.0) | 48 (32.9) |
| Adjuvant therapy | 9 (12.2) | 5 (16.1) | 0 | 2 (6.7) | 2 (8.0) | 0 | 16 (11.0) |
| Neoadjuvant therapy | 2 (2.7) | 0 | 0 | 0 | 0 | 0 | 2 (1.4) |
Data are presented in n (%), unless otherwise specified
Efficacy
At the time of data cutoff on Aug 20, 2020, the median follow-up was 29.5 months (95% CI 27.4–30.8). A total of 146 patients were included in the full analysis set. Five patients with TPS of < 1%, two with TPS of ≥ 1–< 25%, none with TPS of ≥ 25–< 50% and three with TPS of ≥ 50% were still continuing camrelizumab treatment. Subsequent anti-cancer therapy was received by 72 (49.3%) patients, with docetaxel-containing therapy (22, 15.1%) being the most common one.
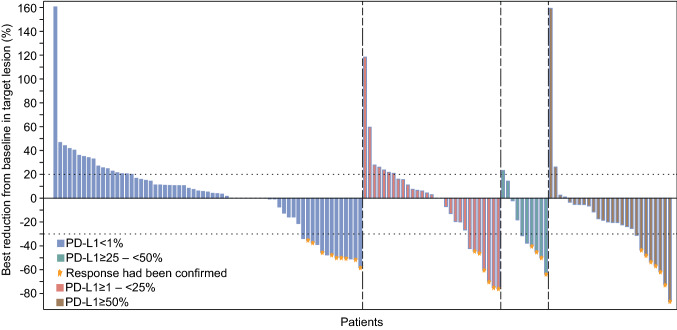
Best change in sum of target lesion dimensions from baseline is shown in Fig. 2. No patient achieved complete response, 26 (17.8%) patients had partial response at their best response, 51 (34.9%) had stable disease, 54 (37.0%) had progressive disease, and overall response of 15 (10.3%) patients was not evaluable (Table 2). The objective response rate was 17.8% (95% CI 12.0–25.0), and disease control rate was 52.7% (95% CI 44.3–61.1). When grouped according to PD-L1 expression, the objective response rate was 12.2% (95% CI 5.7–21.8) in PD-L1 TPS < 1% population, 19.4% (95% CI 7.5–37.5) in TPS ≥ 1–< 25% population, 36.4% (95% CI 10.9–69.2) in TPS ≥ 25–< 50% population, and 23.3% (95% CI 9.9–42.3) in TPS ≥ 50% population. Objective response rate was improved with the increase of PD-L1 expression, and the greatest benefit was seen in patients with a PD-L1 TPS of ≥ 25–< 50%. In patients with TPS ≥ 50%, objective response was observed in 28.0% (95% CI 12.1–49.4) of patients without EGFR mutation, while no response occurred in patients with EGFR mutations. Subgroup analysis showed that objective response rate was promising in nearly all subgroups irrespective of baseline characteristics (Table S1).
Fig. 2.
Tumor response
Table 2.
Summary of key efficacy results
| Cohorts by PD-L1 expression | All patients (n = 146) | ||||||
|---|---|---|---|---|---|---|---|
| < 1% (n = 74) | ≥ 1–< 25% (n = 31) | ≥ 25–< 50% (n = 11) | ≥ 50% | ||||
| Total (n = 30) | EGFR − (n = 25) | EGFR + (n = 5) | |||||
| Best overall response, n (%) | |||||||
| PR | 9 (12.2) | 6 (19.4) | 4 (36.4) | 7 (23.3) | 7 (28.0) | 0 | 26 (17.8) |
| SD | 24 (32.4) | 10 (32.3) | 5 (45.5) | 12 (40.0) | 10 (40.0) | 2 (40.0) | 51 (34.9) |
| PD | 33 (44.6) | 13 (41.9) | 1 (9.1) | 7 (23.3) | 6 (24.0) | 1 (20.0) | 54 (37.0) |
| NE | 8 (10.8) | 2 (6.5) | 1 (9.1) | 4 (13.3) | 2 (8.0) | 2 (40.0) | 15 (10.3) |
| Objective response rate, % (95% CI) | 12.2 (5.7–21.8) | 19.4 (7.5–37.5) | 36.4 (10.9–69.2) | 23.3 (9.9–42.3) | 28.0 (12.1–49.4) | 0 | 17.8 (12.0–25.0) |
| Disease control rate, % (95% CI) | 44.6 (33.0–56.6) | 51.6 (33.1–69.8) | 81.8 (48.2–97.7) | 63.3 (43.9–80.1) | 68.0 (46.5–85.1) | 40.0 (5.3–85.3) | 52.7 (44.3–61.1) |
| Duration of response, months (95% CI) | 8.3 (3.8–22.3) | 27.1 (5.6–NE) | NR (2.8–NE) | 15.1 (2.8–NE) | 15.1 (2.8–NE) | NR | 13.6 (5.6–22.6) |
PR partial response, SD stable disease, PD progressive disease, NE not estimable, NR not reached
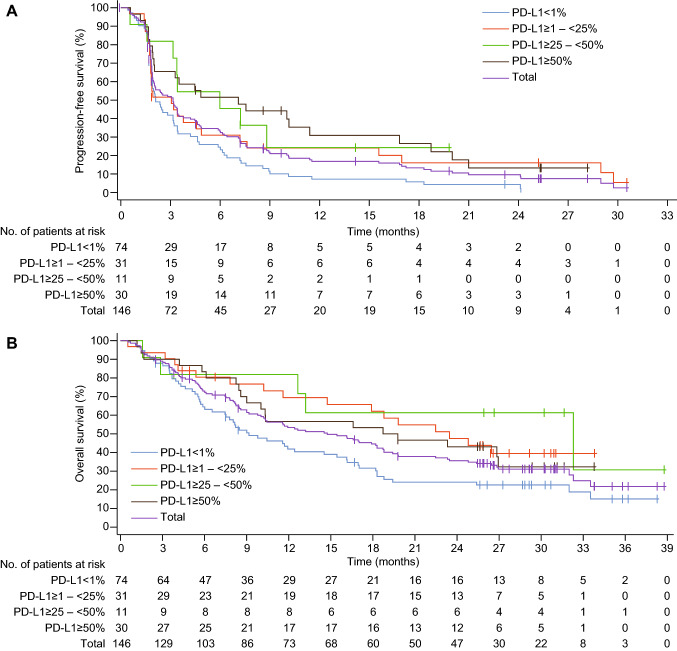
The responders had durable response (median 13.6 months, 95% CI 5.6–22.6; Table 2). A total of 127 (87.0%) patients had disease progression or died by the time of data cutoff. Median progression-free survival was 3.2 months (95% CI 2.0–3.4) (Fig. 3a). In cohorts of patients with different PD-L1 expression, the median progression-free survival was 2.1 months (95% CI 1.9–3.2) in those with TPS < 1%, 3.1 months (95% CI 1.8–4.9) in TPS ≥ 1–< 25%, 6.0 months (95% CI 1.6–not estimable) in TPS ≥ 25–< 50%, and 7.1 months (95% CI 2.0–11.4) in TPS ≥ 50%, indicating that patients with higher PD-L1 expression had numerically longer median progression-free survival. In patients with TPS ≥ 50%, those without EGFR mutation had more favorable median progression-free survival than those with EGFR mutations (7.6 months, 95% CI 2.0–16.8 vs. 1.7 months, 95% CI 1.2–not estimable).
Fig. 3.
Kaplan–Meier estimates of progression-free survival (a) and overall survival (b) in different PD-L1 expression cohorts
A total of 98 (67.1%) deaths occurred in the entire cohort, and the median overall survival was 14.8 months (95% CI 10.2–18.7) (Fig. 3B). In patients with PD-L1 TPS < 1%, ≥ 1–< 25%, ≥ 25–< 50% and ≥ 50%, the median overall survival was 9.2 months (95% CI 6.5–15.4), 23.5 months (95% CI 11.6–not estimable), 32.3 months (95% CI 2.9–not estimable), and 19.3 months (95% CI 9.0–not estimable), respectively. Patients with PD-L1 TPS ≥ 25–< 50% had the longest median overall survival. The estimated 12-month and 18-month survival probability was 53.4% (95% CI 44.8–61.2) and 44.6 (95% CI 36.2–52.6), respectively.
Safety
The median number of cycles of camrelizumab administration was 3.5 (range 0.5–37.0). Treatment-related adverse events of any grade occurred in 87.7% (128/146) of patients (Table 3). The most common treatment-related adverse events were reactive cutaneous capillary endothelial proliferation (RCCEP; 111/146, 76.0%), hypothyroidism (23/146, 15.8%), increased aspartate aminotransferase (22/146, 15.1%), and proteinuria (18/146, 12.3%). Treatment-related adverse events of grade 3 or greater were reported in 21.2% (31/146) patients, with the most common being RCCEP (4/146, 2.7%), dyspnea (4/146, 2.7%), increased gamma-glutamyltransferase (3/146, 2.1%), increased amylase (3/146, 2.1%), and increased lipase (3/146, 2.1%). The percentage of patients with serious treatment-related adverse events was 17.1% (25/146). Treatment-related adverse events led to dose delay or interruption in 15.8% (23/146) of patients and led to treatment discontinuation in 7.5% (11/146) of patients. Five (3.4%) deaths were considered as treatment-related, including two dyspnea, one pneumonitis, one respiratory failure, and one death from unknown cause. For the two patients with pneumonitis and respiratory failure, the possibility of immune-related pneumonitis cannot be excluded. The other two patients with dyspnea could not rule out the possibility of disease progression or pulmonary embolism. The correlation between the death from unknown cause and camrelizumab was unassessable. Therefore, the causes of all five deaths were not definitely related to treatment.
Table 3.
Treatment-related adverse events occurring in > 5% of patients
| All patients (n = 146) | ||
|---|---|---|
| Any grade | Grade 3–5 | |
| All treatment-related adverse events* | 128 (87.7) | 31 (21.2) |
| RCCEP | 111 (76.0) | 4 (2.7) |
| Hypothyroidism | 23 (15.8) | 0 |
| Aspartate aminotransferase increased | 22 (15.1) | 0 |
| Proteinuria | 18 (12.3) | 0 |
| Alanine aminotransferase increased | 17 (11.6) | 0 |
| Decreased appetite | 12 (8.2) | 1 (0.7) |
| Amylase increased | 11 (7.5) | 3 (2.1) |
| Pruritus | 11 (7.5) | 0 |
| Cough | 10 (6.8) | 0 |
| Pyrexia | 10 (6.8) | 0 |
| Asthenia | 10 (6.8) | 0 |
| Anemia | 10 (6.8) | 2 (1.4) |
| Pneumonitis | 9 (6.2) | 3 (2.1) |
| Hyperthyroidism | 9 (6.2) | 0 |
| Lipase increased | 8 (5.5) | 3 (2.1) |
| Hepatic function abnormal | 8 (5.5) | 0 |
Data are presented in n (%). RCCEP, Reactive cutaneous capillary endothelial proliferation. *The treatment-related adverse event refers to the correlation of the event with treatment was “definitely related”, “possibly related”, or “unassessable”
Discussion
To our knowledge, this is the first prospective study using such a trial design, which tests different types of patients or treatments in an innovative and effective way, to confirm the value of PD-L1 expression in the selection of potential patients for immunotherapy in second-line treatment of NSCLC. This is also the first prospective study to enroll EGFR-mutated patients in those with PD-L1 TPS ≥ 50% for immunotherapy and to show that EGFR-positive patients did not respond to camrelizumab. It has been reported that the response rate of first-line chemotherapy for NSCLC was 17–22% and overall survival was 7.4–8.2 months [3], while the response rate of second-line immunotherapy for NSCLC was about 17–30%, and the overall survival was approximately 9.2–13.8 months [10–14]. In our phase II study, the response rate (36.4%) and overall survival (32.3 months) of camrelizumab in higher PD-L1 expression cohorts were far beyond those of immunotherapy without patient selection.
Overall, camrelizumab demonstrated comparable efficacy with that of other immune checkpoint inhibitors in advanced/metastatic NSCLC. The confirmed objective response rate (17.8%, 95% CI 12.0–25.0), progression-free survival (3.2 months, 95% CI 2.0–3.4), and 12-month overall survival rate (53.4%, 95% CI 44.8–61.2) with camrelizumab as second-line therapy were numerically similar as that reported with nivolumab, pembrolizumab, and atezolizumab (objective response rate, 14–18.0%; progression-free survival, 2.8–3.0 months; 12-month overall survival rate, 50–55%) [11, 13, 25], indicating that camrelizumab may be an efficacious treatment option for pre-treated advanced/metastatic NSCLC.
As to the association between PD-L1 expression and efficacy of immunotherapy drugs in second-line and beyond setting, conclusions from previous studies have been discrepant [9, 10, 12–14]. In this study, we compared efficacies of camrelizumab across different PD-L1 TPS cohorts. Numerically, patients with positive PD-L1 expression derived greater benefit from camrelizumab compared with historical data of the second-line chemotherapy, while efficacy of camrelizumab in patients with TPS < 1% was similar to that of second-line chemotherapy [11–13]. The observation that benefit from camrelizumab improved with the increase of PD-L1 expression was also echoed with other anti-PD-1/PD-L1 drugs in patients with advanced NSCLC [10, 12–14, 25–27]. In this study, we found patients with PD-L1 TPS ≥ 25–< 50% achieved higher objective response rate, median overall survival, and 12-month overall survival rate than other PD-L1 expression groups, and the correlation of progression-free survival and PD-L1 TPS was not fully synchronized with the correlation of overall survival and PD-L1 TPS in this population, since patients in PD-L1 TPS ≥ 50% group had the longest progression-free survival than other groups. Two possible reasons might lead to such phenomenon. Firstly, TPS ≥ 50% group included five patients with EGFR-positive. They did not response to camrelizumab and progressed rapidly, resulting in a reduction in the efficacy of camrelizumab in TPS ≥ 50% group. Secondly, the number of patients varies among different PD-L1 TPS groups and TPS ≥ 25–< 50% group had only 11 patients. Therefore, a larger randomized, controlled, confirmative study is further needed to validate this result.
There is no evidence in this study suggesting EGFR-positive patients could obtain overall response to camrelizumab treatment. But among these patients with EGFR mutations, one with EGFR exon 20 insertion had a best overall response of stable disease, a progression-free survival of 8.7 months, and an overall survival of 24.8 months, while another stable disease patient with EGFR L858R showed a progression-free survival of 4.5 months and an overall survival of 26.4 months. This indicated that patients harboring some specific mutation types might achieve benefit to camrelizumab, and further studies are needed to verify this hypothesis.
Camrelizumab demonstrated a manageable safety profile, which was similar to other PD-1/PD-L1 inhibitors [11, 26], except for RCCEP. The occurrence frequency of RCCEP was consistent with that of other studies of camrelizumab monotherapy [23, 28, 29]. RCCEP is characterized by a tripartite growth cycle of proliferation, plateau and involution, and is not life-threatening or function-impairing. RCCEP is expected to disappear after termination of treatment [30, 31]. It was reported that patients developed RCCEP seemed to have higher response rate than those without RCCEP, but the conclusion needs further validation [30].
There are several limitations of this study. One limitation of this study is the lack of control group, which makes it difficult to assess the correlation of PD-L1 expression and clinical benefits from camrelizumab versus chemotherapy. In addition, bias might be introduced owing to the unbalanced number of patients in each cohort.
Conclusions
In conclusion, camrelizumab improved efficacy compared with historical data of the second-line chemotherapy in previously treated advanced/metastatic NSCLC with positive PD-L1 expression. Patients with EGFR mutations may not benefit from camrelizumab even with PD-L1 > 50%. Camrelizumab has a manageable safety profile. This phase II data suggested that camrelizumab monotherapy might be a feasible option as second-line treatment for patients with previously treated advanced/metastatic NSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families and acknowledge the contributions of all investigators in this trial. We would also like to acknowledge Tengfei Zhang (PhD, Medical Writer, Jiangsu Hengrui Pharmaceuticals Co., Ltd.) for medical writing support according to Good Publication Practice Guidelines.
Abbreviations
- CI
Confidence interval
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EMA
European Medicines Agency
- HR
Hazard ratio
- NCI-CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- NSCLC
Non-small cell lung cancer
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand 1
- RCCEP
Reactive Cutaneous Capillary Endothelial Proliferation
- RECIST
Response Evaluation Criteria In Solid Tumors
- TPS
Tumor proportion score
Authors' contribution
JZ and YW helped in conception and design of the study; JY, CH, YF, HP, JF, LJ, XL, XL, JX, YZ, YC, RM, JW, YW, YL, DL, WS collected and assembled the clinical data; TW analyzed the data; WS, JZ, YW interpreted the results; all authors wrote and revised the manuscript. All authors approved the final manuscript.
Funding
This study was funded by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Yi-Long Wu reports advisory board participance for AstraZeneca, Roche, BMS; honorarium from AstraZeneca, Roche, Eli Lilly, BMS, Pfizer and Boehringer Ingelheim; contracted/support research grant from Roche and Boehringer Ingelheim; consultant for AstraZeneca, Roche and BMS. Tao Wang, Wei Shi and Jianjun Zou are employees of Jiangsu Hengrui Pharmaceuticals Co., Ltd. All remaining authors have declared no conflict of interest.
Ethical approval
The study protocol was approved by institutional review boards or ethic committees at each center, and the study was conducted in accordance with the protocol, Good Clinical Practice guidelines and Declaration of Helsinki.
Informed consent
Patients were required to provide written informed consent before the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch FR, Suda K, Wiens J, Bunn PA., Jr New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388(10048):1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 6.Reckamp KL. Targeted therapy for patients with metastatic non-small cell lung cancer. J Natl Compr Canc Netw. 2018;16(5S):601–604. doi: 10.6004/jnccn.2018.0046. [DOI] [PubMed] [Google Scholar]
- 7.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: checkmate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 13.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 15.Gettinger S, Herbst RS. B7–H1/PD-1 blockade therapy in non-small cell lung cancer: current status and future direction. Cancer J. 2014;20(4):281–289. doi: 10.1097/PPO.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 16.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 20.Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 21.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2020;9(3):305–314. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Wu J, Chen X, Lin T, Cao J, Liu Y, et al. A Single-arm, multicenter, phase ii study of camrelizumab in relapsed or refractory classical hodgkin lymphoma. Clin Cancer Res. 2019;25(24):7363–7369. doi: 10.1158/1078-0432.CCR-19-1680. [DOI] [PubMed] [Google Scholar]
- 24.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 25.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 26.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 27.Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer. 2020;148(9):2313–2320. doi: 10.1002/ijc.33399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24(6):1296–1304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 29.Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119(5):538–545. doi: 10.1038/s41416-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Ma L, Wang X, Mo H, Wu D, Lan B, et al. Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol Med. 2019;16(1):173–181. doi: 10.20892/j.issn.2095-3941.2018.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng Y, Guo R, Sun J, Jiang Y, Liu Y. Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol. 2019;58(3):388–389. doi: 10.1080/0284186X.2019.1567935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.