Abstract
Plastic polymers are non-degradable solid wastes that have become a great threat to the whole world and degradation of these plastics would take a few decades. Compared with other degradation processes, the biodegradation process is the most effective and best way for plastic degradation due to its non-polluting mechanism, eco-friendly nature, and cost-effectiveness. Biodegradation of synthetic plastics is a very slow process that also involves environmental factors and the action of wild microbial species. In this plastic biodegradation, fungi play a pivotal role, it acts on plastics by secreting some degrading enzymes, i.e., cutinase`, lipase, and proteases, lignocellulolytic enzymes, and also the presence of some pro-oxidant ions can cause effective degradation. The oxidation or hydrolysis by the enzyme creates functional groups that improve the hydrophilicity of polymers, and consequently degrade the high molecular weight polymer into low molecular weight. This leads to the degradation of plastics within a few days. Some well-known species which show effective degradation on plastics are Aspergillus nidulans, Aspergillus flavus, Aspergillus glaucus, Aspergillus oryzae, Aspergillus nomius, Penicillium griseofulvum, Bjerkandera adusta, Phanerochaete chrysosporium, Cladosporium cladosporioides, etc., and some other saprotrophic fungi, such as Pleurotus abalones, Pleurotus ostreatus, Agaricus bisporus and Pleurotus eryngii which also helps in degradation of plastics by growing on them. Some studies say that the degradation of plastics was more effective when photodegradation and thermo-oxidative mechanisms involved with the biodegradation simultaneously can make the degradation faster and easier. This present review gives current knowledge regarding different species of fungi that are involved in the degradation of plastics by their different enzymatic mechanisms to degrade different forms of plastic polymers.
Keywords: Biodegradation, Plastic polymers, Plastic degradation, Fungi, Degrading enzymes
Introduction
Plastic is considered as one of the threatful elements in the environment because of its slow degradation in the environment which seriously takes some decades, so it’s considered a non-degrading material. These non-degradable plastics accumulated considered as solid waste on the earth's surface which is assumed as food by terrestrial animals, such as cows, buffaloes, and consuming it which causes the death of animals (Singh 2005). These plastics which form particulate matter by UV irradiation and weathering increase surface area and mobility and, therefore, easily incorporate into the food chain causing serious effects to all the living organisms (Bonhomme et al. 2003; Sen and Raut 2015). Disposing of the plastic waste in oceans leads accumulation of toxic chemicals, such as polychlorinated biphenyl (PCB’s), nonylphenol (NP), dichlorodiphenyltrichloroethane (DDT), polycyclic aromatic hydrocarbons (PAH), polybrominated diphenyl esters (PBDE), and bisphenol A (BPA) (Bryant et al. 2016), which have been found as a serious problem of indigestion, gastrointestinal blockages and reproductive problems in marine organisms. Due to this plastic pollution in the marine environment minimum of 267 species are being affected which includes sea turtles (86%) and seabirds (44%) (Coe et al. 1997). The worldwide annual production of non-degradable plastic ranges from 350 to 400 million tons out of that yearly 5–13 million tons of waste plastic are released into oceans which damages the ecological environment. They are various forms of plastics, i.e., nylon, polycarbonate, polyethylene terephthalate, polyethylene, polypropylene, polystyrene, polytetrafluoroethylene, polyurethane, and polyvinyl chloride (Usha et al. 2011). To degrade these plastics, there are different methods, such as photodegradation, thermo-oxidative degradation, hydrolytic degradation, and biodegradation. Photodegradation and thermo-oxidation are categorized under abiotic degradation, whereas biotic degradation involves the action of microbes. Photodegradation involves continuous exposure of UV light from the sun or artificial source on plastic material which eventually incorporates oxygen molecules in between the structure which leads to breaking the complex polymers into simple molecules, while thermo-oxidative involves exposure of heat on the plastic polymers (Geweret et al. 2015). However, exposure to high temperatures leads burning of plastic produces toxic gases into the environment and poses health hazards by causing lung diseases and cancer after inhalation (Pramila and Vijaya Ramesh 2011). Compared with other degradations, the biodegradation method is mostly preferred due to its pollution-free mechanism and eco-friendly process. In biodegradation, the process is initiated by micro-organisms, i.e., bacteria and fungi. In general, this biodegradation of plastics involves the growth of fungi on the surface of plastic, where plastic is consumed as a food source by the fungi under the influence of environmental factors, such as temperature and pH. These fungi will secrete enzymes, such as cutinase, lipase, and proteases, carboxylesterases, esterases, lignocellulolytic enzymes, and some pro-oxidant ions which will degrade the plastics. By oxidation/hydrolysis enzyme improves the hydrophilicity of polymers and consequently degrade the high molecular weight polymer into low molecular weight. As high molecular weight is a large compound that cannot be transported across the cellular membrane of the fungi thus it primarily depolymerizes it into small monomers before they cross the cell membrane (Shah et al. 2008). The enzyme activity is mainly dependent on the solvent properties and the enzyme activity increases with polarity and decreases with the viscosity of the solvent in the biodegradation of polymers (Patel et al. 2013). The process becomes more effective when photodegradation and thermo-oxidative degradation is followed by biodegradation, because as by photodegradation and thermo-oxidative degradation, the plastic debris will be broken-down from complex to simple material so biodegradation on such material will be easy and does not require much time. Even practicing different methods, plastic degradation takes sufficient time to complete the process. The best solution for decreasing this plastic pollution is using biodegradable plastics. These biodegradable polymers are designed to degrade quickly by the microbes due to their ability to degrade organic and inorganic materials, such as lignin, starch, cellulose, and hemicelluloses (Kumar et al. 2013). In this review, we described various fungi involved in the biodegradation of different types of plastic polymers and summarized recent studies on enzymes that are produced by various fungi for the biodegradation of plastics. In additional, we discussed the effective degradation of fungi on selective plastics and plastic degradation by edible fungi.
Enzymes that involve in plastic degradation
Cutinases
Cutinases are a subclass of esterase enzyme which is identified by their ability to hydrolyze polyesters with high molar mass (Chen et al. 2013). They are α/β hydrolases or carboxylic ester hydrolases which were observed in plant pathogenic fungi, i.e., Fusarium solani pisi (Kolattukudy and Brown 1975; Kolattukudy et al. 1981; Heredia 2003). Cutinases are produced by Fusarium solani (Alisch-Mark et al. 2006; O’Neill et al. 2007), Penicillium citrinum (Liebminger et al. 2007), Pichia pastoris (Munari 2019) Aspergillus oryzae, Humicola insolens. The activities of Fusarium solani pisi cutinase (FsC) and Humicola insolens cutinase (HiC) were shown to be capable of degrading low crystallinity PET film with 97% weight loss being observed within 96 h (Ronqvist et al. 2009). PET-hydrolases belong to the cutinases group which also has promising results in the biodegradation of PET. An enzyme that is similar to cutinase in function, i.e., isolated from Cryptococcus sp. Strain S-2 was identified for its effective degradation of high molecular weight plastic, i.e., polylactic acid (PLA) based plastic (Gemeren et al. 1998). Cutinase 2p from Arxula adeninivorans showed enzymatic decomposition (hydrolysis/oxidation) of electrospun polycaprolactone fiber mats (Furukawa et al. 2019).
Lipases
Lipases are enzymes that catalyze the hydrolysis of lipids they are also the subclass of esterases enzyme. Some fungal species that are well known to produce lipases and are involved in the degradation of plastics, i.e., Rhizopus delemer, Candida antarctica (Vertommen et al. 2005), Thermomyces lanuginosus (Eberl et al. 2009), Candida rugosa were degrading poly (butylene succinate-co-hexamethylene succinate) copolymer. (Pereira et al. 2001). Rhizopus delemer lipase degraded 53% of the polyester type-polyurethanes (ES-PU) film after 24 h reaction (Tokiwa and Calabia 2009). A lipase enzyme extracted from the yeast Cryptococcus sp, exhibited hydrolysis of polybutylene succinate (PBS) and polybutylene succinate-co-adipate (PBSA) (Thirunavukarasua et al. 2008). Lipase B from Candida antarctica was effectively hydrolyzing PET to TPA (Carniel et al. 2017), lipase FE-01 from Thermomyces languinosus showed enzymatic decomposition of electrospun polycaprolactone fiber (Furukawa et al. 2019).
Proteases
Proteases are enzymes that cleave the long peptide chain to short peptides or break down proteins to polypeptide chains by hydrolysis this process is termed to be proteolysis. Aspergillus, Trichoderma, Paecelomyces, Penicillium, Alternaria, Fusarium (Loredo-Treviño et al. 2011; Cosgrove et al. 2007), Phaenarochete (Shimao 2001), Pestalotiopsis (Russell 2011) Rhizopus, Mucor, Humicola, Thermoascus, Thermomyces (Souza et al. 2015) are some of the important fungal species which are producing proteases to degrade plastics. Anthrobotrys oligospora synthesis serine protease that can degrade polylactic acid (Ozsagiroglu et al. 2012).
Esterases
Esterases are hydrolase enzymes that split esters into alcohols and acids by the addition of water molecules. Esterases are also involved in plastic degradation which is produced by both bacteria and fungi, esterase from Comamonas acidovorans is helpful in the degradation of low molecular weight PLA (plastic obtained from renewable resources). Purpureocillium lilacinum and Curvularia senegalensis are a group of fungi that degrade poly (butylene succinate-co-adipate) and polyurethane (Yamamoto-Tamura et al. 2015). Aspergillus flavus, Aspergillus tubingensis are assumed that they secrete esterases that are responsible for the degradation of plastics (Khan et al. 2017; Tokiwa et al. 2009). Esterase, derived from Xepiculopsis graminea, and Penicillium griseofulvum reported degrading polyurethane (Brunner et al. 2018). Lipase and esterase also degrade polycaprolactone polymers (Ganesh et al. 2017).
Laccase
Laccases are multi-copper oxidases that catalyze the oxidation of phenolic compounds. It utilizes molecular oxygen as a co-substrate and produces water and by-products (Nunes and Kunamneni 2018). The special ability of these laccases is they oxidize lignin so they involve in degrading lignin (Osma et al. 2010). Laccase can also involve in the oxidation of the hydrocarbon backbone of polyethylene (Sivan 2011). Cochliobolus sp., a specific fungus that is degrading PVC by laccases (Sumathi et al. 2016). Bjerkandera adusta TBB-03 was identified for its ability to degrade HDPE under lignocellulose substrate treatment by laccase production (Bo Ram Kang 2019). Trametes versicolor, Pleurotus ostreatus, Streptomyces, P. ostreatus and T. pubescens produce laccase that degrades polyethylene (Osma et al. 2010). In general, the Ligninolytic enzyme families include phenol oxidase (laccase), heme peroxidases, lignin peroxidase (LiP), manganese peroxidase (MnP), and versatile peroxidase (VP) (Dashtban et al. 2010). Papain and urease are the two proteolytic enzymes that were found to degrade medical polyester polyurethane by hydrolysis of urethane and urea linkages by producing free amine and hydroxyl groups (Phua et al. 1987). Penicillium-derived laccase potentially involves in PE breakdown (Abd El-Rehim et al. 2004).
Peroxidases
Peroxidases are enzymes that fall under the oxidoreductase class which catalyzes oxidation–reduction reactions by the action of free radicals on compounds to form oxidized and polymerized compounds. They are also called catalases; peroxidases of fungi are regarded to be more efficient in converting lignin. This class includes lignin peroxidases (LiP), manganese peroxidases (MnP), and versatile peroxidases (VP) which are mostly found in white-rot fungi (Hofrichter and Ullrich 2006). Phanerochaete chrysosporium, and Trametes versicolor showed effective degradation of high-molecular-weight polyethylene, where MnP/Manganese peroxidases is the key enzyme in polyethylene degradation (Iiyoshi et al. 1998). Phanerochaete chrysosporium, Pleurotus ostreatus and S22 showed a high capacity for lignin degradation at pH 9.0–11. Ligninolytic enzymes, includes LiP, MnP, and laccase (Wu et al. 2005). Fusarium graminearum showed polyethylene degradation by producing peroxidase (Ganesh et al. 2017). Aspergillus flavus, Aspergillus niger, and Fusarium graminearum produce manganese peroxidase, lignin peroxidase and these enzymes induce the biodegradation of PCB (polyethylene carry bag). The maximum range of lignin peroxides are produced in Aspergillus niger and Aspergillus flavus (Bholay et al. 2012).
Pro-oxidant ions
Pro-oxidant ions are chemical elements that induce oxidative stress by releasing reactive oxygen species or by inhibiting the antioxidant system. These elements can be as transition metals, such as Fe, Co (Weiland et al. 1995), and Mn (Jakubowicz 2003), and can enhance the photo- and thermo-oxidation, PE chains may lead to radical reactions that lead to cleavage in polymer chains (Koutny et al. 2006). The pro-oxidants are transient metal ions, added in form of stearate or other organic ligand complexes, mostly stearates of Fe3+, Mn2+ (Jakubowicz 2003), or Co2+ (Weiland et al. 1995). Fe3+ complex involves in the photo-oxidation process as a source of radicals for reaction initiation, the Mn2+ or Co2 is necessary for oxidation without the influence of light, when they catalyze the decomposition of peroxides associated with chain cleavage. It was identified that trace amounts of metals such as Co, Mn, Fe, Cu, and Ni showed increasing the rate of oxidation (Gorghiu et al. 2004), these facilitate cleavage of molecules into smaller fragments containing hydrophilic oxygenated groups that can be easily degraded by microbes (Shang et al. 2003).
Enzymatic mechanisms involved in plastic biodegradation by fungi
The mechanism of biodegradation involves the action of microbial enzymes on the surface of the plastics. The microbes such as bacteria and fungi attach to the plastic film and inert the enzymes and grow on it by utilizing it as substrate and source of nutrition. Therefore, the polymers slowly get depolymerized and degradation will be compiled by mineralization process, where H2O (water), CO2 (carbon dioxide), CH2 (methane) are end products (Frazer 1994; Montazer et al. 2019). The ability of fungi was they can invade substrates using enzymes that can detoxify pollutants. Fungi can also produce some surface-active proteins, i.e., hydrophobins to coat hyphae to hydrophobic substrates. The growth of many fungi can also cause small-scale swelling and bursting, as the fungi penetrate the polymer solids (Griffin 1980). The degradation of plastics by some fungi occurs through the intracellular and extracellular enzymatic systems. The intracellular enzymatic system acts as an internal mechanism for detoxification and plays a major role in fungal adaption (Jeon et al. 2016; Olicón-Hernández et al. 2017; Schwartz et al. 2018; Shin et al. 2018). This system is mediated by the cytochrome P450 family (CYP), Phase I enzyme epoxidase and, Phase II enzyme transferases which involve oxidation and conjugation reactions. Cytochrome P450 family are heme-containing mono-oxygenases that are involved in catalyzing various enzymatic reactions (Shin et al. 2018). Cytochrome P450 enzymes are important for primary metabolism, enabling protection of the hyphal wall integrity and the formation of the spore outer wall (Črešnar and Petrič 2011). CYP isoforms are anchored in the membrane of the endoplasmic reticulum, having their active sites connected to both the cytosolic and membrane environments so they can uptake substrate from both surroundings (Šrejber et al. 2018). CYP contains three cofactors (NADPH+, H+, FAD, and Heme) and two enzymes (NADPH: CYP reductase and cytochrome P-450 hydrolase). The extracellular enzymatic system consists of a hydrolytic system that produces hydrolases that are involved in polysaccharide degradation and the unspecific oxidative system involved in breaking down complex structures, such as lignin degradation (Sánchez 2009). The unspecific oxidative system can oxidize a wide range of substrates. It is formed mainly by nonspecific oxidoreductases, including enzymes, such as class II peroxidases (manganese peroxidase, lignin peroxidase, and versatile peroxidase), laccases, and unspecific peroxygenases. These enzymes transfer electrons from organic substrates to molecular oxygen (laccases) by oxidation–reduction reactions using H2O2 as an electron-accepting co-substrate or by epoxidation, aromatic preoxygenation, and sulfoxidation (Karich et al. 2017). This enzymatic complex is produced mainly by wood-degrading fungi, such as basidiomycetes (Sánchez 2009). The action of fungi on the surface of plastics can be affected by environmental factors such as moisture, pH, temperature, etc. sufficient moisture is required for activation of fungi, appropriate pH environment is required for the action of enzymes on plastic polymer and equally, temperature plays a vital role in this biodegradation process, polymers of high melting point take more time to degrade than polymers of low melting point (Fig. 1).
Fig. 1.
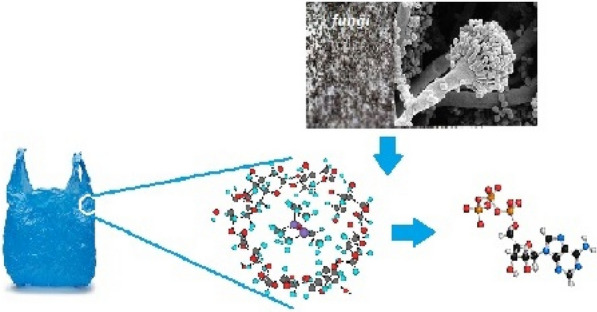
Complex polymer chains of polythene are converted to simple polymer chains by action of fungi (biodegradation)
Aerobic and anaerobic plastic biodegradation
In aerobic biodegradation, the degradation involves in presence of oxygen which is also known as aerobic respiration. Aerobic fungi use oxygen as an electron acceptor, and breakdown complex organic compounds into smaller organic compounds often producing co2 and water as end products (Seymour et al. 1899), formation of carbon dioxide (Sturm test) are good indicators for polymer degradation and are the most often used methods to measure biodegradation in laboratory tests. Anaerobic degradation occurs in absence of oxygen. Degradation of complex polymers into smaller units by microbes with CH2, CO2, H2O, and biomass as their by-products. Anaerobic fungi in the absence of oxygen utilize other sources as their electron acceptor, such as sulfate, nitrate, iron, manganese, and carbon dioxide for biodegradation (Alshehrei 2017). Two Pestalotiopsis microspora isolates were observed to grow on PUR as the sole carbon source under both aerobic and anaerobic conditions, effective degradation activity was observed under anaerobic growth using PUR (Jonathan and Russell 2011).
Biodegradation of various plastics
Polythene is mostly used plastics in daily life due to its easy processing for products, such as plastic bags, plastic films, packing food materials, textiles (Arutchelvi et al. 2021). Polyethylene is chemically represented as (C2H4) n. PE is a combination of polymers of ethylene with different values of n. They are low-density polyethylene and high-density polyethylene. Low-density polyethylene is processed by applying high pressure (1000–5000 atm) and high temperature (520 kelvins), whereas high-density polyethylene requires low pressure (6–7 atm) and low temperature (333–343 K) (Lee et al. 1991). It is reported that Polyethylene constitutes 64% of total synthetic plastics as it is been used for manufacturing bottles, carry bags, disposable articles, garbage containers, margarine tubs, milk jugs, and water pipes. Annually 500 billion to 1 trillion polythene bags are being used daily all over the world. The usage of polythene is increased at a rate of 12% annum and approximately 140 million tonnes of synthetic plastic polymers are produced worldwide annually (Roy et al. 2008). Phanerochaete chrysosporium is fungal species that degrade high molecular weight polyethylene under nitrogen-limited and carbon-limited conditions (Shimao 2001). Aspergillus, Cladosporium, Fusarium, Penicillium, Phanerochaete have been reported for polyethylene degradation (Danso et al. 2019; Glaser 2019; Restrepo-Florez et al. 2014). Additives free Polyethylene degradation was identified in Pencillium. simplicissimum (Esmaeili et al. 2013), Aspergillus niger, Aspergillus japonicas and Fusarium. sp (Raaman et al. 2012). Penicillium chrysogenum NS10 (KU559907), Penicillium oxalicum NS4 (KU559906) were identified for degrading HDPE and LDPE (Ojha et al. 2017).
Polyethylene terephthalate/PET
Polyethylene terephthalate is a semicrystalline, thermoplastic, strong and durable, chemically and thermally stable, has low gas permeability, and is easily processed. PET is used as fibers, sheets and films, electronics, automotive parts, houseware, lighting products, power tools, sports goods, photographic applications, X-ray sheets and textiles, and in food and beverage packaging (especially, soft-drink and water bottles) (Awaja and Pavel 2005; Kint and Muñoz-Guerra 1999; Levchik and Weil 2004; Bergeret et al. 2009). Polyethylene and polypropylene represent about 92% of the synthetic plastics produced, and they are used for the production of plastic bags, disposable containers, bottles, packaging materials, etc. (Byuntae et al. 1991). The enzymes involved in the degradation (e.g., PET hydrolase and tannase, MHETase) are typical serine hydrolases, such as cutinases, lipases, and carboxylesterases (Wei et al. 2014). Fungal cutinases of Fusarium and Humicola were identified for their degradation in PET. Even lipase CalB from Candida antarctica was also used in the PET degradation process (Carniel et al. 2017). Aspergillus sp., Penicillium sp., and Fusarium sp are used as biological agents to degrade PET and PS foam (Umamaheswari and Murali 2013). Aspergillus oryzae, C. antarctica, and Penicillium citrinum are among other fungal enzymes that have been investigated for activity on PET (Zimmermann and Billig 2010; Kawai et al. 2019).
Polypropylene/PP
Polypropylene is also referred to as polypropene, a thermoplastic polymer. Polypropylene belongs to partially crystalline polyolefins, mostly used polypropylene is isotactic. PP is used in manufacturing rugs, mats, carpets, ropes, and chairs. It is also used in manufacturing laboratory-required equipment, such as wash bottles, centrifuge tubes, Eppendorf tubes, tips for pipettes, etc. The surfaces of polypropylene are hydrophobic, because it has CH and CH2 groups along its backbone and a CH3 pendant group. To make the polymer surface more hydrophilic, pre-treatments such as UV radiation, gamma sterilization, or thermal treatments are followed (Koutny et al. 2006; Halina et al. 2005; Alariqi et al. 2006). Bjerkandera adusta (Butnaru et al. 2016), Lasiodiplodia theobromae (SanaSheik et al. November 2015), Coriolus versicolor, (Kord et al. 2021) fungi are identified for their ability in the degradation of Polypropylene.
Polyvinylchloride/PVC
Polyvinylchloride is a strong polymer, composed of repeating chloroethyl units (Fischer et al. 2014), these are low cost, and show biological and chemical resistance. PVC comes in two forms, i.e., rigid and flexible. Pure PVC is soluble in tetrahydrofuran and insoluble in alcohols. PVC is similar to the structure of chlorophenol compounds. Compared with PET and PS, polyvinylchloride (PVC) is considered to be a hard plastic for biodegradation. Some fungal species which showed PVC degradation are Cochliobolus sp. (Sumathi et al. 2016), Phanerochaete chrysosporium, Aspergillus niger (Ali et al. 2014), Penicillium funiculosum ATCC 9644, Trichoderma viride ATCC 13631, Paecilomyces variotii CBS 62866, Aspergillus niger (ATCC 6275) (Whitney 1996), Aureobasidium pullulans (Webb et al. 2020), Chaetomium globosum (ATCC 16021) (Vivi et al. 2018). Some yeast-like fungi Rhodotorula aurantiaca and Kluyveromyces spp also showed some degrading properties towards polyvinylchloride.
Polystyrene (PS)
Polystyrene is a thermoplastic polymer, aromatic hydrocarbon polymer which is composed of monomers, i.e., styrene (John Scheirs 2003). They are in the form of solid or foamed, its chemical formula is (C8H8) n. PP is degraded by acetone, chlorinated solvents, and aromatic hydrocarbon solvents. It is used for protective packaging such as packing food items and jewel cases and used for manufacturing cases for CDs, DVDs, containers, lids, bottles, trays, tumblers, etc. Degrading polystyrene ability was identified in Cephalosporium spp. and Mucor spp. Gloeophyllum striatum DSM 9592 and Gloeophyllum trabeum DSM 1398 strains causing almost 50% reduction in molecular weight of polystyrene. White rot fungi Pleurotus ostreatus, Phanerochaete chrysosporium, and Trametes versicolor, and the brown-rot fungi Gloeophyllum trabeum were capable of depolymerization of polystyrene when coincubated together with lignin (Krueger et al. 2015; Milstein et al. 1992). Aspergillus sp., Penicillium sp., and Fusarium sp. were also identified for their ability to degrade PET and PS foam (Umamaheswari and Murali 2013) Curvularia species hyphae had adhered to and penetrated the polymer’s structure (Motta et al. 2009). Geomyces, Mortierella species also involve in degrading polystyrene (Oviedo-Anchundia et al. 2021).
Polyurethane/PUR
Polyurethane is a polymer composed of organic units which are joined by carbamate (urethane) links. They are in rigid and flexible foam forms, varnishes and coatings, adhesives, electrical compounds, and fibers, such as spandex and polyurethane laminate (Gama et al. 2018). Polyurethanes are a type of plastic that has wide use in industries, they are synthesized from polyols and polyisocyanates. This polyurethane is degraded by the following fungi, i.e., Gliocladium roseum, Aspergillus spp., Emericella spp., Fusarium spp., Penicillium spp., Trichoderma spp., Gliocladium pannorum, Nectria gliocladiodes, Penicillium ochrochloron, Aureobasidium pullulans, Rhodotorula aurantiaca, and Kluyvermyces spp (Cosgrove et al. 2007; Lagauskas and Pečiulytė 2009; Webb et al. 2000). Some fungi such as Plectosphaerella, Nectria, Neonectria, Phoma, and Alternaria also showed their ability for PU biodegradation. Aspergillus niger was also identified for its quite slow growth with visible signs of deterioration occurring only after 30 days (Russell 2011). Pestalotiopsis microspora, utilizing polyurethane as a carbon source with an enzyme serine hydrolase and degrade it within a few days (Russell 2011). Comamonas acidovorans produce polyurethane esterase that degrades polyurethane and low and high molecular weight polylactic acid (Bhardwaj et al. 2012). In degradation of polyurethane, proteases are more effective than esterases (Ozsagiroglu et al. 2012). Recent studies identified Candida ethanolica (Zafar et al. 2013), and Candida rugosa (Gautam et al. 2007) as PUR degraders. Cladosporium pseudo cladosporioides, Cladosporium tenuissimum, Cladosporium asperulatum, Cladosporium montecillanum, Aspergillus fumigatus, Penicillium chrysogenum were also reported for degrading PUR (Álvarez-Barragán et al. 2016; Mathur and Prasad 2012).
Polycarbonate/PC
Polycarbonate is a thermoplastic polymer that contains carbonate groups (−O−(C=O). its rigid and strong polymer. It is used in manufacturing electronic components such as TVs screens computer screens, compact discs, DVDs, automotive, aircraft, and some security elements such as bulletproof sheets, eyeglasses/lenses. Degradation of polycarbonates is typical in-process due to its rigid structure and usually takes years. Phanerochaete chrysosporium NCIM 1170 a white-rot fungus showed degrading properties towards polycarbonates (Artham and Doble 2010). Geotrichum spp., are identified by their aryl alcohol oxidases and Mn2+-oxidizing peroxidase enzymes and degrading polycarbonates (Romero et al. 2007). Some common fungal species which are showing biodegradation of polycarbonates are Fusarium, Ulocladium, Chrysosporium, and Penicillium (Arefian et al. 2013).
Plastic polymer biodegradation by edible fungi
Edible fungi/Edible Mushrooms which are considered macrofungi are fleshy and fruit bodies which is rich in their nutritional benefits. They are artificially cultivated on a suitable substrate, such as straw, husk, sugarcane residues, leaves, etc. The mushrooms grow on the surface of the substrate by absorbing the nutrients from the substrate. In the place of other substrates, plastic films/sheets are used as substrate. Some important fungi species which showed their ability to absorb nutrients from the plastic polymers they are Pleurotus abalones, Pleurotus ostreatus, Agaricus bisporus these species are by secretion of enzyme laccases utilized the polyethylene and polystyrene as carbon sources and showed growth by degrading the plastics. (Hock et al. 2019). Pleurotus eryngii, Lentinula edodes, fungi showed the ability to degrade BPA (Bisphenol-A) and DEHP (di-2-ethyhexylphthalate) plastics by secretion of manganese peroxidase (MnP) enzyme (Hock et al. 2019). The capability of degrading green polyethylene and oxo-biodegradable (D2W) plastics without prior physical treatment was observed in Pleurotus ostreatus. The formation of mushrooms from the plastic as a substrate is a very new approach to control plastic pollution, and the productivity of mushrooms can be increased by altering the composition of the substrate (Luz et al. 2013).
Conclusions
The increase in plastic pollution greatly affects living organisms, biodegradation of plastics by fungi can help to decrease the problem. Biodegradation of plastic polymers has been one of the current focussed areas of research on solving plastic pollution. The review provides eminent information on various fungi which are involved in degrading different types of plastic polymers, and specific degrading enzymes produced by various fungi which are involved in the biodegradation mechanism. Though many studies identified the degrading abilities in fungi very few shows effective biodegradation. Genetic engineering could be a preferred strategy to enhance the ability of fungi in biodegradation of plastic polymers. Several strategies, such as random mutagenesis and site-directed mutagenesis, genome editing, advanced computational modelling, computational genomics, are the recent strategies to address enzyme engineering. In addition, genetically engineered robust enzyme systems could be an effective strategy to reduce plastic waste.
Acknowledgements
Not applicable.
Author contributions
MS designed the study, and was a major contributor in writing the manuscript. TSRSS wrote a part of the manuscript and polished the manuscript along with reviewing. KS provided the required and relevant information and literature. SG reviewed overall manuscript preparation and supported the study. All authors read and approved the final manuscript.
Funding
No funding required.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El-Rehim HA, Hegazy El-Sayed A, Ali AM, Rabie AM. Synergistic effect of combining UV-sunlight–soil burial treatment on the biodegradation rate of LDPE/starch blends. J Photoch Photobio A. 2004;163:547–556. doi: 10.1016/j.jphotochem.2004.02.003. [DOI] [Google Scholar]
- Alariqi SAS, Pratheep Kumar A, Rao BSM, Singh RP. Biodegradation of γsterilised biomedical polyolefins under composting and fungal culture environments. Polym Degrad Stabil. 2006;91:1105–1116. doi: 10.1016/j.polymdegradstab.2005.07.004. [DOI] [Google Scholar]
- Ali MI, Ahmed S, Robson G, et al. Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J Basic Microbiol. 2014;54(1):18–27. doi: 10.1002/jobm.201200496. [DOI] [PubMed] [Google Scholar]
- Alisch-Mark M, Herrmann A, Zimmermann W. Increase of the hydrophilicity of polyethylene terephthalate fibers by hydrolases from Thermomonospora fusca and Fusarium solani f. sp. pisi. Biotechnol Lett. 2006;28:681–685. doi: 10.1007/s10529-006-9041-7. [DOI] [PubMed] [Google Scholar]
- Alshehrei F. Biodegradation of synthetic and natural plastic by microorganisms. J Appl Environ Microbiol. 2017;5:8–19. [Google Scholar]
- Álvarez-Barragán J, Domínguez-Malfavón L, Vargas-Suárez M, González-Hernández R, Aguilar-Osorio G, Loza-Tavera H. Biodegradative activities of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl Environ Microbiol. 2016;82:5225–5235. doi: 10.1128/AEM.01344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefian M, Zia M, Tahmourespour A, et al. Polycarbonate biodegradation by isolated molds using clear-zone and atomic force microscopic methods. Int J Environ Sci Technol. 2013;10:1319–1324. doi: 10.1007/s13762-013-0359-0. [DOI] [Google Scholar]
- Artham T, Doble M. Biodegradation of physicochemically treated polycarbonate by fungi. Biomacromol. 2010;11(1):20–28. doi: 10.1021/bm9008099. [DOI] [PubMed] [Google Scholar]
- Arutchelvi J, Sudhakar M, Arkatkar AD, Mukesh B, Sumit U, Parasu V. Biodegradation of polyethylene and polypropylene. IJBT. 2021;7(1):9–22. [Google Scholar]
- Awaja F, Pavel D. Recycling of PET. Eur Polym J. 2005;41(1453–1477):39. [Google Scholar]
- Behzad K, Nadir A, Mohammad, Dahmardeh G, Mohammad D (2021) Effect of fungal degradation on technological properties of carbon nanotubes reinforced polypropylene/rice straw composites. Poylm Polym Composit 29(5): 303–310
- Bergeret A, Ferry L, Ienny P. Influence of the fibre/matrix interface on ageing mechanisms of glass fibre reinforced thermoplastic composites (PA-6,6, PET, PBT) in a hygrothermal environment. Polym Degrad Stabil. 2009;94:1315–1324. doi: 10.1016/j.polymdegradstab.2009.04.009. [DOI] [Google Scholar]
- Bhardwaj H, Gupta R, Tiwari A. Microbial population associated with plastic degradation. Open Access Sci Rep. 2012;1:1–4. [Google Scholar]
- Bholay AD, Borkhataria V, Jadhav U, Palekar S, Dhalkari V, Nalawade PM, et al. Bacterial lignin peroxidase: a tool for biolecching and biodegradation of industrial effluents. Univ J Environ Res Technol. 2012;2:58–64. [Google Scholar]
- Bo RK, Soo BK, Hyun AS, Tae KL. Accelerating the biodegradation of high-density polyethylene (HDPE) using Bjerkandera adusta TBB-03 and Lignocellulose Substrates. MDPI Microorganisms. 2019;7:304. doi: 10.3390/microorganisms7090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme S, Cuer A, Delort A, Lemaire J, Sancelme M, Scott G. Environmental biodegradation of polyethylene. Polym Degrad Stab. 2003;81:441–452. doi: 10.1016/S0141-3910(03)00129-0. [DOI] [Google Scholar]
- Brunner I, Fischer M, Rüthi J, Stierli B, Frey B. Ability of fungi isolated from plastic debris floating in the shoreline of a lake to degrade plastics. PLoS ONE. 2018;13(8):1–14. doi: 10.1371/journal.pone.0202047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JA, Clemente TM, Viviani DA, Fong AA, Thomas KA, Kemp P, Karl DM, White AE, DeLong EF. Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre. mSystems. 2016;1(3):e00024–e116. doi: 10.1128/mSystems.00024-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butnaru E, Darie-Niţă RN, Zaharescu T, Balaeş T, Tănase C, Hitruc G, et al. Gamma irradiation assisted fungal degradation of the polypropylene/biomass composites. Radiat Phys Chem. 2016;125:134–144. doi: 10.1016/j.radphyschem.2016.04.003. [DOI] [Google Scholar]
- Byuntae L, Anthony LP, Alfred F, Theodore BB. Biodegradation of degradable plastic polyethylene by Phanerocheate and Streptomyces species. Appl Environ Microbiol. 1991;3:678–688. doi: 10.1128/aem.57.3.678-685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel A, Valoni É, Nicomedes J, Gomes ADC, Castro AMD. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017;59:84–90. doi: 10.1016/j.procbio.2016.07.023. [DOI] [Google Scholar]
- Chen S, Su L, Chen J, Wu J. Cutinase: characteristics, preparation and application. Biotechnol Adv. 2013;31:1754–1767. doi: 10.1016/j.biotechadv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Coe JM, Andersson S, Rogers DB (1997) Marine debris in the Caribbean Region. In: Coe JM, Rogers DB (eds) Marine debris. Springer Series on Environmental Management. Springer, New York, NY. 10.1007/978-1-4613-8486-1_4
- Cosgrove L, McGeechan PL, Robson GD, Handley PS. Fungal communities associated with degradation of polyester polyurethane in soil. Appl Environ Microbiol. 2007;73:5817–5824. doi: 10.1128/AEM.01083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Črešnar B, Petrič Š. Cytochrome P450 enzymes in the fungal kingdom. Bbaproteins Proteom. 2011;1814:29–35. doi: 10.1016/j.bbapap.2010.06.020. [DOI] [PubMed] [Google Scholar]
- da Luz JMR, Paes SA, Nunes MD, da Silva MdCS, Kasuya MCM. Degradation of oxo-biodegradable plastic by Pleurotus ostreatus. PLoS ONE. 2013;8(8):e69386. doi: 10.1371/journal.pone.0069386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso D, Chow J, Streit WR. Plastics: environmental and bio technological perspectives on microbial degradation. Appl Environ Microbiol. 2019;85:1–14. doi: 10.1128/AEM.01095-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtban M, Schraft H, Syed TA. Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mol Biol. 2010;1(1):36–50. [PMC free article] [PubMed] [Google Scholar]
- Eberl A, Heumann S, Brueckner T, Araujo R, Cavaco-Paulo A, Kaufmann F, et al. Enzymatic surface hydrolysis of poly(ethylene terephthalate) and bis(benzoyloxyethyl)terephthalate by lipase and cutinase in the presence of surface active molecules. J Biotechnol. 2009;143:207–212. doi: 10.1016/j.jbiotec.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Esmaeili A, Pourbabaee AA, Alikhani HA, Shabani F, Esmaeili E. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. PLoS ONE. 2013;8:717–720. doi: 10.1371/journal.pone.0071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I, Schmitt WF, Porth H, Allsopp MW, Vianello G (2014) Poly (vinyl chloride). In; Ullmann’s encyclopedia of industrial chemistry. Wiley, Germany
- Frazer AC. O-methylation and other transformations of aromatic compounds by acetogenic bacteria. In: Drake HL, editor. Acetogenes. New York: Chapman & Hall; 1994. p. 1994. [Google Scholar]
- Furukawa M, Kawakami N, Tomizawa A, et al. Efficient degradation of poly (ethylene terephthalate) with Thermobifida fusca cutinase exhibiting improved catalytic activity generated using mutagenesis and additive-based approaches. Sci Rep. 2019;9:16038. doi: 10.1038/s41598-019-52379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama N, Ferreira A, Barros-Timmons A. Polyurethane foams: past, present, and future. Materials. 2018;11(10):1841. doi: 10.3390/ma11101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh P, Dineshraj D, Yoganathan K. Production and screening of depolymerising enzymes by potential bacteria and fungi isolated from plastic waste dump yard sites. Int J Appl Res. 2017;3(3):693–695. [Google Scholar]
- Gautam R, Bassi AS, Yanful EK. Candida rugosa lipase-catalyzed polyurethane degradation in aqueous medium. Biotechnol Lett. 2007;29:1081–1086. doi: 10.1007/s10529-007-9354-1. [DOI] [PubMed] [Google Scholar]
- Geweret B, Plassmann MM, MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Processes Impacts. 2015;17:1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- Glaser JA (2019) Biological degradation of polymers in the Environment. Plastic Environ IntechOpen
- Gorghiu LM, Jipa S, Zaharescu T, Setnescu R, Mihalcea I. The effect of metals on thermal degradation of polyethylenes. Polym Degrad Stab. 2004;84(1):7–11. doi: 10.1016/S0141-3910(03)00265-9. [DOI] [Google Scholar]
- Griffin GJL. Synthetic polymers and the living environment. Pure Appl Chem. 1980;52:399–407. doi: 10.1351/pac198052020399. [DOI] [Google Scholar]
- Halina K, Dagmara O, Przemysław M, Hanna C. Effect of short wavelength UVirradiation on ageing of polypropylene/cellulose compositions. Polym Degrad Stabil. 2005;88:189–198. doi: 10.1016/j.polymdegradstab.2004.04.017. [DOI] [Google Scholar]
- Heredia A. Biochemical and biophysical characteristics of cutin, a plant barrier biopolymer. Biochim Biophys Acta. 2003;1620:1–7. doi: 10.1016/S0304-4165(02)00510-X. [DOI] [PubMed] [Google Scholar]
- Hock OG, Lum HW, De Qin D, Kee WK, Shing WL (2019) The growth and laccase activity of edible mushrooms involved in plastics degradation, Researchgate. Toxicology. 15
- Hofrichter M, Ullrich R. Heme-thiolate haloperoxidases: versatile biocatalysts with biotechnological and environmental significance. Appl Microbiol Biotechnol. 2006;71(3):276–288. doi: 10.1007/s00253-006-0417-3. [DOI] [PubMed] [Google Scholar]
- Iiyoshi Y, Tsutsumi Y, Nishida T. Polyethylene degradation by lignin-degrading fungi and manganese peroxidase. J Wood Sci. 1998;44:222–229. doi: 10.1007/BF00521967. [DOI] [Google Scholar]
- Jakubowicz I. Evaluation of degradability of biodegradable polyethylene (PE) Polym Degrad Stabil. 2003;80:39–43. doi: 10.1016/S0141-3910(02)00380-4. [DOI] [Google Scholar]
- Jeon H, Durairaj P, Lee D, Ahsan MM, Yun H. Improved NADPH regeneration for fungal cytochrome P450 monooxygenase by co-expressing bacterial glucose dehydrogenase in resting-cell biotransformation of recombinant yeast. Microbiol Biotechnol J. 2016;26:2076–2086. doi: 10.4014/jmb.1605.05090. [DOI] [PubMed] [Google Scholar]
- John Scheirs, Duane P (2003) Modern styrenic copolymers. Wiley, pp. 3. ISBN 978-0-471-49752-3
- Karich A, Ullrich R, Scheibner K, Hofrichter M. Fungal unspecific peroxygenases oxidize the majority of organic EPA priority pollutants. Front Microbiol. 2017;8:1463. doi: 10.3389/fmicb.2017.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Kawabata T, Oda M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl Microbiol Biotechnol. 2019;103:4253–4268. doi: 10.1007/s00253-019-09717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Nadir S, Shah ZU, Shah AA, Karunarathna SC, Xu J, Khan A, Munir S, Hasan F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ Pollut. 2017;225:469–480. doi: 10.1016/j.envpol.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Kint D, Muñoz-Guerra S. A review on the potential biodegradability of poly(ethylene terephthalate) Polym Int. 1999;48:346–352. doi: 10.1002/(SICI)1097-0126(199905)48:5<346::AID-PI156>3.0.CO;2-N. [DOI] [Google Scholar]
- Kolattukudy PE, Brown L. Fate of naturally occurring epoxy acids: a soluble epoxide hydrase, which catalyzes cis hydration, from Fusarium solani pisi. Arch Biochem Biophys. 1975;166(2):599–607. doi: 10.1016/0003-9861(75)90425-7. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Purdy RE, Maiti IB. Cutinases from fungi and pollen. In: Lowenstein JM, editor. Methods in enzymology. New York: Academic Press; 1981. p. 652. [Google Scholar]
- Koutny M, Lemaire J, Delort AM. Biodegradation of polyethylene films with pro-oxidant additives. Chemosphere. 2006;64:1243–1252. doi: 10.1016/j.chemosphere.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Krueger MC, Hofmann U, Moeder M, Schlosser D. Potential of wood-rotting fungi to attack polystyrene sulfonate and its depolymerisation by Gloeophyllum trabeum via hydroquinone-driven Fenton chemistry. PLoS ONE. 2015;10:e0131773. doi: 10.1371/journal.pone.0131773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Das ML, Rebecca J, Sharmila S. Isolation and identification of LDPE degrading fungi from municipal solid waste. J Chem Pharm Res. 2013;5(3):78–81. [Google Scholar]
- Lagauskas, Levinskaitė L, Pečiulytė D. Micromycetes as deterioration agents of polymeric materials. Int Biodeterior Biodegrad. 2009;52:233–242.
- Lee B, Pometto AL, Fratzke A, Bailey TB. Biodegradation of degradable plastic polyethylene by Phanerochaete and Streptomyces species. Appl Environ Microbiol. 1991;57:678–685. doi: 10.1128/aem.57.3.678-685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchik SV, Weil ED. A review on thermal decomposition and combustion of thermoplastic polyesters. Polym Adv Technol. 2004;15:691–700. doi: 10.1002/pat.526. [DOI] [Google Scholar]
- Liebminger S, Eberl A, Sousa F, Heumann S, Fischer-Colbrie G, Cavaco-Paulo A, et al. Hydrolysis of PET and bis-(benzoyloxyethyl) terephthalate with a new polyesterase from Penicillium citrinum. Biocatal Biotransform. 2007;25:171–177. doi: 10.1080/10242420701379734. [DOI] [Google Scholar]
- Loredo-Treviño A, García G, Velasco-Téllez A, Rodríguez-Herrera R, Aguilar CN. Polyurethane foam as substrate for fungal strains. Adv Biosci Biotechnol. 2011;2(2):52–58. doi: 10.4236/abb.2011.22009. [DOI] [Google Scholar]
- Mathur G, Prasad R. Degradation of polyurethane by Aspergillus flavus (ITCC 6051) isolated from soil. Appl Biochem Biotechnol. 2012;167:1595–1602. doi: 10.1007/s12010-012-9572-4. [DOI] [PubMed] [Google Scholar]
- Milstein O, Gersonde R, Huttermann A, Chen MJ, Meister JJ. Fungal biodegradation of lignopolystyrene graft copolymers. Appl Environ Microbiol. 1992;58:3225–3232. doi: 10.1128/aem.58.10.3225-3232.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazer Z, Habibi Najafi MB, Levin DB. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can J Microbiol. 2019;65:1–11. doi: 10.1139/cjm-2018-0335. [DOI] [PubMed] [Google Scholar]
- Motta O, Proto A, De Carlo F, De Caro F, Santoro E, Brunetti L, Capunzo M. Utilization of chemically oxidized polystyrene as co-substrate by filamentous fungi. Int J Hyg Environ Health. 2009;212(1):61–66. doi: 10.1016/j.ijheh.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Munari (2019) Enzymatic hydrolysis of poly (1,4-butylene 2,5-thiophenedicarboxylate) (PBTF) and poly(1,4-butylene 2,5-furandicarboxylate) (PBF) films: a comparison of mechanisms, Environ Int 130: 104852 [DOI] [PubMed]
- Nunes CS, Kunamneni A (2018) “Chapter 7—laccases—properties and applications. In: Nunes CS, Kumar V (eds) Enzymes in Human and Animal Nutrition. Academic Press, Cambridge. pp. 133–161. 10.1016/b978-0-12-805419-2.00007-1.
- O’Neill A, Araújo R, Casal M, Guebitz G, Cavaco-Paulo A. Effect of the agitation on the adsorption and hydrolytic efficiency of cutinases on polyethylene terephthalate fibres. Enzym Microb Technol. 2007;40(7):1801–1805. doi: 10.1016/j.enzmictec.2007.02.012. [DOI] [Google Scholar]
- Ojha N, Pradhan N, Singh S, Barla A, Shrivastava A, Khatua P, Rai V, Bose S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci Rep. 2017;7:39515. doi: 10.1038/srep39515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olicón-Hernández DR, González-López J, Aranda E. Overview on the biochemical potential of filamentous fungi to degrade pharmaceutical compounds. Front Microbiol. 2017;8:1792. doi: 10.3389/fmicb.2017.01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osma JF, Toca-Herrera JL, Rodríguez-Couto S. Uses of laccases in the food industry. Enzyme Res. 2010;2010:918761. doi: 10.4061/2010/918761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Anchundia R, del Castillo DS, Naranjo-Moran J, Francois N, Alvarez-Barreto J, Alarcon A, Villafuerte JS, Barcos-Arias M. Analysis of the degradation of polyethylene, polystyrene and polyurethane mediated by three filamentous fungi isolated from the Antarctica. Afr J Biotechnol. 2021;20(2):66–76. doi: 10.5897/AJB2020.17200. [DOI] [Google Scholar]
- Ozsagiroglu E, Iyisan B, Guvenilir YA. Biodegradation and Characterization studies of different kinds of polyurethenes with several enzyme solutions. Pol J Environ Studies. 2012;6:1777–1782. [Google Scholar]
- Patel C, Yadav S, Rahi S, Dave A. Studies on biodiversity of fungal endophytes of indigenous monocotaceous and dicotaceous plants and evaluation of their enzymatic potentialities. Int J Sci Res Publ. 2013;3:1–5. [Google Scholar]
- Pereira EB, De Castro HF, De Moraes FF, Zanin GM. Kinetic studies of lipase from Candida rugosa. Appl Biochem Biotechnol. 2001;91:739. doi: 10.1385/ABAB:91-93:1-9:739. [DOI] [PubMed] [Google Scholar]
- Phua SK, Castillo E, Anderson JM, Hiltner A. Biodegradation of a polyurethane in vitro. J Biomed Mater Res. 1987;21:231–246. doi: 10.1002/jbm.820210207. [DOI] [PubMed] [Google Scholar]
- Pramila R, Vijaya Ramesh K. Biodegradation of low density polyethylene (LDPE) by fungi isolated from municipal landfill area. J Microbiol Biotech Res. 2011;1(4):131–136. [Google Scholar]
- Raaman N, Rajitha N, Jayshree A, Jegadeesh R. Biodegradation of plastic by Aspergillus spp. isolated from polythene polluted sites around Chennai. J Acad Ind Res. 2012;1:313–316. [Google Scholar]
- Restrepo-Florez JM, Bassi A, Thompson MR. Microbial degrada tion and deterioration of polyethylene—a review. Int Biodeterior Biodeg Radation. 2014;88:83–90. doi: 10.1016/j.ibiod.2013.12.014. [DOI] [Google Scholar]
- Romero E, Speranza M, García-Guinea J, Martínez ÁT. María Jesús Martínez, an anamorph of the white-rot fungus Bjerkandera adusta capable of colonizing and degrading compact disc components. FEMS Microbiol Lett. 2007;275(1):122–129. doi: 10.1111/j.1574-6968.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- Ronqvist ÅM, Xie W, Lu W, Gross RA. Cutinase-catalyzed hydrolysis of poly(ethyleneterephthalate) Macromolecules. 2009;42:5128–5138. doi: 10.1021/ma9005318. [DOI] [Google Scholar]
- Roy PK, Titus S, Surekha P, Tulsi E, Deshmukh C, Rajagopal C. Degradation of abiotically aged LDPE flms containing pro-oxidant by bacterial consortium. Polym Degrad Stab. 2008;93:1917–1922. doi: 10.1016/j.polymdegradstab.2008.07.016. [DOI] [Google Scholar]
- Russell JR. Biodegradation of polyester polyurethane by endophytic fungi. Am Soc Microbiol Appl Environ Microbiol. 2011;77(17):6076–6084. doi: 10.1128/AEM.00521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JR. Biodegradation of polyester polyurethane by endophytic fungi. Appl Environ Microbiol. 2011;77:6076–6084. doi: 10.1128/AEM.00521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanaSheik KR, Chandrashekar K, Swaroop HM. Somashekarappa, Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int Biodeterior Biodegrad. 2015;105:21–29. doi: 10.1016/j.ibiod.2015.08.006. [DOI] [Google Scholar]
- Sánchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv. 2009;27:85–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Perrot T, Aubert E, Dumarçay S, Favier F, Gérardin P, Morel-Rouhier M, Mulliert G, Saiag F, Didierjean C, Gelhaye E. Molecular recognition of wood polyphenols by phase II detoxification enzymes of the white rot Trametes versicolor. Sci Rep. 2018;8:8472. doi: 10.1038/s41598-018-26601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen SK, Raut S. Microbial degradation of low density polyethyl-ene (LDPE): a review. J Environ Chem Eng. 2015;3:462–473. doi: 10.1016/j.jece.2015.01.003. [DOI] [Google Scholar]
- Seymour RB. Polymer science before and after 1899: notable developments during the lifetime of Maurtis Dekkar. J Macromol Sci Chem. 1989;26(1989):1023–1032. doi: 10.1080/00222338908052032. [DOI] [Google Scholar]
- Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: a comprehensive review. Biotechnol Adv. 2008;26:246–265. doi: 10.1016/j.biotechadv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Shang J, Chai M, Zhu Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environ Sci Technol. 2003;37(19):4494–4499. doi: 10.1021/es0209464. [DOI] [PubMed] [Google Scholar]
- Shimao M. Biodegradation of plastics. Curr Opin Biotechnol. 2001;12:242–247. doi: 10.1016/S0958-1669(00)00206-8. [DOI] [PubMed] [Google Scholar]
- Shin J, Kim JE, Lee YW, Son H. Fungal Cytochrome P450s and the P450 complement (CYPome) of Fusarium graminearum. Toxins. 2018;10:112. doi: 10.3390/toxins10030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B (2005) Harmful effect of plastic in animals. The Indian Cow.
- Sivan A. New perspectives in plastic biodegradation. Curr Opin Biotechnol. 2011;22:422–426. doi: 10.1016/j.copbio.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Souza PMD, Bittencourt MLDA, Caprara CC, Freitas MD, Almeida RPCD, Silveira D, et al. A biotechnology perspective of fungal proteases. Braz J Microbiol. 2015;46(2):337–346. doi: 10.1590/S1517-838246220140359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šrejber M, Navrátilová V, Paloncýová M, Bazgier V, Berka K, Anzenbacher P, Otyepka M. Membrane-attached mammalian cytochromes P450: an overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J Inorg Biochem. 2018;183:117–136. doi: 10.1016/j.jinorgbio.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Thirunavukarasua K, Edwinolivera NG, DuraiAnbarasana S, Gowthamana MK, Iefujib H, Kamini NR. Removal of triglyceride soil from fabrics by a novel lipase from Cryptococcus sp. S-2. Process Biochem. 2008;43:701–706. doi: 10.1016/j.procbio.2008.02.011. [DOI] [Google Scholar]
- Tirupati S, Buddolla V, Akula SL, SaiGopal DVR. Production of laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem Res Int. 2016;2016:10. doi: 10.1155/2016/9519527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa Y, Calabia BP. Biodegradability of plastics. Int J Mol Sci. 2009;10:3722–3742. doi: 10.3390/ijms10093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa Y, Calabia BP, Ugwu CU, Aiba S. Biodegradability of plastics. Int J Mol Sci. 2009;10(9):3722–3742. doi: 10.3390/ijms10093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umamaheswari S, Murali M. FTIR spectroscopic study of fungal degradation of poly(ethylene terephthalate) and polystyrene foam. Elixir Chem Engg. 2013;64:19159–19164. [Google Scholar]
- Usha R, Sangeetha T, Palaniswamy M. Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agric Res Center J Int. 2011;2(4):200–204. [Google Scholar]
- Van Gemeren IA, Beijersbergen A, Van den Hondel CAMJJ, Verrips CT. Expression and secretion of defined cutinase variants by Aspergillus awamori. Appl Environ Microbiol. 1998;64:2794–2799. doi: 10.1128/AEM.64.8.2794-2799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertommen MAME, Nierstrasz VA, van der Veer M, Warmoeskerken MMCG. Enzymatic surface modification of poly(ethylene terephthalate) J Biotechnol. 2005;120:376–386. doi: 10.1016/j.jbiotec.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Vivi VK, Martins-Franchetti SM, Attili-Angelis D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2018 doi: 10.1007/s12223-018-0621-4. [DOI] [PubMed] [Google Scholar]
- Webb JS, Nixon M, Eastwood IM, Greenhalgh M, Robson GD, Handley PS. Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl Environ Microbiol. 2000;66:3194–3200. doi: 10.1128/AEM.66.8.3194-3200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JS, Nixon M, Eastwood IM, Greenhalgh M, Robson GD, Handley PS (2020) Fungal colonization and biodeterioration of plasticized polyvinyl chloride. ASM J Appl Environ Microbiol 66(8) [DOI] [PMC free article] [PubMed]
- Wei R, Oeser T, Then J, Kuhn N, Barth M, Schmidt J, Zimmermann W. Functional characterization and structural modeling of synthetic polyester-degrading hydrolases from Thermomonospora curvata. AMB Express. 2014;4:44. doi: 10.1186/s13568-014-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland M, Daro A, David C. Biodegradation of thermally oxidized polyethylene. Polym Degrad Stabil. 1995;48:275–289. doi: 10.1016/0141-3910(95)00040-S. [DOI] [Google Scholar]
- Whitney PJ. A comparison of two methods for testing defined formulations of PVC for resistance to fungal colonisation with two methods for the assessment of their biodegradation. Int Biodeterior Biodegrad. 1996;37(3–4):205–213. doi: 10.1016/S0964-8305(96)00012-1. [DOI] [Google Scholar]
- Wu J, Xiao Y, Yu H. Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm. Biores Technol. 2005;96(12):1357–1363. doi: 10.1016/j.biortech.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Tamura K, Hiradate S, Watanabe T, et al. Contribution of soil esterase to biodegradation of aliphatic polyester agricultural mulch film in cultivated soils. AMB Expr. 2015;5:10. doi: 10.1186/s13568-014-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar U, Houlden A, Robson GD. Fungal communities associated with the biodegradation of polyester polyurethane buried under compost at different temperatures. Appl Environ Microbiol. 2013;79:7313–7324. doi: 10.1128/AEM.02536-13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W, Billig S (2010) Enzymes for the biofunctionalization of poly (ethylene terephthalate). In: Nyanhongo GS, Steiner W, Gübitz G (eds) Biofunctionalization of polymers and their applications. Springer, New York, pp. 97–120. 10.1007/10_2010_87
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


