Abstract
Macrophages (MΦs) are an abundant component in the multiple myeloma (MM) environment and contribute to MM drug resistance. We previously showed that interleukin-32 (IL-32) is highly expressed in MM patients and induces the immunosuppressive function of MΦs. The present study was designed to explore the role of IL-32 in MΦ-mediated MM drug resistance and the underlying mechanism. Our analysis revealed that IL-32 expression was upregulated in relapsed MM patients and associated with CD206+ M2 MΦ infiltration. Subsequently, we found that the most active isoform, IL-32γ, promoted MΦs to protect MM cells from drug-induced apoptosis both in vitro and in vivo. Furthermore, by evaluating many parameters, including surface markers, cytokines, metabolic enzymes and characteristic molecules, IL-32γ was verified to induce the polarization of M2 MΦs, a function that was partly dependent on increasing the expression of colony-stimulating factor 1 (CSF1). Taken together, the results of our study indicate that IL-32γ promotes MΦ-mediated MM drug resistance and modifies MΦs toward the M2 phenotype, providing a crucial theoretical basis for targeted MΦ immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03241-1.
Keywords: Interleukin-32, Multiple myeloma, Chemoresistance, Macrophages, Polarization
Introduction
Multiple myeloma (MM) is a B-lymphocyte tumor characterized by many malignant plasma cells accumulating in the bone marrow (BM). The clinical manifestations are anemia, bone destruction and renal damage [1]. The development of new drugs has significantly improved the treatment response and overall survival of MM patients, but drug resistance remains a major challenge [2]. The supportive BM microenvironment is considered one of the main causes of MM resistance and recurrence [3].
Macrophages (MΦs) are an abundant cellular component in the BM microenvironment of MM [4]. In contrast to normal MΦs, MΦs in the MM BM microenvironment promote tumor proliferation and blood vessel formation [5]. Additionally, MΦs protect MM cells from apoptosis induced by chemotherapeutic drugs and increase the drug resistance of MM [6]. MΦs are mainly divided into two polarization subtypes, classically activated (M1) MΦs and alternatively activated (M2) MΦs [7, 8]. M1 MΦs express the surface markers CD80 and CD86, highly express inducible nitric oxide synthase (iNOS) and can kill foreign antigens and tumor cells. The main markers of M2 MΦs are CD163, CD206 and Dectin-1, and these cells highly express arginase 1 (Arg1) and phosphorylated adenylate-activated protein kinase (p-AMPK), which function in immunosuppression and tumor promotion. The two MΦ subtypes are extremely plastic. Currently, MΦs in early-stage tumors are thought to primarily comprise the M1 type, exerting antitumor functions; however, following interactions with tumor cells, MΦs differentiate into the M2 type, promoting tumor growth and metastasis [9]. In the MM BM microenvironment, MΦs are mainly the M2 type and increase in number with disease progression [10]. How the phenotype and function of MΦs are regulated remains under investigation.
Interleukin-32 (IL-32) is a newly discovered inflammatory factor produced by NK cells, monocytes, T lymphocytes, endothelial cells, and mast cells [11]. There are nine splice variants of IL-32, and IL-32γ has the strongest activity [12]. IL-32 is highly expressed in various tumors, but its role in different tumors varies [13]. Studies have reported that IL-32 promotes the progression of liver cancer, pancreatic cancer, gastric cancer and lung cancer [14–17]; however, in colon cancer, prostate cancer and cervical cancer, IL-32 exhibits antitumor function [18–20]. Our team previously found that IL-32 expression in the BM and peripheral blood of MM patients is significantly higher than that in the same tissues of normal controls [21]. Furthermore, IL-32 regulates the expression of various inflammatory factors and chemokines, such as IL-6 and IL-10, in bone marrow stromal cells (BMSCs), thereby promoting the proliferation of MM cells [21]. We also revealed that IL-32 plays a crucial role in forming the immunosuppressive MM microenvironment [22]. In this study, we further explored the specific effects of IL-32 on MΦ-induced MM drug resistance and MΦ polarization and the underlying mechanism.
Materials and methods
Cell culture
The human MM cell lines ARP-1 and H929 were generously provided by Dr. Qing Yi (Center for Hematologic Malignancy, Research Institute, Houston Methodist, Houston, TX, USA) and cultured in RPMI-1640 medium containing 1% l-glutamine and 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA).
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors after obtaining informed consent. Human MΦs were generated from PBMCs as previously described [4]. Monocytes were cultured in 6-well plates for 1–2 h; nonadherent cells were removed, and the adherent monocytes were incubated for 7 days in RPMI-1640 medium containing M-CSF (20 ng/ml; R&D Systems, Minneapolis, MN, USA).
MM cells were cocultured with MΦs at a 1:1 ratio for 24 h in RPMI-1640 medium containing bortezomib (Bor; 10 nM; Selleckchem, TX, USA). Next, the MM cells in suspension were collected for flow cytometry to detect the number of apoptotic cells. In inhibitory experiments, MΦs were pretreated with a CSF1 receptor inhibitor (500 nM BLZ945 or 10 μM GW2580) for 2 h and then stimulated with IL-32γ (20 ng/mL) for 24 h.
Flow cytometry
The expression of CD206, Dectin-1, CD86 and CD11b was measured by direct staining using the human antibodies PE-conjugated anti-CD206, PE-conjugated anti-Dectin-1, APC-conjugated anti-CD86, and FITC-conjugated anti-CD11b (BioLegend, CA, USA, 1:600). The corresponding isotype controls were adopted to exclude nonspecific signals. Apoptotic cells were detected by staining with Annexin V-FITC/propidium iodide (PI) (Dojindo, Kumamoto, Japan). The data were acquired using a FACScan flow cytometer (BD Biosciences, San Diego, CA, USA) and analyzed using FlowJo 7.6.1.
Quantitative real-time PCR and RNA-seq
Total mRNA was isolated from cells using RNAiso™ Plus (TaKaRa, Shiga, Japan) and then was reverse transcribed using the PrimeScript™ RT Reagent Kit with DNA Eraser (TaKaRa). qRT-PCR was performed using SYBR® Premix Ex TaqTM II (TaKaRa) and Bio-Rad CFX96 real-time system. The primer sequences are shown in the Supplementary Materials. The 2−ΔCt method was used to normalize the expression to GAPDH. RNA-seq was conducted following the Illumina mRNA Sequencing Sample Preparation Guide (Illumina, San Diego, CA, USA) as previously described [22]. Total RNA was extracted using Redzol reagent (SBS Genetech Inc., Beijing, China) from MΦs (biological samples from two donors) stimulated with IL-32γ (10 or 40 ng/ml) for 24 h. The analysis pipeline for RNAseq procedure is shown in the Supplementary Materials.
Western blotting analysis
Cells were harvested and extracted in lysis buffer containing a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then were transferred to polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA). The membranes were blocked with 5% bovine serum albumin for 1–2 h and then were incubated with the indicated primary antibodies overnight at 4 °C. The membranes were washed three times with Tween (TBST) buffer and then were incubated with HRP-conjugated anti-rabbit or anti-mouse antibodies (1:5000) for 1 h at room temperature. The membranes were washed three times with TBST, and then, the proteins were detected using the ChemiDoc™ MP Imaging System (Bio-Rad) and an enhanced chemiluminescence detection kit (Biological Industries, Beit Haemek, Israel). Primary antibodies against Arg1, iNOS, p-AMPK, CSF1, PARP, Caspase-3 and GAPDH were purchased from Cell Signaling Technology (Danvers, MA, USA, 1:1000).
Cytokine chip and enzyme-linked immunosorbent assay (ELISA)
MΦs were stimulated with different concentrations of IL-32γ, and then, the culture supernatant was collected after 24 h. The Bio-Plex Pro Human Cytokine Grp I Panel 27-plex was purchased from Huaying Biotechnology Company (Shanghai, China) and was used according to the manufacturer’s instructions. The plate was finally placed in a Bio-Plex instrument for detection. Human IL-10 and CSF1 ELISA kits were purchased from DAKEWE (Beijing, China). The levels of IL-10 and CSF1 in culture supernatants were detected following the manufacturer’s instructions.
MM xenograft model
This experiment was approved by the Animal Experimental Ethical Inspection of the First Affiliated Hospital, School of Medicine, Zhejiang University. NSG mice (4 weeks old, male) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China) and housed in the animal facility of Zhejiang University School of Medicine.
ARP-1 cells (5 × 106/mouse) were injected subcutaneously into the right flank of mice. After approximately 1 week, when palpable tumors (> 5 mm) were detected, the mice were randomly divided into four groups (Control, Bor, Bor + MΦs, and Bor + IL-32γ-educated MΦs). MΦs were injected intratumorally only at the time of the first Bor administration. The Bor dosage was 0.1 mg/kg/every 3 days. Tumor size was monitored every other day using calipers; following sacrifice, the subcutaneous tumor was removed. Part of the tumor was immersed in 4% paraformaldehyde (for immunofluorescence detection). The other part of the tumor was placed in culture medium (for flow cytometry).
Immunohistochemistry and immunofluorescence
We detected IL-32 and CD206 expression in formalin-fixed, paraffin-embedded BM biopsy samples from MM patients by immunohistochemistry. All the patients provided consent for their participation, and the study was approved by the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. Immunohistochemistry and semiquantitative analyses were performed as previously reported [22]. An anti-human IL-32 monoclonal antibody (Abcam, Cambridge, UK, 1:150) and an anti-human CD206 monoclonal antibody (Proteintech, Chicago, USA, 1:2000) were used in the present study. Immunofluorescence staining was performed as previously described [22] using anti-CD138 (1:300), anti-cleaved caspase-3 (1:200), and anti-Ki67 (1:500) antibodies purchased from Proteintech.
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) and SPSS Statistics 19 (SPSS Inc., Chicago, IL, USA) were used for all statistical analyses. Two-tailed Student’s t test was adopted to determine the significance of differences between experimental groups. Comparison was analyzed by Fisher’s exact test. The data are presented as the mean ± standard error of mean (SEM). All P values < 0.05 were considered statistically significant.
Results
IL-32 is highly expressed in relapsed MM and correlated with CD206+ M2 MΦs
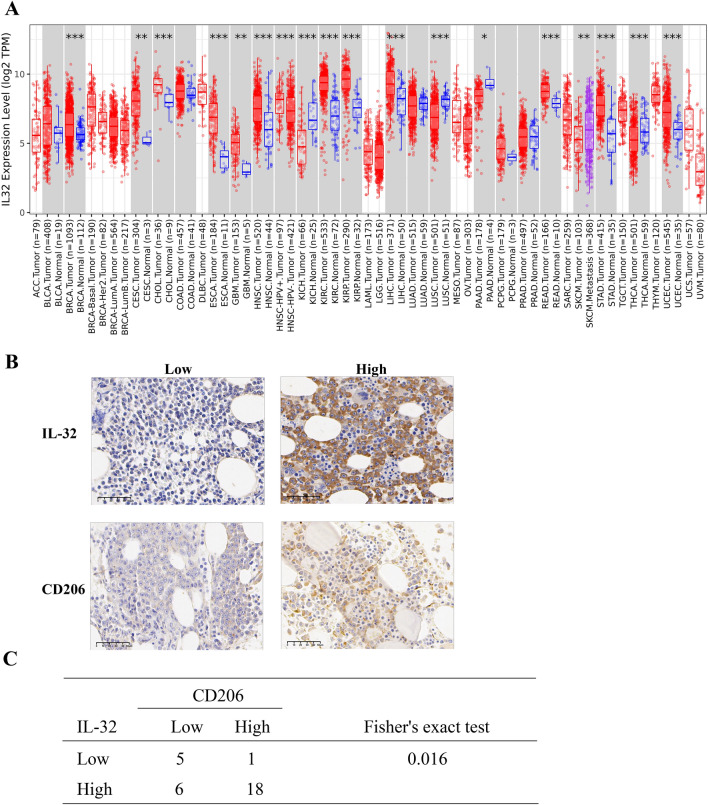
We used the TIMMER database to analyze the expression of IL-32 in different cancer cells. The results showed that IL-32 expression was obviously higher in multiple types of cancer cells than in normal tissues (Fig. 1a). In our previous study, we verified that IL-32 expression was increased in MM patients [21]. Furtherly, we detected the protein level of IL-32 in newly diagnosed MM patients and relapse MM patients. Results revealed that IL-32 was upregulated in relapsed MM patients [23]. Moreover, we evaluated the expression of IL-32 and CD206+ M2 MΦs in BM biopsies from MM patients based on immunohistochemistry. In the BM biopsies, the IL-32 protein was distributed diffusely and was mainly found in the cytoplasm, while the CD206 protein was primarily expressed in the cellular membrane (Fig. 1b). IL-32 and CD206 exhibited high expression rates in MM patients, with values of 80% (24/30) and 63.3% (19/30), respectively. Fisher’s exact test showed a positive correlation between the expression of IL-32 and CD206 (Fig. 1c). These results indicate that IL-32 expression is correlated with M2 MΦs in the BM of MM patients.
Fig. 1.
IL-32 is highly expressed in relapsed MM patients and correlated with CD206+ M2 MΦ infiltration. a IL-32 mRNA expression was abnormal in various cancer cells as determined by TIMER database analysis. ACC Adrenocortical carcinoma; BLCA Bladder Urothelial Carcinoma; BRCA Breast invasive carcinoma; CESC Cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL Cholangiocarcinoma; COAD Colon adenocarcinoma; DLBC Lymphoid Neoplasm Diffuse Large B-cell Lymphoma; ESCA Esophageal carcinoma; GBM Glioblastoma multiforme; HNSC Head and Neck squamous cell carcinoma; KICH Kidney Chromophobe; KIRC Kidney renal clear cell carcinoma; KIRP Kidney renal papillary cell carcinoma; LAML Acute Myeloid Leukemia; LGG Brain Lower Grade Glioma; LIHC Liver hepatocellular carcinoma; LUAD Lung adenocarcinoma; LUSC Lung squamous cell carcinoma; MESO Mesothelioma; OV Ovarian serous cystadenocarcinoma; PAAD Pancreatic adenocarcinoma; PCPG Pheochromocytoma and Paraganglioma; PRAD Prostate adenocarcinoma; READ Rectum adenocarcinoma; SARC Sarcoma; SKCM Skin Cutaneous Melanoma; STAD Stomach adenocarcinoma; TGCT Testicular Germ Cell Tumors; THCA Thyroid carcinoma; THYM Thymoma; UCEC Uterine Corpus Endometrial Carcinoma; UCS Uterine Carcinosarcoma; UVM Uveal Melanoma. b Representative immunohistochemistry images of IL-32 and CD206 in BM biopsies from MM patients. Scale bars, 50 μm. c Positive correlation between the expression of IL-32 and CD206 based on Fisher’s exact test. Samples from 30 MM patients were used
IL-32γ increases MΦ-mediated MM drug resistance
How IL-32 affects the relapse of MM patients remains unclear. As we previously reported, IL-32 had no direct effect on MM cells but exerted a certain influence on MΦs [22]; thus, we sought to further determine whether IL-32 influences the effect of MΦ-mediated MM drug resistance. We confirmed that IL-32γ is the main and most active isoform in MM [22]; thus, our subsequent analyses mainly focused on IL-32γ.
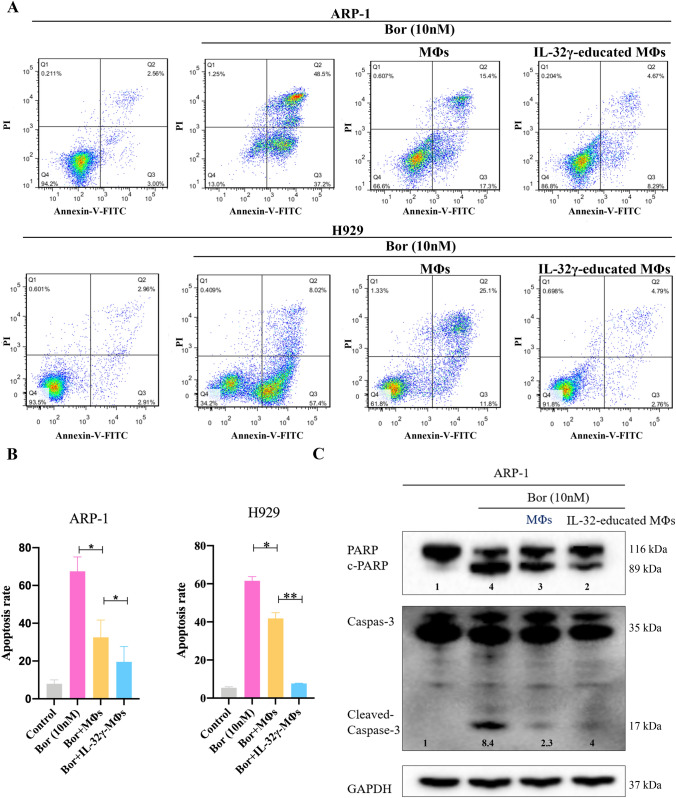
MΦs were treated with IL-32γ for 24 h to generate IL-32γ-educated MΦs and then were cocultured with MM cells and Bor. MM cells were collected for Annexin V/PI-based apoptosis detection. When ARP-1 cells were cocultured with MΦs, bortezomib induced the apoptosis of fewer MM cells (Fig. 2a). Notably, the apoptosis rate of ARP-1 cells cocultured with IL-32γ-educated MΦs was significantly lower than that in the group cocultured with MΦs (p < 0.05). We verified this effect using the H929 cell line (Fig. 2a, b). Additionally, we detected the expression of apoptotic proteins in MM cells by Western blotting. The expression of the apoptotic proteins c-PARP and c-Caspase3 in MM cells cocultured with IL-32γ-educated MΦs was significantly lower than that in those cocultured with MΦs (Fig. 2c, Supplementary Fig. 1A), a finding that was consistent with the flow cytometry results. These results indicate that IL-32γ enhances the protective effect of MΦs on MM cells and induces drug resistance in MM.
Fig. 2.
IL-32γ enhances the protective effect of MΦs on MM cells. a MΦs were stimulated with IL-32γ for 24 h and then cocultured with MM cells (ARP-1 or H929 cells), and Bor was added for 24 h to induce apoptosis. A representative flow cytometry analysis shows the apoptosis of MM cells. b Summarized results from at least three independent experiments. Values are presented as means ± SEM. c Western blotting was used to detect apoptotic proteins (c-PARP and c-Caspase3) in ARP-1 cells. The quantified density is shown below the bands. The data are representative of at least three independent experiments with similar results. *p < 0.05, **p < 0.01
IL-32γ induces the polarization of M2 MΦs
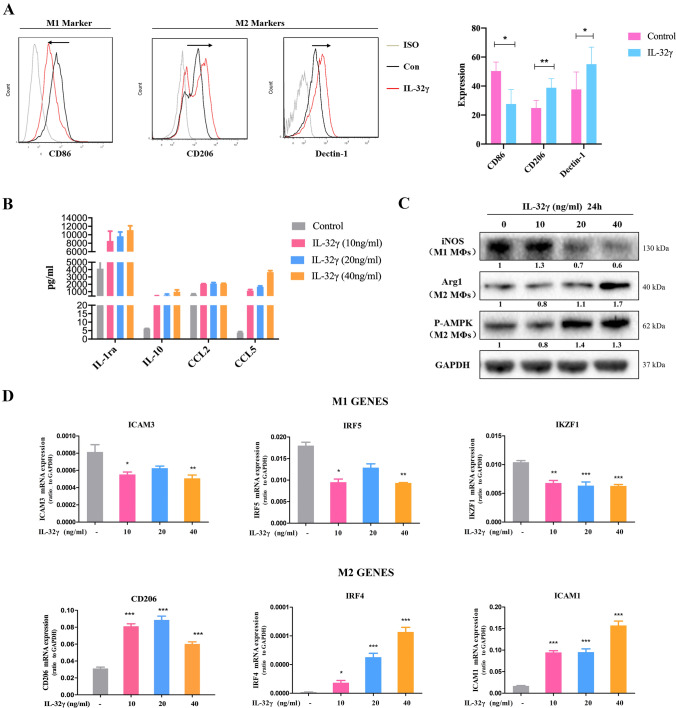
The above results suggest that IL-32γ affects MM drug sensitivity by regulating the immune characteristics of MΦs. We cultured MΦs with IL-32γ and analyzed the global transcriptional profile of the cells by RNA-seq. Compared with control cells, IL-32γ-treated MΦs expressed significantly higher levels of M2 MΦ-related molecules and lower levels of M1 MΦ-related genes (Supplementary Fig. 1B). We then verified the effect of IL-32γ on MΦ polarization by evaluating four aspects: surface markers, cytokines, metabolic enzymes and characteristic molecules. After stimulation with IL-32γ, MΦs expressed higher levels of CD206 and Dectin-1 (classic surface markers of M2 MΦs) and decreased levels of CD86 (classic surface marker of M1 MΦs) (Fig. 3a and Supplementary Fig. 1C). Additionally, a cytokine microarray showed that IL-32γ increased the secretion of IL-1ra, IL-10, CCL2 and CCL5, classic inflammation factors secreted from M2 MΦs (Fig. 3b). Some other cytokines also changed under the stimulation of IL-32γ (Supplementary Fig. 1D). The change in the IL-10 level was further confirmed by ELISA (Supplementary Fig. 1E). However, representative M1 MΦ cytokines, such as IL-12P40 and IL-12P70, were not detected. Next, we explored the metabolism of MΦs based on western blot analysis, revealing that MΦs with IL-32γ stimulation expressed higher levels of Arg1 and p-AMPK (M2 MΦ metabolic markers) and lower levels of iNOS (M1 MΦ metabolic enzyme) (Fig. 3c). Consistent with the above results, qRT-PCR showed that IL-32γ increased the expression of the M2 MΦ-related molecules (CD206, IRF4, ICAM1, CCL17, CCL24 and CD163) but decreased the level of M1 MΦ-related molecules (ICAM3, IRF5 and IKZF1) (Fig. 3d, Supplementary Fig. 1F). These results indicate that IL-32γ induces the polarization of MΦS toward the M2 phenotype, a finding that is consistent with the above effect of promoting MM drug resistance.
Fig. 3.
IL-32γ induces the polarization of M2 MΦs. a MΦs were stimulated with IL-32γ (20 ng/mL) for 24 h, and flow cytometry was used to detect the expression of CD86, CD206 and Dectin-1 on the surface of the MΦs. The summarized results are from at least three independent experiments. Values are presented as means ± SEM. b MΦs (biological samples from two donors) were stimulated with IL-32γ (10, 20 or 40 ng/mL) for 24 h, and a cytokine chip was adopted to detect the expression of IL-1a, IL-10, CCL2 and CCL5 in the cell culture supernatant. c Western blotting was used to detect the expression of iNOS, Arg1 and p-AMPK. The quantified density is shown below the bands. The data are representative of at least three independent experiments with similar results. d qRT-PCR was adopted to evaluate the expression of M1 and M2 MΦ-related molecules. Data are presented as means ± SEM of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
CSF1 is crucial in IL-32γ-induced M2 MΦ polarization and drug resistance
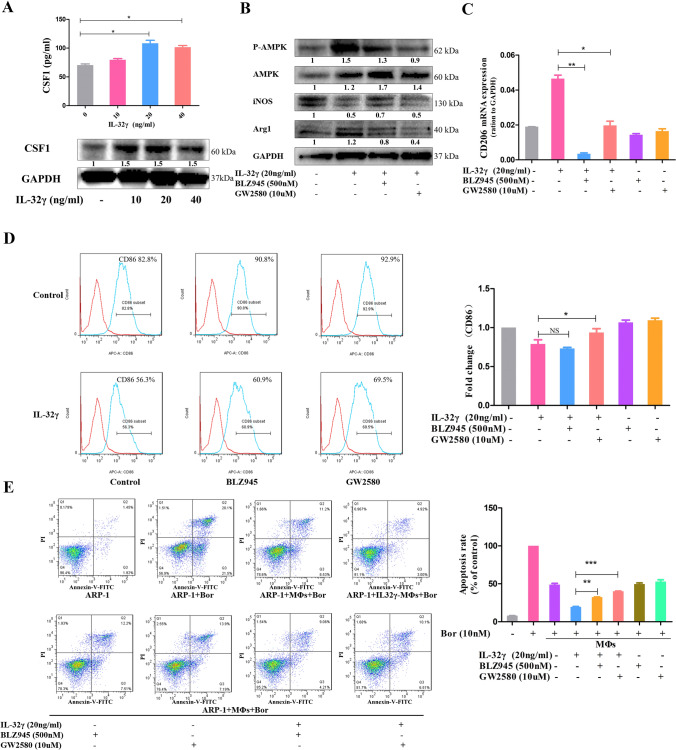
Colony-stimulating factor 1 (CSF1, M-CSF) is a key molecule in the development of MΦs, and IL-32γ upregulated CSF1 expression in MΦs, as revealed by ELISA and Western blotting (Fig. 4a). To directly clarify the role of CSF1 in IL-32γ-induced M2 MΦ polarization, two inhibitors of the CSF1 receptor (BLZ945 and GW2580) were used. The survival and activity of MΦs were not affected at the experimental concentration of inhibitors (Supplementary Fig. 2A). The promotive effects of IL-32γ on p-AMPK and Arg1 expression and reductive effects on iNOS expression in MΦs were weakened by the two inhibitors (Fig. 4b). Additionally, qRT-PCR revealed that the effect of IL-32γ increasing CD206 expression in MΦs could be completely reversed by BLZ945 or GW2580 (Fig. 4c). Flow cytometry showed that the ability of IL-32γ to reduce CD86 expression was impaired after blocking CSF1 (Fig. 4d). Our previous research demonstrated that IL-32γ significantly activates the STAT3 and NF-κB pathways in MΦs [22]. In this study, we found that inhibitors of these two pathways could weaken the effect of IL-32γ on IL-10 secretion but had no apparent inhibitory effects on MΦ subtype characteristic molecules (Supplementary Fig. 2B-D). These results suggest that CSF1 plays a crucial role in the process of IL-32γ-induced M2 MΦ polarization.
Fig. 4.
CSF1 is crucial in IL-32γ-induced M2 MΦ polarization and drug resistance. a MΦs were stimulated with different concentrations of IL-32γ (10, 20 and 40 ng/mL) for 24 h, ELISA was used to detect the content of CSF1 in the cell culture supernatant, and Western blotting was adopted to evaluate the protein level of CSF1 in MΦs. Summarized results from at least three independent experiments are shown. Values are presented as means ± SEM. b MΦs were pretreated with a CSF1 receptor inhibitor (500 nM BLZ945 or 10 μM GW2580) for 2 h and then stimulated with IL-32γ (20 ng/mL) for 24 h. The expression of iNOS, p-AMPK and Arg1 was detected by Western blotting. The quantified density is shown below the bands. Similar results were obtained in three independent experiments. c qRT-PCR was used to detect changes in CD206 expression, and summarized results from at least three experiments are shown. Values are presented as means ± SEM. d Representative and summarized results for flow cytometry analysis of CD86 expression. The summarized results are from at least three independent experiments. Values are presented as means ± SEM. e MΦs were treated as above and then cocultured with ARP-1 cells in suspension and Bor (10 nM) for 24 h. The ARP-1 cells were collected, and apoptosis was detected by flow cytometry. Similar results were obtained in three independent experiments. Values are presented as means ± SEM. NS, not statistically significant; *p < 0.05; **p < 0.01
We further explored the effect of CSF1 on the enhanced protective effect of IL-32γ-educated MΦs. MΦs were first pretreated with the above inhibitors for 2 h and then were stimulated with IL-32γ (20 ng/mL) for 24 h, and finally cocultured with ARP-1 cells and Bor (10 nM) for another 24 h. Flow cytometry showed that the number of apoptotic ARP-1 cells in the inhibitor + IL-32γ-educated MΦ group was significantly higher than that in the IL-32γ-educated MΦ group (Fig. 4e). This result demonstrated that the effect of IL-32γ-educated MΦs on inducing MM drug resistance was partly dependent on CSF1.
IL-32γ induces M2 MΦ polarization and MM drug resistance in vivo
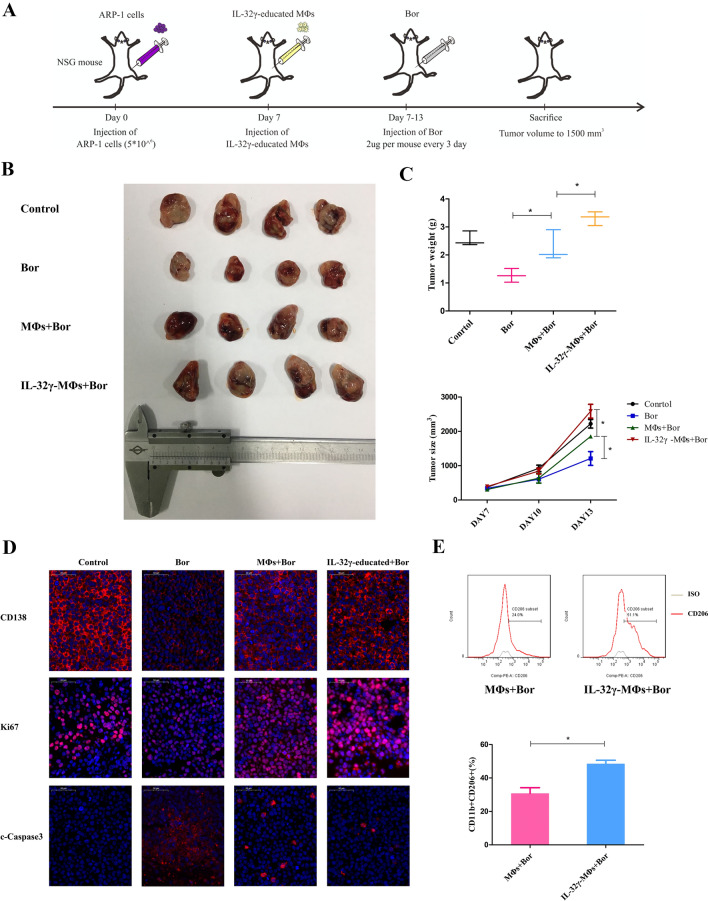
We next adopted an MM cell xenograft model to determine whether IL-32γ plays a crucial role in promoting MΦs to protect MM cells in vivo. The workflow of the vivo experiment is shown in Fig. 5a. After subcutaneous ARP-1 tumor formation in NSG mice, either IL-32γ-educated MΦs or MΦs was injected into the tumor mass, and then, Bor was administered every three days. On the 7th day after treatment, the mice were sacrificed, and the tumors were removed for related experiments (Fig. 5b). The tumor volume and weight of the IL-32γ-educated MΦ group were significantly larger than those of the MΦ group (Fig. 5c), a finding consistent with the results of the in vitro experiment. To further explore the proliferation and apoptosis of tumor cells, we used immunofluorescence to detect the expression of CD138, Ki67 and c-Caspase3 in tumor tissue. The expression of the plasma cell surface marker CD138 and proliferation marker Ki67 in the IL-32γ-educated MΦ group was higher than that in the MΦ group, while the expression of the apoptotic protein c-Caspase3 was lower than that in the MΦ group (Fig. 5D). Additionally, we mechanically disassociated tumor tissue to obtain a tumor cell suspension and detected the number of CD11b+CD206+ M2 MΦs in the tumor cell suspension by flow cytometry. The IL-32γ-educated MΦ group had more M2 MΦs than the MΦ group (Fig. 5e). The above in vivo experiments confirmed that IL-32γ enhanced the protective effect of MΦs on MM cells and increased the tumor load.
Fig. 5.
a Workflow of the in vivo experiment. NSG mice were subcutaneously inoculated in the flank with ARP-1 cells (5 × 106/mouse). When palpable tumors (> 5 mm) were detected, the mice were randomly divided into four groups (Control, Bor, Bor + MΦs, and Bor + IL-32γ-educated MΦs, n = 4 per group). MΦs were injected intratumorally only at the time of the first Bor administration. The Bor dosage was 2 μg/mouse/every 3 days. b Representative image of tumor volumes at the end point of the experiment. c Statistical results for the tumor weight and volume at the end point of the experiment. Data show the mean ± SEM of at least three mice per group. d Immunofluorescence analysis of CD138, Ki67 and cleaved caspase-3 expression in tumor tissues from mice in different groups. Scale bar, 50 μm. e Representative and summarized results for flow cytometry analysis of the number of CD11b+CD206+ M2 MΦs in tumor tissue. Data show the mean ± SEM of at least three mice per group. *p < 0.05
Discussion
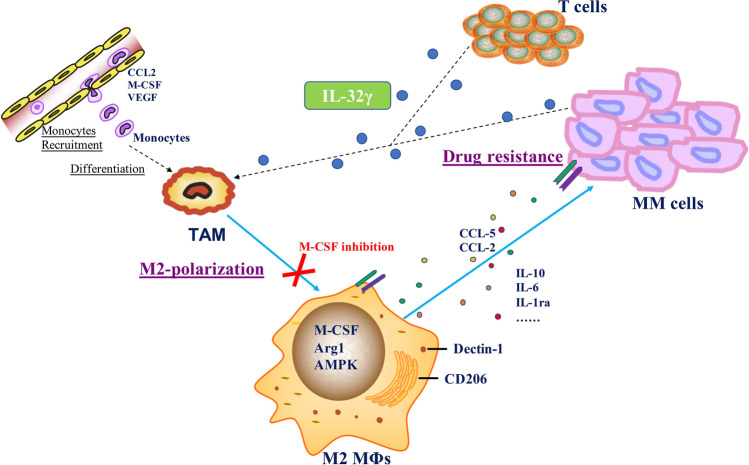
Herein, IL-32 was highly expressed in relapsed MM patients and significantly associated with the infiltration of CD206+ M2 MΦs in patient samples. Notably, the most active isoform, IL-32γ, increased MΦ-mediated MM drug resistance and induced the polarization of M2 MΦs, a function that was partly dependent on CSF1 (Fig. 6).
Fig. 6.
Summary of the main findings revealed in the study. IL-32γ increases MΦ-mediated MM drug resistance and induces the polarization of M2 MΦs, a function that is partly dependent on CSF1
IL‑32, as a proinflammatory cytokine, plays various roles in human cancer pathological processes, including tumor initiation, proliferation and maintenance [13]. In MM, we previously demonstrated that IL-32 promoted the proliferation of MM cells by increasing various inflammatory chemokines in BMSCs [21]. Furthermore, we revealed that IL-32γ induced the immunosuppressive microenvironment of MM by increasing the expression of the immunosuppressive molecule indoleamine 2,3-dioxygenase (IDO) in MΦs [22]. Another group reported that IL-32 promoted osteoclast differentiation and was associated with bone disease and inferior survival in MM [24]. Our recent research showed that IL-32 was highly expressed in relapsed MM patients [23]. In the present study, IL-32 expression was significantly associated with the CD206+ M2 MΦs in the BM of MM patients. These results revealed the clinical significance of IL-32 in MM treatment, and further study is needed in more patient samples.
The infiltration of MΦs in the tumor microenvironment is correlated with a poor prognosis and is associated with chemotherapy resistance in most cancers [9]. In MM, MΦs were reported to promote the growth of MM cells and protect them from apoptosis induced by chemotherapeutic drugs, thereby inducing drug resistance in MM cells [25]. In the present study, we found that, after MM cells were cocultured with MΦs, the number of cells killed by Bor was significantly reduced, a finding that was consistent with previous report findings [4]. Furthermore, the number of apoptotic MM cells cocultured with IL-32γ-educated MΦs was lower than that cocultured with MΦs, a finding that was further confirmed by evaluating the expression of the apoptotic proteins c-PARP and c-Caspase3. These results indicate that IL-32γ is important for the protective effect of MΦs on MM cells. This tumor-promoting feature of IL-32γ-educated MΦs suggests that IL-32γ may modify the MΦ phenotype to M2.
The biological function of IL-32 is being revealed gradually, and an increasing number of studies have revealed its effect on immune cell function. For example, IL-32 can enhance the ability of NK cells to kill tumor cells [19]. Additionally, IL-32 induced differentiation of peripheral blood monocytes to MΦs and dendritic cell (DC)-like cells with phagocytic function [26]. Other studies have reported that IL-32 promoted the differentiation of monocytes into CD1c+ DCs and CD163+CD68+ MΦs [27]; however, whether IL-32 affects the polarization of MΦs remains unclear. In this study, we detected the expression of MΦ surface molecules, secreted cytokines, characteristic metabolic enzymes and molecules, confirming that IL-32γ could induce the polarization of M2 MΦs. The MΦs in the BM of MM patients mainly comprise the M2 type [28]. Additionally, soluble CD206 in MM is an independent prognostic marker of poor overall survival. Consistent with this finding, we found that IL-32 was positively associated with CD206+ M2 MΦs in MM patient samples.
The phenotype and function of MΦs in tumors are different from those of normal MΦs and are regulated by various immune signals in the tumor microenvironment [29]. Previous studies have shown that tumor-related MΦs mainly exhibit an M2 phenotype, which can be mediated by cytokines such as IL-4 and IL-13 and signaling molecules such as STAT3, IRF4 and SOCS1 [7]. Our previous studies demonstrated that IL-32γ significantly activates the STAT3 and NF-κB pathways in MΦs [22]. In the present study, we found that inhibitors of these two pathways could weaken the effect of IL-32γ on IL-10 secretion but had no apparent inhibitory effects on MΦ subtype characteristic molecules. Thus, STAT3 and NF-κB were not considered key pathways involved in M2 MΦ polarization induced by IL-32γ. CSF1 is a well-known molecule that is key for the maintenance of MΦ survival and upregulated by IL-32γ [30]. We pretreated MΦs with two inhibitors of the CSF1 receptor (BLZ945 and GW2580) and ensured that the survival and activity of MΦs were not affected at the experimental concentration. Both inhibitors weakened the polarization of M2 MΦs induced by IL-32γ and the protective effect of IL-32γ-educated MΦs on MM cells, indicating that CSF1 plays a certain role in the effect of IL-32γ on MΦs. Notably, treatment that targets the CSF1 receptor to modify MΦ function has shown a good therapeutic effect in many tumors [31, 32]. Recent studies have reported that inhibitors of the CSF1 receptor can reverse M2 polarization to the M1 phenotype, thus inhibiting the proliferation and survival of MM cells [33].
Immunodeficient mice are a common animal model to study the occurrence and development of tumors [34]. NSG mice are highly immunodeficient mice and are suitable for human cell transplantation modeling [35]. In this experiment, we subcutaneously transplanted ARP-1 cells into 4-week-old NSG mice. Because IL-32 is not expressed in rodents, we pretreated MΦs with IL-32γ in vitro and injected IL-32γ-educated MΦs or MΦs into the mice. The tumor burden of the IL-32γ-educated MΦ group was significantly higher than that of the MΦ group, indicating that the protective effect of IL-32γ-educated MΦs on MM was stronger than that of MΦs. These results were consistent with those of the vitro experiment. Because rodents do not express IL-32, researchers have constructed IL-32-overexpressing transgenic mice for in vivo studies related to inflammation and tumors [36]. Our group constructed transgenic mice overexpressing IL-32γ and will further explore the content of this study in follow-up experiments.
In summary, our study demonstrated that IL-32 was highly expressed in relapsed MM patients and correlated with the CD206+ M2 MΦ infiltration level. Additionally, IL-32γ induced the polarization of M2 MΦs and enhanced the protective effect of MΦs on MM cells by upregulating CSF1 expression. This study provides new insight into the role of IL-32γ in MM drug resistance, providing an important theoretical basis for targeted MΦ immunotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contributions
HY and JQ contributed to conceptualization, formal analysis, validation and writing—original draft. YL and RX contributed to data curation and writing—review and editing. HG, DH and YL contributed to investigation, software and methodology. EZ, YZ and JH contributed to visualization, project administration, and resources, JC and ZC contributed to funding acquisition, supervision, project administration, and validation.
Funding
This work was supported by grants from the National Natural Science Foundation of China [Grant numbers 81900209 and 81872322], Natural Science Foundation of Zhejiang Province [Grant number LQ22H080001] and Zhejiang Key Research and Development Project [Grant number 2020C03014].
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haimeng Yan and Donghua He are contributed equally to this work.
References
- 1.Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, Gay F, Anderson KC. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Li Y, Gu H, Dong M, Cai Z. Emerging agents and regimens for multiple myeloma. J Hematol Oncol. 2020;13:150. doi: 10.1186/s13045-020-00980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawano Y, Roccaro AM, Ghobrial IM, Azzi J. Multiple myeloma and the immune microenvironment. Curr Cancer Drug Targets. 2017;17:806–818. doi: 10.2174/1568009617666170214102301. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berardi S, Ria R, Reale A, De Luisi A, Catacchio I, Moschetta M, Vacca A. Multiple myeloma macrophages: pivotal players in the tumor microenvironment. J Oncol. 2013;2013:183602. doi: 10.1155/2013/183602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, He D, Chen Q, Guo X, Yang L, Lin X, Li Y, Wu W, Yang Y, He J, Zhang E, Yi Q, Cai Z. BAFF is involved in macrophage-induced bortezomib resistance in myeloma. Cell Death Dis. 2017;8:e3161. doi: 10.1038/cddis.2017.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment, F1000prime reports, 6:13. [DOI] [PMC free article] [PubMed]
- 8.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassetta L. Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer, Nature reviews. Drug Discovery. [DOI] [PubMed]
- 10.Panchabhai S, Kelemen K, Ahmann G, Sebastian S, Mantei J, Fonseca R. Tumor-associated macrophages and extracellular matrix metalloproteinase inducer in prognosis of multiple myeloma. Leukemia. 2016;30:951–954. doi: 10.1038/leu.2015.191. [DOI] [PubMed] [Google Scholar]
- 11.Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. doi: 10.4049/jimmunol.148.2.597. [DOI] [PubMed] [Google Scholar]
- 12.Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH (2017) Interleukin 3. Inflammation Cancer Pharmacol Therapeutics (2017). [DOI] [PubMed]
- 13.Yan H, He D, Huang X, Zhang E, Chen Q, Xu R, Liu X, Zi F, Cai Z. Role of interleukin-32 in cancer biology. Oncol Lett. 2018;16:41–47. doi: 10.3892/ol.2018.8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, Jang YJ, Ahn DK, Kim JW, Song EY. Dysregulation of overexpressed IL-32alpha in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-kappaB and Bcl-2. Cancer Lett. 2012;318:226–233. doi: 10.1016/j.canlet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH, Lin KH. Interleukin-32 increases human gastric cancer cell invasion associated with tumor progression and metastasis. Clin Cancer Res Official J Am Assoc Can Res. 2014;20:2276–2288. doi: 10.1158/1078-0432.CCR-13-1221. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang L, Huang W, Huang L, Wang Q. Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine. 2014;65:24–32. doi: 10.1016/j.cyto.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Park ES, Yoo JM, Yoo HS, Yoon DY, Yun YP, Hong J. IL-32gamma enhances TNF-alpha-induced cell death in colon cancer. Mol Carcinog. 2014;53(Suppl 1):E23–35. doi: 10.1002/mc.21990. [DOI] [PubMed] [Google Scholar]
- 19.Park MH, Song MJ, Cho MC, Moon DC, Yoon do Y, Han SB, Hong JT. Interleukin-32 enhances cytotoxic effect of natural killer cells to cancer cells via activation of death receptor 3. Immunology. 2012;135:63–72. doi: 10.1111/j.1365-2567.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 21.Lin X, Yang L, Wang G, Zi F, Yan H, Guo X, Chen J, Chen Q, Huang X, Li Y. Interleukin-32α promotes the proliferation of multiple myeloma cells by inducing production of IL-6 in bone marrow stromal cells. Oncotarget. 2017;8:92841–92854. doi: 10.18632/oncotarget.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan H, Dong M, Liu X, Shen Q, He D, Huang X, Zhang E, Lin X, Chen Q, Guo X, Chen J, Zheng G, Wang G, He J, Yi Q, Cai Z. Multiple myeloma cell-derived IL-32γ increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett. 2019;446:38–48. doi: 10.1016/j.canlet.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Yan H, Gu H, Zhang E, He J, Cao W, Qu J, Xu R, Cao L, He D, Zhang J, Hou Y, Cai Z. Myeloma-derived IL-32gamma induced PD-L1 expression in macrophages facilitates immune escape via the PFKFB3-JAK1 axis. Oncoimmunology. 2022;11:2057837. doi: 10.1080/2162402X.2022.2057837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahoor M, Westhrin M, Aass KR, Moen SH, Misund K, Psonka-Antonczyk KM, Giliberto M, Buene G, Sundan A, Waage A. Hypoxia promotes IL-32 expression in myeloma cells, and high expression is associated with poor survival and bone loss. Blood Adv. 2017;1:2656. doi: 10.1182/bloodadvances.2017010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu R, Li Y, Yan H, Zhang E, Huang X, Chen Q, Chen J, Qu J, Liu Y, He J, Yi Q, Cai Z. CCL2 promotes macrophages-associated chemoresistance via MCPIP1 dual catalytic activities in multiple myeloma. Cell Death Dis. 2019;10:781. doi: 10.1038/s41419-019-2012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohmatsu H, Humme D, Gonzalez J, Gulati N, Möbs M, Sterry W, Krueger JG (2016) IL-32 induces indoleamine 2,3-dioxygenase+CD1c+dendritic cells and indoleamine 2,3-dioxygenase+CD163+macrophages: relevance to Mycosis Fungoides progression. OncoImmunology. [DOI] [PMC free article] [PubMed]
- 28.Chen X, Chen J, Zhang W, Sun R, Liu T, Zheng Y, Wu Y. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget. 2017;8:112685–112696. doi: 10.18632/oncotarget.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronte V, Murray PJ. Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat Med. 2015;21:117–119. doi: 10.1038/nm.3794. [DOI] [PubMed] [Google Scholar]
- 30.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100:481–489. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carola H. Ries, Michael A. Cannarile, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Leon P. Pradel, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Ingo H. Gorr, Walz A, Abiraj K, Philippe A. Cassier, Sica A, Gomez-Roca C, Karin E. de Visser, Italiano A, Le Tourneau C, Delord J-P, Levitsky H, BlayJ-Y, Rüttinger D (2014) Targeting tumor-associated macrophages with Anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25:846–859. [DOI] [PubMed]
- 32.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Lu Y, Li R, Jiang Y, Zheng Y, Qian J, Bi E, Zheng C, Hou J, Wang S, Yi Q. Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma. Leukemia. 2018;32:176–183. doi: 10.1038/leu.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paton-Hough J, Chantry AD, Lawson MA. A review of current murine models of multiple myeloma used to assess the efficacy of therapeutic agents on tumour growth and bone disease. Bone. 2015;77:57–68. doi: 10.1016/j.bone.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Xia X, Li H, Satheesan S, Zhou J, Rossi JJ (2019) Humanized NOD/SCID/IL2rgammanull (hu-NSG) mouse model for HIV replication and latency studies. J Visualized Exp JoVE. [DOI] [PMC free article] [PubMed]
- 36.Lee DH, Kim DH, Hwang CJ, Song S, Han SB, Kim Y, Yoo HS, Jung YS, Kim SH, Yoon DY, Hong JT. Interleukin-32gamma attenuates ethanol-induced liver injury by the inhibition of cytochrome P450 2E1 expression and inflammatory responses. Clin Sci. 2015;128:695–706. doi: 10.1042/CS20140576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.