Abstract
While PD-1/L1 inhibitors are characterized by durable tumor control, they also prolong survival without prolongation of progression-free survival (PFS) in part of patients. However, little is known about the factors and mechanisms involved in this. Between December 2015 and September 2018, 106 patients with advanced non-small cell lung cancer treated with ICI monotherapy were enrolled in a prospective-observational study. Sixty-nine of whom progressed or died within 6 months after ICI initiation were defined as patients without durable clinical benefit (NDBs). Clinical factors and 39 serum proteins before ICI initiation and at the time of progressive disease (PD) were explored for an association with overall survival (OS) and OS after PD (OS-PD). As a result, median PFS, OS, and OS-PD were 44 days [95% confidence interval (CI): 39–56), 211 days (95% CI: 158–425), and 193 days (95% CI: 118–349), respectively. By multivariate analysis for OS, CRP (> 1.44 mg/dl) [HR 2.59 (95% CI:1.33–5.04), P = 0.005] and follistatin (> 685 pg/ml) [HR 2.29 (95% CI:1.12–4.69), P = 0.023] before ICI initiation were significantly predictive. Notably, no serum protein at the time of PD was predictive for OS-PD. There were also no serum predictive factors of OS in the 33 patients with durable clinical benefit. In conclusion, serum levels of CRP and follistatin before ICI initiation, not at the time of PD, are predictive for OS in NDBs, suggesting long-term survivor in NDBs are predetermined by the immune status before ICI initiation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03141-4.
Keywords: Non-small cell lung cancer, Immune checkpoint inhibitor, CRP, Follistatin, PD-1
Introduction
Immune checkpoint inhibitors (ICI), such as PD-1/L1 inhibitors, are characterized by their long-term tumor-suppressive effects and are now indispensable in the treatment of advanced non-small cell lung cancer (NSCLC) [1–4]. Such long-term “durable” efficacy has not been reported from any other chemotherapeutic agents, although a wide range of research has been conducted to elucidate the mechanisms of the durable clinical benefit and to explore related factors.
Another important feature of ICI is that they prolong overall survival (OS) more than they prolong progression-free survival (PFS). In an integrated analysis of five-year follow-up data from Checkmate 017 and Checkmate 057, phase III trials that demonstrated better clinical benefits of PD-1/L1 inhibitors to docetaxel in the second-line treatment of NSCLC, the hazard ratio (HR) for OS was 0.68 (0.59–0.78), but the HR for PFS was 0.79 (0.6–0.92) [1]. In similar studies, KEYNOTE010 [2] and OAK [3], the HRs of OS were 0.69 (0.60–0.80) and 0.73 (0.62–0.87), but the HRs of PFS were 0.83 (0.72–0.96), and 0.95 (0.82–1.10), respectively, showing deviations not experienced with chemotherapy. Furthermore, in the OAK study, patients who had progressive disease (PD) at their best response had a two-year survival rate of 17% when treated with atezolizumab, which was better than the 7% in patients who had PD when treated with docetaxel [3] Even in a comparison of patients whose best response to treatment was PD, atezolizumab was shown to significantly prolong survival [HR 0.76 (0.59–0.98)] [4]. These results also suggest that ICI can prolong OS even in patients without PFS prolongation with ICI. However, the factors and mechanisms involved in this have not been widely understood until now.
The relationship between the effect of ICIs and serum protein levels has been increasingly reported in recent years. Muller et al. combined the results of mass spectrometry analysis of serum proteins with clinical data and classified it into three groups by machine learning with 289 patients, which resulted in clear difference of ICI efficacy among three groups [5]. Basal levels and/or changes of serum IL-6, IL-8, CRP, and follistatin after ICI administration were also shown to be associated with the efficacy of ICI [6–11]. In patients who did not durably suppress tumor with ICI, it remains unknown whether such serum protein concentrations are predictive for OS. Furthermore, exploring the association between serum protein levels (before ICI initiation and at the time of PD) and OS will be a clue to the understanding of whether the long-term survival after PD with ICIs is due to the immune status before or after ICI commencement.
This study, therefore, focuses upon patients without durable clinical benefit, and explores for serum proteins and clinical factors related to OS, PFS, and OS after PD (OS-PD) in such poor-PFS populations with ICI.
Materials and methods
Patients, data collection, and study design
Between December 2015 and September 2018, 106 patients with advanced NSCLC began treatment with ICI monotherapy (nivolumab, pembrolizumab, or atezolizumab) at Wakayama Medical University Hospital. They were enrolled in a prospective biomarker study (UMIN000024414) which prespecified the timing of blood collection and radiological evaluation [12]. The primary objective of the observational study was to explore factors associated with the efficacy of ICIs. All participants provided written informed consent. Peripheral blood was collected several times, including just prior to initiation of ICI treatment and at the time of PD. Treatment efficacy was evaluated through radiological examination every 6–8 weeks according to RECIST version 1.1. Results from the initial biomarker study have been published by Akamatsu et al. and others [6, 7, 12]. This time, additional analyses were made to those of the biomarker study.
Of the 106 cases, 104 were treated with ICI monotherapy for the first time. Sixty-nine of them who progressed or died within six months of the ICI initiation were classified as patients without durable clinical benefit (NDBs). Of the other 35 patients, 33 patients who did not die and had image confirmation of no tumor progression for more than 6 months were considered to have had durable clinical benefit (DBs). OS was defined as the period from the day of ICI initiation to death from any cause or to the latest visit. PFS was defined as the period from the day of ICI initiation to the time of progression or death. We also calculated OS-PD, the period from the time of progression with ICI to either death or to the last visit. OS, PFS, and OS-PD curves were calculated by Kaplan–Meier method and were compared by log-rank test.
Biomarker analysis
In the prospective observational study, 25 mL of peripheral blood was collected, and 39 multiplexed serum proteins were quantified using Luminex 200 analyzer (Luminex, Austin, TX) with Milliplex MAP system (Millipore, Billerica, MA). Assays were performed in accordance with the manufacturer’s instructions with human cytokine/chemokine panel 1, human angiogenesis/growth factor panel 1 and a multi-species TGF-β panel, as shown previously [7, 8]. Briefly, standards or serum samples were mixed with antibody-bound, chemically dyed beads, and incubated overnight at 4 °C. Beads were washed and then incubated with the biotinylated detection antibody for one hour at room temperature. After washing, the beads were incubated with phycoerythrin-labeled streptavidin for 30 min at room temperature and the median fluorescent intensities were quantified. All samples were measured in duplicate. PD-L1 immunohistochemistry was done using tumor tissue specimen with 22C3 pharmDx antibody (clone 22C3; Dako North America, Inc., Carpinteria, CA) in accordance with recommended methods. Some of the results have been previously published [7, 8, 13].
Statistical analysis
To explore factors associated with OS, we performed univariate analysis of the following baseline covariates in Cox proportional hazards models: age, Eastern Cooperative Oncology Group performance status (PS) (0–1 vs 2–4), sex, smoking history (yes vs no), PD-L1 tumor proportion score (TPS) (≥ 50% vs not or unknown), treatment line of ICI monotherapy (1st or 2nd vs ≥ 3rd), presence of brain metastasis (yes vs no), presence of liver metastasis (yes vs no), history of radiation therapy (yes vs no), neutrophil to lymphocyte ratio (NLR), CRP and levels of the 39 measured serum proteins (high vs low). NLR, CRP and 39 serum protein levels were divided into two groups depending on being higher or lower than each median. To explore predictive factors of OS and OS-PD, an association between factors before ICI initiation and OS and OS-PD, and between factors at the time of PD and OS-PD were separately analyzed in the univariate Cox proportional hazard models. Factors with significant (P < 0.05) association in the univariate analysis were additionally evaluated in multivariate models, in which all the factors were adjusted for age, sex, and PS. PD-L1 TPS is well known to be a factor associated with the efficacy of ICI treatment for patients with metastatic non-small cell lung cancer; however it was not included as a mandatory adjustment factor here, because the role in NDBs is unclear. The measured serum protein levels were evaluated for their correlation by Spearman's rank correlation coefficient. Factors with correlation coefficients above 0.7 or below − 0.7 were defined as correlated. Statistical analyses were conducted with JMP version 14.0 (SAS Inc., Cary, USA). For all analyses, P values were two-sided, and P < 0.05 was considered statistically significant. This study conforms with the principles of the Declaration of Helsinki and with approval by the ethics committee in Wakayama Medical University.
Results
Clinical characteristics of patients without durable clinical Benefit
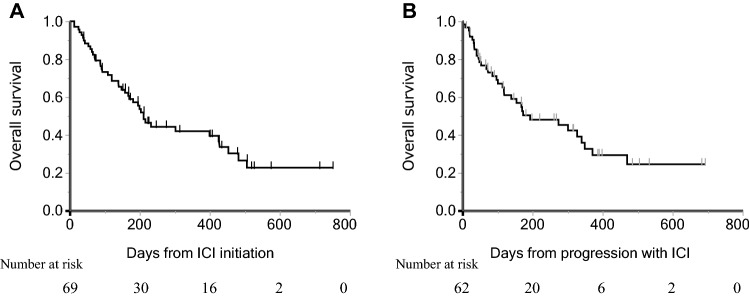
Characteristics of the 69 NDBs are shown in Table 1. Median age was 70 (range, 31–91), and 50 patients (72%) were men. Forty-nine patients (71%) were PS 0–1 and 50 patients (72%) had non-squamous cell carcinoma. Forty-five patients (65%) received 1st or 2nd line ICI monotherapy. The PD-L1 TPS was ≥ 50% in 30 patients (43%). Regarding the ICIs administered, 34, 27, and 8 patients were treated with nivolumab, pembrolizumab, and atezolizumab, respectively. Median PFS was 44 days (95% confidence interval (CI): 39–56) (Fig. 1), and median OS and OS-PD were 211 (95% CI: 158–425) and 193 days (95% CI: 118–349), respectively (Fig. 2A, B). The best tumor response to ICI treatment was partial response in six patients, stable disease in 18 patients, PD in 41 patients and four patients were not evaluable. The one-year survival rate for all enrolled patients was 24.6%. Thirty-six patients received systemic therapy after ICI, and the anticancer agents administered immediately after ICI treatment were platinum plus pemetrexed with or without bevacizumab in six patients, docetaxel combined with ramucirumab in six patients, docetaxel in five patients, S-1 in nine patients, pemetrexed in three patients, other platinum doublet (cisplatin and gemcitabine, carboplatin and nab-paclitaxel) in two patients, and five other patients received other anticancer agents.
Table 1.
Patient Characteristics
| Number of cases | 69 |
| Age, years [median (range)] | 70 (31–91) |
| Sex [n (%)] | |
| Male | 50 (72) |
| Female | 19 (28) |
| ECOG PS [n (%)] | |
| 0–1 | 49 (71) |
| 2–4 | 20 (29) |
| Smoking history [n (%)] | |
| Current or Ex | 53 (76) |
| Never | 16 (23) |
| Tumor histology [n (%)] | |
| Non-squamous cell carcinoma | 50 (72) |
| Squamous cell carcinoma | 19 (28) |
| PD-L1 TPS [n (%)] | |
| ≥ 50% | 20 (29) |
| 1–49% | 11 (16) |
| < 1% | 8 (12) |
| Unknown | 30 (43) |
| Targetable driver mutations [n (%)] | |
| EGFR | 10 (14) |
| ALK | 3 (4) |
| ROS1 | 1 (1) |
| None | 41 (59) |
| Not examined | 14 (20) |
| Previous treatment [n (%)] | |
| 0 or 1 | 45 (65) |
| ≥ 2 | 24 (35) |
| ICI agents [n (%)] | |
| Nivolumab | 34 (49) |
| Pembrolizumab | 27 (39) |
| Atezolizumab | 8 (12) |
| Brain metastasis, yes [n (%)] | 24 (35) |
| Liver metastasis, yes [n (%)] | 11 (16) |
ALK Anaplastic lymphoma kinase, ECOG PS Astern Cooperative Oncology Group performance status, EGFR Epidermal growth factor receptor, ICI Immune checkpoint inhibitor, PD-L1 Programmed cell death ligand 1, ROS1 ROS Proto-oncogene 1, TPS Tumor proportion score
Fig. 1.
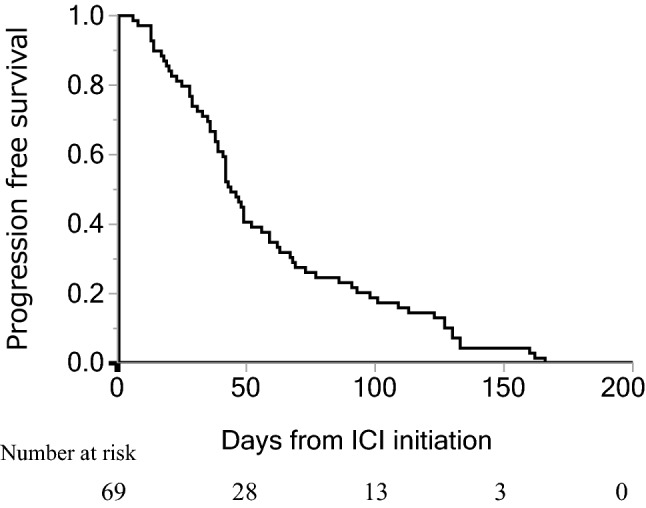
Progression-free survival in patients without durable clinical benefit
Fig. 2.
Kaplan–Meyer curves of A Overall survival from the start of immune-checkpoint inhibitors and B Overall survival from progression with immune-checkpoint inhibitors in patients without durable clinical benefit
Predictive factor analysis for survival from ICI initiation and disease progression
In univariate analysis for OS with factors before ICI start, baseline NLR, CRP, follistatin, leptin, placental growth factor, growth-related oncogene, and IL-8 were negatively associated with OS (Supplemental Table 1). Correlation analysis of all measured serum proteins and NLR revealed that there are correlations for several factors, but no significant correlations were found for these seven factors selected above (Supplementary Fig. S1). According to multivariate analysis for the seven factors adjusted for age, sex, and PS (0–1 or not) before ICI start, CRP (> 1.44 mg/dl) [HR 2.59 (95% CI:1.33–5.04), P = 0.005], and follistatin (> 685 pg/ml) [HR 2.29 (95% CI:1.12–4.69), P = 0.023] had significant association (Table 2). CRP was also associated with OS-PD (HR 2.53, P = 0.014) (Supplementary Table 2). Factors at the time of PD were also analyzed to explore the predictivity for OS-PD. In 47 patients with serum protein measurement at the time of PD, NLR, vascular endothelial growth factor-A, IL-10, and platelet-derived growth factor-AB, BB were significant in univariate analysis, although no factors were significant in the multivariate analysis when adjusted with the clinical factors shown above (Supplementary Table 3, 4). Correlations were also evaluated for these selected factors, and again, no factors had correlation (Supplemental Table 5). Comparison of serum protein levels before ICI start between patients with and without PD-L1 TPS≧50 is shown in Supplemental Table 6. Endoglin, HGF, VEGF-A, and GM-CSF had significant difference while CRP and follistatin did not.
Table 2.
Multivariate analysis of factors before ICI start to predict overall survival in patients without durable clinical benefit
| HR | 95%CI | P value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| NLR high (vs. low) | 1.636 | 0.843 | 3.165 | 0.146 |
| CRP high (vs. low) | 2.586 | 1.326 | 5.042 | 0.005 |
| Follistatin high (vs. low) | 2.294 | 1.122 | 4.686 | 0.023 |
| Leptin high (vs. low) | 0.474 | 0.224 | 1.004 | 0.051 |
| PLGF high (vs. low) | 1.725 | 0.886 | 3.359 | 0.109 |
| GRO high (vs. low) | 1.666 | 0.845 | 3.287 | 0.141 |
| IL-8 high (vs. low) | 1.797 | 0.848 | 3.810 | 0.126 |
CRP C-reactive protein, CI Confidence interval, GRO Growth-related oncogene, HR Hazard ratio, ICI Immune checkpoint inhibitor, IL-8 Interleukin-8, NLR Neutrophil-to-Lymphocyte rate
Survival and progression-free survival curves in patients without durable clinical benefit
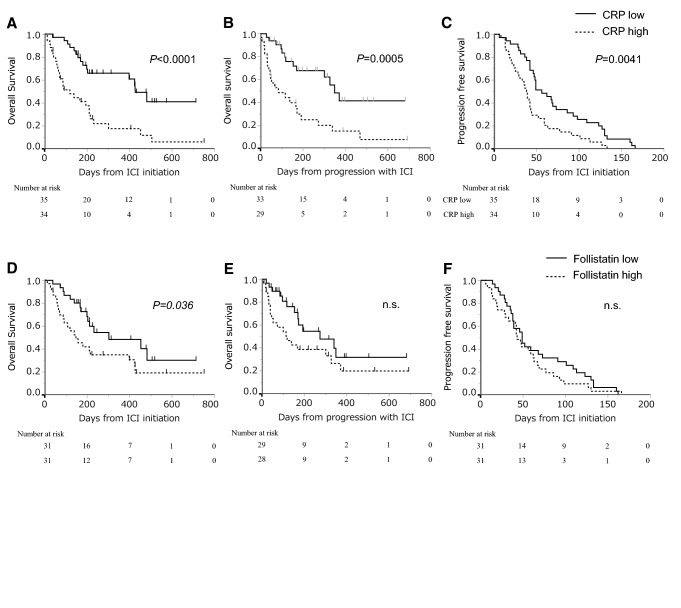
NDBs were next divided into two groups according to whether CRP and follistatin were higher or lower than each median, and OS, PFS and OS-PD were compared between these groups. PFS, OS and OS-PD were significantly longer in patients with low CRP (≤ 1.44 mg/dl) than in patients with high CRP [median PFS: 56 (95% CI: 46–73) days vs 38.5 (95% CI: 25–43) days, P = 0.004 (Fig. 3A), median OS: 428 (95% CI: 203–not reached) days vs 120 (95% CI: 66–211) days, P < 0.0001 (Fig. 3B), and median OS-PD: 349 (95% CI: 170–not reached) days vs 85 (95% CI: 40–173) days, P = 0.0005 (Fig. 3C)]. In patients with low follistatin (≤ 685 pg/ml), OS was significantly longer than in patients with high follistatin [median OS: 302 (95% CI: 199–not reached) days vs 148 (95% CI: 71–399) days, P = 0.036 (Fig. 3E)], while PFS and OS-PD were not significantly different from patients with high follistatin [P = 0.303, P = 0.084, respectively (Fig. 3D, F)].
Fig. 3.
Comparison between patients with low and high CRP, and between patients with low and high serum follistatin. A, D Overall survival from the start of immune-checkpoint inhibitors; B, E Overall survival from progression with immune-checkpoint inhibitors; C, F progression-free survival, in patients without durable clinical benefit
Analysis in patients with durable clinical benefit
In 33 DBs, median age was 68 years (range, 52–90), and 28 patients (85%) were men. Twenty-nine patients (88%) were PS 0–1 and 23 patients (66%) had non-squamous cell carcinoma. PD-L1 TPS ≧50 was observed in 12 patients (36%). The median PFS for these was 16.5 months (95% CI 9.77—NR), and the median OS was not reached (95% CI 9.77–NR) (Supplemental Fig. S2 and S3). We also performed the same analysis as NDBs; however no factors, including CRP and follistatin were significantly associated with OS (data not shown). To confirm no association between CRP or follistatin with OS, additional multivariate analysis was performed, but again, no statistically significant difference was found [follistatin (high): HR 0.81, 95%CI 0.126–5.21, P = 0.823; CRP (high): HR 0.88, 95%CI 0.141–5.50, P = 0.892].
Discussion
This study was conducted to explore the factors related to the mechanism of the OS prolongation in patients without apparent, durable disease suppression, which is an event of interest in ICIs. Finding the predictive factors of OS in this population would also be useful for determining the treatment strategy after PD with ICIs. In the current study there are two important points. The first is that serum levels of protein (CRP and follistatin) before ICI start were associated with OS in NDBs while not in DBs. The second was that these serum factors only before ICI administration, not at the time of PD, were predictive for OS.
CRP is an indicator of systemic inflammation that is excited by IL-6, and IL-6 is a common inflammatory cytokine and is known to be pleiotropic. Although IL-6 is protective against infection, it also functions as an immune-suppressant through various mechanisms, including induction of regulatory T cell, myeloid-derived suppressor cells, and type 2 macrophages, or exhaustion of lymphocytes [14]. Follistatin, a secreted protein, is ubiquitously expressed in humans, and functions as a potent activin and bone morphogenetic protein binding protein, thereby antagonizing these proteins [7]. Our results showed for the first time that serum CRP and follistatin were negatively associated with survival in NDBs while not with survival in DBs. Considering easiness of measurement, serum proteins like CRP and follistatin would be of interest as a promising subject for future research in this population. Furthermore, it also should be noted our results suggest predictive factors may be different between DBs and NDBs.
To date, it has been unclear why the improvement of OS is better than the improvement of PFS in most clinical trials in ICI monotherapy [1, 2, 4, 15, 16]. One possible explanation is the enhanced antitumor effect of posttreatment. Schvartsman et al. extracted 28 patients who received single-agent chemotherapy after ICI at third or later line from thoracic GEMINI database and conducted analysis. The results showed a good response rate of 39%, with a median PFS of 4.7 months and a median OS of 9.0 months, despite 46.4% of the patients being in the fifth or later lines [17]. Park et al. reported that in 73 patients treated with PD-1/L1 inhibitors followed by chemotherapy, the response rate with systemic chemotherapy immediately after PD-1/L1 inhibitors was significantly better than with chemotherapy before PD-1/L1 inhibitors (53.4% vs 34.9%, P = 0.03). The difference was seen with both non-platinum and platinum-based regimens [18]. In this study, 36 patients received systemic chemotherapy after PD-1/L1 inhibitors and a single agent (including 6 of docetaxel combined with ramucirumab) was administered in 28, of which the response rate was as good as 26.1%. There has been no clear explanation for the mechanism about this efficacy enhancement of the sequence chemotherapy until now. As serum proteins prior to the start of ICI, but not at the time of PD, were associated with OS in this population with short PFS, survival and therapeutic efficacy after ICI treatment are, at least to some extent, predetermined by immune status prior to the start of ICI treatment.
In the analysis of DBs, no obvious serum proteins were found to be associated with OS, including CRP and follistatin, which were associated with survival in NDBs. Analysis is limited by the small number of cases, 33, and death during the observed period, only 8 owing to the good efficacy of ICIs in DBs. Nonetheless, the results indicate that predictors of ICI efficacy may differ between the patients with good and poor PFS when treated with ICI.
There are several limitations to our study. First, the size of this study was small, so we cannot be conclusive. However, very few studies have prospectively enrolled patients, collected sera both pre-treatment and at the time of PD as planned, and comprehensively assessed approximately 40 serum proteins. Analysis of 69 NDBs can thus be considered sufficient to demonstrate the significance of studying this population, the clinical importance of pre-treatment serum protein analysis, and the need for further research focusing on this population. A second limitation of this study is that data about patients treated with chemotherapy without ICI administration was unavailable. A possibility that CRP and serum follistatin are prognostic factors, meaning the same thing happens in patients treated with chemotherapy alone, cannot be completely ruled out. However, CRP and follistatin at the time of PD with ICI did not impact on OS-PD, suggesting the efficacy of post-ICI treatment may not be related to post-ICI CRP and follistatin. Further analysis including the chemotherapy group for comparison is warranted.
Despite there being several limitations, this is the first study to explore OS-associated factors with a focus on patients with poor direct benefit from ICIs. More studies should be conducted in NDBs to elucidate unknown, indirect mechanisms of anti-cancer effects in ICI monotherapy.
Conclusion
In NDBs, serum CRP and follistatin before ICI commencement, not at the time of PD, are predictive for survival time. Long survival after PD with ICI may be predetermined according to the immune status before commencement of ICI treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research received no specific grants from funding agencies in the public, commercial, or not-for-profit sectors. We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center, Wakayama Medical University.
Abbreviations
- CI
Confidence interval
- HR
Hazard ratio
- ICI
Immune-checkpoint inhibitors
- NDBs
Patients without durable clinical benefit
- NLR
Neutrophil to lymphocyte ratio
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- OS-PD
OS after progressive disease
- PD
Progressive disease
- PD-1
Programmed cell death protein-1
- PD-L1
Programmed cell death ligand-1
- PFS
Progression-free survival
- PS
Eastern Cooperative Oncology Group performance status
- TPS
Tumor proportion score
Author contributions
HY and YO were involved in collection of clinical data, data quality control, statistical data analysis and interpretation of results. HY, YO, EM and KS were involved in collection of clinical data. JO analyzed samples. EM, JO and KS were involved in collection of samples. T.Y, HY and YO were involved in statistical data analysis. HA, RS, TS, KF, ST, NT, AH, HU, MN, YK, NY, EM and KS were involved in patient enrollments. HA, RS, TS, KF, ST, NT, AH, HU, MN, YK, NY, HY and YO were involved in manuscript writing. HA, RS, TS, KF, ST, NT, AH, HU, MN, YK and NY were involved in manuscript editing. All authors have approved the manuscript’s final version.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
Yuichi Ozawa has served on speakers’ bureaus for Chugai Pharmaceuticals, MSD, and Ono Pharmaceutical. Hiroaki Akamatsu has served on speakers’ bureaus for Bristol-Myers Squibb, Chugai Pharmaceutical, Ono Pharmaceutical, Taiho Pharmaceutical, and received research funding from Chugai Pharmaceutical and MSD. Shunsuke Teraoka has served on speakers’ bureaus for Chugai Pharmaceutical, Ono Pharmaceutical, and Taiho Pharmaceutical. Nahomi Tokudome has served on speakers’ bureaus for Chugai Pharmaceutical. Yasuhiro Koh has served on speakers’ bureaus for Chugai Pharmaceutical, and received research funding from Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb. Nobuyuki Yamamoto has served on speakers’ bureaus for Chugai Pharmaceutical, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Bristol-Myers Squibb, and received research funding from Chugai Pharmaceutical, MSD, Ono Pharmaceutical, Taiho Pharmaceutical. All remaining authors declare no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of the Wakayama Medical University, Wakayama, Japan (No. 1734), and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from each patient before enrollment to the prospective observational study. (UMIN000024414).
Footnotes
In patients without durable clinical benefit by ICIs, serum CRP and follistatin before starting ICI, not at the time of PD, are predictors of survival. Long-term survivor in this population may be predetermined by immune status.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borghaei H, Gettinger S, Vokes EE, et al. Five-Year outcomes from the randomized, phase III trials checkmate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol : Off J Am Soc Clin Oncol. 2021;39:723–733. doi: 10.1200/jco.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Garon EB, Kim DW, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol : Off J Am Soc Clin Oncol. 2020;38:1580–1590. doi: 10.1200/jco.19.02446. [DOI] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London, England) 2017;389:255–265. doi: 10.1016/s0140-6736(16)32517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Pawel J, Bordoni R, Satouchi M et al. (2019) Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer (Oxford, England : 1990). 107: 124–32. 10.1016/j.ejca.2018.11.020 [DOI] [PubMed]
- 5.Muller M, Hummelink K, Hurkmans DP, et al. A serum protein classifier identifying patients with advanced non-small cell lung cancer who derive clinical benefit from treatment with immune checkpoint inhibitors. Clin Cancer Res : An Off J Am Assoc Cancer Res. 2020;26:5188–5197. doi: 10.1158/1078-0432.ccr-20-0538. [DOI] [PubMed] [Google Scholar]
- 6.Sanmamed MF, Perez-Gracia JL, Schalper KA, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol : Off J Euro Soc Med Oncol. 2017;28:1988–1995. doi: 10.1093/annonc/mdx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyanagi J, Koh Y, Sato K, et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung cancer (Amsterdam, Netherlands) 2019;132:107–113. doi: 10.1016/j.lungcan.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Ozawa Y, Amano Y, Kanata K, et al. Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol (Northwood, London, England) 2019;36:33. doi: 10.1007/s12032-019-1255-3. [DOI] [PubMed] [Google Scholar]
- 9.Keegan A, Ricciuti B, Garden P, et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–692. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen KC, Liu LF, Gupta V, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26:693–698. doi: 10.1038/s41591-020-0860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abiko K, Matsumura N, Hamanishi J, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akamatsu H, Murakami E, Oyanagi J, et al. Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncologist. 2020;25:e679–e683. doi: 10.1634/theoncologist.2019-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 15.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 2019;393:1819–1830. doi: 10.1016/s0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 17.Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2017;112:90–95. doi: 10.1016/j.lungcan.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13:106–111. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.