Abstract
Acute myeloid leukemia (AML) is considered as one of the most malignant conditions of the bone marrow. Over the past few decades, despite substantial progresses in the management of AML, relapse remission remains a major problem. Natural killer cells (NK cells) are known as a unique component of the innate immune system. Due to swift tumor detection, distinct cytotoxic action, and extensive immune interaction, NK cells have been used in various cancer settings for decades. It has been a growing knowledge of therapeutic magnitudes ranging from adoptive NK cell transfer to chimeric antigen receptor NK cells, aiming to achieve better therapeutic responses in patients with AML. In this article, the potentials of NK cells for treatment of AML are highlighted, and challenges for such therapeutic methods are discussed. In addition, the clinical application of NK cells, mainly in patients with AML, is pictured according to the existing evidence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03217-1.
Keywords: NK cells, AML, Natural killer cells, Acute myeloid leukemia, Cancer immunotherapy, Immune escape
Introduction
Acute myeloid leukemia (AML) is a malignant disorder of the bone marrow, characterized by clonal expansion of the progenitor cells with abnormal differentiation of the hematopoietic stem cells [1]. Caused by the propagation of the immature myeloid cells, AML is a form of cancer with the highest mortality and shortest 5-year survival rate (SR) among all leukemia subtypes. It is estimated that by the end of 2022, approximately 20,050 new adults will be diagnosed with AML, and 11,540 individuals will die [2].
Since the cytogenetic heterogeneity of AML has been recognized for about 40 years, it is considered a type of cancer with variable therapeutic responses [3, 4]. Despite the rapid diagnosis and directed therapy, which are vital for a better AML management, relapse rates are still high [5]. Many therapeutic methods have been conducted for years [6–8]; however, finding a proper approach to reach a better treatment for AML is still a great challenge and remained to be addressed.
Natural killer (NK) cells are innate lymphocytes of the immune system that monitor and recognize tumor cells by an unusual expression of cell surface markers [9]. First discovered in 1975, NK cells can lyse specific tumor cells without previous exposure, nor substantial damage to the patient’s tissues [10, 11].
Due to rapid tumor marker detection, fast anti-tumor action, and immune system response elevation, NK cells have been utilized to treat various types of cancer, AML in particular [12–14]. On the other hand, AML malignant cells can develop several mechanisms to escape from NK cells’ immunity [15, 16] and makes the therapeutic outcome even more challenging.
In this article, the potentials of NK cells for the treatment of AML are highlighted, and challenges for such therapeutic methods are discussed. In addition, the clinical application of NK cells, mainly in patients with AML, is pictured according to the existing evidence.
Natural killer cells
Overview
As a member of the innate lymphoid cells (ILCs) family, NK cells are large granular bone marrow-derived lymphocytes with prevailing cytotoxic capacity. The term “natural killer” is characterized by the natural ability to directly kill virally infected or cancerous cells, without any need for pre-activation or previous exposure [17, 18]. After B and T lymphocytes, NK cells represent the third-largest lymphocytes population in the peripheral blood circulation, yet unlike other lymphocytes can identify a wide range of tumor mediators [11, 19].
Since their scientific establishment in the 1970s, NK cells have been drawing much attention for their fascinating properties in cancer immunotherapy. NK cells are first discovered in the adult mice spleen via an unintended experiment aimed at distinguishing the T cell-associated cytotoxicity [10, 20] and then characterized as naturally occurring non-T lymphoid cells [21]. In the same year, their functionality was observed in humans, which introduced them as the non-thymus-derived lymphoid cells that can make impulsive lymphocyte-associated cytotoxicity [21, 22].
Before entering the peripheral blood circulation, NK cells are differentiated in the bone marrow, spleen, thymus, liver, and lymph nodes [23]. NK cell differentiation is a linear process of turning functionally suboptimal and highly proliferative immature organoids into a large granular population of effectors, and finally, terminally differentiated cytotoxic cells [9, 17]. The procedure is mediated by numerous intermediates along with a remarkable expression of specific cell markers [23, 24]. Despite T cells activity that mostly leads to host tissue damages, NK cells will die after their cytotoxic attack, which can be a privilege to be used as a therapeutic method [25].
NK cells are primarily categorized into two subtypes based on the CD56 and CD16 expression level. About 90% of the total NK cell population of the peripheral blood are capable of applying cytolytic activity, which are called CD56dimCD16+ NK cells [26, 27]. The cytolytic capacity is mainly provided through the secretion of cytotoxic granules, such as granzymes and perforin. Following the NK cells’ activation, perforin is released and forms several pores in the tumor cell’s membrane. This process is followed by the secretion of granzymes, which are believed to deliver inside the cell through the disrupted membrane to induce cell apoptosis [27, 28]. The other 10% of the NK cell population in the peripheral blood, also known as CD56brightCD16−, are largely presented in the lymph nodes to produce and release immunoregulatory cytokines. Interferon (IFN)-γ, interleukin (IL)-10, and tumor necrosis factor (TNF)-α/β are the examples of such cytokines [26, 29].
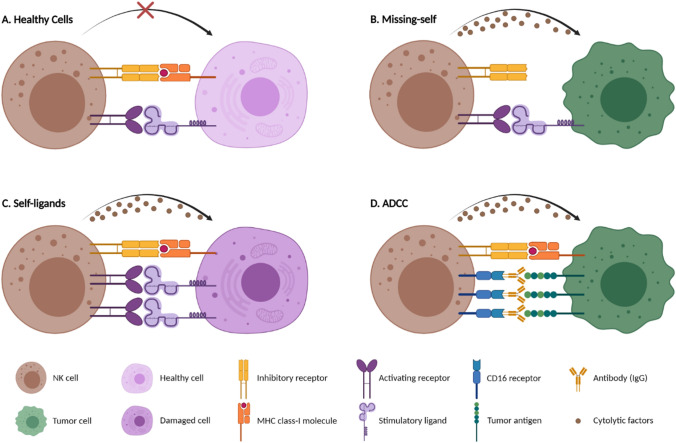
To recognize any modification of the host cells caused by malignant alterations or viral infections, two major types of polymorphic yet germline-encoded receptors are expressed on the NK cells’ surface [30]. The first type are activating receptors that are responsible for recognizing pathological ligands and interacting with signaling pathways, based on effector activities. Natural cytotoxicity receptors (NCRs), activating killer immunoglobulin-like receptors (KIRs), CD94/natural killer group 2C (NKG2C), NKG2D, and DNAX accessory molecule-1 (DNAM-1) are of the activating receptors [31, 32]. The second type, inhibitory receptors, are designed to identify major histocompatibility complex (MHC) class I molecules, leukocyte immunoglobulin-like receptors (LIRs), inhibitory KIRs, and NKG2A are considered as NK cell inhibitory receptors [31, 33] (Fig. 1).
Fig. 1.
Schematic representation of NK cells cytotoxic function
Properties
Cytotoxic function
The cytotoxic functionality of the NK cells is regulated by a compound array of activating and inhibitory receptors that carry stimulatory and suppressive signals, respectively [30]. Because of their natural ability to kill various cells, a particular type of regulation is defined to evade self-cell injuries (Fig. 1A). This feature is mainly acquired through encountering inhibitory receptors (KIRs, for example) with the host MHC class I molecules on the surface of the cells [34]. When NK cells meet the healthy cells, activating receptor functions will be hindered by inhibitory receptor signals; hence, host cells are less likely to be attacked by NK cells [35, 36].
Conversely, activating receptors are stimulated by the molecular changes in the surface of the normal cells. Due to the downregulation of MHC class I ligands by virally infected or cancerous cells, known as the “missing-self” phenomenon (Fig. 1B), the inhibitory response will be surpassed by the activating signals. The same process will happen when the expression of activating ligands is upregulated due to damage or stress to the healthy cells, also known as the induced “self-ligands” phenomenon (Fig. 1C) [37, 38]. As the result, NK cells immunity is provoked against non-self cells by secreting cytolytic granules, cytokines, and inducing death receptor-associated apoptosis, which in turn leads to massive cellular destruction [32, 39]. These mechanisms are encountered through diverse clinical experiments, which result in effective cytotoxic function of NK cells against various cancers, AML for example [40–42].
A. NK cells can recognize healthy cells through a dynamic balance of delivery signals via inhibitory and activating receptors. B. NK cells can detect downregulated MHC class I molecules as “missing-self” and release cytotoxic granules to lyse non-self cells. C. Stressed or damaged cells are recognized by activating receptors of NK cells due to the overexpression of the induced stimulatory “self-ligands.” D. NK cells can also elicit cell-mediated cytotoxicity through tumor antigen-specific (IgG) antibody binding to CD16 receptor, also known as antibody-dependent cell-mediated cytotoxicity (ADCC). Provided by Biorender.com.
There are other ways in which NK cells can contribute to the cytotoxic activities. Similar to direct tumor cell exposure (mostly via NKG2D and NCRs), neoplastic cells can also be killed by antibody-dependent cell-mediated cytotoxicity (ADCC) (Fig. 1D). This antibody-dependent process is mediated via CD32 (FcγRIIC) and mainly CD16 (FcγRIIIA) receptors, which are bind to a variety of antibodies (IgG, for example). After the Fc receptors engagement, cell death is induced through NK cell granulocytic apoptosis via death signaling cytokines [43]. NK cells activity can also be fortified by anticancer drugs such as dinaciclib [44] or other immune components such as activated dendritic cells (DCs) and macrophages through regulatory cytokines [14, 45], which are explained at Supplementary Fig. 2 in detail.
Effector function
Upon NK cell activation, both innate and acquired immunity arms will be impacted. The maturation and activation of macrophages, DCs, and T cells are boosted by NK cells through organization of cell receptor and secretion of cytokines, IFN-γ and TNF-α in particular [46, 47] (Supplementary Fig. 2). Cytokine secretion, particularly by CD56brightCD16− NK cells, holds great promises in effector mediation and cancer immunotherapy. For instance, IFN-γ is shown to mediate Thelper-ITcytotoxic-I (Th1/Tc1) immune reaction, as well as preventing tumor cell’s proliferation [48]. The effector function of Th2/Tc2 is also mediated by IFN-γ, leading to attract other immune cells to collaborate against tumor cells growth [49].
When it comes to antileukemic activity, several cytokines are represented as effective tools. For example, a specific combination of IL-2 and IL-15 was reported to be ideal for the expansion and activation of the NK cells in patients with AML, which resulted in function recovery of NK cells [50]. However, the main problem of their antileukemic effect is mainly described as the short-term persistence of NK cells in the body. Meanwhile, a mixture of cytokines (IL-18, IL-15, IL-12, and type-I IFN) is shown to stimulate NK cells’ activity for weeks that results in significant amplification of antileukemic responses [51, 52] (Supplementary Fig. 2).
Upon priming by different soluble factors (IL-12, IL-15, IL-18, and type-I IFN), NK cells can boost the maturation and activation of DCs, macrophages, and T cells, via a combination of cytokines and cell surface receptors (IFN-γ and TNF-α). Besides, NK cells can also destroy immature DCs (iDC), activated T cells (CD4+), and hyper-activated macrophages. These NK cell-based regulatory functions are mainly held in check by the recognition of fundamentally expressed self-molecules via the inhibitory receptors, such as inhibitory KIRs and CD94/NKG2A. Provided by Biorender.com.
Despite unique abilities of NK cells to overcome cancer evasion, several cytokines such as IL-10 and transforming growth factor (TGF)-β are released from tumor cells, which results in NK cells dysfunction [53, 54]. While the combination of IL-10 and IL-15 is shown to increase the cytotoxicity of NK cells [55], secretion of IL-10 by regulatory T cells (Tregs) can negatively affect NK cells activity [56], which is further explained at Supplementary Fig. 3 [57–59]. Activating signals from NKG2D and NKp30 are also downregulated via TGF-β secretion [60], as a result, leading to facilitate tumor escape from the NK cells’ immune surveillance.
Cancer immune surveillance
A connection between tumor suppression and NK cells activity is well documented based on animal experiments, where the deficiency of the NK cells had resulted in a higher rate of tumor growth [61, 62]. The lack of NK cells functionality, which is determined via tumor infiltration, is also shown to be associated with worse outcomes of patients with cancer [63]. Therefore, relative observations of primary NK cells dysfunction and deficiency toward cancer incidence have suggested the role of NK cells in cancer immune surveillance [64]. The idea, which is originally embodied in Burnet and Thomas’s theory [65], has been considerably improved since its introduction, and through time, developed as the cancer immunoediting hypothesis [66].
The theory of immunoediting is designed to describe the three major phases of cancer immunity [67]. In the first phase, also known as elimination, tumor cells will be detected and removed by the immune system. Due to releasing cytotoxic granules and inducing death receptors, circulating cancerous cells are eliminated by NK cells to prevent possible metastasis [68]. The process is finalized when all of the tumor cells are eradicated; however, if cancerous cells are not completely removed, cancer immunoediting will be shifted into the equilibrium phase [69]. During this phase, tumor growth is generally controlled via both adaptive and innate immune systems, while tumor cells, AML cells for example, are provided with an exquisite opportunity for acquiring DNA mutations and heterogeneity within cancer microenvironment [70, 71]. As this dynamic balance goes on, tumor cells will be developed to suppress, avoid, or resist anti-tumor immunity. These cells will be selected, proliferated, and finally, colonized as progressive tumors to escape from the NK cells’ immune surveillance in the final phase, which is also known as the tumoral escape [72, 73].
Cytotoxic T lymphocytes (CTLs), the predominant lymphocytes around tumor cells, can be attracted via chemokines and cytokines secreted by NK cells. Meanwhile, tumor cells are detected and destroyed by CTLs through a complex MHC class I identification [74]. As an answer, the remained tumor cells, including AML blasts, can downregulate the MHC class I ligands to hide from CTLs’ inflammatory response [75, 76]. As mentioned earlier, NK cells’ activating signals are repressed by inhibitory responses through a self-MHC-I suppression mechanism. When MHC-I is downregulated, activating responses will override the inhibitory signals, and therefore, tumor cells will be identified and destroyed by NK cells [37, 77]. NK cells are also designed to detect and kill cancer stem-like cells (CSCs), which are relatively accountable for chemotherapy-resistant malignancies and uncontrolled tumor growth [78].
Acute myeloid leukemia
As the most common subtype of acute leukemia in adults, AML is considered a clinically heterogeneous disorder of the bone marrow. While there are remarkable progresses in approving new drugs or therapeutic methods for AML treatment, the outcomes are still far from expectations [5, 13]. Despite cytogenetic heterogeneity of AML, the malignant cells can be recognized, eliminated, or remained in a dynamic equilibrium by both innate and adaptive immune arms [79]. AML blasts are predisposed to be attacked by T lymphocytes and NK cells via expression of MHC class I molecules, leukemia-associated antigens (LAAs), or NK cell activating ligands [80]. Nevertheless, AML can predominantly evade NK cells’ immune surveillance through several mechanisms focused on antileukemic dysfunction of the immune responses [81, 82]. An overview of such mechanisms is given comprehensively via Supplementary Fig. 3.
NK cell dysfunction
The antileukemic immune response of NK cells is provided by direct cytotoxicity or activation of other immune components [83, 84]. In AML, however, impaired killing capacity of NK cells is found due to altered expression of both ligands and receptors (Supplementary Fig. 3). Since the function of the NK cells is tightly regulated by the inhibitory and activating signals, the imbalanced expression of receptors will lead to the NK cells dysfunction [81, 85, 86].
Activating receptors downregulation
Expression of NCRs such as NKp46, NKp44, and NKp30 is shown to be downregulated in NK cells of the majority of the AML patients [87, 88]. Besides, DNAM-1 expression is diminished on the surface of the NK cells in AML patients compared with the control group [89]. Along with NKG2D, other activating receptors are also represented to detect and kill leukemic target cells [90, 91]. However, among diagnosed AML patients the ones with a lower amount of NCRs were found to live shorter than others, suggesting the pivotal role of activating receptors regulation in AML-associated responses [92].
Inhibitory receptor upregulation
Along with activating receptors, high expression of various inhibitory receptors is shown to play a significant role in AML evasion from NK cells’ immune surveillance [93, 94]. The association between KIR inhibitory receptors and AML immune escape is controvertibly demonstrated with several studies in the haploidentical hematopoietic stem cell transplant (HSCT) setting. Several studies have suggested a mismatch association between recipients’ leukocyte ligands and donor KIRs, which results in relapse rates reduction of AML patients [95–97]. In the same setting, CD94/NKG2A upregulation is also indicated to be linked with lower NK cell cytotoxicity against AML blasts [98], all of which represent the regulatory role of inhibitory receptors concerning with AML immune escape.
AML immunosuppression
As mentioned above, downregulation of activating receptors as well as upregulation of inhibitory receptors can lead to cytotoxic dysregulation of NK cells (Supplementary Fig. 3). NK cells’ antileukemic responses can also be compromised through various AML receptor–ligand interactions or immunosuppressive factors secretion [86, 99], which are explained here in detail.
Receptor–ligand interactions
Due to an absence or reduction of ligand expression for activating receptors, AML cells can develop a chronic stimulus system to escape from NK cells attack. Shedding NKG2D ligands by AML cells can result in reduction of NK cells’ activating responses and, consequently, impair cytotoxic functionalities [100, 101]. Downregulation of DNAM-I on NK cells besides lower expression of DNAM-I ligands on the leukemic blasts further supports such interactions [102].
Increased expression of inhibitory ligands on AML, CD137L and glucocorticoid-induced TNFR-related protein–ligand (GITRL) for example, is also shown to be associated with receptor-ligand interactions. Both CD137L and GITRL overexpression are found to impair NK cells’ killing capacity or IFN-γ secretion, through either direct GITR ligation on the NK cells or indirect IL-10 secretion by AML blasts [103, 104]. Upregulation of CD200 on the surface of AML cells is another evidence to decline antileukemic responses of NK cells [105], altogether leading to a poorer prognosis for AML patients.
Immunosuppressive factor secretion
Aside from interactions between activating/inhibitory ligands and NK cell receptors, AML cells can also evade NK cells’ immune surveillance through secretion of specific immunosuppressive factors [106]. By using apoptosis resistance facilities, AML cells are capable of hiding from CD95 and TNF-based killing pathways, which are basically designed for NK cells to attack and kill malignant blasts [107, 108].
Multiple mechanisms contribute to the AML immune escape from NK cells either through NK cells’ alteration, AML target cells’ expression, or via third-party immune cells’ contribution. NK cells of patients with AML often show modifications in receptor phenotypes compared to the NK cells of healthy group. Shedding of ligands and low expression of NK cells activating receptors are also impair AML cells recognition. Besides, secretion of specific immunosuppressive factors by AML cells can directly affect NK cells cytotoxicity and also induce Tregs, which in turn diminish NK cells’ activating responses. The later effect may be further increased due to suppression of DCs function and reduction of immature dendritic cells (iDC) destruction by NK cells, which can further induce activity of Tregs. IS, immunosuppressive; AR, activating receptor; IR, inhibitory receptor. Provided by Biorender.com.
Other immune cells contribution
In addition to NK cell dysfunction and AML-induced immunosuppression, AML immune escape is also facilitated through various interactions between AML cells, NK cells, and other immune system components (Supplementary Fig. 3).
Dendritic cells (DCs)
As mentioned earlier, immature dendritic cells (iDCs) are destroyed by NK cells to limit the subsequent unwanted inflammation. Impaired destruction of iDCs by NK cells is vastly demonstrated in AML patients, which can further induce toleration against tumor antigens presented by iDCs [14, 109]. During the development of leukemia in mice, stimulation of DCs activity by T cells was also suppressed due to a poorer cytotoxicity of NK cells [110].
Regulatory T cells (Tregs)
The number of Tregs, which is associated with inhibition of NK cells cytotoxicity, was demonstrated to be higher in AML patients than in healthy controls or patients under complete remission (CR) [111, 112]. Tregs of leukemic samples were shown to be more suppressive toward NK cells and responder T cells through either direct cell interaction or soluble factor secretion, such as IL-10 and TGF-β1 [112, 113]. Besides, in an animal experiment, the reduction of Tregs resulted in more cytotoxic activity of NK cells [114]. These findings together with previous results indicate the importance of constant investigation on the interactions between NK cells and AML within the immune system to have a clearer understanding of disease development and better clinical achievements.
NK cells against AML
A robust rationale for investigating NK cells as antileukemic agents is established through explicit data from in vitro experiments, animal models, and clinical trials [12, 115, 116]. The combination of signal regulation in NK cells activity and underlying AML characteristics of the immune escape capacity provides essential clues on how to harness maximum potentials of NK cells against AML [13, 117]. However, when it comes to clinical applications, various laboratory procedures and comprehensive clinical circumstances need to be executed to meet the sophisticated regulatory requirements.
Adoptive NK cell transfer
Autologous NK cells
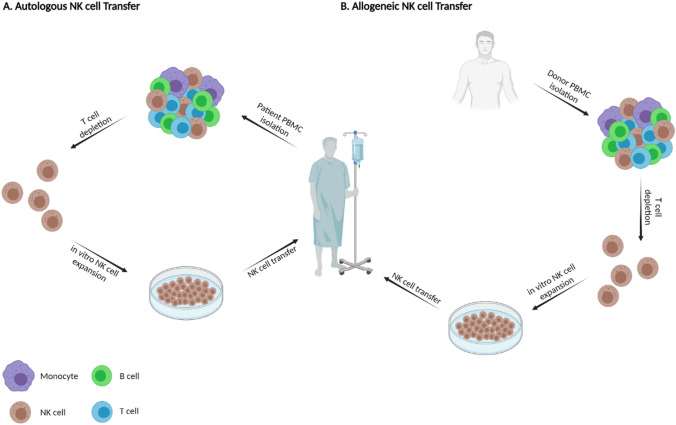
As a solution to enhance NK cells’ immune surveillance, cell lineages from patients’ peripheral blood can be expanded ex vivo and transduced back to their own body as autologous NK cell [118, 119]. Due to the use of the patient’s own blood as a convenience source of cells, independency from immunosuppressive therapies, and low possibility of graft versus host disease (GvHD), autologous NK cell transfer is known as the first major effort of using NK cells for cancer immunotherapy [40, 120–122] (Fig. 2A).
Fig. 2.
Adoptive NK cell transfer for AML patients. A In autologous NK cell transfer, endogenous NK cells are needed to be harvested from patient’s own peripheral blood. For this purpose, peripheral blood mononuclear cells (PBMC) of patient is isolated, and T cells are depleted. Purified NK cells are then expanded and proliferated in vitro to infuse to patient’s own blood. B. In allogeneic NK cell transfer, however, NK cells are isolated from PBMC of a healthy HLA-matched or haploidentical donor. Following T cell depletion and in vitro NK cell expansion, allogeneic NK cells will be infused to the AML patients. Provided by Biorender.com
Despite the considerable in vivo expansion of infused cells, a significant therapeutic outcome is often failed, owed to the inhibitory effects by self-human leukocyte antigen (HLA) ligands in some type of tumors, AML in particular [123, 124]. Furthermore, since autologous NK cells are attained from profoundly pre-treated patients, the expansion and the functional capacities are less likely to be as equal as expectations [123].
Various strategies are being developed to overcome current checkpoints of autologous NK cell linages. Lirilumab, as an anti-KIR antibody, is established to block inhibitory signals by avoiding interactions with MHC class I ligands, leading to upregulation of NK cells’ killing capacity [125]. Moreover, the use of combination therapy, consisting of autologous NK cells and various anti-tumor agents, is shown to enhance therapeutic responses against tumors [126].
On the other hand, many clinical applications of autologous NK cells within the cancer settings are yielded disappointing results. In a study designed to pre-activate NK cells with IL-2, despite a considerable in vitro cytolytic activity and in vivo persistence of NK cells, no significant clinical responses was perceived. However, NK cells’ persistent mediated ADCC without in vitro cytokine reactivation suggest that combination of monoclonal antibodies with autologous adoptive NK cell transfer deserves more evaluation [124]. Similar to the AML setting, patients who received unmodified NK cells have shown a poorer outcome than others with alloreactive NK cell transfer [124, 127], which provides a strong basis for shifting from autologous NK cells to allogeneic NK cell transfer.
Allogeneic NK cells
In this method, NK cells are acquired from healthy HLA-matched or haploidentical (partly matched) donors, expanded ex vivo similar to autologous expansion methods, and undergone exclusion of T cells to prevent possible GvHD [128] (Fig. 2B). Adoptive allogeneic NK cell transfer can also be promoted from umbilical cord blood (UCB) through pre-activation and expansion with IL-2 to be performed in both HSCT and non-HSCT settings [123, 129].
In 2002, the first evidence of allogeneic NK cell transplant was elucidated by Ruggeri et al. in the HSCT setting, where AML diagnosed patients who received mismatched KIR and HLA-C allogeneic bone marrow transplants were remarkably expressed lower relapse rates [130]. Due to alloreactive cytotoxicity pre-cultured in IL-2 for 2 days, results suggested the mediation role of alloantigen-specific NK cells against AML blasts in mice (survival rate of 100%) without the risk of GvHD [131]. A few years later, haploidentical NK cell infusions outside the HSCT setting were revealed to induce complete remissions in 5 of 19 poor prognosis diagnosed AML patients. This remission rate was significantly higher (CR, 75%) when KIR–ligand mismatched donors where applied [132]. These findings are also replicated in additional cohort studies [133, 134]. Besides, the current reports of the recent clinical trials on the application of adoptive NK cells for AML patients in both HSCT and non-HSCT settings are shown at Table 1.
Table 1.
Ongoing clinical trials of adoptive NK cell therapy in AML
| Identifier | Phase | Conditions | NK cells’ source | Other interventions | Outcome measures |
|---|---|---|---|---|---|
| Hematopoietic Stem Cell Transplant (HSCT) setting | |||||
| NCT05247957 | I | AML | CAR-NK cell | – | DLT, MTD, LFS |
| NCT05272293 | I/II | AML | Expanded haploidentical NK cells | K562-mbIL21-41BBL | LFS, OS, Persistence of infused NK cells, Number of T, B, and activated NK cells |
| NCT05256277 | I | AML | Cytokine-induced memory-like NK cells | CT101a | ORR, OS, DOR, TTP, LFS, safety |
| NCT04632316 | I/II | AML | PBMC-derived NK cell | Cyclophosphamide-Fludarabine (Cy/Flu) | AE, MRD, Response, Survival (EFS), QOL |
| NCT01904136 | I/II | AML, MDS, CML | PBMC-derived NK cell | Cyclophosphamide, Fludarabine, Melphalan, Mycophenolate Mofetil, Tacrolimus | MTD, AE, Survival (OS and RFS) |
| NCT01823198 | I/II | High-risk AML or MDS | PBMC-derived NK cell | IL-2, Busulfan, Fludarabine Phosphate | NK cell dose, Survival (OS and DFS), AE (GvHD and Toxicity) |
| NCT04166929 | II | AML, MDS | PBMC-derived NK cell | – | Relapse |
| NCT03300492 | I/II | AML, MDS | PBMC-derived NK cell | NK-DLI | AE, Survival (PFS), Response, NK cell dose |
| NCT02809092 | I/II | R/R AML | PBMC-derived NK cell | IL-21 | MTD, Response, NK cell expansion |
| NCT04836390 | II | AML | PBMC-derived NK cell | IL-21 | Survival (RFS), Success rate, AE (GvHD) |
| NCT01619761 | I | AML, MDS, et al | UCB-derived HSPC-NK cell | Cyclophosphamide, Fludarabine Phosphate, Lenalidomide, Melphalan, Mycophenolate Mofetil, Tacrolimus | AE (GvHD and TRM), Survival (OS and DFS) |
| NCT02727803 | II | AML, MDS, et al | UCB-derived HSPC-NK cell | Busulfan, Clofarabine, Cyclophosphamide, Fludarabine Phosphate, Melphalan, Rituximab | Survival (PFS and OS), AE (GvHD, Infection, and TRM) |
| NCT04024761 | I | Relapsed AML, MDS, or MPN | Cytokine Induced Memory-like NK Cell | IL-2, Fludarabine, Cyclophosphamide | AE (IR, IS, and GVHD), Response, Survival (LFS and OS) |
| NCT03068819 | I/II | Relapsed AML | Cytokine Induced Memory-like NK Cell | CD3 + T Cell Product Infusion | Survival (OS and LFS), Response, AE (GvHD) |
| NCT02782546 | II | R/R AML | Cytokine Induced Memory-like NK Cell | ALT-803, Tacrolimus, Mycophenolate mofetil, G-CSF | Survival (LFS and OS), Response |
| Non-Hematopoietic Stem Cell Transplant (non-HSCT) setting | |||||
| NCT04209712 | Early Phase I | AML with MRD | PBMC-derived NK cell | IL-2 | MRD, AE |
| NCT02890758 | I | AML, MDS, et al | PBMC-derived NK cell | ALT-803 | MTD, AE (GvHD), in vivo NK cell levels, Survival (OS and RFS), Response, DOR |
| NCT04221971 | I | R/R AML | PBMC-derived NK cell | Chemotherapy | AE, Response, NK cells’ metabolism, migration and reconstruction, Recovery, Relapse |
| NCT03955848 | N/A | AML in Remission | PBMC-derived NK cell | IL-2 | Survival (RFS and OS) |
| NCT04220684 | I | R/R AML or MDS | PBMC-derived NK cell | IL-21, Cytarabine Hydrochloride, Fludarabine | MTD, AE, Response, Survival (LFS and RFS), DOR, patients proceeding transplantation, Recovery |
| NCT04327037 | I | AML | PBMC-derived NK cell | IL-2 | AE, Days of persistence of NK cells, Recovery |
| NCT04347616 | I/II | R/R AML | UCB-derived HSPC-NK cell | IL-2 | MRD, Life span and Functional activity of NK cells, Plasma cytokine concentrations |
| NCT04310592 | I | AML | Placental-derived HSPC-NK cell | CYNK-001 | MTD, AE, MRD, TTP, Response, Survival (OS and PFS) |
| NCT01898793 | I/II | R/R AML or MDS | Cytokine Induced Memory-like NK Cell | ALT-803, IL-2, Fludarabine, Cyclophosphamide, | MT/TD, Response, AE, DOR, TTP, Survival (DFS and OS) |
| NCT04354025 | II | R/R AML | Cytokine Induced Memory-like NK Cell | IL-2, Fludarabine, Ara-C, G-CSF | Patients able to proceed to SCT, Response, MRD, AE, DOR, TTP, Survival (DFS and OS) |
AE adverse event; AML acute myeloid leukemia; CML chronic myeloid leukemia; DFS disease-free survival; DLT dose-limiting toxicity; DOR duration of response; EFS event-free survival; GvHD graft versus host disease; HSPC hematopoietic stem and progenitor cell; IL interleukin; IR immune reactions; IS immune suppressants; LFS leukemia free survival; MDS myelodysplastic syndrome; MPN myeloproliferative neoplasm; MRD minimal residual disease; MTD maximum tolerated doses; MT/TD maximal tolerated or tested dose; N/A not applicable; NK natural killer cell; ORR overall response rate; OS overall survival; PBMC peripheral blood mononuclear cell; PFS progression-free survival; QOL quality of life; RFS relapse-free survival; R/R relapsed/refractory; SCT stem cell therapy; TRM treatment-related mortality; TTP time to progression; UCB umbilical cord blood
Similar to autologous NK cell transfer, allogeneic NK cell-based therapy is associated with the challenges of timely ex vivo expansion, low clinical-grade activation, and lack of in vivo persistence [135, 136]. A combination of cytokines can play an essential role in the activation (IL-18 and IL-21), proliferation (IL-2 and IL-15), and effector function (IFN-γ and TNF-α) of NK cells [46, 50, 137]. IL-15 super agonist complex, ALT-803, is identified as a safe agent at the first inhuman phase I study focusing on elderly AML patients who relapsed after the HSCT [138]. The act of using ALT-803 for AML patients after HSCT as a promising relapse prophylactic agent is also under investigation (NCT02989844).
Furthermore, engineered AML cells with the encoding gene to express IL-15 or IL-12 are constructed to decrease the toxicity of systemic administration of cytokines [139, 140]. A phase I clinical trial is recruiting to test the safety and tolerability of a novel treatment based on modification of AML cells to express IL-12 in a non-HSCT setting (NCT02483312). In brief, the importance of applying genetic modification methods on facing challenges of AML-based therapy seems almost inevitable
Genetic modifications
Increasing activation and proliferation
Different methods have been investigated to enhance both activity and proliferation of NK cells. Anti-tumor activity of NK cells can be amplified through upregulation of activating receptors, which results in increasing sensitivity to tumor activating ligands [141, 142]. NKG2D, as one of the main NK cell activating receptors, is physiologically associated with DNAX-activating protein (DAP)-10 in humans and DAP10/12 in mice [143, 144]. When NKG2D ligand (NKG2D-L) on AML cells are recognized by NKG2D receptors on NK cells, cytotoxic capacity of NK cells will be activated, cytokine release will be induced, and cellular proliferation will be promoted via the promotion and stabilization of NKG2D expression by DAP10 [145].
Through NKG2D/CD3ζ/DAP10 signaling pathway, a wide range of tumor cells including leukemic blasts are predisposed to substantial cytotoxic activity of NK cells, while self-hematopoietic cells remain almost intact. Despite the hampering potential of tumor cells to inhibit activating ligands, NKG2D-L is less likely to be shed by AML cells due to the high expression of NKG2D receptors on NK cells [146, 147]. The result of an unexpected targeting potential of NK cells toward myeloid-derived suppressor cells (MDSCs) via NKG2D/CD3ζ receptors is also indicated in another study [148].
Lower expression of inhibitory NK cell receptors has been tested as another genetic-based method to activate the anti-tumor capacity of NK cells. As mentioned earlier, AML cells can develop several suppressive mechanisms to evade NK cells’ immune surveillance [15]. As a response to IFN-γ secretion, highly expressed HLA-E by AML blasts can also lead to impaired cytolytic capacity of the NK cells [98]. CD94-NKG2D receptors, which are designed for inhibitory signaling transduction by HLA-E ligands, can be targeted via anti-NKG2A expression blocker proteins. As a result, cytolytic capacity of NK cells will be augmented due to lower expression of NKG2A surface receptors [149].
Explicit pieces of evidence are indicated the association between population of cytotoxic NK cells and antileukemic capacity in various AML settings [150–153]. NK cell expansion can be promoted via cytokine-encoding genes such as membrane-bound IL-15 (mbIL-15). Through an animal experiment, mbIL-15 expansion is shown to sustain the propagation, as well as the lifetime of infused NK cells [154]. These studies collected with previous results provide a robust rationale toward the pivotal role of enhancing NK cells activation and proliferation to defeat AML-based immunosuppression.
Chimeric antigen receptors (CAR) NK cells
The immune response of the NK cells against AML is tightly bounded with signaling balance between transferred NK cells and associated target cell ligands [115, 155]. Since the introduction of CAR-T cells, a successful therapy against lymphoid and leukemic disorder subtypes, lots of attention has been appealed to the cytolytic enhancement capacity of chimeric-based techniques [156–158]. Despite several promising results at the refractory stage [159, 160], adverse effects such as cytokine release syndrome (CRS) remained to be resolved for CAR-T cell therapy against AML [161–163]. Instead, NK cells are considered as promising alternatives to T cells due to their shorter lifespan, favorable cytotoxic profile, and lower manufacturing costs [12, 164, 165].
NK cells can be readdressed with CAR-based methods toward tumor cell surface ligands. Since some of the cell markers of AML are shared with the hematopoietic stem cells (HSCs), collecting optimal choices of leukemia-specific markers that can be specifically identified and attacked by CAR-NK cells is a huge obstacle [166, 167]. Derived from NK cell lines (NK-92 cells) or UBC, CAR-NK cells can be used as an efficient “off-the-shelf” product [168]. The substantial benefit of using off-the-shelf CAR-NK cells in refractory/relapsed CD19+ leukemia supports their application for further clinical trials [169].
CAR-NK cells have several advantages; however, still some problems such as loss of targeted antigen, a hostile tumor microenvironment, and tumor heterogeneity remained to be addressed to maximize the CAR-NK related AML immunotherapy [170]. Further research has described a Cas9/ribonucleoprotein-based complexes method for gene targeting in NK cells. Using CRISPR/Cas9 gene editing, targeted genes have been inserted into a safe-harbor locus in combination with an adeno-associated virus-mediated gene delivery approach was used to create primary CD33 CAR-NK cells with enhanced anti-AML activity [171]. CD33 is expressed on a restricted group of terminally differentiated cell lines and is not found on primitive stem cells. It is also detected on AML cells as well as leukemia stem cells (LSCs) of around 90% of patients, therefore, can be a promising target receptor for CAR-NK cells [171–174]. In addition, a targeting effect of specific human NK cell lines toward CD33+ AML blasts [175], as well as the first inhuman report on the safety of CD33 CAR-NK-92 cells infusion are discovered in patients with AML [176]. CD4 is another AML-based ligand, which is not highly expressed on the hematopoietic cells. To support this notion, the role of CD4+ AML blasts elimination is investigated as CD4/CD28/4-1BB/CD3ζ CAR-NK-92 cells [177].
Currently, the application of CAR-NK cells is extensively studied in various cancer settings [178–180], but harnessing their potential as a promising therapeutic method toward AML needs to be further investigated, once their application is relatively limited to the preclinical studies [181]. However, the knowledge acquired from CAR-T cell and CAR-NK cell therapy is worth to be applied to CAR-NK-based therapies for AML patients in the future.
Antibodies
Antibodies targeting tumor-associated antigens
As an attractive method of cancer immunotherapy, antibodies targeting tumor-associated antigens playing a great part in the induction of ADCC by NK cells. The outcomes for unconjugated antibodies are generally poor when used alone [182, 183]; however, the effects could be improved by engineering Fc parts of the antibodies to enhance binding to CD16 or integrating with other therapeutic methods [184, 185]. Preclinical studies have investigated the efficiency of new Fc-optimized antibodies directing against various possible antigens such as IL-1 receptor accessory protein (IL1RAP), CD33, CD133, and CD157 [186–189]. Besides, new antibodies regimens combined with NK cell transfer methods have shown promising outcomes, and therefore, these valuable strategies could be used in upcoming clinical trials [190, 191].
Antibody–radio conjugates (ARCs) and antibody–drug conjugates (ADCs) are favorable strategies to augment the antibodies' potency, while they could have better clinical outcomes on AML patients [192–194]. The combination of antineoplastic calicheamicin agent with anti-CD33 antibody and gemtuzumab ozogamicin (GO), is currently the only ADC antibody approved by the Food and Drug Administration (FDA) for the treatment of R/R CD33+ and newly diagnosed AML patients [195, 196]. Preliminary findings of different ADCs targeting CD33, CD37, leukocyte immunoglobulin-like receptor subfamily B4 (LILRB4), C-type lectin-like molecule 1 (CLL-1), and FLT3 highlight further clinical potential of using antibodies targeting tumor-associated antigens for the treatment of AML [197–201].
Antibodies targeting NK cell inhibitory receptors
Inhibitory receptors of NK cells play a critical role in the immune escape of tumor cells, making them great targets for cancer immunotherapy. Over the last decades, the number of identified NK cells inhibitory receptors has been increasing. Aside from MHC-I inhibitory LIRs, KIRs, and CD94/NKG2A receptors, other NK cell immune checkpoints have shown to cause cellular dysfunction [202]. Besides the benefits of KIR–ligand mismatch between recipients and donors in improving the results of HSCT, pharmacologic blockage of KIR via anti-KIR antibodies can potentially prevent the interaction of KIR-HLA-C and augment the function of NK cells. IPH-2101 and IPH-2102 (lirilumab) are antibodies that target KIR2D and have reported to be safe in elderly AML patients in their first CR [203, 204]; however, in a phase II study, the leukemia-free survival (LFS) of lirilumab did not compare satisfactorily to the placebo [205]. The combination of azacitidine with lirilumab also did not exhibit substantial development in R/R AML patients in terms of median survival (overall survival, OS 4.2 months) or response rate (overall response rate, ORR 14%). Thus, due to unsatisfactory results, the relevant clinical trial was terminated (NCT02399917) [206]. Besides, blockade of LIR-1 or NKG2A have resulted in amplified cytotoxicity of NK cells against AML blasts, suggesting a necessary option of the combination of anti-NKG2A, anti-KIR, anti-LIR-1 antibodies for a better effectiveness in patients with AML [207, 208].
BiKE and TriKE
As the recombinant factors of single-chain variable fragments (scFv), Bi-specific killer cell engager (BiKE) and tri-specific killer cell engager (TriKE), function as immunologic synapses within NK cells and tumor cells. Maintaining the specificity of the antibodies, they minimize the size of the antibodies to increase their distribution. CD16-binded BiKE and TriKE can activate NK cells through specified CD16 signaling pathway against tumor cells with targeted antigens in such an effective manner [209]. Scientists designed a novel BiKE (CD16*33 BiKE) that binds to both CD16 and CD33. CD16*33 BiKE specifically triggers cytotoxicity and cytokine release of NK cells when combined with CD33+ and primary AML cell lines. Besides, NK cells’ effector function were significantly enhanced when co-cultured with a disintegrin and metalloprotease-17 (ADAM-17) inhibitor to prevent shedding of CD16 [210]. The same research group later designed a TriKE by integrating a modified human IL-15 into the CD16*33 BiKE, which provided a signal for proliferation and activation of NK cells [211]. A phase I/II clinical trial of using CD16*33*IL-15 TriKE (GTB-3550) for CD33+ R/R AML treatment is under investigation (NCT03214666).
Through linking anti-CD16 to either scFv alongside the same antigen (CD16*33*33 TriKE, for example) or scFv alongside two different antigens (CD16*33*123 TriKE, for example), TriKEs displayed a greater binding affinity and NK cell cytotoxicity toward AML malignant cells compared to BiKEs [212, 213]. Since CD33 is largely expressed on the surface of healthy myeloid cells, NKG2DLs have become promising targets. Compared to the engineered NKG2D-Fc protein, CD16*NKG2D BiKE displayed an increased attraction to CD16 while induced larger leukemia cell killing capacity [214]. Moreover, CD16*CLL-1*IL-15 TriKE presented a robust activity of NK cells against AML both in vivo and in vitro [215]. Altogether, these molecules became potential candidates for AML personalized immunotherapy based on clinical and preclinical findings.
Other therapeutic methods
Cytokines
As mentioned earlier, Cytokines play an essential role in proliferation, activation, and function of NK cells. Ex vivo stimulation with 50 ng/mL of IL-15 or 10 ng/mL of IL-2 was reported to be ideal for expansion and enabling NK cells of AML patients via promoting the function of activating receptors such as NKG2D, NKp46, and NKp30 [50, 216]. On the other hand, IL-2 alone may not be clinically effective as a monotherapy for AML patients [217–219]; however, in combination with histamine di-hydrochloride, IL-2 has been reported to be effective as a maintenance therapy in patients with AML, resulting in leukemia-free survival improvement [220, 221]. The mechanism of this method may partly be due to a remarkable expansion of CD56bright subpopulations of NK cells [222].
In order to increase proliferation and expansion of NK cells, cytokines have been extensively used in the context of NK cell transfer as a “priming or arming” process. However, their short-lasting effect and short-term persistence within patients’ blood might limit their clinical application. Therefore, despite inhibitory KIR-KIR ligand interactions, a mixture of cytokines (IL-18, IL-15, and IL-12) are presented as an amplified antileukemia response and re-stimulation form several weeks to few months [51, 52, 223]. These cytokine-induced memory like (CIML) NK cells along with adaptive immune properties provide a promising method to improve adoptive NK cell transfer efficiency. The first inhuman clinical trial of CIML NK cells transfer in elderly AML patients revealed an effective remission (ORR = 67%) without the cause of GvHD, CRS, or neurotoxicity. Conversely, patients outcome were negatively related to CD8α+ NK cells frequency and NKG2A expansion on CIML NK cells within AML patients [116, 224]. Further preliminary data also encourage scientists with more ongoing clinical trials of using CIML NK cells transfer for R/R AML patients (NCT02782546, NCT04354025, NCT03068819, and NCT01898793) [225, 226].
Drugs (with immunomodulatory function)
To improve NK cells function against AML, many anti-tumor drugs with immunomodulatory properties have been designed in the past years. Since AML malignant cells can resist NK cell-mediated killing capacity (through either changing their surface ligand expression or immunosuppressive factor secretion), drug that can restore ligand expressions of AML cells can make them more vulnerable to NK cell attacks [227]. Azacitidine and decitabine, as hypomethylating agents, can upregulate NKG2DL expression on AML cell surface, resulting in improved NK cell immunity via the immune-mediated recognition by engagement of NKG2D-NKG2DL [228]. Simultaneously, they can upregulate the expression of tissue inhibitor of metalloproteinases-3 (TIMP3), an inhibitor of ADAM17, which can decrease soluble NKG2DLs shedding from AML cells [229]. Differentiation-promoting drugs (all-trans-retinoic acids, bryostatin 1, and vitamin D3) [228], histone deacetylase inhibitors (valproic acid and trichostatin A) [230, 231], and hydroxyurea [232] all have shown to potentially upregulate NKG2DLs expression on AML cells. On the other hand, dinaciclib-treated AML cells are linked to lowering inhibitory NK ligand (HLA-E) expression on AML cells, resulting to induce anti-tumor immunity of NK cells. Moreover, pomalidomide and lenalidomide can exert antileukemic effects directly or through NK cell-based immune-modulatory activities besides to HLA class I downregulation on AML blasts [44, 233].
The combination therapy including the above-mentioned drugs are widely used in both clinical practice and clinical trials for AML. Natural compounds such as casticin [234], safrole [235], ouabain [236], and α-phellandrene [237] can also promote NK cells functionality against AML blasts. Moreover, novel immunomodulatory agents were suggested in several researches, providing a therapeutic implication in patients with AML. For example, vascular endothelial growth factor receptor (VEGFR)-3 antagonist re-established NK cells killing cytotoxicity with increasing IFN-γ level [238, 239]. Also, through galunisertib, a TGF-β receptor kinase inhibitor, therapeutic efficiency of adoptive NK cell transfer can be improved significantly [240]. With the clarification of anti-tumor drugs’ mechanisms, merging pharmacologic methods with other NK cell-related immunotherapies can potentially strengthen the safety and efficiency of clinical outcomes for patients with AML.
Conclusion and future perspective
NK cells represented a promising immunotherapy strategy that is equipped with rapid tumor marker detection, fast anti-tumor action, and immune response elevation capacities. Despite numerous immune escape mechanisms, a pivotal role of NK cells in AML immune surveillance has been visualized owed to their explicit cytolytic capacity and distinguished effector function. From adoptive NK cell transfer, cytokines and immunomodulatory drugs to antibodies and genetically modified methods, NK cells have notably paved the road to a better management of AML.
Several challenges are remained to augment NK cell potentials in the setting of AML immunotherapy. Blocking inhibitory receptors, eliminating Tregs activities, and neutralizing immunosuppressive cytokines are some of the existing issues. Providing essential cytokines and growth factors, which are required for activation, propagation, and persistence of NK cells are another challenges remained to be addressed. Finally, to extend current therapeutic methods, a better understanding of NK cells dysfunction mechanisms and immunotherapy-based methods against AML are required to the alternative therapies in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author’s contribution
SR conceptualized the title, collected the data, designed the figures, and prepared the first draft of the manuscript. NY edited and revised the manuscript and finalized the draft. NR critically revised the manuscript, edited and finalized the draft, and supervised the project. All the authors have read and approved the final draft of the manuscript.
Funding
The authors did not receive fund, grant, and support from any organization for the submitted work.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. New Engl J Med. 2018;378(13):1189–99. doi: 10.1056/NEJMoa1716863. [DOI] [PubMed] [Google Scholar]
- 6.Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19(12):2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 7.Shaffer BC, Gillet J-P, Patel C, Baer MR, Bates SE, Gottesman MM. Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist Updates. 2012;15(1):62–69. doi: 10.1016/j.drup.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Löwenberg B, Pabst T, Maertens J, Gradowska P, Biemond BJ, Spertini O, et al. Addition of lenalidomide to intensive treatment in younger and middle-aged adults with newly diagnosed AML: the HOVON-SAKK-132 trial. Blood Adv. 2021;5(4):1110–1121. doi: 10.1182/bloodadvances.2020003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huntington ND, Cursons J, Rautela J. The cancer–natural killer cell immunity cycle. Nat Rev Cancer. 2020;20(8):437–454. doi: 10.1038/s41568-020-0272-z. [DOI] [PubMed] [Google Scholar]
- 10.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 11.Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol. 2002;3(1):6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- 12.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discovery. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Niu T. Natural killer cell-based immunotherapy for acute myeloid leukemia. J Hematol Oncol. 2020;13(1):167. doi: 10.1186/s13045-020-00996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 15.Lion E, Willemen Y, Berneman ZN, Van Tendeloo VFI, Smits ELJ. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26(9):2019–2026. doi: 10.1038/leu.2012.87. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Xiao Y, Guan W, Wang M, Chen J, Zhang L, et al. Acute myeloid leukemia immune escape by epigenetic CD48 silencing. Clin Sci. 2020;134(2):261–271. doi: 10.1042/CS20191170. [DOI] [PubMed] [Google Scholar]
- 17.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaemdoust F, Keshavarz-Fathi M, Rezaei N. Natural killer cells and cancer therapy, what we know and where we are going. Immunotherapy. 2019;11(14):1231–1251. doi: 10.2217/imt-2019-0040. [DOI] [PubMed] [Google Scholar]
- 19.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11(10):645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5(2):117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 21.Pross HF, Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975;21(2):226–35. [PMC free article] [PubMed] [Google Scholar]
- 22.Jondal M, Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- 23.Huntington ND, Vosshenrich CAJ, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7(9):703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 24.Crinier A, Dumas P-Y, Escalière B, Piperoglou C, Gil L, Villacreces A, et al. Single-cell profiling reveals the trajectories of natural killer cell differentiation in bone marrow and a stress signature induced by acute myeloid leukemia. Cell Mol Immunol. 2020;18:1290–1304. doi: 10.1038/s41423-020-00574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ames E, Murphy WJ. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother. 2014;63(1):21–28. doi: 10.1007/s00262-013-1469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 27.Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E, et al. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia. 2005;19(5):835–840. doi: 10.1038/sj.leu.2403704. [DOI] [PubMed] [Google Scholar]
- 28.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15(2):251–262. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 29.Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M, et al. Human CD56<sup>bright</sup> NK cells: an update. J Immunol. 2016;196(7):2923–2931. doi: 10.4049/jimmunol.1502570. [DOI] [PubMed] [Google Scholar]
- 30.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 31.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132(3):536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chester C, Fritsch K, Kohrt HE. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol. 2015;6:601. doi: 10.3389/fimmu.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89(2):216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 34.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Ljunggren H-G, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 36.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 37.Cornel AM, Mimpen IL, Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel) 2020;12(7):1760. doi: 10.3390/cancers12071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bessoles S, Grandclément C, Alari-Pahissa E, Gehrig J, Jeevan-Raj B, Held W. Adaptations of natural killer cells to Self-MHC class I. Front Immunol. 2014;5:349. doi: 10.3389/fimmu.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SEA, Yagita H, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42(4):501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 41.Mancusi A, Ruggeri L, Urbani E, Pierini A, Massei MS, Carotti A, et al. Haploidentical hematopoietic transplantation from KIR ligand–mismatched donors with activating KIRs reduces nonrelapse mortality. Blood. 2015;125(20):3173–3182. doi: 10.1182/blood-2014-09-599993. [DOI] [PubMed] [Google Scholar]
- 42.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun HD, Schirm DK, Felices M, Miller JS, Eckfeldt CE. Dinaciclib enhances natural killer cell cytotoxicity against acute myelogenous leukemia. Blood Adv. 2019;3(16):2448–2452. doi: 10.1182/bloodadvances.2019000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force”. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 46.Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, et al. Effector and regulatory events during natural killer–dendritic cell interactions. Immunol Rev. 2006;214(1):219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 47.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5(2):112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 48.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, et al. Tumor cell responses to IFNγ affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9(1):25–34. doi: 10.1016/S1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 49.Haghshenas M, Khademi B, Ashraf M, Ghaderi A, Erfani N. Helper and cytotoxic T-cell subsets (Th1, Th2, Tc1, and Tc2) in benign and malignant salivary gland tumors. Oral Dis. 2016;22(6):566–572. doi: 10.1111/odi.12496. [DOI] [PubMed] [Google Scholar]
- 50.Decot V, Voillard L, Latger-Cannard V, Aissi-Rothé L, Perrier P, Stoltz JF, et al. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38(5):351–362. doi: 10.1016/j.exphem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235(1):297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HM, Kim KS, Kim J. A comparative study of the effects of inhibitory cytokines on human natural killer cells and the mechanistic features of transforming growth factor-beta. Cell Immunol. 2014;290(1):52–61. doi: 10.1016/j.cellimm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Park JY, Lee SH, Yoon SR, Park YJ, Jung H, Kim TD, et al. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol Cells. 2011;32(3):265–272. doi: 10.1007/s10059-011-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Littwitz-Salomon E, Malyshkina A, Schimmer S, Dittmer U. The cytotoxic activity of natural killer cells is suppressed by IL-10(+) regulatory T cells during acute retroviral infection. Front Immunol. 2018;9:1947. doi: 10.3389/fimmu.2018.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118(19):5084–5095. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan Y, Zhang C, Xu Y, Wang M, Rao Q, Xing H, et al. Hyperfunction of CD4 CD25 regulatory T cells in de novo acute myeloid leukemia. BMC Cancer. 2020;20(1):472. doi: 10.1186/s12885-020-06961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mougiakakos D (2019) The Induction of a Permissive Environment to Promote T Cell Immune Evasion in Acute Myeloid Leukemia: The Metabolic Perspective. Front Oncol, 9 [DOI] [PMC free article] [PubMed]
- 60.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci. 2003;100(7):4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Street SEA, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24(11):603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1957;1(5023):841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 67.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32(2):135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284(1):1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 71.Barrett AJ. Acute myeloid leukaemia and the immune system: implications for immunotherapy. Br J Haematol. 2020;188(1):147–158. doi: 10.1111/bjh.16310. [DOI] [PubMed] [Google Scholar]
- 72.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 74.Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy. 2011;3(10):1143–1166. doi: 10.2217/imt.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53(10):844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 77.Woan K, Reddy V. Potential therapeutic role of natural killer cells in cancer. Expert Opin Biol Ther. 2007;7(1):17–29. doi: 10.1517/14712598.7.1.17. [DOI] [PubMed] [Google Scholar]
- 78.Grossenbacher SK, Canter RJ, Murphy WJ. Natural killer cell immunotherapy to target stem-like tumor cells. J Immunother Cancer. 2016;4(1):19. doi: 10.1186/s40425-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 80.Barrett AJ, Le Blanc K. Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol. 2010;161(2):223–232. doi: 10.1111/j.1365-2249.2010.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tratkiewicz JA, Szer J. Loss of natural killer activity as an indicator of relapse in acute leukaemia. Clin Exp Immunol. 1990;80(2):241–246. doi: 10.1111/j.1365-2249.1990.tb05241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lowdell MW, Craston R, Samuel D, Wood ME, O'Neill E, Saha V, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol. 2002;117(4):821–827. doi: 10.1046/j.1365-2141.2002.03495.x. [DOI] [PubMed] [Google Scholar]
- 83.Pross HF, Lotzová E. Role of natural killer cells in cancer. Nat Immun. 1993;12(4–5):279–292. [PubMed] [Google Scholar]
- 84.Tajima F, Kawatani T, Endo A, Kawasaki H. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia. 1996;10(3):478–482. [PubMed] [Google Scholar]
- 85.Bryceson YT, March ME, Ljunggren H-G, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99(5):836–847. doi: 10.3324/haematol.2013.087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costello RGT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci M-J, Reviron D, et al. Defective expression and function of natural killer cell–triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–7. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 88.Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother CII. 2010;59(1):73–79. doi: 10.1007/s00262-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90(1):109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 90.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, et al. Analysis of the receptor-ligand interactions in the natural killer–mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105(5):2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 91.Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T, et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189(3):1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 92.Fauriat C, Just-Landi S, Mallet FO, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2006;109(1):323–30. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 93.Verheyden S, Bernier M, Demanet C. Identification of natural killer cell receptor phenotypes associated with leukemia. Leukemia. 2004;18(12):2002–2007. doi: 10.1038/sj.leu.2403525. [DOI] [PubMed] [Google Scholar]
- 94.Sandoval-Borrego D, Moreno-Lafont MC, Vazquez-Sanchez EA, Gutierrez-Hoya A, López-Santiago R, Montiel-Cervantes LA, et al. Overexpression of CD158 and NKG2A inhibitory receptors and underexpression of NKG2D and NKp46 activating receptors on NK cells in acute myeloid leukemia. Arch Med Res. 2016;47(1):55–64. doi: 10.1016/j.arcmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 97.Verheyden S, Demanet C. NK cell receptors and their ligands in leukemia. Leukemia. 2008;22(2):249–257. doi: 10.1038/sj.leu.2405040. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen S, Beziat V, Dhedin N, Kuentz M, Vernant JP, Debre P, et al. HLA-E upregulation on IFN-γ-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2009;43(9):693–699. doi: 10.1038/bmt.2008.380. [DOI] [PubMed] [Google Scholar]
- 99.Nowbakht P, Ionescu M-CS, Rohner A, Kalberer CP, Rossy E, Mori L, et al. Ligands for natural killer cell–activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105(9):3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 100.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee H-G, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102(4):1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 101.Baragaño Raneros A, Martín-Palanco V, Fernandez AF, Rodriguez RM, Fraga MF, Lopez-Larrea C, et al. Methylation of NKG2D ligands contributes to immune system evasion in acute myeloid leukemia. Genes Immun. 2015;16(1):71–82. doi: 10.1038/gene.2014.58. [DOI] [PubMed] [Google Scholar]
- 102.Kearney CJ, Ramsbottom KM, Voskoboinik I, Darcy PK, Oliaro J. Loss of DNAM-1 ligand expression by acute myeloid leukemia cells renders them resistant to NK cell killing. OncoImmunology. 2016;5(8):e1196308. doi: 10.1080/2162402X.2016.1196308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baessler T, Charton JE, Schmiedel BJ, Grünebach F, Krusch M, Wacker A, et al. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood. 2010;115(15):3058–3069. doi: 10.1182/blood-2009-06-227934. [DOI] [PubMed] [Google Scholar]
- 104.Baessler T, Krusch M, Schmiedel BJ, Kloss M, Baltz KM, Wacker A, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein ligand subverts immunosurveillance of acute myeloid leukemia in humans. Can Res. 2009;69(3):1037–1045. doi: 10.1158/0008-5472.CAN-08-2650. [DOI] [PubMed] [Google Scholar]
- 105.Coles SJ, Wang ECY, Man S, Hills RK, Burnett AK, Tonks A, et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25(5):792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim SH, Worman CP, Jewell A, Goldstone AH. Production of tumour-derived suppressor factor in patients with acute myeloid leukaemia. Leuk Res. 1991;15(4):263–268. doi: 10.1016/0145-2126(91)90129-H. [DOI] [PubMed] [Google Scholar]
- 107.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK cells and cancer. J Immunol. 2007;178(7):4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 108.Min YJ, Lee J-H, Choi S-J, Chi H-S, Lee J-S, Kim W-K, et al. Prognostic significance of Fas (CD95) and TRAIL receptors (DR4/DR5) expression in acute myelogenous leukemia. Leuk Res. 2004;28(4):359–365. doi: 10.1016/j.leukres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 109.Fauriat C, Moretta A, Olive D, Costello RGT. Defective killing of dendritic cells by autologous natural killer cells from acute myeloid leukemia patients. Blood. 2005;106(6):2186–8. doi: 10.1182/blood-2005-03-1270. [DOI] [PubMed] [Google Scholar]
- 110.Ebata K, Shimizu Y, Nakayama Y, Minemura M, Murakami J, Kato T, et al. Immature NK cells suppress dendritic cell functions during the development of leukemia in a mouse model. J Immunol. 2006;176(7):4113–4124. doi: 10.4049/jimmunol.176.7.4113. [DOI] [PubMed] [Google Scholar]
- 111.Wang X, Zheng J, Liu J, Yao J, He Y, Li X, et al. Increased population of CD4+CD25high regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75(6):468–476. doi: 10.1111/j.1600-0609.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 112.Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, et al. Elevated frequencies of CD4+CD25+CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2011;129(6):1373–1381. doi: 10.1002/ijc.25791. [DOI] [PubMed] [Google Scholar]
- 113.Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15(10):3325–3332. doi: 10.1158/1078-0432.CCR-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hallett WHD, Ames E, Álvarez M, Barao I, Taylor PA, Blazar BR, et al. Combination therapy using IL-2 and Anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplant. 2008;14(10):1088–1099. doi: 10.1016/j.bbmt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carlsten M, Järås M. Natural killer cells in myeloid malignancies: immune surveillance, NK cell dysfunction, and pharmacological opportunities to bolster the endogenous NK cells. Front Immunol. 2019;10:2357. doi: 10.3389/fimmu.2019.02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiossone L, Dumas P-Y, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 118.Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13(1):277. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y, Zhang Y, Hughes T, Zhang J, Caligiuri MA, Benson DM, et al. Fratricide of NK Cells in daratumumab therapy for multiple myeloma overcome by <em>Ex Vivo</em>–expanded autologous NK cells. Clin Cancer Res. 2018;24(16):4006–4017. doi: 10.1158/1078-0432.CCR-17-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 121.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Handgretinger R, Lang P, André MC. Exploitation of natural killer cells for the treatment of acute leukemia. Blood. 2016;127(26):3341–3349. doi: 10.1182/blood-2015-12-629055. [DOI] [PubMed] [Google Scholar]
- 123.Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol. 2017;8:631. doi: 10.3389/fimmu.2017.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17(19):6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 127.Malmberg K-J, Schaffer M, Ringdén O, Remberger M, Ljunggren H-G. KIR-ligand mismatch in allogeneic hematopoietic stem cell transplantation. Mol Immunol. 2005;42(4):531–534. doi: 10.1016/j.molimm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 128.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 129.Kärre K. Immunology. A perfect mismatch. Science. 2002;295(5562):2029–2031. doi: 10.1126/science.1070538. [DOI] [PubMed] [Google Scholar]
- 130.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 131.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115(21):4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 133.Mills CD, North RJ. Expression of passively transferred immunity against an established tumor depends on generation of cytolytic T cells in recipient. Inhibition by suppressor T cells. J Exp Med. 1983;157(5):1448–60. doi: 10.1084/jem.157.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lupo KB, Matosevic S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers (Basel) 2019;11(6):769. doi: 10.3390/cancers11060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Franks SE, Wolfson B, Hodge JW. Natural born killers: NK cells in cancer therapy. Cancers (Basel) 2020;12(8):2131. doi: 10.3390/cancers12082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Granzin M, Wagner J, Köhl U, Cerwenka A, Huppert V, Ullrich E. Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front Immunol. 2017;8:458. doi: 10.3389/fimmu.2017.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang J, Liu Y, Au BC, Barber DL, Arruda A, Schambach A, et al. Preclinical validation: LV/IL-12 transduction of patient leukemia cells for immunotherapy of AML. Mol Ther Methods Clin Dev. 2016;3:16074. doi: 10.1038/mtm.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi Y, Dincheva-Vogel L, Ayemoba CE, Fung JP, Bergamaschi C, Pavlakis GN, et al. IL-15/IL-15Rα/CD80-expressing AML cell vaccines eradicate minimal residual disease in leukemic mice. Blood Adv. 2018;2(22):3177–3192. doi: 10.1182/bloodadvances.2018019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 142.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 144.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci USA. 2005;102(21):7641–7646. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu Z, Zhang H, Wu M, Peng G, He Y, Wan N, et al. Targeting the NKG2D/NKG2D-L axis in acute myeloid leukemia. Biomed Pharmacother. 2021;137:111299. doi: 10.1016/j.biopha.2021.111299. [DOI] [PubMed] [Google Scholar]
- 146.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73(6):1777–1786. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 147.Kamiya T, Chang YH, Campana D. Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res. 2016;4(7):574–581. doi: 10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 148.Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. 2019;7(3):363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest. 2019;129(5):2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Venton G, Labiad Y, Colle J, Fino A, Afridi S, Torres M, et al. Natural killer cells in acute myeloid leukemia patients: from phenotype to transcriptomic analysis. Immunol Res. 2016;64(5):1225–1236. doi: 10.1007/s12026-016-8848-0. [DOI] [PubMed] [Google Scholar]
- 151.van der Ploeg K, Le Luduec J-B, Stevenson PA, Park S, Gooley TA, Petersdorf EW, et al. HLA-A alleles influencing NK cell function impact AML relapse following allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4(19):4955–4964. doi: 10.1182/bloodadvances.2020002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsirogianni M, Grigoriou E, Dagla K, Pappa V, Konsta E, Kapsimali-Vaiopoulou V, et al. NK cytotoxicity in vitro and NK/ MDSCs/Tcell subpopulations in AML/MDS patients on 5-azacytidine treatment. Blood. 2016;128(22):5123. doi: 10.1182/blood.V128.22.5123.5123. [DOI] [Google Scholar]
- 153.Arvindam US, van Hauten P, Hallstrom C, Vallera DA, Dolstra H, Miller JS, et al. CD16-IL15-CLEC12A trispecific killer engager (TriKE) drives NK cell expansion, activation, and antigen specific killing of cancer stem cells in acute myeloid leukemia. Blood. 2018;132(Supplement 1):1454. doi: 10.1182/blood-2018-99-117150. [DOI] [Google Scholar]
- 154.Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SM, Coustan-Smith E, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124(7):1081–1088. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 155.Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14(1):7. doi: 10.1186/s13045-020-01014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21(11):2122–2129. doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Q-S, Wang Y, Lv H-Y, Han Q-W, Fan H, Guo B, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–91. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cummins KD, Gill S. Chimeric antigen receptor T-cell therapy for acute myeloid leukemia: how close to reality? Haematologica. 2019;104(7):1302–1308. doi: 10.3324/haematol.2018.208751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yáñez L, Sánchez-Escamilla M, Perales M-A. CAR T cell toxicity: current management and future directions. Hemasphere. 2019;3(2):e186-e. doi: 10.1097/HS9.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mardiana S, Gill S. CAR T cells for acute myeloid leukemia: state of the art and future directions. Front Oncol. 2020;10:697. doi: 10.3389/fonc.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klingemann H. Are natural killer cells superior CAR drivers? OncoImmunology. 2014;3(4):e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 166.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 167.Sutherland H, Blair A, Zapf R. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood. 1996;87(11):4754–4761. doi: 10.1182/blood.V87.11.4754.bloodjournal87114754. [DOI] [PubMed] [Google Scholar]
- 168.Hu Y, Tian Z-G, Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin. 2018;39(2):167–76. doi: 10.1038/aps.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J (2020) CAR-NK cells: a promising cellular immunotherapy for cancer. eBioMedicine, 59 [DOI] [PMC free article] [PubMed]
- 171.Naeimi Kararoudi M, Likhite S, Elmas E, Schwartz M, Sorathia K, Yamamoto K, et al. CD33 targeting primary CAR-NK cells generated by CRISPR mediated gene insertion show enhanced anti-AML activity. Blood. 2020;136(Supplement 1):3. doi: 10.1182/blood-2020-142494. [DOI] [Google Scholar]
- 172.Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth M-T, Fritsch G, et al. Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Invest. 2007;37(1):73–82. doi: 10.1111/j.1365-2362.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 173.Sperr WR, Florian S, Hauswirth AW, Valent P. CD33 as a target of therapy in acute myeloid leukemia: current status and future perspectives. Leuk Lymphoma. 2005;46(8):1115–1120. doi: 10.1080/10428190500126075. [DOI] [PubMed] [Google Scholar]
- 174.Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an Anti-CD33 calicheamicin immunoconjugate: presented in part at the 1997 annual meeting of the American Society of clinical oncology, Denver, CO; the 1997 European cancer conference, hamburg, germany; and the 1997 Annual meeting of the American Society of hematology, San Diego, CA. Blood. 1999;93(11):3678–3684. doi: 10.1182/blood.V93.11.3678. [DOI] [PubMed] [Google Scholar]
- 175.Schirrmann T, Pecher G. Specific targeting of CD33+ leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk Res. 2005;29(3):301–306. doi: 10.1016/j.leukres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 176.Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–1089. [PMC free article] [PubMed] [Google Scholar]
- 177.Salman H, Pinz KG, Wada M, Shuai X, Yan LE, Petrov JC, et al. Preclinical targeting of human acute myeloid leukemia using CD4-specific chimeric antigen receptor (CAR) T cells and NK cells. J Cancer. 2019;10(18):4408–4419. doi: 10.7150/jca.28952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Albinger N, Hartmann J, Ullrich E (2021) Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Therapy [DOI] [PMC free article] [PubMed]
- 179.Marofi F, Rahman HS, Thangavelu L, Dorofeev A, Bayas-Morejón F, Shirafkan N, et al. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther. 2021;12(1):200. doi: 10.1186/s13287-021-02251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Basar R, Daher M, Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Blood Adv. 2020;4(22):5868–5876. doi: 10.1182/bloodadvances.2020002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kloess S, Oberschmidt O, Dahlke J, Vu XK, Neudoerfl C, Kloos A, et al. Preclinical assessment of suitable natural killer cell sources for chimeric antigen receptor natural killer-based "Off-the-Shelf" acute myeloid leukemia immunotherapies. Hum Gene Ther. 2019;30(4):381–401. doi: 10.1089/hum.2018.247. [DOI] [PubMed] [Google Scholar]
- 182.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O'Connor J, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23(18):4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 183.He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID, et al. A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk Lymphoma. 2015;56(5):1406–1415. doi: 10.3109/10428194.2014.956316. [DOI] [PubMed] [Google Scholar]
- 184.Kayser S, Heitmann JS, Dörfel D, Thol F, Heuser M, Märklin M, et al. Interim results of a first in man study with the Fc-optimized FLT3 antibody flysyn for treatment of acute myeloid leukemia with minimal residual disease. Blood. 2019;134:3928. doi: 10.1182/blood-2019-125327. [DOI] [Google Scholar]
- 185.Riether C, Pabst T, Höpner S, Bacher U, Hinterbrandner M, Banz Y, et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med. 2020;26(9):1459–1467. doi: 10.1038/s41591-020-0910-8. [DOI] [PubMed] [Google Scholar]
- 186.Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, Palffy SV, et al. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci. 2015;112(34):10786–91. doi: 10.1073/pnas.1422749112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koerner SP, André MC, Leibold JS, Kousis PC, Kübler A, Pal M, et al. An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia. Leukemia. 2017;31(2):459–469. doi: 10.1038/leu.2016.194. [DOI] [PubMed] [Google Scholar]
- 188.Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858–mediated natural killer ADCC against AML blasts. Blood. 2016;127(23):2879–2889. doi: 10.1182/blood-2015-11-680546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Venditti A, Buccisano F, Maurillo L, Del Principe MI, Coppola A, Palomba P, et al. MEN1112/OBT357, an anti Bst1/CD157 humanized antibody inducing acute myelogenous leukemia (AML) blast depletion in an autologous ex vivo assay: a potential new targeted therapy for AML. Blood. 2015;126(23):788. doi: 10.1182/blood.V126.23.788.788. [DOI] [Google Scholar]
- 190.Krupka C, Lichtenegger FS, Köhnke T, Bögeholz J, Bücklein V, Roiss M, et al. Targeting CD157 in AML using a novel. Fc-Eng Antibody Const Oncotarget. 2017;8(22):35707–35717. doi: 10.18632/oncotarget.16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mani R, Rajgolikar G, Nunes J, Zapolnik K, Wasmuth R, Mo X, et al. Fc-engineered anti-CD33 monoclonal antibody potentiates cytotoxicity of membrane-bound interleukin-21 expanded natural killer cells in acute myeloid leukemia. Cytotherapy. 2020;22(7):369–376. doi: 10.1016/j.jcyt.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Daver NG, Erba HP, Papadantonakis N, DeAngelo DJ, Wang ES, Konopleva MY, et al. A phase I, first-in-human study evaluating the safety and preliminary antileukemia activity of IMGN632, a novel CD123-targeting antibody-drug conjugate, in patients with relapsed/refractory acute myeloid leukemia and other CD123-positive hematologic malignancies. Blood. 2018;132:27. doi: 10.1182/blood-2018-99-112955. [DOI] [Google Scholar]
- 193.Jurcic JG, Ravandi F, Pagel JM, Park JH, Smith BD, Douer D, et al. Phase I trial of α-particle therapy with actinium-225 (225Ac)-lintuzumab (anti-CD33) and low-dose cytarabine (LDAC) in older patients with untreated acute myeloid leukemia (AML) J Clin Oncol. 2015;33(15_suppl):7050. doi: 10.1200/jco.2015.33.15_suppl.7050. [DOI] [Google Scholar]
- 194.Narayan R, Blonquist TM, Emadi A, Hasserjian RP, Burke M, Lescinskas C, et al. A phase 1 study of the antibody-drug conjugate brentuximab vedotin with re-induction chemotherapy in patients with CD30-expressing relapsed/refractory acute myeloid leukemia. Cancer. 2020;126(6):1264–1273. doi: 10.1002/cncr.32657. [DOI] [PubMed] [Google Scholar]
- 195.Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie J-N, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 196.Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- 197.Anami Y, Deng M, Gui X, Yamaguchi A, Yamazaki CM, Zhang N, et al. LILRB4-targeting antibody-drug conjugates for the treatment of acute myeloid leukemia. Mol Cancer Ther. 2020;19(11):2330–2339. doi: 10.1158/1535-7163.MCT-20-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jiang Y-P, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J, et al. CLT030, a leukemic stem cell–targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv. 2018;2(14):1738–1749. doi: 10.1182/bloodadvances.2018020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kovtun Y, Noordhuis P, Whiteman KR, Watkins K, Jones GE, Harvey L, et al. IMGN779, a novel CD33-targeting antibody-drug conjugate with DNA-alkylating activity, exhibits potent antitumor activity in models of AML. Mol Cancer Ther. 2018;17(6):1271–1279. doi: 10.1158/1535-7163.MCT-17-1077. [DOI] [PubMed] [Google Scholar]
- 200.Pereira DS, Guevara CI, Jin L, Mbong N, Verlinsky A, Hsu SJ, et al. AGS67E, an Anti-CD37 monomethyl Auristatin E antibody-drug conjugate as a potential therapeutic for B/T-cell malignancies and AML: a new role for CD37 in AML. Mol Cancer Ther. 2015;14(7):1650–1660. doi: 10.1158/1535-7163.MCT-15-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zheng B, Yu S-F, del Rosario G, Leong SR, Lee GY, Vij R, et al. An anti–CLL-1 antibody-drug conjugate for the treatment of acute myeloid leukemia. Clin Cancer Res. 2019;25(4):1358–1368. doi: 10.1158/1078-0432.CCR-18-0333. [DOI] [PubMed] [Google Scholar]
- 202.Khan M, Arooj S, Wang H (2020) NK Cell-Based Immune Checkpoint Inhibition. Frontiers in Immunology, 11 [DOI] [PMC free article] [PubMed]
- 203.Vey N, Bourhis J-H, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 204.Vey N, Bourhis J-H, Recher C, Etienne A, Charbonnier A, Andre P, et al. Repeated dosing Of anti-KIR (IPH2101) as maintenance therapy in ederly patients with acute myeloid leukemia. Blood. 2013;122(21):2696. doi: 10.1182/blood.V122.21.2696.2696. [DOI] [Google Scholar]
- 205.Vey N, Dumas P-Y, Recher C, Gastaud L, Lioure B, Bulabois C-E, et al. Randomized phase 2 trial of lirilumab (anti-KIR monoclonal antibody, mAb) as maintenance treatment in elderly patients (pts) with acute myeloid leukemia (AML): results of the effikir trial. Blood. 2017;130:889. doi: 10.1182/blood.V130.Suppl_1.889.889. [DOI] [Google Scholar]
- 206.Daver N, Boddu P, Garcia-Manero G, Ravandi F, Jabbour EJ, Borthakur G, et al. Phase IB/II study of lirilumab with azacytidine (AZA) in relapsed AML. Blood. 2017;130:2634. [Google Scholar]
- 207.Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S, et al. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor–negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16(5):612–621. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Loredana R, Elena U, Pascale A, Antonella M, Antonella T, Fabiana T, et al. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. 2016;101(5):626–633. doi: 10.3324/haematol.2015.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11(12):2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 × 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19(14):3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016;22(14):3440–3450. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150(5):574–586. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- 213.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33(6):599–608. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 214.Märklin M, Hagelstein I, Koerner SP, Rothfelder K, Pfluegler MS, Schumacher A, et al. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J Immunother Cancer. 2019;7(1):143. doi: 10.1186/s40425-019-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Arvindam US, van Hauten PMM, Schirm D, Schaap N, Hobo W, Blazar BR, et al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia. 2021;35(6):1586–1596. doi: 10.1038/s41375-020-01065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sanchez-Correa B, Bergua JM, Pera A, Campos C, Arcos MJ, Bañas H, et al. (2017) In vitro culture with interleukin-15 leads to expression of activating receptors and recovery of natural killer cell function in acute myeloid leukemia patients. Front Immunol, 8 [DOI] [PMC free article] [PubMed]
- 217.Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrózek K, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: cancer and leukemia group b study 9720. J Clin Oncol. 2008;26(30):4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 219.Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C, et al. Maintenance therapy with interleukin-2 for childhood AML: results of ELAM02 phase III randomized trial. Hemasphere. 2018;2(6):e159-e. doi: 10.1097/HS9.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann W-K, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108(1):88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 221.Nilsson MS, Hallner A, Brune M, Nilsson S, Thorén FB, Martner A, et al. Immunotherapy with HDC/IL-2 may be clinically efficacious in acute myeloid leukemia of normal karyotype. Hum Vaccin Immunother. 2020;16(1):109–111. doi: 10.1080/21645515.2019.1636598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cuapio A, Post M, Cerny-Reiterer S, Gleixner KV, Stefanzl G, Basilio J, et al. Maintenance therapy with histamine plus IL-2 induces a striking expansion of two CD56 bright NK cell subpopulations in patients with acute myeloid leukemia and supports their activation. Oncotarget. 2016;7:29. doi: 10.18632/oncotarget.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rosario M, Romee R, Schneider SE, Leong JW, Sullivan RP, Fehniger TA. Human cytokine-induced memory-like (CIML) NK cells are active against myeloid leukemia in vitro and in vivo. Blood. 2014;124(21):1117. doi: 10.1182/blood.V124.21.1117.1117. [DOI] [Google Scholar]
- 224.Berrien-Elliott MM, Cashen AF, Cubitt CC, Neal CC, Wong P, Wagner JA, et al. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 2020;10(12):1854–1871. doi: 10.1158/2159-8290.CD-20-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bednarski JJ, Zimmerman C, Cashen AF, Desai S, Foster M, Schappe T, et al. Adoptively transferred donor-derived cytokine induced memory-like NK cells persist and induce remission in pediatric patient with relapsed acute myeloid leukemia after hematopoietic cell transplantation. Blood. 2019;134(Supplement_1):3307. doi: 10.1182/blood-2019-126982. [DOI] [Google Scholar]
- 226.Foltz JA, Berrien-Elliott MM, Neal C, Foster M, McClain E, Schappe T, et al. (2019) Cytokine-induced memory-like (ML) NK cells persist for > 2 months following adoptive transfer into leukemia patients with a MHC-compatible hematopoietic cell transplant (HCT). Blood, 134(Supplement1), 1954
- 227.Mastaglio S, Wong E, Perera T, Ripley J, Blombery P, Smyth MJ, et al. Natural killer receptor ligand expression on acute myeloid leukemia impacts survival and relapse after chemotherapy. Blood Adv. 2018;2(4):335–346. doi: 10.1182/bloodadvances.2017015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar-Filipowicz A. Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res. 2007;31(10):1393–1402. doi: 10.1016/j.leukres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 229.Baragaño Raneros A, Minguela A, Rodriguez RM, Colado E, Bernal T, Anguita E, et al. Increasing TIMP3 expression by hypomethylating agents diminishes soluble MICA, MICB and ULBP2 shedding in acute myeloid leukemia, facilitating NK cell-mediated immune recognition. Oncotarget. 2017;8:19. doi: 10.18632/oncotarget.16657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111(3):1428–1436. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 231.Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23(4):641–648. doi: 10.1038/leu.2008.354. [DOI] [PubMed] [Google Scholar]
- 232.Lu X, Ohata K, Kondo Y, Luis Espinoza J, Qi Z, Nakao S. Hydroxyurea upregulates NKG2D ligand expression in myeloid leukemia cells synergistically with valproic acid and potentially enhances susceptibility of leukemic cells to natural killer cell-mediated cytolysis. Cancer Sci. 2010;101(3):609–615. doi: 10.1111/j.1349-7006.2009.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Le Roy A, Prébet T, Castellano R, Goubard A, Riccardi F, Fauriat C, et al. (2018) Immunomodulatory drugs exert anti-leukemia effects in acute myeloid leukemia by direct and immunostimulatory activities. Front Immunol, 9 [DOI] [PMC free article] [PubMed]
- 234.Lai K-C, Lu H-F, Chen K-B, Hsueh S-C, Chung J-G, Huang W-W, et al. Casticin promotes immune responses, enhances macrophage and NK cell activities, and increases survival rates of leukemia BALB/c mice. Am J Chin Med. 2019;47(01):223–236. doi: 10.1142/S0192415X19500113. [DOI] [PubMed] [Google Scholar]
- 235.Yu F-S, Yang J-S, Yu C-S, Chiang J-H, Lu C-C, Chung H-K, et al. Safrole suppresses murine myelomonocytic leukemia WEHI-3 cells in vivo, and stimulates macrophage phagocytosis and natural killer cell cytotoxicity in leukemic mice. Environ Toxicol. 2013;28(11):601–608. doi: 10.1002/tox.20756. [DOI] [PubMed] [Google Scholar]
- 236.Shih Y-L, Shang H-S, Chen Y-L, Hsueh S-C, Chou H-M, Lu H-F, et al. Ouabain promotes immune responses in WEHI-3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ Toxicol. 2019;34(5):659–665. doi: 10.1002/tox.22732. [DOI] [PubMed] [Google Scholar]
- 237.Lin J-J, Lu K-W, Ma Y-S, Tang N-Y, Wu P-P, Wu C-C, et al. Alpha-phellandrene, a natural active monoterpene, influences a murine WEHI-3 leukemia model in vivo by enhancing macrophague phagocytosis and natural killer cell activity. In Vivo. 2014;28(4):583–588. [PubMed] [Google Scholar]
- 238.Lee JY, Park S, Kim DC, Yoon J-H, Shin SH, Min W-S, et al. A VEGFR-3 antagonist increases IFN-γ expression on low functioning NK cells in acute myeloid leukemia. J Clin Immunol. 2013;33(4):826–837. doi: 10.1007/s10875-013-9877-2. [DOI] [PubMed] [Google Scholar]
- 239.Lee JY, Park S, Min W-S, Kim H-J. Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett. 2014;354(2):281–289. doi: 10.1016/j.canlet.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 240.Otegbeye F, Ojo E, Moreton S, Mackowski N, Lee DA, de Lima M, et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE. 2018;13(1):e0191358. doi: 10.1371/journal.pone.0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.