Abstract
Background
Talimogene laherparepvec (T-VEC) is a genetically modified herpes simplex type 1 virus and known as an effective oncolytic immunotherapy for injectable cutaneous, subcutaneous and nodal melanoma lesions in stage IIIB-IVM1a patients. This study set out to identify prognostic factors for achieving a complete response that can be used to optimize patient selection for T-VEC monotherapy.
Methods
Patients with stage IIIB-IVM1a melanoma, treated with T-VEC at the Netherlands Cancer Institute between 2016–12 and 2020–01 with a follow-up time > 6 months, were included. Data were collected on baseline characteristics, responses and adverse events (AEs). Uni- and multivariable analyses were conducted, and a prediction model was developed to identify prognostic factors associated with CR.
Results
A total of 93 patients were included with a median age of 69 years, median follow-up time was 16.6 months. As best response, 58 patients (62%) had a CR, and the overall response rate was 79%. The durable response rate (objective response lasting > 6 months) was 51%. Grade 1–2 AEs occurred in almost every patient. Tumor size, type of metastases, prior treatment with systemic therapy and stage (8Th AJCC) were independent prognostic factors for achieving CR. The prediction model includes the predictors tumor size, type of metastases and number of lesions.
Conclusions
This study shows that intralesional T-VEC monotherapy is able to achieve high complete and durable responses. The prediction model shows that use of T-VEC in patients with less tumor burden is associated with better outcomes, suggesting use earlier in the course of the disease.
Supplementary Information
The online version contains supplementary material available at (10.1007/s00262-020-02839-7).
Keywords: Talimogene laherparepvec, Complete response, Durable response, Prediction model, Tumor load, Stage IIIB-IVM1a melanoma
Introduction
T-VEC is a genetically modified herpes simplex type 1 virus (HSV-1), which is used as oncolytic immunotherapy. Since approval by the Food and Drug Administration (FDA) and the EMA, it is used as intralesional monotherapy for cutaneous, subcutaneous and nodal lesions in stage IIIB/C and IVM1a melanoma patients [1]. T-VEC has a dual mechanism of action: a local effect in which it replicates in the infiltrated tumor cells, thereby causing cell death as well as a systemic effect which induces the patient’s immune response [2, 3].
As T-VEC shows only mild side effects, compared to treatment with systemic immunotherapy, it has become a popular alternative for patients with early metastatic melanoma. The phase III OPTiM trial was the first to show the therapeutic benefit of T-VEC with an overall response rate (ORR) of 26% [4]. Subsequently, several real-world studies demonstrated superior results, with complete response (CR) rates ranging between 39% and 61.5% [5–7]. Response rates vary substantially due to differences in patient- and tumor selection, and the DRR is often not calculated due to a short follow-up time.
Although these outcomes are promising, there is still a group of patients without tumor response or even progressing to distant metastases during treatment. These patients end treatment with T-VEC and are usually referred to a different therapy, i.e., surgery, radiotherapy, systemic therapy or isolated limb perfusion. It remains unclear what causes the dissimilarity in response between patients.
In our center, selecting treatment for patients with stage IIIB/C and IVM1a disease is performed in a multidisciplinary setting, taking into account various characteristics such as age and performance score of the patient, tumor characteristics and previous treatments. In order to make such decisions, it is convenient to be aware of predictive factors for a complete response. To date, several independent factors for response on T-VEC have been reported by previous studies, including lesion size, prior treatment with systemic therapy and clinical substage. However, more factors might still be unknown and a clinically applicable predictive model for the selection of patients for treatment with T-VEC is, to our knowledge, still lacking. This could guide both clinicians and patients in shared decision making toward their preferred treatment option.
Therefore, the aim of this study was to identify predictive factors for a CR in melanoma patients that were treated with T-VEC. We also set out to build a prediction model that could be used to predict a CR in patients, allowing for a more accurate selection of stage IIIB/C and IVM1a melanoma patients.
Patients and methods
Patients
From January 2017, until June 2020, a total of 128 patients with stage IIIB-D or IVM1a melanoma were treated with T-VEC monotherapy at the Netherlands Cancer Institute—Antoni van Leeuwenhoek. However, for this study, we only included patients treated from January 2017 to January 2020, all with a follow-up time beyond 6 months from the start of treatment. To be eligible for treatment with T-VEC, patients had to have injectable cutaneous, subcutaneous or lymph node metastases. This study was performed in accordance with the institutional ethical guidelines. A database was prospectively maintained with patient-, tumor- and treatment characteristics and follow-up data obtained from patient records.
T-VEC treatment protocol
The first dose of T-VEC consisted of 106 plaque-forming units (PFU)/ml and all doses thereafter consisted of 108 PFU/ml. Treatment with T-VEC required repeat administration every 2 weeks, except for the second dose which was administered 3 weeks after the initial dose. The maximum injection volume per treatment session is 4.0 ml and the volume that is injected depends on the size of the lesion(s) [8, 9].
Patients were clinically evaluated before each administration: metastatic lesions were counted, measured and photographed. For this study, when dividing the treated metastases of patients into types, we classified patients with subcutaneous as well as cutaneous metastases, as subcutaneous metastases. Likewise, all patients with cutaneous and/or subcutaneous as well as lymph node metastases were classified as lymph node metastases. Prior to the start of T-VEC, the HSV infection status was determined with a serologic test (IgG). Blood count, lactate dehydrogenase (LDH), tumor marker S100B and infection parameters were assessed by routine laboratory tests. Three monthly whole body positron emission tomography/computed tomography (PET/CT) were performed for response evaluation. When a complete response was suspected, histological biopsies of the remaining lesions were taken for pathological confirmation.
Data and statistical analyses
Best response rates were divided into four groups according to the World Health Organization criteria and in case of pathological evaluation as described by Tetzlaff et al. [10, 11]. The few patients with stable disease, who showed neither response nor progression, were added to the PR group.
ORR was defined as all patients with CR, nearCR or PR as their best response. Durable response rate (DRR) was defined the percentage of patients with a CR or PR lasting longer than 6 months continuously and beginning within 12 months after initiating treatment. Statistical analyses were performed using IBM SPSS Statistics 26.0 and R 3.6.1. P values < 0.05 were considered statistically significant. Fisher’s exact test and Mann–Whitney U test were used for comparison of categorical and continuous data between groups, and the Mann–Whitney U test was used for comparison of continuous data between groups, respectively. The ‘non-CR’ group included all patients that had a nearCR, PR or PD as their best response. Uni- and multivariable analyses were performed by logistic regression to identify variables associated with achieving a CR on T-VEC. For all variables, the cutoff value that corresponded to the most significant difference in outcome was selected. This also applies for using continuous or categorical variables. We generated Kaplan–Meier survival curves to assess progression-free survival (duration of time from the commencement of T-VEC, in which the patient shows no signs of progression; PFS), overall survival (duration of time from the commencement of T-VEC that a patient is still alive; OS) and relapse-free survival (duration of time from the cessation of T-VEC, in which the patient develops no relapse; RFS). A risk model was developed using predictors that were selected on statistical significance and clinical importance. The predictive accuracy of the model was assessed through calculation of overall performance, discriminative ability and calibration. Overall performance was calculated with the Brier score. Discriminative ability was assessed by calculating the area under the receiver operating characteristic (ROC) curve. This value can range from 0.5 to 1.0, the first indicating no discriminative ability and the latter indicating perfect discrimination. For a fair discriminative ability, the value must be above 0.7. The model was internally validated through calibration by generating a calibration plot. Bootstrapping analyses with 1000 samples were done to internally validate the model, thereby reducing the overfit bias. A nomogram is presented as graphical representation of the model.
Results
A total of 93 patients, with a median follow-up time of 16.6 months, were included in this study. More patients were female (57%), and the median age of all patients was 69 years. Most patients had metastases on their extremities (73%), and 60%, 32%, 8% of patients had 8th AJCC stage IIIC, IIIB, IIID + IVM1a disease, respectively.
The median time to best response was 3.9 months (6–7 treatments). Fifty-eight patients (62%) had a CR and 3 (3%), 13 (14%) and 19 (20%) had a near CR, PR and PD, respectively, to T-VEC as their best response. The ORR was 79%, and the DRR was 51%.
Significant differences in distribution of age, melanoma substage, diameter of largest metastases, type of metastases and pre-treatment with systemic therapy were found between the CR and non-CR group. Baseline characteristics and their correlation with CR or non-CR as best response, are summarized in Table 1.
Table 1.
Clinical features of 93 patients treated with T-VEC and their correlations with a CR or non-CR as best response
| Number of patients per response | |||||
|---|---|---|---|---|---|
| CR | Non-CR | Total | p value | ||
| Sex (%) |
Female Male |
37 (70) 21 (53) |
16 (30) 19 (48) |
53 40 |
0.130 |
| Mean age in years (range) | 72 (30–90) | 65 (35–97) | 69 (30–97) | 0.044 | |
| Elevated S100B at baseline (%) |
No (< 0.10) Yes (> 0.10) |
53 (66) 5 (38) |
27 (34) 8 (62) |
80 13 |
0.069 |
| HSV status at baseline (%) |
Positive Negative Unknown |
40 (68) 15 (50) 3 (75) |
19 (32) 15 (50) 1 (25) |
59 30 4 |
0.211 |
| Substage (AJCC 8) (%) |
IIIB IIIC IIID + IVM1a |
25 (83) 30 (54) 3 (43) |
5 (17) 26 (46) 4 (57) |
30 56 7 |
0.010 |
| Mutation status (%) |
Wildtype BRAF mut NRAS mut Other or unknown |
8 (53) 22 (55) 14 (70) 14 (70) |
7 (47) 18 (45) 6 (30) 4 (22) |
15 40 20 18 |
0.290 |
| Location metastases (%) |
Extremity Trunk Head/neck |
43 (63) 8 (62) 7 (58) |
25 (37) 5 (38) 5 (42) |
68 13 12 |
0.941 |
| Number of metastases (%) |
< 20 lesions > 20 lesions |
48 (66) 10 (50) |
25 (34) 10 (50) |
73 20 |
0.206 |
| Mean diameter of the largest metastases in mm (range) | 14 (0.5–65) | 29 (5–100) | 20 (0.5–100) | 0.001 | |
| Type of metastases (%) |
Cutaneous only Subcutaneous Lymph nodes |
23 (72) 34 (64) 1 (13) |
9 (28) 19 (36) 7 (88) |
32 53 8 |
0.008 |
| Pre-treatment metastases (%) | |||||
| Radiotherapy |
No Yes |
56 (63) 2 (50) |
33 (37) 2 (50) |
89 4 |
0.630 |
| Systemic therapy |
No Yes |
53 (68) 5 (31) |
24 (31) 11 (69) |
77 16 |
0.009 |
| Perfusion |
No Yes |
42 (60) 16 (70) |
28 (40) 7 (30) |
70 23 |
0.466 |
| Resection (surgery) |
No Yes |
3 (60) 55 (63) |
2 (40) 33 (38) |
5 88 |
1.000 |
| Mean number of AE per patient (range) | 4 (0–12) | 4 (0–14) | 4 (0–14) | 0.798 | |
The ‘non-CR’ group includes all patients with a near CR, PR or PD as best response
Data are expressed as n (%) unless otherwise specified
The p values for age, tumor diameter and number of AE were determined by Mann–Whitney U tests, while other p values were determined by Fisher’s exact test. Bold values indicate statistical significance (p < 0.05)
CR complete response, PR partial response, PD progressive disease, HSV herpes simplex virus, AJCC American Joint Committee on Cancer
Patients had a median of 8 treatments before cessation of T-VEC therapy. All patients with a PR as best response, eventually had to stop treatment with T-VEC due to primary or secondary progression (n = 29) or insufficient further response to T-VEC (n = 3). Most patients with PD developed locoregional or distant metastases. The majority of these progressive patients were treated with checkpoint inhibitors. Of the 61 patients with a CR or nearCR, 28% (n = 17) developed a relapse during follow-up and most of them were locoregional. Seven of these patients had in-transit metastases that were re-treated with T-VEC and again leading to a CR in five patients (Table 2).
Table 2.
Patients with PD or insufficient response during treatment or relapse during follow-up and their following treatment
| Progressive disease or insufficient response during/on treatment (total patients included = 93) |
Relapse during follow-up (total patients with CR or near CR = 61) |
|
|---|---|---|
| N (%) | N (%) | |
|
Total no. of patients No. of patients with PD No. of patients with insufficient (locoregional) response |
32 (34) 29 (32) 3 (3) |
17 (18) – – |
| Location | ||
| Locoregional | 12 (13) | 11 (12) |
| Regional | 7 (8) | 2 (2) |
| Distant | 13 (14) | 4 (4) |
| Treatment | ||
| Surgery | 1 (1) | 3 (3) |
| Surgery and adjuvant therapy | – | 1 (1) |
| Surgery and radiotherapy | – | 1 (1) |
| Radiotherapy | 3 (3) | 3 (3) |
| Checkpoint inhibitors | 19 (20) | 3 (13) |
| Targeted therapy | 4 (4) | – |
| TIL therapy | 2 (2) | – |
| T-VEC | – | 6 (7) |
| ILP | 1 (1) | – |
| No therapy | 2 (2) | – |
CR complete response, BRAF/MEKi BRAF/MEK inhibitors, TIL tumor-infiltrating lymphocyte, T-VEC talimogene laherparepvec; ILP isolated limb perfusion
AE was seen in almost all patients. The three most common AE’s were influenza-like symptoms, such as illness, fatigue and chills. One patient had a serious AE, a grade 3 colitis. He was treated with steroids and recovered, after which he restarted treatment with T-VEC after missing 4 cycles, without developing further AE’s (Supplementary table).
Univariable and multivariable analyses
Univariable logistic regression analyses indicated that substage according to the 8th AJCC (IIIC OR 0.23; 95% CI 0.08–0.69, p = 0.009 and IIID+IVM1a OR 0.15; 95% CI 0.03–0.89, p = 0.037), diameter of the largest metastases (per unit increase OR 0.96; 95% CI 0.94–0.99, p = 0.002), type of metastases (cutaneous OR 17.89; 95% CI 1.92–166.78, p = 0.011 and subcutaneous OR 12.53; 95% CI 1.43–109.62, p = 0.022) and pre-treatment with systemic therapy (OR 0.21; 95% CI 0.06–0.66, p = 0.008) were independent predictors of a CR. Multivariable analyses showed that substage (IIIC OR 0.13; 95% CI 0.02–0.86. p = 0.034), diameter of largest metastases (OR 0.95; 95% CI 0.92–0.98, p = 0.002) and type of metastases (cutaneous OR 19.41; 95% CI 1.37–275.00, p = 0.028) were associated with a CR (Table 3).
Table 3.
Predictive factors for CR estimated by univariable and multivariable logistic regression analyses
| Univariable analyses | Multivariable analyses | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Sex (%) | Female | 1 | |||||
| Male | 0.48 | 0.20–1.12 | 0.090 | ||||
| Mean age in years (range) | 1.03 | 1.00–1.07 | 0.050 | ||||
| Elevated S100B at baseline (%) | No (< 0.10) | 3.14 | 0.94–10.53 | 0.064 | |||
| Yes (> 0.10) | 1 | ||||||
| HSV status at baseline (%) | Positive | 2.11 | 0.86–5.18 | 0.105 | |||
| Negative | 1 | ||||||
| Unknown | |||||||
| Substage (AJCC 8) (%) | IIIB | 1 | 1 | ||||
| IIIC | 0.23 | 0.08–0.69 | 0.009 | 0.20 | 0.05–0.75 | 0.017 | |
| IIID + IVM1a | 0.15 | 0.03–0.89 | 0.037 | 0.33 | 0.03–3.24 | 0.344 | |
| Mutation status (%) | Wildtype | 1 | 0.145 | ||||
| BRAF mut | 1.07 | 0.33–3.52 | |||||
| NRAS mut | 2.04 | 0.51–8.23 | 0.316 | ||||
| Other or unknown | 3.06 | 0.68–13.79 | 0.912 | ||||
| Location metastases (%) | Extremity | 1 | |||||
| Trunk | 0.93 | 0.27–3.16 | 0.747 | ||||
| Head/neck | 0.81 | 0.23–2.84 | 0.908 | ||||
| Number of metastases (%) | < 20 | 1 | |||||
| > 20 | 0.52 | 0.19–1.42 | 0.202 | ||||
| Diameter of the largest metastases in mm (range) | 0.96 | 0.94–0.99 | 0.002 | 0.95 | 0.92–0.98 | 0.002 | |
| Type of metastases (%) | Cutaneous only | 17.89 | 1.92–166.78 | 0.011 | 19.41 | 1.37–275.00 | 0.028 |
| Subcutaneous | 12.53 | 1.43–109.62 | 0.022 | 9.92 | 0.83–118.39 | 0.070 | |
| Lymph nodes | 1 | – | – | 1 | – | – | |
| Pre-treatment (%) | |||||||
| Radiotherapy |
No Yes |
1 0.59 |
– 0.08–4.38 |
– 0.605 |
|||
| Systemic therapy |
No Yes |
1 0.21 |
– 0.06–0.66 |
– 0.008 |
0.39 | 0.10–1.49 | 0.167 |
| Perfusion |
No Yes |
1 1.52 |
– 0.56–4.18 |
0.413 | |||
| Excision |
No Yes |
1 1.11 |
0.18–7.00 | 0.911 | |||
| Number of AE per patient | 0.96 | 0.83–1.11 | 0.582 | ||||
Bold values indicate statistical significance (p < 0.05)
OR odds ratio, HSV herpes simplex virus, AJCC American Joint Committee on Cancer, AE adverse event
Survival
Median PFS was 17 months for all patients. Median PFS was not yet reached in the CR group and 4 months in the non-CR group, and the difference between these two groups was significant (p < 0.001). Median OS was not reached for any group. However, there was a significant difference in median OS between the non-CR and CR group (p < 0.001). Median RFS (only for CR and nearCR patients) was not yet reached (Fig. 1).
Fig. 1.

a Kaplan–Meier plot of OS in patients who achieved a CR versus patients who did not achieve a CR versus all patients; b Kaplan–Meier plot of PFS in patients who achieved a CR versus patients who did not achieve a CR versus all patients; c Kaplan–Meier plot of RFS in patients with a nearCR or CR as best response
Prediction model
A combination of known and statistically significant predictors was used to set up a model for predicting CR in patients that were treated with T-VEC. These predictors consisted of number of metastases (categorical), diameter of largest metastases (continuous) and type of metastases (categorical). Statistically significant in this model was: diameter of the largest metastases (per unit increase OR 0.96; 95% CI 0.93–0.98, p = 0.002) and cutaneous (OR 23.96; 95% CI 2.25–252.94, p = 0.008) or subcutaneous metastases (OR 9.94; 95% CI 1.04–95.09, p = 0.046). Two statistically significant variables were not included in the model: substage, due to high correlation with the variable ‘type of metastases’, and prior treatment with systemic therapy, as most of the patients were treatment-naive.
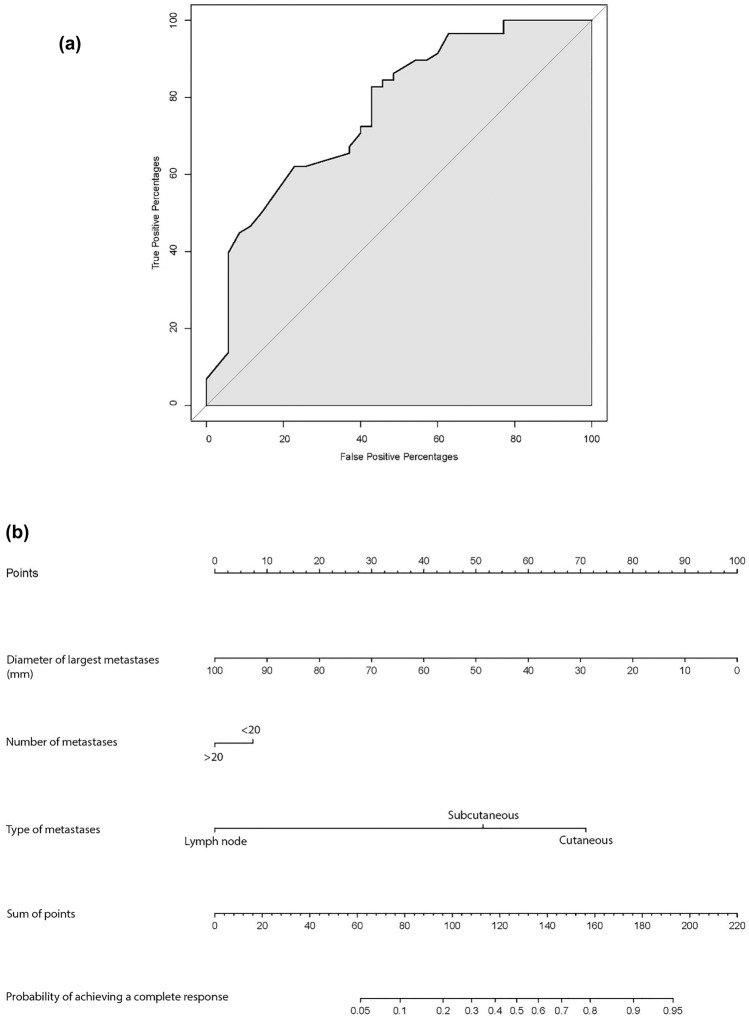
The Brier score of the model was 0.182, indicating an overall good performance. The ROC curve had an area under the curve (AUC) of 0.767, which indicated that the nomogram had a fair discriminatory capability (Fig. 2a). The calibration plot, based on internal validation with a bootstrap resampling frequency of 1000, showed underestimation for predicted probabilities < 0.55 and mostly overestimation for predicted probabilities > 0.55 (Supplementary figure). In order to easily estimate the probability of achieving a CR on T-VEC per patient, a nomogram is provided in Fig. 2b.
Fig. 2.
a ROC curve, the area under the curve is 0.767; b Nomogram of prediction model. The probability is calculated by drawing a vertical line from each predictor to the ‘points’ axis. The points corresponding to each predictor should then be summed. Subsequently, the sum on the ‘sum of points’ axis can be located and vertically projected onto the bottom probability scale. For example, patient with 4 subcutaneous tumor lesions of which the largest has a diameter of 15 mm. Probability of achieving a complete response to T-VEC is 70%
Discussion
To our knowledge, this real-world cohort of patients demonstrates the highest CRR and DRR for treatment with T-VEC monotherapy, published to date. Moreover, this study is the first to develop and report a prediction model which estimates the chance of achieving a CR based on three easily accessible tumor characteristics.
Previous studies that calculated the DRR for T-VEC monotherapy reported outcomes of 30% and 40% [6, 12]. While these studies included patients with visceral disease, all patients in the present study were staged as stage IIIB, IIIC, IIID or IVM1a melanoma. The OPTiM trial also showed more pronounced differences for patients that were treated with T-VEC compared to GM-CSF: patients with stage IIIB or IIIC melanoma had DRR’s of 33% versus 0%, while those of patients with stage IVM1b and IVM1c melanoma were 3% versus 4% and 7% versus 3%, respectively [4]. Interestingly, in our current series, all patients that achieved CR’s or nearCR’s had durable responses.
For the nomogram, we chose to focus fully on tumor characteristics, combining known and statistically significant predictors. Three real-world studies, with cohorts of varying size, have investigated predictive factors for achieving a CR in patients treated with T-VEC and their results were broadly in accordance with ours [6, 12, 13]. Bulky disease was a consistent negative predictive factor for CR and overall response, and Zhou et al. [12] reported a significant association with OS too. Bulky disease is often only measured by the maximum lesion diameter, yet we believe that number of metastases, although not associated with CR in our analyses (neither categorical nor continuous), also contributes to the patient’s tumor load. Therefore, both factors were added to the model. Our model suggests that the patients with a low tumor burden have the highest probability of achieving a CR. Thus, we may conclude that T-VEC monotherapy may need to be used earlier on in the course of the disease when tumor lesions are still small. This would mean a change in the old dogma to resect small in-transit metastases.
The neo-adjuvant use of T-VEC versus surgery already shows evidence to support this change in mindset, as a randomized phase 2 trial of T-VEC + surgical resection versus surgical resection alone showed improved RFS for the combined treatment group, suggesting more durable benefit when adding in T-VEC to the treatment paradigm [14, 15]. At our institute we will shortly commence a single arm phase 2 trial, investigating the neo-adjuvant combination of T-VEC + nivolumab for patients with resectable in-transit metastases ± lymph node metastases (NIVEC trial, NCT04330430).
This current study is the first to find an association between the different type of metastases and the clinical response to T-VEC. We determined that our patients with subcutaneous metastases often had a higher tumor burden, because either the tumor(s) were larger or patients had more lesions, as this group also included those with cutaneous and subcutaneous metastases. The final analyses of the OPTiM trial were the first to report that a lower tumor burden was a predictor of clinical response [16]. It is also possible that the different tumor sites (cutaneous and subcutaneous) are biologically heterogeneous, leading to aberrant antitumor responses. T-cells activated by a specific tumor site, might preferentially migrate to the same anatomic site, thereby determining a difference in efficacy of therapy [17, 18]. Thirdly, subcutaneous as well as nodal metastases are less superficial than cutaneous metastases and we hypothesize that for the latter, metastasis through the lymphatic route or extravasation to distant anatomic sites occurs easier and faster, leading to PD. Finally, intratumoral injection directly into subcutaneous and nodal metastases is sometimes more difficult to achieve, although guidance by ultrasound already makes this less challenging.
Patients that were treated with systemic therapy prior to treatment with T-VEC had worse outcomes in our study. This could be the result of the tumor having the opportunity to grow over time in those with insufficient response to prior systemic therapy (e.g., anti-PD1). However, as most of our patients started T-VEC for newly developed lesions, it is more likely that their tumors had the time to develop immunologic escape mechanisms or had a low cell proliferative rate, which is shown to be linked to a less successful viral growth [19, 20]. Nevertheless, combination studies investigating concurrent use of T-VEC and systemic immunotherapies are currently under investigation. In a phase 1b study, Ribas et al. [21] discovered a potential synergistic effect when combining pembrolizumab and T-VEC, resulting in clinical outcomes beyond what would be expected with either therapy alone. The phase III sequel is still ongoing (Masterkey 265, NCT02263508), but a phase II study evaluating ipilimumab + T-VEC has already found a greater antitumor effect for the combination than ipilimumab alone, in injected as well as noninjected lesions [22]. It seems that the enhancement of antitumor response, leading to greater antitumor activity, only occurs when the therapies are given simultaneously. Most of our patients with a PR as best response developed distant metastases during treatment and were forced to switch therapy. Especially for these patients, an improved systemic response, induced by combination therapy, might be the solution for a better outcome.
Although this study shows useful results, it is monocentric and limited by the relatively small patient cohort with limited follow-up. A larger independent cohort is needed, preferably multicenter, whereby the prediction model can be externally validated and possibly incorporate more (continuous) predictive factors. Although our model was internally validated by bootstrap method, cautious conclusions should be drawn when using the model and only external validation will ensure accurate use by clinicians.
In conclusion, this study demonstrates high response rates and four predictive factors for achieving a CR in patients with early metastatic melanoma. We developed a prediction model, which can be used to select patients for treatment with T-VEC. In general, patients with a low tumor burden have the best outcomes, suggesting T-VEC should perhaps be used earlier on in the course of the disease.
Supplementary Information
Abbreviations
- AE
Adverse event
- AUC
Area under the curve
- AJCC
American Joint Committee on Cancer
- CR
:Complete response
- DRR
Durable response rate
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- HSV-1
Herpes simplex type 1 virus
- LDH
Lactate dehydrogenase
- ORR
Overall response rate
- OS
Overall survival
- PR
Partial response
- PFU
Plaque-forming units
- PET/CT
Positron emission tomography/computed tomography
- PD
Progressive disease
- PFS
Progression-free survival
- RFS
Relapse-free survival
- ROC
Receiver operating characteristic
- T-VEC
Talimogene laherparepvec
Author contributions
Conceptualization: ES, AvA; Methodology: ES, AvA; Formal analysis and investigation: ES; Writing—original draft preparation: ES; Writing—review and editing: ES, VF, CZ, WK, BvdH, BvdW, MW, YS, WvH; Funding acquisition: AvA; Resources: ES, VF, CZ, WK, BvdH, BvdW, MW, YS, WvH; Supervision: AvA.
Funding
No funding was received for conducting this study.
Data availability
Data and material presented in this study are available on request.
Compliance with ethics standards
Conflict of interest
Winan van Houdt declares advisory board/consultancy agreement and research grant received from Amgen. Michel Wouters declares a research grant received from Novartis. Alexander van Akkooi declares advisory board/consultancy agreements for Bristol-Myers Squibb, Novartis, MSD – Merck, Merck – Pfizer, 4SC and Amgen and a research grant received from Bristol-Myers Squibb, Novartis and Amgen. All other others have no relevant financial or non-financial interests to disclose.
Consent to participate
No identifiable patient information has been published warranting individual consent from patients. The requirement for individual patient informed consent for the purpose of data collection and publication was waived as per the regulations covered under the respective institutional IRBs.
Ethical approval
The study was performed with the approval of the Institutional Review Board (IRB) Netherlands Cancer Institute – Antoni van Leeuwenhoek and in accordance with the declaration of Helskini.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaufman HL, Bines SD. OPTIM trial: a phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6(6):941–949. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman HL, Amatruda T, Reid T, Gonzalez R, Glaspy J, Whitman E, Harrington K, Nemunaitis J, Zloza A, Wolf M, Senzer NN. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J Immunother Cancer. 2016;4:12. doi: 10.1186/s40425-016-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4(1):53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/jco.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 5.Perez MC, Miura JT, Naqvi SMH, Kim Y, Holstein A, Lee D, Sarnaik AA, Zager JS. Talimogene laherparepvec (TVEC) for the treatment of advanced melanoma: a single-institution experience. Ann Surg Oncol. 2018;25(13):3960–3965. doi: 10.1245/s10434-018-6803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louie RJ, Perez MC, Jajja MR, Sun J, Collichio F, Delman KA, Lowe M, Sarnaik AA, Zager JS, Ollila DW. Real-world outcomes of talimogene laherparepvec therapy: a multi-institutional experience. J Am Coll Surg. 2019;228(4):644–649. doi: 10.1016/j.jamcollsurg.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Franke V, Berger DMS, Klop WMC, van der Hiel B, van de Wiel BA, Ter Meulen S, Wouters M, van Houdt WJ, van Akkooi ACJ. High response rates for T-VEC in early metastatic melanoma (stage IIIB/C-IVM1a) Int J Cancer. 2019;145(4):974–978. doi: 10.1002/ijc.32172. [DOI] [PubMed] [Google Scholar]
- 8.Amgen (2015) Imlygic (talimogene laherperepvec) suspension for intralesional injection: US prescribing information
- 9.Harrington KJ, Michielin O, Malvehy J, Pezzani Gruter I, Grove L, Frauchiger AL, Dummer R. A practical guide to the handling and administration of talimogene laherparepvec in Europe. OncoTargets Ther. 2017;10:3867–3880. doi: 10.2147/OTT.S133699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tetzlaff MT, Messina JL, Stein JE, Xu X, Amaria RN, Blank CU, van de Wiel BA, Ferguson PM, Rawson RV, Ross MI, Spillane AJ, Gershenwald JE, Saw RPM, van Akkooi ACJ, van Houdt WJ, Mitchell TC, Menzies AM, Long GV, Wargo JA, Davies MA, Prieto VG, Taube JM, Scolyer RA. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861–1868. doi: 10.1093/annonc/mdy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geneva W, Organization WH, Organization WH. WHO Handbook for reporting results of cancer treatment. Cambridge: WHO Offset Publication; 1979. [Google Scholar]
- 12.Zhou AY, Wang DY, McKee S, Ye F, Wen CC, Wallace DE, Ancell KK, Conry RM, Johnson DB. Correlates of response and outcomes with talimogene laherperpvec. J Surg Oncol. 2019;120(3):558–564. doi: 10.1002/jso.25601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masoud SJ, Hu JB, Beasley GM, Stewart JHT, Mosca PJ. Efficacy of talimogene laherparepvec (T-VEC) Therapy in Patients with in-transit melanoma metastasis decre. Ann Surg Oncol. 2019;26(13):4633–4641. doi: 10.1245/s10434-019-07691-3. [DOI] [PubMed] [Google Scholar]
- 14.Dummer R, Gyorki DE, Hyngstrom JR, Berger AC, Conry RM, Demidov LV, Sharma A, Treichel S, Faries MB, Ross MI. One-year (yr) recurrence-free survival (RFS) from a randomized, open label phase II study of neoadjuvant (neo) talimogene laherparepvec (T-VEC) plus surgery (surgx) versus surgx for resectable stage IIIB-IVM1a melanoma (MEL) J Clin Oncol. 2019;37(15_suppl):9520–9520. doi: 10.1200/JCO.2019.37.15_suppl.9520. [DOI] [Google Scholar]
- 15.Andtbacka RHI, Dummer R, Gyorki DE, Berger AC, Conry RM, Demidov LV, Chan E, Treichel S, Faries MB, Ross MI. Interim analysis of a randomized, open-label phase 2 study of talimogene laherparepvec (T-VEC) neoadjuvant treatment (neotx) plus surgery (surgx) vs surgx for resectable stage IIIB-IVM1a melanoma (MEL) J Clin Oncol. 2018;36(15_suppl):9508–9508. doi: 10.1200/JCO.2018.36.15_suppl.9508. [DOI] [Google Scholar]
- 16.Andtbacka RHI, Collichio F, Harrington KJ, Middleton MR, Downey G, Ӧhrling K, Kaufman HL. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7(1):145. doi: 10.1186/s40425-019-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CJ, Tai KF, Roffler S, Hwang LH. The immunization site of cytokine-secreting tumor cell vaccines influences the trafficking of tumor-specific T lymphocytes and antitumor efficacy against regional tumors. J Immunol. 2004;173(10):6025–6032. doi: 10.4049/jimmunol.173.10.6025. [DOI] [PubMed] [Google Scholar]
- 18.Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, van den Braber M, Rozeman EA, Haanen J, Blank CU, Horlings HM, David E, Baran Y, Bercovich A, Lifshitz A, Schumacher TN, Tanay A, Amit I. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 2019;176(4):775–789.e18. doi: 10.1016/j.cell.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 20.Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH, Federoff HJ, Fong Y. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) Faseb j. 1999;13(11):1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 21.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, Logan TF, Hauschild A, Lebbe C, Chen L, Kim JJ, Gansert J, Andtbacka RHI, Kaufman HL. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced unresectable melanoma. J Clin Oncol. 2018;36(17):1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and material presented in this study are available on request.