Abstract
We found that RNA 2 of the four ilarviruses sequenced to date encodes an additional conserved open reading frame (ORF), 2b, that overlaps the 3′ end of the previously known ORF, 2a. A novel RNA species of 851 nucleotides was found to accumulate to high levels in plants infected with spinach latent virus (SpLV). Further analysis showed that RNA 4A is a subgenomic RNA of RNA 2 and encodes all of ORF 2b. Moreover, a protein species of the size expected for SpLV ORF 2b was translated in vitro from the RNA 4A-containing virion RNAs. The data support the suggestion that the SpLV 2b protein is translated in vivo. The 2b gene of ilarviruses, which is not encoded by alfamoviruses and bromoviruses, shares several features with the previously reported cucumovirus 2b gene; however, their encoded proteins share no detectable sequence similarities. The evolutionary origin of the 2b gene is discussed.
The Bromoviridae family of plant RNA viruses contains four genera: Alfamovirus, Bromovirus, Cucumovirus, and Ilarvirus (16). All members of these genera have a tripartite single-stranded messenger-sense RNA genome. Alfalfa mosaic virus (AMV), brome mosaic virus, and cucumber mosaic virus are among the most extensively studied RNA viruses and have long served as model systems for examination of the genome structure, expression, and replication of plant RNA viruses. In contrast, although Ilarvirus, containing 16 virus species, is the largest genus of the family, its members have been little studied. Thus, until the publication of the complete RNA 3 sequence for prune dwarf virus in 1994 (2), the only genomic sequence determined was that of RNA 3 of tobacco streak virus (TSV; reference 5), the type member of the genus. However, a large amount of genomic sequence data have recently become available for the ilarviruses with the publication of sequences for the complete genomes of citrus leaf rugose virus (CLRV) (10, 19, 20) and elm mottle virus (EMoV) (the virus for which the complete genomic sequence was determined as described in reference 12 has recently been shown to be EMoV [18a]), the complete RNA 1 of prune dwarf virus (15), and the complete RNA 3 of apple mosaic virus (25), citrus variegation virus (19), prunus necrotic ringspot virus (14, 17), lilac ring mottle virus (21), and hydrangea mosaic virus (11). Analysis of these sequence data (18, 19) has shown that (i) the genome organization of members of the genus Ilarvirus is typical of the more extensively studied genera in Bromoviridae and includes four genes, (ii) the encoded proteins share significant sequence similarities with the corresponding proteins encoded by the other member viruses of Bromoviridae, and (iii) ilarviruses are more closely related to AMV than to either cucumoviruses or bromoviruses, thus confirming the classical relationships between the four genera of Bromoviridae (16).
We reported (8) that RNA 2 of CLRV encodes an additional open reading frame (ORF), 2b, that overlaps the 3′ end of ORF 2a, which encodes the 2a protein. Importantly, this overlapping ORF, which is conserved in the second completely sequenced ilarvirus, EMoV (12), shares several features with the recently discovered 2b gene of the cucumoviruses (7). We report here that ORF 2b is conserved in all four of the ilarviruses sequenced to date, including TSV and spinach latent ilarvirus (SpLV), and that SpLV ORF 2b is highly likely to be expressed in SpLV-infected plants.
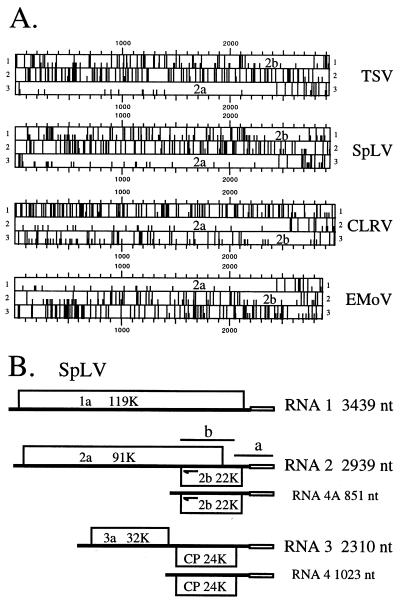
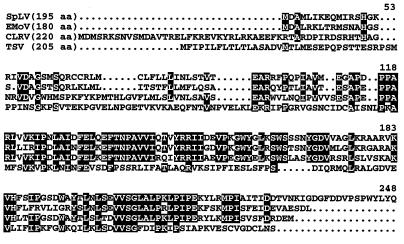
Sequence analysis of the complete genomic sequences of TSV (5, 22) and SpLV (23) showed that RNA 2 of both viruses encodes an ORF 2b (Fig. 1A and Table 1). As found previously for CLRV (8) and EMoV (12), the ORF 2b of TSV and SpLV is also encoded in the +1 reading frame of ORF 2a and overlaps its 3′ end. Amino acid sequence comparisons using the Wisconsin GCG package (6) revealed that the encoded putative 2b proteins are conserved among the four ilarviruses (Fig. 2) with percent identities ranging from 28.7 to 63.6% (percent similarity, 38.0 to 72.2%). The percent sequence identities (57.3 to 63.6%) are particularly high among the 2b proteins of CLRV, EMoV, and SpLV, which are all members of ilarvirus subgroup 2, whereas TSV (with lower percent identity) belongs to subgroup 1 (16).
FIG. 1.
Genome organization of the ilarviruses. (A) Diagram of the three triplet codon phases of plus-strand RNA 2 of the four ilarviruses. The AUG triplet and the three stop codons are shown as short and long vertical lines, respectively. (B) SpLV genome organization. Proteins 1a, 2a, and 3a are translated from genomic RNAs 1, 2, and 3, while the CP and the 2b protein are translated via subgenomic RNAs 4 and 4A. The open box represents the highly conserved 3′-terminal sequence of 163 nucleotides (nt). The target positions of the two probes (a and b) used in Northern blot analysis, as well as the approximate binding position of primer Xin-44 (arrows), are indicated. K, 103.
TABLE 1.
Structural features of RNA 2 of the ilarviruses
| Virus | Length (nucleotides) and position (in parentheses) of region
|
||||
|---|---|---|---|---|---|
| 5′ NTRa | ORF 2a | ORF 2b | RNA 4A | 3′ NTRb | |
| SpLV | 71 | 2394 (72–2465) | 588 (2158–2745) | 851 (2089–2939) | 194 |
| EMoV | 75 | 2370 (76–2445) | 543 (2141–2683) | ? | 191 |
| CLRV | 73 | 2499 (74–2572) | 663 (2139–2801) | ? | 189 |
| TSV | 41 | 2403 (42–2444) | 618 (2170–2787) | ? | 139 |
5′-NTR, 5′ nontranslated region.
3′-NTR, 3′ nontranslated region after ORF 2b.
FIG. 2.
Alignment of the amino acid (aa) sequences of the putative 2b proteins encoded by the four ilarviruses (10, 12, 22, 23). The alignment was obtained by using the Pileup program of the Wisconsin GCG package (6) implemented at the Bioinformatic Center of the National University of Singapore. Residues identical in three or more 2b proteins are highlighted. Notably, the VVSG motif is absolutely conserved in all four ilarvirus 2b proteins.
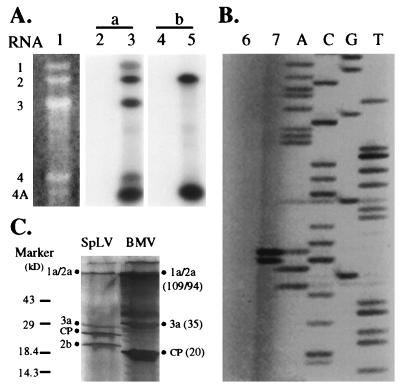
Conservation of ORF 2b among the four ilarviruses suggests that it is likely to be functionally important and expressed in vivo. RNA 3 of all of the members of Bromoviridae is bicistronic, and in vivo expression of the CP-encoding gene located 3′ of the 3a-encoding gene is via a subgenomic mRNA (RNA 4) (Fig. 1B). As ilarvirus ORF 2b is located in the 3′ portion of RNA 2 (e.g., nucleotides 2158 to 2745 of SpLV RNA 2, [Table 1]), its in vivo expression is also likely to be via a subgenomic RNA. Thus, using Northern blot analysis with SpLV RNA-specific probes, we first determined if a subgenomic RNA corresponding to ORF 2b was produced in SpLV-infected plants. Ten days after inoculation, total RNAs were extracted from either healthy (mock-inoculated) or SpLV-infected Nicotiana tabacum cv. Xanthi-nc plants and subjected to agarose gel electrophoresis (under denaturing conditions), followed by Northern blot analysis, as previously described (8). Two probes, a and b (Fig. 1B), were used in the Northern blot analysis. Probe a was derived from the 3′-terminal region of RNA 2 (nucleotides 2639 to 2939) containing the 3′-untranslated region highly conserved among all SpLV RNAs (Fig. 1B). In SpLV-infected plants, this probe detected RNAs 1 to 4, as well as an abundant novel RNA species with a molecular weight lower than that of RNA 4 (Fig. 3A, lane 3). Probe b, derived from nucleotides 2008 to 2777 of SpLV RNA 2, targeted the ORF 2b coding sequence of SpLV RNA 2 (Fig. 1B). As expected, this probe hybridized to RNA 2 but not to RNA 1, 3, or 4, indicating its specificity (Fig. 3A, lane 5). The novel RNA species also hybridized strongly with this ORF 2b-specific probe (Fig. 3A, lane 5). Therefore, this novel RNA species, designated RNA 4A (Fig. 3A), was 3′ coterminal with RNA 2, contained the ORF 2b coding sequence, and is thus most likely to be the subgenomic mRNA for ORF 2b.
FIG. 3.
Characterization of expression of the SpLV 2b gene. (A) SpLV RNA 4A detection. Total RNAs were extracted from SpLV virions (lane 1) or from mock-inoculated (lanes 2 and 4) or SpLV-infected (lanes 3 and 5) N. tabacum (cv. Xanthi) plants, fractionated, and blotted onto an Amersham Hybond-N+ membrane as previously described (8). SpLV virion RNAs were visualized by ethidium bromide staining (lane 1). Alternatively, duplicate membranes were hybridized with either probe a, which is specific for all SpLV RNAs (lanes 2 and 3), or probe b, which is specific for the ORF 2b coding sequence only (lanes 4 and 5). The positions of SpLV RNAs 1, 2, 3, 4, and 4A are indicated. (B) Mapping of the 5′ end of SpLV RNA 4A. Total RNAs from healthy plants (lane 6) or plants infected with SpLV (lane 7) were annealed with primer Xin-44, reverse transcribed by avian myeloblastosis virus reverse transcriptase, and analyzed on a sequencing gel. Primer Xin-44 was also used to sequence a full-length cDNA clone of SpLV RNA 2 by using the Sequenase kit, version 2.0 (U.S. Biochemicals), to provide a sequence ladder (lanes A, C, G, and T). (C) In vitro translation. SpLV virion RNAs were translated in vitro by using the Promega wheat germ extract in accordance with the manufacturer’s instructions. The translational products from BMV RNAs provided by the manufacturer were also analyzed, and their sizes (in kilodaltons) are in parentheses. The positions of the 14C-labeled low-range protein molecular weight standards from Gibco Bethesda Research Laboratories are on the left.
Primer extension experiments were performed to map the 5′ end of RNA 4A (Fig. 3B). Primer Xin-44 is complementary to nucleotides 2164 to 2185 of SpLV RNA 2 and thus will bind to both RNAs 2 and 4A (arrows in Fig. 1B). However, only the runoff cDNA species reverse transcribed from the RNA 4A template can be resolved in sequencing gels, and thus, its length can be determined when it is run alongside a sequence ladder used as size markers. cDNAs synthesized from total RNAs of mock-inoculated (lane 6) or SpLV-infected (lane 7) plants using Xin-44 were separated in a sequencing gel. The dideoxynucleotide sequencing reaction products (lanes A, C, G, and T) generated by using Xin-44 as the primer and a full-length cDNA clone of SpLV RNA 2 (26) as the template were run alongside the primer extension products of RNA 4A (Fig. 3B). Two strong bands, which were considered to represent the runoff cDNAs from the RNA 4A template present in SpLV-infected plants, were detected (Fig. 3B, lane 7). However, such cDNA species were not detected when total RNAs from uninfected healthy plants were used as the templates (Fig. 3B, lane 6). The lower band (lane 7) is likely to represent the position of the full-length cDNA of RNA 4A synthesized by using primer Xin-44, whereas the upper band, extended by an additional nucleotide, may be due to the 5′-end cap structure (1); the 5′ termini of both genomic and subgenomic RNAs are known to be capped for member viruses of Bromoviridae (16). These results mapped the 5′ end of RNA 4A to nucleotide 2089 of RNA 2. Accordingly, RNA 4A is predicted to be 851 nucleotides long, with a 5′-terminal sequence of 5′-GTCTGAAGA … -3′, and to encode all of ORF 2b. The 5′ and 3′ untranslated regions of RNA 4A are 69 and 194 nucleotides long, respectively (Table 1). Similarly, the 5′ end of RNA 4 was mapped to nucleotide 1288 of RNA 3 by using a primer complementary to nucleotides 1390 to 1409 of RNA 3 (data not shown). Thus, RNA 4 is 1023 nucleotides long (Fig. 1B) and 172 nucleotides longer than RNA 4A, which agrees well with their relative migration rates observed by denaturing agarose gel electrophoresis (Fig. 3A, lanes 1 and 3). Notably, the 5′-terminal sequences of both subgenomic RNAs 4 and 4A start with GTCT (bold in the following sequence) and this tetranucleotide is immediately preceded in both genomic RNAs 2 and 3 with a highly conserved sequence, GAGAGCAAGGGTGTCT (nucleotides 2077 to 2092 of RNA 2). The bases underlined represent positions where nucleotide substitutions were observed between RNAs 2 and 3. This sequence conservation suggests that both subgenomic RNAs may be produced by a similar mechanism, i.e., by transcription from their respective genomic RNAs, as proposed for RNAs 4 and 4A of the cucumoviruses (7).
All five known SpLV RNAs, including RNA 4A, are encapsidated in virions (Fig. 3A, lane 1); the encapsidation of an RNA species smaller than RNA 4 had been noted before (4), and we confirmed that this RNA was RNA 4A by Northern blot analysis (data not shown). Moreover, total virion RNA preparations of CLRV, EMoV, and TSV all contain an RNA species that is smaller than RNA 4 but approximately the same size as the putative RNA 4A of the respective viruses (18a). In vitro translation of the total SpLV virion RNAs in wheat germ extracts produced four major protein bands (filled circles in Fig. 3C) visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% separation gel). The top band may contain proteins 1a and 2a, with predicted Mrs of 119,000 and 91,000, respectively; proteins 1a and 2a of the control bromovirus brome mosaic virus (BMV) also migrated as one band (lane BMV, Fig. 3C). The lower two bands, migrating slightly faster than the 35,000-Mr 3a protein of BMV, are likely to represent protein 3a and the CP. The predicted Mrs of the SpLV 3a protein and CP are 32,000 and 24,000, respectively. The smallest protein, with an Mr of 21,000, which was nearly as abundant as the CP, is most likely the translational product of ORF 2b from RNA 4A since its size is in good agreement with the Mr of 21,000 predicted from the sequence (Fig. 3C). It must be pointed out that conclusive identification of the SpLV 2b protein requires either a specific antibody or protein sequencing. Nevertheless, since (i) RNA 4A, encoding all of ORF 2b, accumulated to high levels in SpLV-infected plants; (ii) a protein species of the size expected for ORF 2b was translated in vitro from virion RNAs containing RNA 4A; and (iii) we have shown recently that RNA 4A is associated with polysomes in infected tobacco plants (26), it is reasonable to deduce that the SpLV 2b protein is translated in vivo.
We have recently reported that the genome of the cucumoviruses encodes a 2b gene (7, 24) in addition to the four-genes genome structure known for many years as one of the hallmarks for member viruses of Bromoviridae (16). Functional analyses indicated that cucumoviral 2b is important for both systemic virus spread and virulence determination (8, 9). The 2b genes of cucumoviruses and ilarviruses share several important features which were recognized previously (8) and are further confirmed here. These include that both (i) are encoded at the 3′ end of RNA 2 in the +1 reading frame of ORF 2a, (ii) partially overlap the 3′ end of ORF 2a, and (iii) are expressed in vivo through a subgenomic mRNA. However, we detected no significant sequence similarities between the 2b proteins of cucumo- and ilarviruses. In addition, the ilarvirus 2b gene (543 to 663 nucleotides; Table 1) is almost twice as large as the cucumovirus 2b gene (288 to 333 nucleotides; reference 7).
Among the four genera of Bromoviridae, AMV and the ilarviruses differ from bromo- and cucumoviruses in requiring the presence of either the CP or subgenomic RNA 4 in addition to the three genomic RNAs for initiation of infection (3). Indeed, the CPs of AMV and some ilarviruses are interchangeable in their ability to activate viral genomes (13). Moreover, bromo- and cucumoviruses have a conserved 3′-terminal tRNA-like structure which can be aminoacylated with tyrosine, whereas the 3′ termini of AMV and the ilarviruses cannot be aminoacylated. Phylogeny analyses of viral replicase proteins 1a and 2a also indicated a two-cluster structure for Bromoviridae; i.e., AMV and the ilarviruses form one closely related cluster, while bromo- and cucumoviruses form another (10, 18, 20). Intriguingly, the 2b gene is not encoded by either AMV or the bromoviruses (7), indicating that whether or not to encode the 2b gene is not correlated with relatedness between the genomes of the viral genera within Bromoviridae. Furthermore, as summarized above, the 2b genes of cucumoviruses and ilarviruses share several features, although no similarities could be detected at the primary amino acid sequence level. Thus, it is likely that the 2b gene is either an ancestral gene of Bromoviridae that has been subsequently lost in AMV and the bromoviruses or an independent de novo creation in cucumoviruses and ilarviruses. Although our present data do not appear to favor one possibility over the other, the 2b gene unique to each of the two clusters must have played an important role in the evolution of the Bromoviridae family. For cucumoviruses, we have shown that the 2b gene encodes a host-specific long-distance movement function, suggesting that the 2b gene could provide an important genetic basis for the wide host range of the cucumoviruses (8). We are currently analyzing the functional role of the ilarvirus 2b gene, and it will be of great interest to see if it is important in determining some of the unique biological properties of the ilarviruses.
Acknowledgments
We acknowledge Andrew P. Lucy for critical reading of the manuscript and Wan-Xiang Li for excellent technical support.
REFERENCES
- 1.Ahlquist P, Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984;4:2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachman E J, Scott S W, Xin G, Vance V B. The complete nucleotide sequence of prune dwarf ilarvirus RNA 3: implications for coat protein activation of genome replication in ilarviruses. Virology. 1994;201:127–131. doi: 10.1006/viro.1994.1272. [DOI] [PubMed] [Google Scholar]
- 3.Bol J F, van Vloten-Doting L, Jaspars E M. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971;46:73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- 4.Bos L. Spinach latent virus. CMI/ABB descriptions of plant viruses, no. 281. Warwick, United Kingdom: Association of Applied Biologists; 1984. [Google Scholar]
- 5.Cornelissen B J C, Janssen H R, Zuidema D, Bol J F. Complete nucleotide sequence of tobacco streak virus RNA 3. Nucleic Acids Res. 1984;12:2427–2437. doi: 10.1093/nar/12.5.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding S W, Anderson B J, Haase H R, Symons R H. New overlapping gene encoded by the cucumber mosaic virus genome. Virology. 1994;198:593–601. doi: 10.1006/viro.1994.1071. [DOI] [PubMed] [Google Scholar]
- 8.Ding S W, Li W X, Symons R H. A naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 1995;14:5762–5772. doi: 10.1002/j.1460-2075.1995.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding S W, Shi B J, Li W X, Symons R H. An interspecies hybrid RNA virus is significantly more virulent than either parental virus. Proc Natl Acad Sci USA. 1996;93:7470–7474. doi: 10.1073/pnas.93.15.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge X, Scott S W. The nucleotide sequence of citrus leaf rugose ilarvirus RNA-2. J Gen Virol. 1994;75:2841–2846. doi: 10.1099/0022-1317-75-10-2841. [DOI] [PubMed] [Google Scholar]
- 11.Ge X, Scott S W. The nucleotide sequence of hydrangea mosaic virus RNA 3 exhibits similarity with the RNA 3 of tobacco streak virus. Virus Res. 1996;40:57–63. doi: 10.1016/0168-1702(95)01251-6. [DOI] [PubMed] [Google Scholar]
- 12.Ge X, Scott S W, Zimmerman M T. The complete sequence of the genomic RNAs of spinach latent virus. Arch Virol. 1997;142:1213–1226. doi: 10.1007/s007050050153. [DOI] [PubMed] [Google Scholar]
- 13.Gonsalves D, Garnsey S M. Functional equivalence of an RNA component and coat protein for infectivity of citrus leaf rugose virus. Virology. 1975;64:23–31. doi: 10.1016/0042-6822(75)90075-6. [DOI] [PubMed] [Google Scholar]
- 14.Guo D, Maiss E, Adam G, Casper R. Prunus necrotic ringspot ilarvirus: nucleotide sequence of RNA 3 and the relationship to other ilarviruses based on coat protein comparison. J Gen Virol. 1995;76:1073–1079. doi: 10.1099/0022-1317-76-5-1073. [DOI] [PubMed] [Google Scholar]
- 15.Rampitsch C, Eastwell K C. The complete nucleotide sequence of prune dwarf ilarvirus RNA-1. Arch Virol. 1997;142:1911–1918. doi: 10.1007/s007050050210. [DOI] [PubMed] [Google Scholar]
- 16.Rybicki E P. Bromoviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. The sixth report of the International Committee on Taxonomy of Viruses 1995. Vienna, Austria: Springer-Verlag; 1995. pp. 450–457. [Google Scholar]
- 17.Sanchez-Navarro J A, Pallas V. Evolutionary relationships in the ilarviruses: nucleotide sequence of prunus necrotic ringspot virus RNA 3. Arch Virol. 1997;142:749–763. doi: 10.1007/s007050050116. [DOI] [PubMed] [Google Scholar]
- 18.Scott S W. Xth International Congress of Virology Abstracts PW17-22. 1996. A re-appraisal of the taxonomy of the genus ilarvirus and an examination at the molecular level of the relationship between members of this genus and alfalfa mosaic virus; p. 147. [Google Scholar]
- 18a.Scott, S. W. Unpublished data.
- 19.Scott S W, Ge X. The complete nucleotide sequence of the RNA 3 of citrus leaf rugose and citrus variegation ilarviruses. J Gen Virol. 1995;76:957–963. doi: 10.1099/0022-1317-76-4-957. [DOI] [PubMed] [Google Scholar]
- 20.Scott S W, Ge X. The nucleotide sequence of citrus leaf rugose virus RNA 1. J Gen Virol. 1995;76:3233–3238. doi: 10.1099/0022-1317-76-12-3233. [DOI] [PubMed] [Google Scholar]
- 21.Scott S W, Ge X. The complete nucleotide sequence of the RNA 3 of lilac ring mottle ilarvirus. J Gen Virol. 1995;76:1801–1806. doi: 10.1099/0022-1317-76-7-1801. [DOI] [PubMed] [Google Scholar]
- 22.Scott, S. W., X. Ge, and M. T. Zimmerman. The sequence of the RNA 1 and RNA 2 of tobacco streak virus: additional evidence for the inclusion of alfalfa mosaic virus in the genus Ilarvirus. Arch. Virol., in press. [DOI] [PubMed]
- 23.Scott S W, Ge X, Zimmerman M T. The complete sequence of the genomic RNAs of spinach latent virus. 1997. GenBank accession no. U93192, U93193, and U93194. [DOI] [PubMed] [Google Scholar]
- 24.Shi B J, Ding S W, Symons R H. In vivo expression of an overlapping gene encoded by the cucumoviruses. J Gen Virol. 1997;78:237–241. doi: 10.1099/0022-1317-78-1-237. [DOI] [PubMed] [Google Scholar]
- 25.Shiel P J, Alrefai R H, Domier L L, Korban S S, Berger P H. The complete nucleotide sequence of apple mosaic virus RNA-3. Arch Virol. 1995;140:1247–1256. doi: 10.1007/BF01322750. [DOI] [PubMed] [Google Scholar]
- 26.Xin, H. W., and S. W. Ding. Unpublished data.