Abstract
Background
The objective of this study was to investigate the association between the onset of TD and treatment efficacy in NSCLC patients who initiated anti-PD-1 blockade (Nivolumab®) and to assess the impact of TD severity and subtype on nivolumab efficacy.
Materials and methods
This study was performed at a referral oncology center between July 20, 2015 and June 30, 2018. Patients with histologically confirmed stage IIIB/IV NSCLC in progression after one or two lines of treatment and who initiated Nivolumab were included. Thyroid function (TSH ± fT4, fT3) was monitored and patients were classified according to TD status [TD(+) versus TD(−)], severity [moderate thyroid dysfunction: TSH level between 0.1 and 0.4 or 4.0 and 10 mIU/L and severe thyroid dysfunction: TSH ≤ 0.1 or ≥ 10mUI/L) and subtype (isolated hypothyroidism, isolated hyperthyroidism and hyperthyroidism then hypothyroidism)]. Clinical endpoints were overall survival (OS) and progression-free survival (PFS).
Results
Among 194 eligible patients, 134 patients (median age, 63 yo; 70.1% male) were included. Forty (29.9%) patients were classified in TD(+) and had a longer OS of 29.8 months (95% CI 18.8-NR) versus 8.1 months (95% CI 5.5–11.5) in TD(−) group (p < 0.001). PFS was also longer (8.7 months (95% CI 5.3–15.1) in TD(+) versus 1.7 months (95% CI 1.6–1.9) in TD(−) group (p < 0.001). In Cox proportional hazards analysis, TD remained an independent predictive factor of OS/PFS. Severity and subtype of TD were not correlated with OS/PFS.
Conclusions
This study suggested that TD induced by Nivolumab appears to be an independent predictive factor of survival, irrespective of TD severity and subtype.
Keywords: Non-small cells lung cancer, Immune checkpoint inhibitors, PD-1 blockade, Thyroid dysfunction, Prognosis
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, good clinical practice and relevant french regulations regarding ethics and data protection. living patients were informed and gave their non-opposition to participate in the study. the study (29BRC19.0228) was approved by our university hospital’s institutional review board (B2019CE48).
Consent for publication
All authors contributed to drawing up the manuscript and approved this version.
Introduction
In the recent years, a better understanding of the immunological mechanisms involved in oncogenesis has led to the development of immune checkpoint inhibitors (ICI). Nivolumab is a human IgG4 immunoglobulin, which belongs to the ICI anti-programmed cell death 1 (anti-PD-1) family. Phase III trials (CHECKMATE 017 and CHECKMATE 057) of non-small cell lung cancer (NSCLC) patients treated with nivolumab vs. docetaxel demonstrated a significant higher overall survival (OS) in patients who had nivolumab therapy [1, 2]. Nivolumab marketing authorization as a second-line treatment for advanced NSCLC (grades IIIB and IV) was obtained in France in 2015 [3, 4].
ICIs are associated with a new spectrum of toxicity known as immune-related adverse events (IRAEs). One of the most common ICI-induced IRAEs are endocrine IRAEs [5, 6]. The latter include disorders such as ICI-induced diabetes, hypophysitis and thyroid dysfunction (TD). The association between immunotherapy efficacy and the onset of IRAEs is debated across the literature with conflicting results. Most of these studies were in melanoma patients. The association between skin-IRAEs (e.g. vitiligo) and improvement of survival outcomes [e.g. OS, progression-free survival, (PFS)] is reported in melanoma patients [7–9]. However, TD does not seem to be associated with patient’s outcomes in melanoma patients [10]. To date, there is a paucity of literature on the onset of TD as a predictive factor in NSCLC. In NSCLC, the incidence of TD differed across the literature ranging from 4–12% in earlier [1, 2, 11–13] to 21–35.5% in more recent and more specific studies [14–17] and up to 50% in those with combined regimens of immune checkpoints inhibitors [18]. Thyroid function monitoring is now recommended by dosing of TSH and T4L before treatment initiation, then before each cycle of treatment during the first three to six months [19–21].
There is a need for predictors of ICI treatment response (i.e. ICI clinical benefit). To date, despite its technical and methodological limitations, PD-L1 expression level seems to be the only reliable metric in prediction of ICI clinical benefit [22, 23]. Active research for new metrics predictors of cancer treatment response has given rise to biological parameters, such as tumor mutational load, microsatellite instability, tumor-infiltrating lymphocytes [24], most of which cannot be readily used in routine practice. On the other hand, emerging clinical metrics, such as Lung Immune Prognostic Index (LIPI) score, an inflammation marker, can be easily used [25].
Several studies have already addressed the question of the association between IRAEs and response to anti-PD1 immunotherapy in the NSCLC. Haratani et al. found an association between the occurrence of IRAEs and the overall response rate in patients treated with nivolumab [26]. Hasan Ali et al. found an association between skin effects and response in patients treated with nivolumab [27].
Regarding TD induced by ICI, several studies reported an association between TD and response to anti-PD-1 in patients treated with pembrolizumab [14] and nivolumab [16, 17].
We hypothesized that the ICI-induced TD in NSCLC patients treated with nivolumab was associated with better treatment efficacy (i.e. survival outcomes). We further studied the impact of severity and sub-type of TD on survival outcomes.
Materials and methods
This was an observational, retrospective and monocentric study, conducted at the University Hospital of Brest from July 20, 2015, to June 30, 2018.
Population
Patients were screened using the Chimio® software (Computer Engineering, Paris, France) according to indication and treatment, i.e. keywords: "Lung/bronchus", “Nivolumab”.
Inclusion criteria were: patients ≥ 18 years old with a cytologically/histologically confirmed locally advanced (stage IIIB) or metastatic (stage IV) NSCLC with progressive disease (PD); to undergo Nivolumab as second or more line of therapy.
Exclusion criteria were: primary tumor other than lung cancer; history of total thyroidectomy or previous treatment with levothyroxine or thyroid dysfunction before Nivolumab initiation; no thyroid function monitoring during Nivolumab treatment and missing data; patients who expressed their opposition to participate in the study.
The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and relevant French regulations regarding ethics and data protection. Living patients were informed and gave their non-opposition to participate in the study. The study (29BRC19.0228) was approved by our university hospital’s institutional review board (B2019CE48).
Cohort characteristics
The following patient characteristics were retrieved from medical records: age, gender, smoking status (absent, current or former), WHO performance index, tumor characteristics (histology, genetic alteration, staging, history of brain metastasis), prior radiotherapy treatment, prior systemic lines (defined by the number of chemotherapy/immunotherapy regimens used before Nivolumab treatment).
We also recorded data on PD-L1 expression in tumor, LDH levels and the lymphocyte-to-neutrophil ratio (dNLR) summarized in the LIPI score (Lung immune prognostic index). The latter distinguishes three prognosis categories: good (normal LDH, dNLR < 3), intermediate (abnormal LDH or dNLR > 3) and poor (abnormal LDH and dNLR > 3).
Treatment schedule and morphological monitoring
Nivolumab (3 mg/kg) was administered by IV infusion for 30 min every 2 weeks until PD, unacceptable toxicity, or death. Tumor evaluation by computed tomodensitometry (CT) and/or magnetic resonance imaging (MRI) was performed every 2 months until PD according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) or immune (i)RECIST 1.1 criteria.
Thyroid function screening and classification of thyroid dysfunction
Thyroid function screening was performed before (< 3 months) and during treatment with Nivolumab according to ESMO Guidelines for each cycle and then every 6 weeks after cycle 4 [19]. Thyroid function test (TFT) including TSH, free T4 (fT4), free T3 (fT3) were measured by immunochemiluminescence (Centaur XPT Siemens). Antithyroid antibodies (Abs) including anti-thyroid peroxidase antibodies (TPOAb), TSH receptor antibodies (TRAbs) were measured by immunofluorescence (Kryptor Thermo Fischer Brahms). Reference laboratory values were: TSH, 0.4–4.0 mIU/L; fT4, 11.5–22.7 pmol/L; fT3, 3.5–6.5 pmol/L; TPOAb, < 60 kIU/L and TRAb, < 1.8U/L.
Given that most of TD was asymptomatic, we did not use CTCAE criteria for the classification of TD. The following abnormalities of thyroid function tests were taken into consideration:
Patients with abnormalities of TSH level were classified in the group “Thyroid Dysfunction+” (TD(+)) and those with normal TSH level were classified in the group “Thyroid Dysfunction“ (TD(−)). In case of normal TSH with an isolated abnormality of peripheral hormone fT4 and/or fT3, the patient was classified in the “Thyroid Dysfunction“ group (TD(−))
According to the level of peripheral thyroid hormones (i.e. fT3 and fT4): subclinical and overt thyroid dysfunctions were defined as an abnormal TSH level, without and with at least one abnormality of the peripheral hormones (fT3, fT4), respectively.
According to TSH level: thyroid dysfunction was regarded as moderate with TSH level range of 0.1–0.4 mIU/L or 4.0–10 mIU/L and severe with TSH level range of ≤ 0.1 mUI/L or ≥ 10 mUI/L
According to thyroid dysfunction subtype, patients were classified into 3 following categories: isolated hypothyroidism, isolated hyperthyroïdism and hyperthyroidism followed by hypothyroidism (hyper/hypothyroidism).
Clinical endpoints
Patients were followed up until the occurrence of the primary endpoints or until January 1, 2019. The Co-primary clinical endpoints were OS and progression-free survival (PFS) according to TD(+) and TD(−). The secondary clinical endpoints were objective response rate (ORR), disease control rate (DCR) and duration of response according to TD(+) and TD(−). We also performed a subgroup analysis according to the severity and subtype of thyroid dysfunction.
OS was defined as the time from the first administration of nivolumab until death. PFS was defined as the time from the first administration of nivolumab until PD or death, whichever came first. The ORR was defined as either partial or complete response. DCR was defined as the percentage of patients who achieved complete response, partial response and stable disease. The duration of response was defined as the time between the first objective response and PD. A response beyond the 12-month timepoint was regarded as a prolonged response.
Statistics
Quantitative variables were expressed as mean ± standard deviation (SD) or as median with interquartile range (IQR), according to their distribution. They were compared using a non-parametric Mann–Whitney U test. Categorical variables were expressed as percentages and compared using a Chi-square test (or Fisher's exact test). The survival analysis was carried out using the Kaplan–Meier curves and the log-rank test to compare OS and PFS between the two groups. A Cox proportional hazard regression model was used for multivariable analysis to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for OS and PFS, using a backward stepwise selection of variables according to their clinical relevance or if p < 0.2 in univariable analysis. All statistical tests were two-sided and p < 0.05 indicated a statistically significant difference. All analyses were performed on XLSTAT 2019.2.2.
Results
Population and patients characteristics
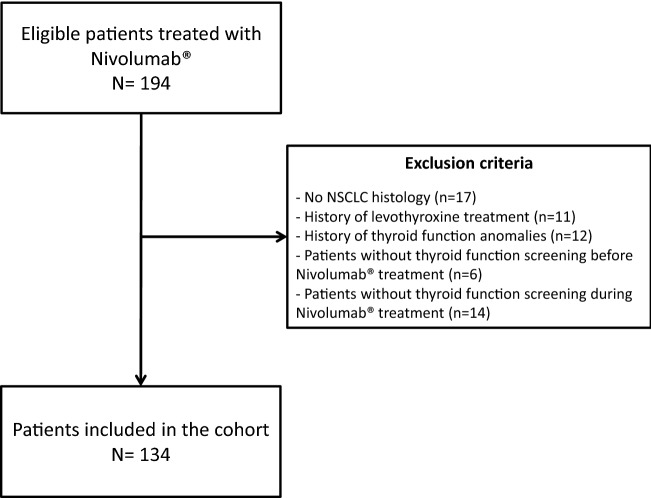
Among the 194 eligible patients, 60 were excluded. One hundred thirty-four (69.1%) were finally included in the study from July 20, 2015 to June 30, 2018. Flowchart of the study is illustrated in Fig. 1.
Fig. 1.
Flowchart of the study
The mean age was 62.5 years (SD: 8.9). Ninety-four patients (70.1%) were male and 97.7% of patients were current or former smokers. The WHO performance score was 0 or 1 for 77.3% of patients. Patient’s pathological subtypes were: squamous cell carcinoma (26.1%), adenocarcinoma (71.6%) and undifferentiated carcinoma (2.2%). Among them, 93.3% of them had a stage IV disease. Brain metastasis was reported in 29.1% of patients. Nivolumab was prescribed in second-line for 75.4% of patients, at least in third-line for others. Half of the patients had already been treated with radiotherapy (51.5%) and 3% had previously received another immunotherapy treatment. PD-L1 status was known in 44 patients (32.8%), with expression > 5% in 22.7% of them. The LIPI score was good in 28% of patients, intermediate in 40.8% and poor in 31.2%. Mean Pre-Nivolumab TSH level was 1.70 mIU/L (SD: 0.98). The patient’s characteristics are presented in Table 1.
Table 1.
Patients characteristics
| Characteristics | Total n = 134 |
TD(+) n = 40 |
TD(−) n = 94 |
p value (TD(+) vs TD(−) |
|---|---|---|---|---|
| Patients | ||||
| Age | ||||
| Mean ± SD | 62.5 ± 8.9 | 63.2 ± 9.2 | 62.2 ± 8.8 | 0.561 |
| ≥ 70 years old (%) | 26 (19.4) | 8 (20) | 18 (19.1) | 0.909 |
| Sex. male (%) | 94 (70.1) | 27 (67.5) | 67 (71.3) | 0.662 |
| Smoking (n = 131) (%) | ||||
| Current or former smoker | 128 (97.7) | 39 (100) | 89 (96.7) | 0.254 |
| WHO Performance Score (n = 119) (%) | ||||
| 0–1 | 92 (77.3) | 33 (86.8) | 59 (72.8) | 0.089 |
| ≥ 2 | 27 (22.7) | 5 (13.2) | 22 (27.2) | |
| Tumor | ||||
| Pathologic subtypes (%) | ||||
| Squamous cells carcinoma | 35 (26.1) | 16 (40) | 19 (20.2) | |
| Non-squamous cells carcinoma | 96 (71.6) | 23 (57.5) | 73 (77.7) | 0.055 |
| Undifferentiated | 3 (2.2) | 1 (2.5) | 2 (2.1) | |
| Stage (%) | ||||
| IIIB | 9 (6.7) | 6 (15) | 3 (3.2) | 0.012 |
| IV | 125 (93.3) | 34 (85) | 91 (96.8) | |
| Brain metastasis (%) | 39 (29.1) | 12 (30) | 27 (28.7) | 0.882 |
| Tumoral PD-L1 expression (n = 44) (%) | ||||
| ≤ 5% | 34 (77.3) | 11 (73.3) | 23 (79.3) | 0.654 |
| > 5% | 10 (22.7) | 4 (26.7) | 6 (20.7) | |
| Past regimens | ||||
| Number of regimens (%) | ||||
| 1 | 101 (75.4) | 33 (82.5) | 68 (72.3) | |
| 2 | 26 (19.4) | 5 (12.5) | 21 (22.3) | 0.409 |
| ≥ 3 | 7 (5.2) | 2 (5) | 5 (5.3) | |
| Radiotherapy (%) | 69 (51.5) | 20 (50) | 49 (52.1) | 0.822 |
| Other ICI (%) | 4 (3) | 1 (2.5) | 3 (3.2) | 0.830 |
| Best therapeutic response (n = 120) (%) | ||||
| Partial or complete response (%) | 27 (22.5) | 6 (16.2) | 21 (25.3) | |
| Stable disease | 45 (37.5) | 15 (40.5) | 30 (36.1) | 0.546 |
| Progression | 48 (40) | 16 (43.2) | 32 (38.6) | |
| Biology | ||||
| LIPI score (dNLR > 3 et LDH > ULN) (n = 93) (%) | ||||
| 0: good | 26 (28) | 14 (41.2) | 12 (20.3) | |
| 1: intermediate | 38 (40.9) | 14 (41.2) | 24 (40.7) | 0.039 |
| 2: poor | 29 (31.2) | 6 (17.6) | 23 (39) | |
|
Pre-Nivolumab TSH level(mIU/L) Mean ± SD |
1.70 ± 0.98 | 1.90 ± 1.06 | 1.61 ± 0.94 | 0.126 |
dNLR; derived neutrophil/leukocyte-lymphocyte ratio; ICI: immune checkpoint inhibitors; LIPI: lung immune prognostic index; PD-L1: programmed cell death ligand 1; TD(+): thyroid dysfunction (+) group
Thyroid dysfunction (+) and thyroid dysfunction (−) groups
Among the 134 patients included, 40 (29.9%) presented with at least one TSH abnormality during follow-up and were assigned to the TD(+) group. Seventy-four patients (70.1%) had no abnormal thyroid function (i.e. normal values of TSH, fT4 and fT3) and 20 patients had isolated abnormalities of fT4 and/or fT3 values and were assigned to the TD(−) group. The median time before the onset of TD was 54 days (IQR 36–104).
There was no significant between group difference in age, sex, WHO performance index, smoking status, past treatment regimens, PD-L1 expression and pre-nivolumab TSH level. However, there was a non-significant difference in histological subtype of cancer [squamous cell carcinoma: 40% in TD(+) vs. 20.2% in TD(−),p = 0.055]. The two groups were significantly different in tumor stage [stage IIIb: 15% in TD(+) vs. 3.2% in TD(−),p = 0.012] and in LIPI score [good LIPI score: 41.2% in TD(+) vs. 20.3% in TD(−),p = 0.039].
The characteristics of the TD(+) and TD(−) groups are presented in Table 1. Non-thyroid IRAEs occurred more frequently in TD(+) patients than TD(−) patients (67.5% vs. 39.4%, p = 0.003). In addition, EGFR (n = 1) mutation, ROS (n = 1) and ALK (n = 1) translocation were observed in TD (−) patients.
Thyroid dysfunction (+) severity and subtypes
In the TD(+), 12 patients (30%) had subclinical and 28 (70%) overt thyroid dysfunction (70%). Subclinical TD included 5 patients with isolated thyrotoxicosis, 7 patients with isolated hypothyroidism and no patient with hyper/hypothyroidism. Overt thyroid dysfunction included 7 patients with isolated thyrotoxicosis, 12 patients with isolated hypothyroidism and 9 patients with hyper/hypothyroidism. According to the TSH level, TD was classified as moderate in 19 patients and severe in 21 patients. Three patients required a temporally discontinuation of the Nivolumab treatment due to transient treatment-induced hyperthyroidism. According to CTCAE classification, there was no grade 4–5 thyroid IRAE.
Survival and response to Nivolumab treatment in TD (+) and TD (−) groups
The median follow-up was 10.4 months. In the entire cohort, median OS was 12.2 months (IQR 4.3–29.1) and median PFS was 2 months (IQR 1.6–9.1). The ORR was 23.1% and the DCR was 40.3%. A prolonged response was observed in 11.2% of patients.
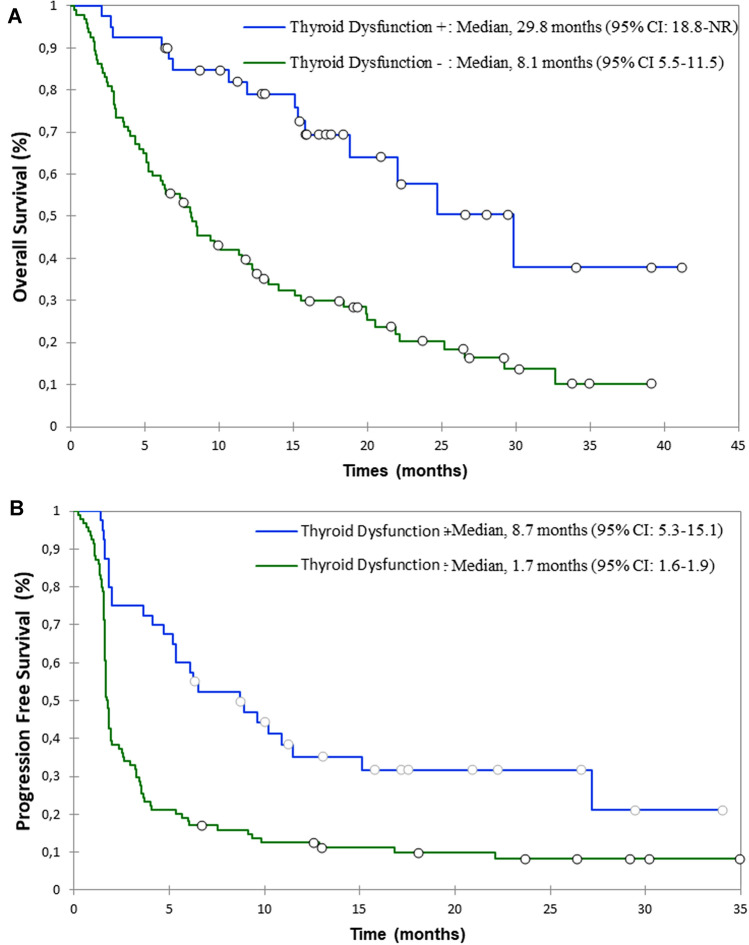
At the database lock, 89 patients (66.4%) had died: 15 patients in the TD(+) (37.5%) and 74 patients (78.7%) in the TD(−) group (p < 0.001). The median OS in TD(+) group was significantly longer than in TD(−) group: 29.8 months (95% CI 18.8-not reached) vs. 8.1 months (95% CI 5.5–11.5), respectively (p < 0.001) (Fig. 2a). In multivariable analysis, the occurrence of a TD remained an independent factor associated with better OS (HR = 0.32 [0.16; 0.62]; p < 0.001; Table 2).
Fig. 2.
OS (a) and PFS (b) in patients according to in TD(+) ad TD(−) group
Table 2.
Univariate and multivariate analyses using Cox regression for overall survival and progression-free survival according to thyroid dysfunction and others characteristics of the cohort
| Overall survival | Progression-free survival | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Univariate | ||||
| Sex (male) | 1.07 [0.64; 1.78] | 0.788 | 1.24 [0.78; 1.96] | 0.366 |
| Age > 70 ans | 1.02 [0.64; 1.61] | 0.944 | 0.9 [0.59; 1.36] | 0.611 |
| WHO Performance Score ≤ 1 | 0.42 [0; 0.69] | < 0.001 | 0.64 [0.4; 1.02] | 0.063 |
| Current or former smoker | 1.27 [0.4; 4.02] | 0.685 | 1.94 [0.61; 6.2] | 0.261 |
| Squamous cell carcinoma positive | 0.99 [0.6; 1.62] | 0.971 | 0.9 [0.59; 1.38] | 0.638 |
| Stade IIIB positive | 0.38 [0.12; 1.19] | 0.096 | 0.49 [0.21; 1.12] | 0.089 |
| Brain metastasis positive | 0.7 [0.43; 1.13] | 0.141 | 0.74 [0.49; 1.13] | 0.164 |
| Number of past regimens (≥ 3) | 0.63 [0.38; 1.05] | 0.075 | 0.8 [0.52; 1.23] | 0.304 |
| History of others ICI (%) | 1.54 [0.56; 4.2] | 0.402 | 1.26 [0.46; 3.44] | 0.647 |
| Radiotherapy positive | 0.96 [0.63; 1.46] | 0.858 | 1.21 [0.83; 1.75] | 0.315 |
| PD-L1 expression > 5% | 1.1 [0.4; 2.97] | 0.858 | 1.1 [0.47; 2.55] | 0.825 |
| LIPI score (poor prognosis) | 1.82 [1.06; 3.13] | 0.029 | 1.59 [0.99; 2.56] | 0.056 |
| Non-thyroid IRAEs positive | 0.32 [0; 0.55] | < 0.001 | 0.4 [0; 0.61] | < 0.001 |
| TD (+) | 0.45 [0; 0.69] | < 0.001 | 0.44 [0; 0.64] | < 0.001 |
| Multivariate | ||||
| WHO Performance Score ≤ 1 | 0.33 [0.17; 0.64] | < 0.001 | 0.37 [0.2; 0.69] | 0.002 |
| Non-thyroid IRAEs positive | – | – | 0.43 [0.26; 0.72] | 0.001 |
| TD (+) | 0.32 [0.16; 0.62] | < 0.001 | 0.36 [0.21; 0.62] | < 0.001 |
Bold indicates p value < 0.05
ICI: immune checkpoint inhibitors; IRAEs: immune related adverse; LIPI: lung immune prognostic index; events; PD-L1: programmed cell death ligand 1; PS: performance score; TD(+): thyroid dysfunction (+) group
During Nivolumab therapy, 27 (67.5%) of TD(+) patients vs. 85 (90.4%) of TD(−) had progressive disease. The median PFS in TD(+) group was significantly longer than in TD(−) group [8.7 months (95% CI 5.3–15.1) vs. 1.7 months (95% CI 1.6–1.9), p < 0.001] (Fig. 2b). In multivariable analysis, the occurrence of a TD remained an independent predictive factor associated with better PFS (HR = 0.36 [0.21; 0.62]; p < 0.001; Table 2).
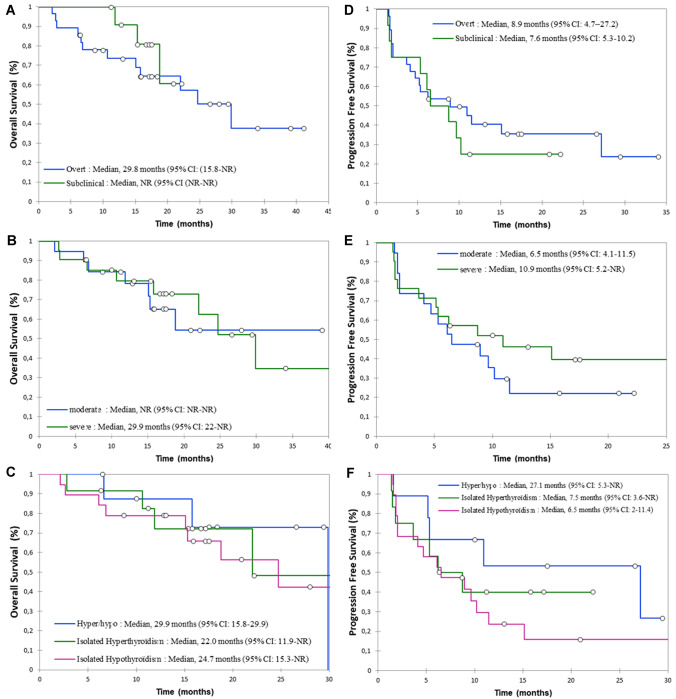
ORR in the TD(+) group was 47.5% versus 12.8% in the TD(−) group (p < 0.001). DCR in the TD(+) group was 70% versus 27.7% in the TD(−) group (p < 0.001). Median treatment duration was longer in TD(+) versus TD(−) group [5.5 months (IQR 3–13.5) versus 1 month (IQR: 1–3) respectively; p < 0.001]. A prolonged response was observed in 20% of patients with the occurrence of TD and 7.4% of patients without (p = 0.035). However, the median duration of response did not differ statistically between the TD(+) and TD(−) groups [15.4 months (IQR: 10.1–21.3) and 9.6 months (IQR 6.9–18.7), respectively, p = 0.330]. In subgroup analysis, median OS and PFS did not differ according to the severity and subtype of TD (Fig. 3). ORR and DCR also did not differ according to the severity and subtype of TD (Table 3).
Fig. 3.
OS and PFS according to thyroid dysfunction subgroups: subclinical and overt thyroid dysfunction (a), moderate and severe thyroid dysfunction (b), isolated hypothyroidism, isolated hyperthyroidism and hyper/hypo (c) for OS and subclinical and overt thyroid dysfunction (d), moderate and severe (e), isolated hypothyroidism, isolated hyperthyroidism and hyper/hypo (f) for PFS
Table 3.
Overall response and disease control rate to Nivolumab according to severity and subtype of thyroid dysfunction
| Overall response rate n = (%) | Disease control rate n = (%) | |||
|---|---|---|---|---|
| p value | p value | |||
| Severity fT3/fT4 levels | ||||
| Subclinical | 5/12 (41.7) | 0.629 | 7/12 (58.3) | 0.292 |
| Overt | 14/28 (50) | 21/28 (75.0) | ||
| Severity TSH levels | ||||
| Moderate | 10/19 (52.6) | 0.536 | 12/19 (63.2) | 0.369 |
| Severe | 9/21 (42.9) | 16/21 (76.2) | ||
| Subtype | ||||
| Isolated hypothyroidism | 9/19 (47.4) | 0.820 | 12/19 (63.2) | 0.365 |
| Isolated hyperthyroidism | 5/12 (41.7) | 8/12 (66.7) | ||
| Hyper/hypothyroidism | 5/9 (55.6) | 8/9 (88.9) | ||
Discussion
Our study showed that patients who experienced a TD induced by nivolumab in second or third-line treatment of advanced NSCLC have a better response and a longer survival irrespective of TD severity and subtype.
These results highlighted a statistically significant association between nivolumab-induced thyroid IRAEs and nivolumab efficacy. The occurrence of TD under nivolumab treatment was a predictor of clinical benefit (i.e. response and OS). Median OS and PFS were longer in TD(+) vs. TD(−): 29.8 months vs. 8.1 months (p < 0.001) and 8.7 months vs. 1.8 months (p < 0.001), respectively. The differences observed were greater than those reported by Kim et al. [16] [median OS:3.9 vs. 2.3 months (p = 0.025); median PFS: 3.9 vs. 2 months (p = 0.014) in patients with and without thyroid dysfunction, respectively]. This can be explained by the between-study difference in the stage of NSCLC (IV in Kim et al. vs. IIIb/IV in our study) and the median follow-up time (89 days in Kim et al. study vs. 10.4 months in our study) [16]. Our results were comparable with those of Yamauchi et al. (median OS: “not reached” vs. 14.2 months (p = 0.025); median PFS: 5.8 vs. 2.3 months (p = 0.01) in NSCLC patients with and without TD, respectively) [17].
Cox proportional multivariable analysis showed that the nivolumab-induced TD and a WHO performance index ≤ 1 were the only two independent protective factors of a longer OS. The latter was consistent with the literature [28, 29]. Our study found additional helpful data on WHO performance correlation and PFS. In line with other studies, the nivolumab-induced non-thyroid IRAEs were associated with PFS in our study [26, 30, 31]. However, other potential prognostic factors, such as the level of PD-L1 expression and LIPI-score, were not independently associated with OS and PFS in our study. We did not take into account the time duration between nivolumab onset and TD onset in our multivariate Cox model. This could lead to a potential bias as patients with bad prognosis have shorter life expectancy to present with IRAEs [32, 33]. However, given our median 54-day time before the onset of TD and the exclusion of patients without TFTs during follow-up, such potential bias might have less impact on our results, in particular in regard with OS. However, given the association between the nivolumab-induced non-thyroid IRAEs and a longer PFS, further research is warranted to obtain the time duration between nivolumab and non-thyroid IRAEs onset. Finally, stage IIIb and a good LIPI score were more frequent in the TD(+) group. However, these two parameters were not associated with OS and PFS in the multivariate analysis.
One of the strengths of our study was the evaluation of a single anti-PD1 drug (i.e. nivolumab) efficacy in NSCLC according to nivolumab-induced TD subtype and severity. To our knowledge, the study by Kim et al. was the only one previously assessing the association between PD-1 blockade drugs (i.e. pembrolizumab and nivolumab) efficacy and PD-1 blockade-induced TD severity in a subgroup of patients with NSCLC [16]. This study demonstrated an improvement in OS and PFS in case of overt TD compared to subclinical TD (p = 0.016 and 0.026, respectively) [16]. The latter results were not confirmed by the results of our study of a larger population and a longer follow-up. Finally, this is the first study assessing specifically the predictive value of TD according to its subtype in NSCLC. Our results show a trend towards an improvement in OS and tumor response in case of thyroiditis but without significance.
TD was defined as at least one abnormal TSH level during nivolumab therapy. Isolated laboratory abnormalities in fT3 and/or fT4 were not regarded as TD in our study. We believe that such anomalies should be considered as equivocal and could be linked to central causes or other drugs effects. With these criteria, the incidence of TD in our cohort was 29.9% and was similar to studies assessing specifically TD in patients with NSCLC (32.7% in Kim et al. study and 29.1% in Yamauchi et al. study [16, 17]). It is important to note that despite the retrospective nature of our study, only a few patients were excluded due to missing TD screening [no thyroid function screening before nivolumab (n = 6) or during nivolumab treatment (n = 14)]. Moreover, patients who did not undergo thyroid function monitoring during Nivolumab® treatment could be regarded as patients with hyperprogressive disease dying before the 2nd or 3rd cycle. This could have led to an overestimation of OS and PFS in our cohort. However, the prognosis and therapeutic response of our cohort were consistent with those of the phase III clinical trials [1, 2] and real-life studies [34, 35].
Understanding the underlying mechanisms of IRAEs and the pathophysiology of the therapeutic response can help at finding optimal predictive factors of immunological agents efficacy. In melanoma patients who received ICI including anti-PD-1 treatment, the skin IRAEs are correlated with survival and therapeutic response [7–9]. This association is thought to be caused by shared antigens between melanoma cells and normal melanocytes [5]. This embryological reasoning can also be hypothesized for the thyroid-IRAEs in NSCLC. Indeed, embryologically, lung and thyroid tissues differentiate from the endoderm. Thyroid transcription factor-1 (TTF-1) controls embryonic development of the thyroid and lung and is expressed in both organs [36, 37]. Koyama et al. study assessed the association between TTF-1 expression in the tumor, thyroid dysfunction occurrence, therapeutic response and survival. TD was associated with therapeutic response and survival but surprisingly the incidence of TD was lower in TTF-1-positive than TTF-1-negative NSCLCs (9% vs. 20%, p = 0.08). Furthermore, PFS was longer (10.3 months) in patients with TTF-1-negative NSCLC with TD compared to those with TTF-1-positive NSCLC with and without TD and TTF-1-negative NSCLC without TD (4.2, 1.4 and 2.4 months, respectively) [38]. Further assessments of the pathophysiology (or mechanism) of ICI therapeutic response in NSCLC are warranted.
There are some limitations to this study. This is a retrospective and monocentric study and our sample size was potentially not sufficient for subgroup analysis in patients with TD. In addition, even though TFTs were performed almost systematically, Abs assays were often missing in our cohort. Given that the Abs could be negative at the time of diagnosis, this limitation may not have affected our results [39]. We chose not to screen for TD according to the results of morphological and functional explorations (i.e., ultrasonography and thyroid scintigraphy). The latter are not systematically performed in all TD subtypes and are mainly done to differentiate iatrogenic thyroiditis and other diagnoses of hyperthyroidism, especially in the case of severe thyrotoxicosis [21]. We can concur with the need for such morphological and functional explorations to better understand ICI-induced TD especially in diagnosis of thyroiditis.
In conclusion, our study showed that nivolumab-induced TD is associated with improvement of OS and response rate in patients with stage IIIb/IV NSCLC irrespective of TD severity and subtype. However, the potential underlying mechanism of such TD remains unclear. Moreover, our findings need to be further confirmed prospectively. Altogether, further relevant, larger randomized studies are needed.
Author contributions
VK, NR are the guarantor of the study. PT, CJ, NR designed the study. PT realized the analysis. PT, CJ, ZA drafted the manuscript. PT, CJ, NR, ZA did the interpretation of data. NR, ZA, GC, GQ, RD, VK revised the manuscript for intellectual content.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors report no conflict of interest in this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HAS, Commission de la transparence Avis du 2 février 2017 (relatif à l’AMM du Nivolumab 20/07/2015)
- 4.HAS, Commission de la transparence Avis du 11 janvier 2017 (relatif à l’AMM du Nivolumab 04/04/2016)
- 5.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Filette J, Andreescu C, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(03):145–156. doi: 10.1055/a-0843-3366. [DOI] [PubMed] [Google Scholar]
- 7.Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 8.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 10.Al Mushref M, Guido PA, Collichio FA, Moore DT, Clemmons DR. Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr Pract. 2019;26(1):36–42. doi: 10.4158/EP-2019-0244. [DOI] [PubMed] [Google Scholar]
- 11.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):599–610. doi: 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 14.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos-Levi AM, Rogado J, Sanchez-Torres JM, Colomer R, Marazuela M. Nivolumab-induced thyroid dysfunction in patients with lung cancer. Endocrinología Diabetes Nutrición. 2019;66(1):26–34. doi: 10.1016/j.endinu.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim HI, Kim M, Lee S-H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. OncoImmunology. 2018;7(1):e1375642. doi: 10.1080/2162402X.2017.1375642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE. 2019;14(5):e0216954. doi: 10.1371/journal.pone.0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morganstein DL, Lai Z, Spain L, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol. 2017;86(4):614–620. doi: 10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- 19.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 20.Illouz F, Drui D, Caron P, Do Cao C. Expert opinion on thyroid complications in immunotherapy. Annales d’Endocrinologie. 2018 doi: 10.1016/j.ando.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Castinetti F, Albarel F, Archambeaud F, et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer. 2018;26(2):G1–G18. doi: 10.1530/ERC-18-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr KM, Hirsch FR. Programmed death ligand-1 immunohistochemistry: friend or foe? Arch Pathol Lab Med. 2016;140(4):326–331. doi: 10.5858/arpa.2015-0522-SA. [DOI] [PubMed] [Google Scholar]
- 23.Califano R, Lal R, Lewanski C, et al. Patient selection for anti-PD-1/PD-L1 therapy in advanced non-small-cell lung cancer: implications for clinical practice. Future Oncol. 2018;14(23):2415–2431. doi: 10.2217/fon-2018-0330. [DOI] [PubMed] [Google Scholar]
- 24.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018;4(3):374. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. OncoImmunology. 2016;5(11):e1231292. doi: 10.1080/2162402X.2016.1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Areses Manrique MC, Mosquera Martínez J, García González J, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res. 2018;7(3):404–415. doi: 10.21037/tlcr.2018.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spigel D, Schwartzberg L, Waterhouse D, et al. P3.02c–026 Is nivolumab safe and effective in elderly and PS2 patients with non-small cell lung cancer (NSCLC)? Results of CheckMate 153: Topic: IT. J Thorac Oncol. 2017;12(1):S1287–S1288. doi: 10.1016/j.jtho.2016.11.1821. [DOI] [Google Scholar]
- 30.Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100–2111. doi: 10.1002/art.41068. [DOI] [PubMed] [Google Scholar]
- 33.Conner SC, Trinquart L. Survivorship bias in analyses of immune checkpoint inhibitor trials. JAMA Oncol. 2019;5(8):1226–1226. doi: 10.1001/jamaoncol.2019.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Audigier-Valette C, Pérol M, Barlesi F, et al. Caractéristiques des patients atteints de cancer bronchopulmonaire non à petites cellules (CBNPC) traités par nivolumab en condition de vie réelle : première analyse de l’étude EVIDENS (lung cancer patients treated with nivolumab : a longitudinal, prospective, observational, multicentric study) Rev Mal Respir. 2018;35:A104–A105. doi: 10.1016/j.rmr.2017.10.229. [DOI] [Google Scholar]
- 35.Geier M, Descourt R, Corre R, et al. Real life second-line nivolumab in advanced non-small cell lung cancer: a French observational multicenter study of 259 patients (ABCT-IMMUNOBZH) Cancer Rep Rev. 2018 doi: 10.15761/CRR.1000164. [DOI] [Google Scholar]
- 36.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270(14):8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 38.Koyama J, Horiike A, Yoshizawa T, et al. Correlation between thyroid transcription factor-1 expression, immune-related thyroid dysfunction, and efficacy of anti-programmed cell death protein-1 treatment in non-small cell lung cancer. J Thorac Dis. 2019;11(5):1919–1928. doi: 10.21037/jtd.2019.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Filette J, Jansen Y, Schreuer M, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439. doi: 10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.