Abstract
Background
We conducted a phase 1 dose escalation study (ACTRN12618000140257 registered on 30/01/2018) to evaluate the safety, tolerability and immunogenicity of a therapeutic human papillomavirus (HPV) DNA vaccine (AMV002) in subjects previously treated for HPV-associated oropharyngeal squamous cell carcinoma (OPSCC).
Methods
Eligible subjects had to have no evidence of recurrent and/or metastatic disease at least 12 weeks following the completion of treatment. Three dosing cohorts each consisted of four subjects: group 1: 0.25 mg/dose, group 2: 1 mg/dose, group 3: 4 mg/dose. AMV002 was delivered intradermally on days 0, 28 and 56. Incidence and severity of treatment-emergent adverse events (TEAE) including local reaction at the injection site, and vaccination compliance were recorded. T cell and antibody responses to HPV16 E6 and E7 were measured by interferon gamma (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assay and enzyme-linked immunosorbent assay (ELISA).
Results
All subjects completed the vaccination programme and experienced mild discomfort at the injection site(s). Pre-immunisation, cell-mediated responses to HPV16 E6 and E7 were evident in all subjects, and E7-specific antibodies were detected in 11 (91.7%), reflecting previous exposure to HPV. Post-vaccination, 10 of 12 (83.3%) subjects responded to one or more of the E6 and/or E7 peptide pools, while 2 (16.7%) did not show additional vaccine-induced cell-mediated responses. Vaccination resulted in a ≥ 4-fold increase in anti-HPV16 E7 antibody titre in one subject in group 3.
Conclusions
AMV002 was well tolerated at all dose levels and resulted in enhanced specific immunity to virus-derived tumour-associated antigens in subjects previously treated for HPV-associated OPSCC.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02720-7) contains supplementary material, which is available to authorized users.
Keywords: Human papillomavirus, Head and neck cancer, Oropharyngeal squamous cell carcinoma, Immunotherapy, DNA vaccine, HPV E6 and E7 oncoproteins
Introduction
Oncogenic “high-risk” genotypes of human papillomavirus (HPV) are increasingly responsible for squamous cell carcinomas originating at squamo-columnar junctions in the anogenital region and the oropharynx. The global annual mortality of high-risk HPV-associated malignant disease is estimated at > 300,000 [1]. The viral oncoproteins E6 and E7 of high-risk HPVs disrupt cell cycle control, leading to intraepithelial hyperplasia [2]. While the majority of high-risk HPV infections resolve within 5 years, ~ 5% of infections persist and convey a significant risk of malignant transformation within the persistently infected epithelium.
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) is rising globally, predominantly due to HPV16 infection [3–5]. In the USA, the annual incidence of HPV-associated OPSCC has increased by 225% since the 1980s and is set to surpass the incidence of cervical cancer [6, 7]. Unlike smoking-related OPSCC, locally advanced HPV-associated OPSCC has a good prognosis with treatment, which typically consists of radiotherapy (RT) with or without concurrent radiosensitising agents or surgery and post-operative RT [8–10]. However, treatment-associated long-term morbidity and limited success in treatment of recurrent or metastatic disease, 20–30%, has led to a search for more effective treatment with less toxicity [11–13].
Despite the implementation in the last decade of many national immunisation programmes to prevent high-risk HPV infection, HPV will continue to add to the global health burden. Global inequalities prevent worldwide vaccine access, and screening and early intervention have proven challenging, particularly for oropharyngeal disease. Prophylactic HPV vaccines induce neutralising antibodies to the HPV major capsid protein, which is not expressed in HPV-transformed cells [14]. Therefore, immunotherapy for established HPV-associated disease requires a different vaccine. HPV-transformed cells express the viral oncoproteins E6 and E7, and can therefore be recognised and killed by cellular immune responses directed at these proteins [15]. We have developed a polynucleotide-based therapeutic HPV vaccine AMV002, comprising plasmids encoding the expression of two variants of a fusion protein of HPV16 E6 and E7; one incorporates a secretory sequence to induce helper T cell responses, and the other incorporates an ubiquitin sequence, facilitating antigen processing and induction of cytotoxic T cells. The genes encoding the fusion proteins are codon optimised for enhanced protein presentation following intradermal delivery. Pre-clinical evaluation has established that intradermal delivery of AMV002 in mice is safe, and induces balanced humoral and cell-mediated immune responses [16]. Furthermore, in mice, AMV002 prevents growth of HPV16 E7-expressing TC-1 tumours, causes remission of established TC-1 tumours, and induces shrinkage of E7-expressing skin grafts [16].
A vaccine targeting genital herpes simplex virus HSV-2, COR-1, using the same technology as AMV002, has been delivered to patients with persistent HSV-2 in two phase 1 clinical studies. COR-1 induced cell-mediated immunity in human subjects, and was safe and well tolerated [17, 18].
We describe here the results of a first in human phase 1, open-label, single centre dose escalation study of AMV002 in patients with treated HPV-associated OPSCC. The primary objective was to examine the safety and tolerability of intradermal (ID) injection of escalating doses of AMV002. A secondary objective was to determine whether AMV002 induced HPV16-specific antibodies and/or a cell-mediated immune response. Administration of up to three doses of 4 mg of AMV002 was found to be safe and well tolerated, and E6- or E7-specific cell-mediated immunity was observed in 10 of 12 subjects. The study indicates a therapeutic potential of AMV002 for treatment of HPV-associated malignant disease.
Materials and methods
Ethics statement
Subjects provided voluntary written informed consent. This clinical trial was approved by the Metro South Hospital and Health Service Human Research Ethics Committee. The study was registered on the ANZCTR: ACTRN12618000140257.
Subjects
This study enrolled the first participant on May 15, 2018 and the last participant on July 11, 2019. Subjects were eligible to participate under the following inclusion criteria: (1) diagnosed with loco-regionally confined HPV-associated OPSCC, (2) positive test for HPV16 DNA or HPV16 mRNA or p16 IHC testing (defined as > 70% malignant cells staining for p16 immunohistochemistry using CINtec® Histology, Hoffmann-La Roche), (3) received curative intent treatment by RT with or without chemotherapy or monoclonal antibody cetuximab or surgery and post-operative RT, (4) completed curative treatment at least 12 weeks prior and underwent re-staging scans confirming loco-regional complete response (CR) and no evidence of distant disease, (5) WHO/ECOG performance status of 0 or 1 at enrolment, (6) able to communicate effectively with study personnel and considered reliable, willing, and cooperative in terms of compliance with the protocol requirements, (7) written informed consent signed prior to study entry, (8) aged > 18 years, (9) males were to refrain from fathering a child until 1 month after last vaccination, (10) females were to use contraceptive measures until 3 months after last vaccination and (11) participant in general good health based on medical history and physical examination. Exclusion criteria were: (1) histologically or cytologically confirmed HNC of any other primary anatomic location in the head and neck, (2) birthmarks, tattoos, wound or skin conditions on forearms that could obscure injection site reactions, (3) inadequate venous access, (4) breastfeeding or pregnant, (5) other current acute or chronic disease, (6) received medication with anti-HPV activity within 28-days of screening, (7) inadequate laboratory blood values, (8) received any prophylactic or therapeutic vaccine, or investigational drug, within 4 weeks of first vaccination, (9) history of severe allergy and reactions to any drugs and (10) had donated blood or plasma within 60 days prior to the screening visit.
Study design and treatment
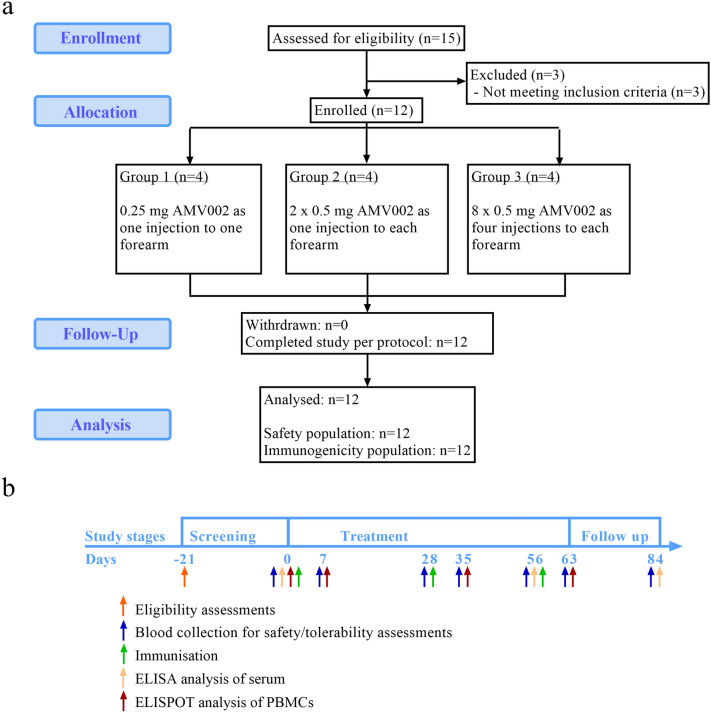
This was a phase 1, single centre, open label, escalating dose study designed to assess the safety, tolerability and immunogenicity of AMV002 for HPV-associated OPSCC when administered to adult subjects in remission after curative treatment. There was no randomisation and blinding in this study. Subjects were recruited from the radiation oncology outpatients’ clinic at the Princess Alexandra Hospital, Brisbane Australia, during routine monitoring. Subjects who were identified as eligible, likely to be compliant with the study and attended outpatients during the recruitment period were offered enrolment. The study was divided into three stages: screening, treatment and follow-up (Fig. 1b). Subjects were enrolled sequentially into three groups. Each treatment group consisted of four subjects which included one sentinel. In the absence of clinically significant safety signals in the sentinel participant within 24 h, the remaining three subjects in the group were vaccinated in a sequential manner. Escalating doses of AMV002 were administered intradermally (ID) to the forearm as follows: group 1: 0.25 mg AMV002 as single ID injection of 0.1 mL; group 2: 1 mg AMV002 as two ID injections of 0.5 mg in 0.2 mL to each forearm; group 3: 4 mg AMV002 as four ID injections of 0.5 mg in 0.2 mL to each forearm. The site of the first injection was ~ 5 cm below the elbow joint on the medial or inside forearm, followed by subsequent injections in lateral direction ~ 5 cm apart. Subjects received three doses of AMV002 vaccine in 4-week intervals on days 0, 28 and 56. Subjects returned to the study site on days 7, 28, 35, 56, 63 and 84 ± 3 days for safety evaluations and efficacy assessments. Blood samples were collected on days -21 (screening) and 0 before immunisation, and on days 7, 28, 35, 56, 63 and 84 after immunisation, and analysed for eligibility, safety and immune responses.
Fig. 1.
a Disposition of subjects. N = number of subjects. b Study schedule of events
Study vaccine
The AMV002 DNA vaccine is a 1:1 mixture of NTC8485-Os-E6E7 and NTC8485-O-UE6E7 plasmids (GenBank accession numbers KY449457 and KY449456, respectively) comprising the NTC8485 expression vector (Nature Technology Corporation, Lincoln Nebraska, USA) minus the enhanced green fluorescent protein sequence [16]. Both plasmids encode a fusion protein of the HPV16 E6 and E7 viral sequences. The E6E7 fusion sequence was constructed by linking HPV16-E6[C70G, Il35T] to HPV16-E7[C24G,E26G] via a Ala-Gly-Ala sequence. The respective mutations were introduced to render the E6 and E7 oncoproteins non-transforming. The sequence was further codon modified using our patented codon preference table (St. Lucia US 2011/0287039 A1). The NTC8485-O-UE6E7 plasmid incorporates a single ubiquitin repeat upstream and in-frame with E6E7. The NTC8485-O-s-E6E7 plasmid incorporates a murine IgK secretory sequence (GenBank: AAH80787.1). AMV002 was manufactured under current good manufacturing practice (cGMP) by VGXI Inc. (Texas, USA) under licence from Jingang Medicine (Australia) Pty Ltd. The plasmids were co-formulated in sterile, isotonic and endotoxin-free 10 mM Tris HCl and 1 mM EDTA, pH 8. AMV002 was supplied in 2 mL vials for injection at a concentration of 2.5 ± 0.2 mg/mL. AMV002 was shipped at a temperature of − 15 to − 25 °C and upon receipt was stored and maintained at − 20° C (± 5 °C) (batch number AMV002.15K017).
Safety and tolerability assessment
All AEs were coded using MedDRA® Version 20.1. All AE summaries were restricted to treatment-emergent adverse events (TEAEs) only. TEAEs were defined as AEs which commenced on or after the time of first vaccine administration through to the end of the study. Reactions considered as TEAEs included injection site reactions [ISR (pain, tenderness, erythema, swelling, ulceration, scabs, redness, induration, ecchymosis, oedema, itching and paraesthesia)], solicited systemic reactions (fatigue, myalgia, malaise, fever, rigours, arthralgia, nausea/vomiting, diarrhoea, light headedness, dizziness, hypersensitivity and headache), laboratory results (haematology, chemistry, coagulation and urinalysis), vital signs (blood pressure [systolic and diastolic], pulse rate, respiratory rate and temperature), electrocardiograms (heart rate, QRS duration, RR interval, PQ (PR) interval, QT interval and QTcF [Fridericia’s correction]) and ECOG Performance Status Scale.
ISRs were captured by photographic record 30 and 60 min post-dosing. Subjects were also requested to complete an ISR/AE diary for each week following the administration of the vaccine, where subjects recorded the presence of pain/tenderness, swelling/induration, erythema and systemic symptoms (hypersensitivity, headache, fatigue, myalgia, malaise, fever, rigours, arthralgia and nausea/vomiting). AEs were reported for the entire study period from screening to follow-up. An AE was defined as any event, side effect, or other untoward medical occurrence that occurred in conjunction with the use of a medicinal product, whether or not considered to have a causal relationship to AMV002 treatment. An AE was therefore any unfavourable and unintended sign, symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product. Treatment-emergent AEs (TEAEs) were evaluated from the time of first dosing (day 0) until day 84 follow-up visit. All AEs were recorded in the participant’s medical records as well as the electronic case report forms. No formal statistical analyses of AEs were undertaken. All TEAEs were tabulated and summarised for description purposes only.
Interferon-γ enzyme-linked immunospot (ELISpot) assay
ELISpot assays were performed by the sponsor [Jingang Medicines (Australia) Pty Ltd] on peripheral blood mononuclear cells (PBMCs) recovered from subjects at day 0 before immunisation (baseline) and on days 7, 35 and 63 to detect the production of interferon gamma (IFN-γ) after stimulation of PBMCs with pools of HPV16 E6 and E7 overlapping peptides or specific E6 and E7 peptides. PBMCs were isolated using SepMate tubes (Stemcell Technologies) and frozen in animal component-free cryopreservation medium (CryoStor CS10 solution, Stemcell Technologies), then stored in liquid nitrogen.
Sterile 96-well plates (Millipore) were coated overnight at 4 °C with monoclonal IFN-γ antibody (1-DK1, Mabtech). After coating, plates were washed once with complete RPMI and blocked for 2 h with complete RPMI containing 10% foetal calf serum (Life Technologies). PBMCs were stimulated with medium alone or 22-mer E6 or E7 overlapping peptides (Supplementary Table 1) (Mimotopes). Full-length 98 amino acid (aa) E7 was divided into 9 E7 peptides with 12 aa overlay, which were combined into one E7 pool. Full length 158 aa E6 was divided into 15 E6 peptides with 12 aa overlay and combined into two pools. The E6 peptide #5 and E7 peptide #5 containing the immune-dominant sequences EVYDFAFRDL and RAHYNIVTF were also tested individually. Positive controls were anti-human monoclonal antibody against CD3 (Mabtech) and CEF peptide pools (Mabtech) containing a mixture of peptides from cytomegalovirus, EBV and influenza peptides. 2.5 × 106 PBMCs/well were incubated with stimulants at 37 °C and 5% CO2 for 18–20 h. Each condition was carried out as technical triplicates. Plates were washed with PBS/0.05% Tween 20. For detection, biotinylated monoclonal antibody (7-B6-1, Mabtech) was used, followed by incubation with streptavidin conjugated with horseradish peroxidase (Mabtech) and DAB substrate. Plates were dried and spots were quantitated using an ELISpot reader system (AID). Values of SFCs were adjusted to 106 PBMCs/well.
The mean of each technical triplicate adjusted SFC/well was considered positive reaction if SFC > 10 after background subtraction. Responders were characterised by evidence of a ≥ 2-fold increase in SFC/well from screening to assessment time points or a negative ELISpot reaction at baseline but a positive reaction post-vaccination.
ELISA
IgG antibodies raised against HPV16 E7 were detected by ELISA in sera collected at day 0 before immunisation (baseline), day 56 and 84. A validated ELISA was performed by TetraQ (Brisbane, Australia). Positive control serum samples (high titre HPV16-E7 immune human serum supplied by sponsor), pooled blank negative control (NC) serum samples (supplied by Golden West Diagnostics, LLC, USA) and a reagent blank (dilution buffer) were included on each assay plate.
Plates (Nunc Maxisorp) were coated with 50 μL of 0.25 μg/ml HPV16 E7 protein (Bioclone; Catalogue no. PN-0943) in carbonate buffer (Sigma; Product no. C3041) per well, and incubated at room temperature for 1 h followed by washing. All washing consisted of four washing steps with 300 μL of PBS-T (Sigma; Product no. P4417 and P7949) using a plate washer (Tecan Hydroflex Platform), followed by dry blotting. Plates were blocked with 250 μL per well of 1% BSA in PBS blocking/dilution buffer (Sigma; Product no. A7030) and incubated for 1 h at 37 °C, followed by washing. Control and test samples were diluted 1/100 in dilution buffer (2 μL serum + 198 μL dilution buffer). To determine antibody titre, additional serial dilutions up to 1:6400 were prepared. Diluted samples were added at 50μL per well and incubated for 1 h at 37 °C. After washing, secondary antibody (Sigma; Product no. A0170) (1:6000 in dilution buffer) was added at 50 μL per well and incubated for 30 min at 37 °C, followed by washing. 100 μL of OPD substrate (SigmaFast, Product no. P9187) was added per well and incubated in the dark at room temperature for 30 min. The reaction was stopped with 25 μL of 3 N HCl (Ajax Finechem; Product code AJA256) per well. The OD was measured at 492 nm in a plate reader (Tecan Sunrise) with 5 s pre-shaking. Data were analysed using the Magellan Tracker software version 7.1 (Tecan). A signal-to-noise ratio was calculated for all samples using the equation OD sample/mean OD NC serum. A positive antibody titre was determined as the highest serum dilution at which the signal-to-noise ratio was above a fixed cut point (determined during validation). The fold change in antibody titre from baseline to days 56 and 84 was determined for each participant. Responders were characterised by evidence of a ≥ 4-fold increase in the ELISA antibody titre from screening to day 56 or 84.
Statistical analysis
No formal statistical analysis was planned in this study. The sample size was based on statistical modelling of previous clinical cancer phase I trials that have examined dose toxicity and maximum tolerated dose, which usually ranges between 6 and 20 patients. This sample size of 12 was considered feasible, likely to accrue in a reasonable time frame, provide sufficient evidence to assess the defined objectives and assist with design of a larger-scale trial examining the oncologic efficacy [19].
Independent review of data
Clinical Network Services (CNS) Pty Ltd. (Brisbane, Australia) compiled the data and prepared the clinical trial report. The study was subject to oversight by a Safety Monitoring Committee (SMC), which reviewed the toxicity data and all clinical relevant information. Enrolment of each subsequent dosing cohort (i.e. dose escalation from group 1 to group 2, and group 2 to group 3) proceeded only with prior approval of the SMC. The SMC conducted an assessment following a minimum 7-day safety period from the first administration of AMV002 on day 0 in the last participant in the prior dosing cohort.
Results
Study cohort
Twelve subjects were enrolled over a 14-month period (Fig. 1a). All enrolled subjects were male with a median age of 63 (range 43–75) years. Participant demographics and tumour characteristics, including TNM staging (American Joint Committee on Cancer 8th Edition) and differentiation, are summarised in Supplementary Tables 1 and 2, respectively.
Consenting patients, with a treated OPSCC that on immunohistochemistry assessment of tumour p16 status demonstrated > 70% of malignant cells staining for p16, were eligible to enrol if deemed to have had a complete response to therapy, based on diagnostic imaging and clinical examination performed at least 12 weeks post-therapy. All subjects had received RT, and some had received concurrent cisplatin (n = 5) or cetuximab (n = 3). The primary tumour site was within the oropharynx in nine subjects, while three had cervical nodal metastatic SCC, with the primary site not identified.
While p16 staining is a common clinical practice to classify HPV-associated malignant disease [20], it does not directly confirm the presence of HPV transcripts. Using RNAscope [21], we therefore additionally analysed the HPV16 E7 mRNA expression in seven subjects for whom suitable biopsy material was available, of which six subjects tested positive. However, while a definite conclusion on HPV status will be important in future trials assessing clinical efficacy, the main purpose of this study was to evaluate the safety and tolerability of the vaccine and measure vaccine-induced immune responses.
Vaccination administration
Subjects were sequentially allocated to three groups, each of four subjects (Fig. 1a, b). All subjects were immunised on three occasions at 4-weekly intervals. Group 1 subjects received one injection of 0.25 mg AMV002 to one forearm at each visit. Group 2 subjects received one injection of 0.5 mg AMV002 to each forearm, resulting in a total dose of 1 mg vaccine, at each visit. Group 3 subjects received four injections of 0.5 mg AMV002 to each forearm, resulting in a total dose of 4 mg vaccine, at each visit. All subjects completed the study as per protocol and contributed data to the safety and immunogenicity analysis.
Safety and tolerability of AMV002
Adverse events (AE) and treatment-emergent AE (TEAE) were coded in accordance with the Medical Dictionary for Regulatory Activities (MedDRA). Study TEAEs are summarised in Supplementary Table 3. All study vaccine-related AEs were considered to be mild in severity. Of 299 TEAEs, 273 were related to the vaccine, and included 271 injection-site reactions (ISRs) and 2 generalised systemic reactions. Vaccine-related TEAE were reported in groups 1 (n = 30, including the two generalised systemic reactions), 2 (n = 48) and 3 (n = 195). All subjects experienced transient pain and erythema at the injection site, two subjects reported discolouration, and one subject developed induration. At study conclusion, resolution was incomplete for erythema (5 subjects), discolouration (2 subjects) and induration (one subject). There were no clinically significant alterations in haematology, biochemistry, coagulation or urinalysis reported for any participant. Minor changes in vital sign parameters following injection were assessed as not clinically significant for all subjects.
Vaccine-induced humoral and cell-mediated immune responses
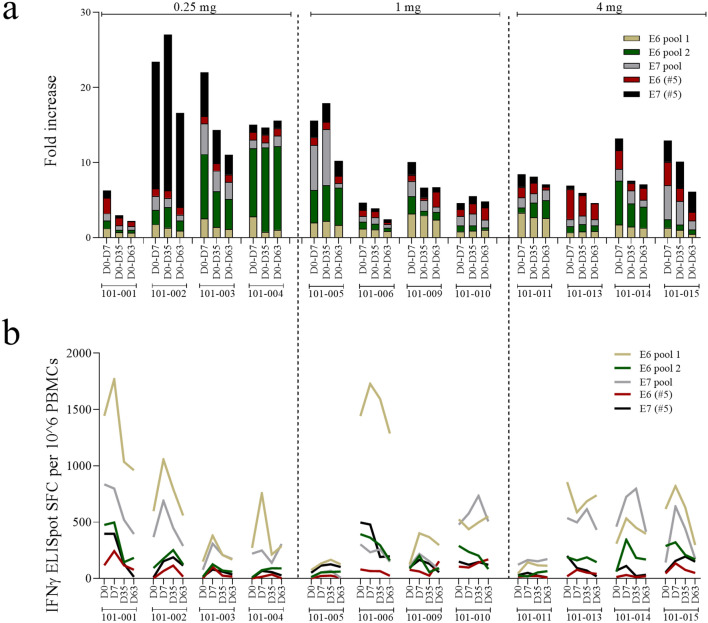
To determine HPV16 E6- and E7-specific T cell immune responses, subject PBMCs were exposed to pools of HPV16 E6 or E7 peptides in an IFNγ ELISPOT, and the frequency of spot-forming cells (SFC) was determined (Fig. 2a, b). All subjects demonstrated T cell responses to at least some HPV16 E6 and E7 peptides at baseline day 0, prior to immunisation (Fig. 2b), as has been previously shown by others for patients with HPV-associated premalignancy [22, 23]. While we only collected absolute evidence of HPV16 status in 6 of 12 patients using RNAscope, the presence of pre-existing HPV16-specific T cell responses in all patients suggests past exposure to HPV16, and, in the context for clinical presentation, of the presence of an HPV16-driven cancer. To determine whether immunisation enhanced existing cell-mediated immune responses, we defined each subject’s response to peptide after immunisation as an enhanced response if there was either conversion of a negative SFC count at baseline to a positive SFC count post-vaccination, or a > 2-fold increase in SFC from baseline to post-vaccination assessment. Based on this definition, 10 of 12 subjects displayed a positive response following vaccination to at least one peptide and at least one time point (Fig. 2c).
Fig. 2.
Cell-mediated immune responses induced by AMV002. a–b T cell responses to HPV16 E6 and E7 peptides were determined using PBMCs collected at baseline day 0 (D0) and at days 7, 35 and 63 after the first, second and third dose of AMV002. PBMCs were restimulated with two pools of E6 peptides or one pool of E7 peptides and cell numbers secreting IFNγ per 10^6 cells were determined by ELISpot. a Cumulated fold increase over pre-immunisation spot-forming cells (SFC) count for each subject at each time point for each peptide pool calculated as SFC (peptide/time point) − SFC (no peptide/time point)/SFC (peptide/pre-immunisation) − SFC (no peptide/pre-immunisation). b Number of SFC per 106 mononuclear cells for each peptide pool at each time point for each study participant, corrected for “no peptide” SFC (SFC (peptide/time point) − SFC (no peptide/time point))
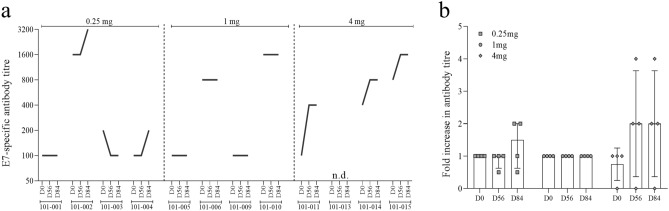
HPV16 E7-specific IgG antibody responses were measured in serum by ELISA. Eleven of 12 subjects displayed pre-existing HPV16 E7-specific serum antibodies at baseline (Fig. 3a, b). An enhanced antibody response was defined as increase in the antibody titre of equal to or > 4-fold from baseline. Based on this definition, 1 (8.3%) of 12 subjects who was treated with the highest dose of AMV002 was classified as a responder at day 56 and 84.
Fig. 3.
Humoral immune responses induced by AMV002. Humoral IgG responses to HPV16 E7 were determined in sera collected at baseline on day 0 and after vaccination on days 56 and 84, which corresponded to 14 days after the second and 28 days after the third dose of AMV002. E7-specific IgG antibody titres were determined by capture ELISA and serial dilutions. The antibody titre was reported as the highest serum dilution which resulted in a signal-to-noise value higher than a fixed cut point determined using HPV-negative pooled sera. a E7-specific IgG titre, b the ratio of post immunisation titre at each time point to pre-immunisation titre for each subject, and the median and interquartile range of the ratio for each dose and time point
Discussion
Here, we describe a first in human study of a potential immunotherapy for HPV-associated cancer. Subjects previously treated for HPV-associated OPSCC were immunised to evaluate the co-endpoints of safety, tolerability and immunogenicity. The immunotherapy was safe and well tolerated at three tested doses. All subjects demonstrated assay responses suggestive of prior immunity to HPV16 E6 or E7 proteins, and 10 of 12 subjects demonstrated enhanced E6- or E7-specific cell-mediated immune responses after immunisation.
While we detected vaccine-induced cell-mediated immunity in 11 of 12 subjects, the induced response dominantly occurred after the first immunisation and waned after subsequent immunisations, suggesting that vaccine-induced cell-mediated immune responses might be short lived, or that repeated immunisation might induce tolerance. Of note, cell-mediated immune responses were measured in peripheral blood, and lack of response in blood to repeated immunisations has been seen in other similar studies with polynucleotide vaccines in human subjects [24], perhaps as secondary T cell response would be expected in the vaccine site draining lymph nodes. Larger studies assessing optimal vaccine administration frequency and timing for anti-tumour efficacy will be required as part of the process of assessing the clinical potential of the vaccine.
Trials of immunotherapy for HPV-associated cancers in human subjects have employed E6 and E7 peptides [25], E6 and E7 proteins [26], bacterial [27] and viral [28] vectors, and DNA [29] and RNA [30] polynucleotides, with or without adjuvants, to generate an E6- and E7-specific immune response. Most HPV DNA vaccines that have entered clinical testing have been delivered intramuscularly, and electroporation has been used to enhance immune responses [15]. The codon usage of the DNA encoding E6 and E7 in the AMV002 immunotherapy was optimised to maximise immune responses [31] and E7 tumour control following intradermal administration in mice [16], as intradermal administration may provide an alternate and more patient acceptable method for delivering DNA vaccines clinically.
HPV-associated OPSCC predominantly affects men (> 90%), and the current study cohort was entirely male. Hence, any gender-associated factors determining the safety and tolerability of AMV002 cannot be excluded in this phase I study [32]. However, a comparable codon-optimised HSV-2 glycoprotein D encoding polynucleotide vaccine with the same plasmid backbone platform was used in phase 1 and phase 2 trials of immunotherapy for genital herpes in which the gender balance was equal [17, 18]. These HSV-2 immunotherapy studies indicated that NTC8485 plasmid-based immunotherapy was well tolerated in both men and women after intradermal delivery.
HPV-associated malignant disease represents a unique opportunity for antigen-specific immunotherapy, as the tumour-associated antigens are non-self, necessary for continued malignant growth, and well characterised. However, persistence of high-risk HPV infections and development of HPV-associated malignant disease, as occurs in ~ 1% of individuals infected with HPV, have at least in part been attributed to genetic determinants linked to the major histocompatibility complex [33], and the persistence of HPV infection and progression of HPV-associated premalignancy to cancer are increased in immunosuppressed individuals. These data suggest that one determinant of development of malignancy following persisting infection may be the inability of the host immune system to respond to the tumour-specific viral antigens, E6 and E7. Thus, the present study evaluated in some detail the immune response induced by these proteins, following their induction by a polynucleotide expression vector for immunotherapy for HPV-associated disease. Subject immune responses to short E6 and E7 peptides measured prior to immunisation were in keeping with the findings of others in patients with cervical and oropharyngeal cancers [22], suggesting that development of HPV16-associated OPSCC is not due to inability of the host immune system to recognise and respond to these antigens, but may rather reflect induction by HPV-associated premalignancy of an antigen-specific tolerogenic or regulatory T cell response [34]. Induction of IFNγ-specific responses, as seen in the current study, suggests induction of Th1 type immune effector function. However, animal studies have shown that IFNγ produced in response to epithelial HPV16 E7 is a local determinant of E7 and E7 transgenic skin graft tolerance [35]. The further enhancement of E6- and E7-specific IFNγ responses following immunisation in the current study may therefore suggest that effective immunotherapy may require additional measures to reprogramme an effector immune response [34].
HPV-associated OPSCC generally has a more favourable prognosis with greater response rates to chemoradiotherapy compared with HPV-associated OPSCC and non-OPSCC [6]. Patients with locally advanced HPV-positive OPSCC have long-term survival rates as high as 80%; however patients are not considered curable following the development of distant metastatic recurrence [8]. The morbidity of loco-regionally recurrent OPSCC is high, and successful salvage with surgery, radiotherapy, or in combination is in the order of 20–30% [36]. Treatment with checkpoint inhibitors in recurrent or metastatic HPV-associated and non-HPV-associated HNC has demonstrated superiority over cytotoxic chemotherapy alone or in combination with cetuximab. However, progression-free survival with checkpoint inhibitors in recurrent or metastatic HNC as either first- or second-line therapy is in the order of 3–5 months [11, 37]. Hence, combination of antigen-specific immunotherapy which can induce a tumour-specific adaptive immune response with checkpoint inhibitors to redirect the effector response to induce cytotoxic effectors may be an alternative strategy to eliminate HPV-transformed malignant cells, both at primary sites and distant metastases. A clinical trial protocol testing the safety and oncologic efficacy of combination therapy of AMV002 with an anti-PDL1 checkpoint inhibitor in recurrent/metastatic OPSCC (DurVax Trial) has been developed and is now open to accrual (Australian New Zealand Clinical Trials Registry: ACTRN12620000406909).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients participating in this study and complying with the protocol. We thank the Queensland Head and Neck Cancer Centre. We thank all relevant staff from the clinical trials unit of the Princess Alexandra Hospital who were involved in carrying out the clinical trial. We thank Clinical Network Services for compiling and analysing data. We thank the staff of TetraQ for the conduction of humoral immunology assays. We also thank Caroline Cooper from Pathology Queensland at the Princess Alexandra Hospital and staff of the Immunohistochemistry Laboratory, Tissue Pathology and Diagnostic Oncology at the Royal Prince Alfred Hospital in Camperdown, NSW, for conducting the RNAscope assay.
Abbreviations
- ELISA
Enzyme-linked immunosorbent assay
- ELISpot
Enzyme-linked immunosorbent spot
- HPV
Human papillomavirus
- HNC
Head and neck cancer
- IFNγ
Interferon gamma
- OPSCC
Oropharyngeal squamous cell carcinoma
- PBMC
Peripheral blood mononuclear cells
- TEAE
Treatment emergent adverse events
Author contributions
SVP, IHF and NF conceptualised the study and acquired funding. SVP recruited patients and oversaw the delivery of the vaccine. SVP verified all clinical data entered onto the case research forms. HYL, MMcG, RL and BP were involved in identifying and recruiting patients to the trial. HYL and MMcG also provided assistance in clinical oversight during the delivery of the vaccine and entering of clinical data. WPW, MB, YX and SH were involved in data curation. JC, WPW and IHF formally analysed data. WPW, YX and SH performed experiments and collected data. WPW and YX developed and designed the methodology. WPW, MB, NF and SVP managed and coordinated trial execution. IHF, SVP and NF supervised the trial execution. JC prepared data visualisation and presentation. JC and WPW prepared the original draft of the manuscript. JC, WPW, IHF and SVP revised and edited the manuscript.
Funding
This study was funded by Admedus Vaccines Pty Ltd, Advance Queensland Ignite Ideas Fund and Jingang Medicine (Australia) Pty Ltd. Assay development and the vaccine technologies used in this study was in part supported by Queensland Government development grants, and by funding from the National Health and Medical Research Council of Australia.
Compliance with ethical standards
Conflict of interest
We have read the journal’s policy and the authors of this manuscript have the following competing interests: WPW, YX and NF are employees of the company that funded the study. WPW, YX and NF hold share options. IHF is a board member of the company that funded the study, is a consultant to the company, is an inventor on the patent US 2011/0287039 A1, “Expression system for modulating an immune response” and WO 02/083181 A1, “Novel compositions and uses” which have been assigned to the company. IHF also holds share options in the company and is a minority shareholder (< 0.01% of the company shares). JC is a consultant to the company that funded the study. SVP reports personal fees from UpToDate, Merck, Celgene and Merck Sharpe & Dome. RL reports honoraria for speaking at symposia at Roche, Merck Serono and Ipsen, holds positions on advisory boards of Roche, Sanofi Aventis, Merck Sharpe & Dohme and Ipsen, and receives financial support for attending symposia and educational programmes at Ipsen and Novartis. We confirm that these commercial affiliations do not alter our adherence to the journals policies on sharing data and materials.
Ethical approval
This clinical trial was approved by the Metro South Hospital and Health Service Human Research Ethics Committee.
Consent to participate and publish
All subjects gave written informed consent for use of their material for research and publication.
Data availability
All relevant data are within the paper and its Supporting Information files.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. Chandra and W. P. Woo contributed equally.
References
- 1.Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol. 2019;9:682. doi: 10.3389/fonc.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Hong A, Lee CS, Jones D, Veillard AS, Zhang M, Zhang X, Smee R, Corry J, Porceddu S, Milross C, Elliott M, Clark J, Rose B. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck. 2016;38(5):743–750. doi: 10.1002/hed.23942. [DOI] [PubMed] [Google Scholar]
- 5.Welfare AIoHa (2017) Cancer in Australia 2017. AUstralian Institute of Health and Welfare, vol Cancer series no.101. Cat. no. CAN 100
- 6.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahal BA, Catalano PJ, Haddad RI, Hanna GJ, Kass JI, Schoenfeld JD, Tishler RB, Margalit DN. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660–1667. doi: 10.1158/1055-9965.EPI-19-0038. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore EJ, Hinni ML. Critical review: transoral laser microsurgery and robotic-assisted surgery for oropharynx cancer including human papillomavirus-related cancer. Int J Radiat Oncol Biol Phys. 2013;85(5):1163–1167. doi: 10.1016/j.ijrobp.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Porceddu SV, Milne R, Brown E, Bernard A, Rahbari R, Cartmill B, Foote M, McGrath M, Coward J, Panizza B. Validation of the ICON-S staging for HPV-associated oropharyngeal carcinoma using a pre-defined treatment policy. Oral Oncol. 2017;66:81–86. doi: 10.1016/j.oraloncology.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM, Burtness B, Ridge JA, Ringash J, Galvin J, Yao M, Koyfman SA, Blakaj DM, Razaq MA, Colevas AD, Beitler JJ, Jones CU, Dunlap NE, Seaward SA, Spencer S, Galloway TJ, Phan J, Dignam JJ, Le QT. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O'Toole L, Al Booz H, Dyker K, Moleron R, Whitaker S, Brennan S, Cook A, Griffin M, Aynsley E, Rolles M, De Winton E, Chan A, Srinivasan D, Nixon I, Grumett J, Leemans CR, Buter J, Henderson J, Harrington K, McConkey C, Gray A, Dunn J, De EHPVTG. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, Lotz B, Melsheimer P, von Knebel DM. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59(24):6132–6136. [PubMed] [Google Scholar]
- 15.Cheng MA, Farmer E, Huang C, Lin J, Hung CF, Wu TC. Therapeutic DNA vaccines for human papillomavirus and associated diseases. Hum Gene Ther. 2018;29(9):971–996. doi: 10.1089/hum.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra J, Dutton JL, Li B, Woo WP, Xu Y, Tolley LK, Yong M, Wells JW, G RL, Finlayson N, Frazer IH, DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J Immunother. 2017;40(2):62–70. doi: 10.1097/CJI.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra J, Woo WP, Dutton JL, Xu Y, Li B, Kinrade S, Druce J, Finlayson N, Griffin P, Laing KJ, Koelle DM, Frazer IH. Immune responses to a HSV-2 polynucleotide immunotherapy COR-1 in HSV-2 positive subjects: a randomized double blinded phase I/IIa trial. PLoS ONE. 2019;14(12):e0226320. doi: 10.1371/journal.pone.0226320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutton JL, Woo WP, Chandra J, Xu Y, Li B, Finlayson N, Griffin P, Frazer IH. An escalating dose study to assess the safety, tolerability and immunogenicity of a Herpes Simplex Virus DNA vaccine, COR-1. Hum Vaccin Immunother. 2016;12(12):3079–3088. doi: 10.1080/21645515.2016.1221872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tighiouart M, Rogatko A, Babb JS. Flexible Bayesian methods for cancer phase I clinical trials. Dose escalation with overdose control. Stat Med. 2005;24(14):2183–2196. doi: 10.1002/sim.2106. [DOI] [PubMed] [Google Scholar]
- 20.Fakhry C, Lacchetti C, Perez-Ordonez B. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement summary of the CAP guideline. J Oncol Pract. 2018;14(10):613–617. doi: 10.1200/JOP.18.00433. [DOI] [PubMed] [Google Scholar]
- 21.Mirghani H, Casiraghi O, Guerlain J, Amen F, He MX, Ma XJ, Luo Y, Mourareau C, Drusch F, Lakdhar AB, Melkane A, St Guily L, Badoual C, Scoazec JY, Borget I, Auperin A, Dalstein V, Vielh P. Diagnosis of HPV driven oropharyngeal cancers: comparing p16 based algorithms with the RNAscope HPV-test. Oral Oncol. 2016;62:101–108. doi: 10.1016/j.oraloncology.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 22.van der Burg SH, Ressing ME, Kwappenberg KM, de Jong A, Straathof K, de Jong J, Geluk A, van Meijgaarden KE, Franken KL, Ottenhoff TH, Fleuren GJ, Kenter G, Melief CJ, Offringa R. Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int J Cancer. 2001;91(5):612–618. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Paaso A, Koskimaa HM, Welters MJ, Grenman S, Syrjanen K, van der Burg SH, Syrjanen S. Cell mediated immunity against HPV16 E2, E6 and E7 peptides in women with incident CIN and in constantly HPV-negative women followed-up for 10-years. J Transl Med. 2015;13:163. doi: 10.1186/s12967-015-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wargowski E, Johnson LE, Eickhoff JC, Delmastro L, Staab MJ, Liu G, McNeel DG. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using Sipuleucel-T and a DNA vaccine. J Immunother Cancer. 2018;6(1):21. doi: 10.1186/s40425-018-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 26.Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, Ng P, O'Connor VM, White O, Wendt N, Martin J, Crowley JM, Edwards SJ, McKenzie AW, Mitchell SV, Maher DW, Pearse MJ, Basser RL. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23(2):172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Basu P, Mehta A, Jain M, Gupta S, Nagarkar RV, John S, Petit R. A randomized phase 2 study of ADXS11-001 listeria monocytogenes-listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28(4):764–772. doi: 10.1097/IGC.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN, Boursnell ME, Rutherford E, Hickling JK, Inglis SC. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 29.Hung CF, Monie A, Alvarez RD, Wu TC. DNA vaccines for cervical cancer: from bench to bedside. Exp Mol Med. 2007;39(6):679–689. doi: 10.1038/emm.2007.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyagi RK, Parmar R, Patel N. A generic RNA pulsed DC based approach for developing therapeutic intervention against nasopharyngeal carcinoma. Hum Vaccin Immunother. 2017;13(4):854–866. doi: 10.1080/21645515.2016.1256518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Gao F, Zhao K, Zhao W, Fernando G, Thomas R, Frazer I. Codon modified human papillomavirus type 16 E7 DNA vaccine enhances cytotoxic T-lymphocyte induction and anti-tumour activity. Virology. 2002;301(1):43–52. doi: 10.1006/viro.2002.1584. [DOI] [PubMed] [Google Scholar]
- 32.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leo PJ, Madeleine MM, Wang S, Schwartz SM, Newell F, Pettersson-Kymmer U, Hemminki K, Hallmans G, Tiews S, Steinberg W, Rader JS, Castro F, Safaeian M, Franco EL, Coutlee F, Ohlsson C, Cortes A, Marshall M, Mukhopadhyay P, Cremin K, Johnson LG, Trimble CL, Garland S, Tabrizi SN, Wentzensen N, Sitas F, Little J, Cruickshank M, Frazer IH, Hildesheim A, Brown MA. Defining the genetic susceptibility to cervical neoplasia-A genome-wide association study. PLoS Genet. 2017;13(8):e1006866. doi: 10.1371/journal.pgen.1006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frazer IH, Chandra J. Immunotherapy for HPV associated cancer. Papillomavirus Res. 2019;8:100176. doi: 10.1016/j.pvr.2019.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattarollo SR, Rahimpour A, Choyce A, Godfrey DI, Leggatt GR, Frazer IH. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J Immunol. 2010;184(3):1242–1250. doi: 10.4049/jimmunol.0902191. [DOI] [PubMed] [Google Scholar]
- 36.Zafereo ME, Hanasono MM, Rosenthal DI, Sturgis EM, Lewin JS, Roberts DB, Weber RS. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 37.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr Psyrri A, Baste N, Neupane P, Bratland A, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, Gonzalez Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D, Investigators K. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.