Abstract
Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII) have been identified as predictors of treatment response in a variety of cancers. We conducted a retrospective analysis to investigate the usefulness of NLR, PLR and SII at baseline and at 6 weeks post-treatment as predictors of response to anti-PD-1/PD-L1 antibody treatment in small cell lung cancer (SCLC). Data of 41 SCLC patients receiving immunotherapy as second- or later-line treatment were analyzed. The overall median progression-free survival (PFS) was 5.1 months (95% CI 3.2–6.2). The median PFS was significantly longer in patients with NLR < 5 than in patients with NLR ≥ 5 at 6 weeks post treatment (HR = 0.29, 95%CI 0.09–0.96, P = 0.04). However, median PFS was comparable between patients with NLR < 5 and patients with NLR ≥ 5 at baseline (HR = 0.75, 95% CI 0.24–2.26, P = 0.56). The median PFS was similar between patients with PLR < 169 and those with PLR ≥ 169 at baseline (HR = 0.67, 95% CI 0.25–1.80, P = 0.43) and at 6 weeks post treatment (HR = 0.69, 95% CI 0.25–1.86, P = 0.46). No statistically different PFS was found between patients with SII < 730 and those with SII ≥ 730 at baseline (HR = 0.70, 95% CI 0.26–1.89, P = 0.48) and at 6 weeks post treatment (HR = 0.38, 95% CI 0.013–1.09, P = 0.07). In conclusion, NLR at 6 weeks after start of treatment appears to be a biomarker of response in the early phase in SCLC patients treated with anti-PD-1/PD-L1 antibodies as second- or later-line treatment.
Keywords: SCLC, Anti-PD-1/PD-L1 antibody, NLR, PLR, SII
Introduction
Small cell lung cancer (SCLC) is an aggressive type of lung cancer with poor prognosis. Standard treatment is platinum-based doublet chemotherapy, but disease progression is common in SCLC patients after frontline platinum-based chemotherapy. Unfortunately, unlike in non-small cell lung cancer (NSCLC), there have been few advances in the treatment of SCLC over the past two decades. The response rate to second line topotecan is just 20–25%, and 1-year survival rate is only 10–30%, so novel efficient treatments for SCLC are urgingly needed [1, 2].
Anti-program cell death receptor 1 (anti-PD-1) monoclonal antibodies and programmed death-ligand 1 blocking antibodies (anti-PD-L1) have demonstrated promising antitumor efficacy in melanoma, NSCLC, head and neck cancers, renal cell cancer, and SCLC [3–6]. In the KEYNOTE-028 study, the disease control rate was 32% in PD-L1-positive extensive-stage SCLC patients receiving pembrolizumab-an anti-PD-1 antibody after failure of standard therapy. Intriguingly, the responders demonstrated ongoing response for over 16 weeks [7]. The IMpower 133 and CASPIAN studies reported significantly longer overall survival (OS) and progression-free survival (PFS) with atezolizumab or durvalumab (both anti-PD-L1 antibody) plus standard chemotherapy as first-line treatment than with chemotherapy alone in extensive-stage SCLC [8, 9]. Recently, the ECOG-ACRIN EA5161 study reported that addition of nivolumab to chemotherapy in first-line treatment significantly improved PFS and OS in extensive-stage (ES) SCLC [10]. The results of KEYNOTE 604 study also revealed significantly improved PFS in ES-SCLC patients treated with pembrolizumab plus etoposide and platinum as first-line therapy, though prolonged OS did not meet significance threshold [11]. These studies showed definite clinical benefit with anti-PD-1/PD-L1 antibody in SCLC patients who respond to treatment. Therefore, identification of patients likely to respond to anti-PD-1/PD-L1 antibody is of crucial importance.
Currently, PD-L1 expression and tumor mutational burden (TMB) were mostly used to identify patients likely to respond to the anti-PD-1/PD-L1 antibody. However, in the CheckMate-032 study, responses were independent of PD-L1 expression [12]. Therefore, PD-L1 expression might not be a reliable biomarker for stratification of SCLC patients. Hellmann et al. found that patients with high TMB were more likely to benefit from nivolumab, and suggested that TMB could be a potential biomarker for identifying responders to anti-PD-1 antibody [13]. However, TMB evaluation needs whole exome sequencing or targeted next-generation sequencing panels, both of which may be unaffordable for SCLC patients struggling to pay for anti-PD-1/PD-L1 antibody treatment. Moreover, TMB evaluation is time-consuming and can only be performed in a handful of medical institutions. Hence, there is a pressing need to find easy-to-use, reliable, and inexpensive biomarkers for identifying SCLC patients likely to respond to anti-PD-1/PD-L1 antibody.
Cancer-related inflammation is a critical determinant of disease progression and survival in most cancers [14]. Systemic inflammation is associated with alteration in peripheral blood leukocytes, and this alternation is captured by the neutrophils-to-lymphocytes ration (NLR). Hematologic parameters such as NLR, platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII) reflect the balance between inflammation and immunoreaction, and have been shown to be useful for predicting outcomes in melanoma and NSCLC patients being treated with immunotherapy [15–19]. An advantage is that hematological tests are routinely performed in cancer patients and so these parameters are readily available to the physician. However, to the best of our knowledge, no study has evaluated the role of NLR, PLR, and SII in SCLC patients receiving anti-PD-1/PD-L1 antibody treatment. The aim of this study was to investigate whether hematologic parameters at baseline and at 6 weeks after treatment could be used for predicting response to anti-PD-1/PD-L1 antibody treatment in SCLC patients.
Patients and methods
Patients
The data of SCLC patients receiving anti-PD-1/PD-L1 antibody after failure of standard chemotherapy at General Hospital of Chinese PLA between June 2015 and August 2018 were retrospectively analyzed. Patients were eligible for inclusion in this study if they (1) had pathologically confirmed SCLC; (2) had received anti-PD-1/PD-L1 antibody after failure of first-line standard chemotherapy; (3) were receiving anti-PD-1/PD-L1 antibody for the first time, and were treated with nivolumab (3 mg/kg every 2 weeks), or pembrolizumab (2 mg/kg every 3 weeks), or atezolizumab (1200 mg every 3 weeks), or toripalimab (240 mg every 3 weeks). The following data were collected from the medical records: age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking status, stage, previous treatments, and treatment response. Blood test results at baseline and at 6 weeks post first administration of anti-PD-1/PD-L1 antibody were also recorded.
Evaluation
Chest computed tomography scans were performed every 8–12 weeks. Clinical responses were assessed by two doctors independently and categorized as either complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to response evaluation criteria in solid tumors (RECIST) v1.1 criteria. PFS was defined as the time from anti-PD-1/PD-L1 antibody initiation until disease progression or death due any cause. Patients with CR or PR or SD were defined as clinical response, while patients with PD were defined as non-clinical response.
Hematological parameters
Total white blood cell count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and platelet count (PLT) at baseline and at 6 weeks after start of treatment were used to calculate the NLR, PLR, and SII. NLR was calculated as the ANC divided by the ALC, and categorized using a threshold value of 5. PLR was defined as the ratio of PLT to ALC. SII was calculated as the PLT multiplied by the NLR. The threshold values of PLR and SII were set as 169 and 730, respectively [20]. ΔNLR was defined as the difference between post-treatment NLR and baseline NLR.
Statistical analysis
Categorical variables are summarized as frequencies and percentages and analyzed using the Fisher exact test. Continuous variables are summarized as median values and standard error and analyzed using the Wilcoxon rank-sum test. The Kaplan–Meier method was used to estimate the probability of PFS, and log rank test was used to investigate the significance of differences between groups. Statistical analyses of composition ratio of patients with NLR ≥ 5 and with NLR < 5 at 6 weeks post treatment were performed using chi-square test. A heatmap was used to demonstrate the trends of NLR. Positive and negative values were marked red and green, respectively, and the color intensity was adjusted to indicate the ΔNLR value. Two-sided P ≤ 0.05 indicated statistical significance. Graphpad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) and SPSS 20 (IBM Corp., Armonk, NY, USA) were used for statistical analysis.
Results
Clinical characteristics of patients
Table 1 summarizes the characteristics of the patients. A total of 41 patients (36 men, 5 women; median age, 61 years, 95% CI 42–80) were included in the study. There were 35 (85.4%) smokers and 6 (14.6%) nonsmokers. While 7 (17.1%) patients had limited stage disease, 34 (82.9%) had extensive-stage disease. ECOG PS was 0 for 2 patients, 1 for 37 patients, and 2 for 1 patient. Treatment was with anti-PD-1/PD-L1 antibody plus chemotherapy for 29 (70.7%) patients, and with anti-PD-1/PD-L1 antibody alone for 12 (29.3%) patients. The agents used were nivolumab (19 patients), pembrolizumab (19 patients), atezolizumab (2 patients), and toripalimab (1 patient). Before the immunotherapy, all 41 patients had received one or more lines of treatment: 19 had received one frontline treatment and 22 had received two or more lines of treatment. Follow-up ended on August 12th 2019. The overall median PFS was 5.1 months (95% CI 3.2–6.2).
Table 1.
Clinical characteristics of the 41 patients included in our study
| Characteristics (n = 41) | N (%) |
|---|---|
| Age-years | |
| Median | 61 |
| Range | 42–80 |
| < 60 | 17 (41) |
| ≥ 60 | 24 (59) |
| Sex | |
| Female | 5 (12) |
| Male | 36 (88) |
| Smoking | |
| Yes | 35 (85) |
| No | 6 (15) |
| ECOG | |
| 0 | 2 (5) |
| 1 | 37 (90) |
| 2 | 2 (5) |
| Stage | |
| Limited disease | 7 (17) |
| Extended disease | 34 (83) |
| Combined | |
| Yes | 29 (71) |
| No | 12 (29) |
| Agent | |
| PD-1 antibody | 39 (95) |
| PD-L1 antibody | 2 (5) |
| Previous line of treatment | |
| 1 | 19 (46) |
| ≥ 2 | 22 (54) |
| Baseline NLR | |
| < 5 | 30 (73) |
| ≥ 5 | 11 (27) |
| 6 weeks NLR | |
| < 5 | 28 (68) |
| ≥ 5 | 13 (32) |
| Baseline PLR | |
| < 169 | 20 (49) |
| ≥ 169 | 21 (51) |
| 6 weeks PLR | |
| < 169 | 19 (46) |
| ≥ 169 | 22 (54) |
| Baseline SII | |
| < 730 | 23 (56) |
| ≥ 730 | 18 (44) |
| 6 weeks SII | |
| < 730 | 22 (54) |
| ≥ 730 | 19 (46) |
Association between response to anti-PD-1/PD-L1 treatment and NLR at baseline and at 6 weeks post treatment
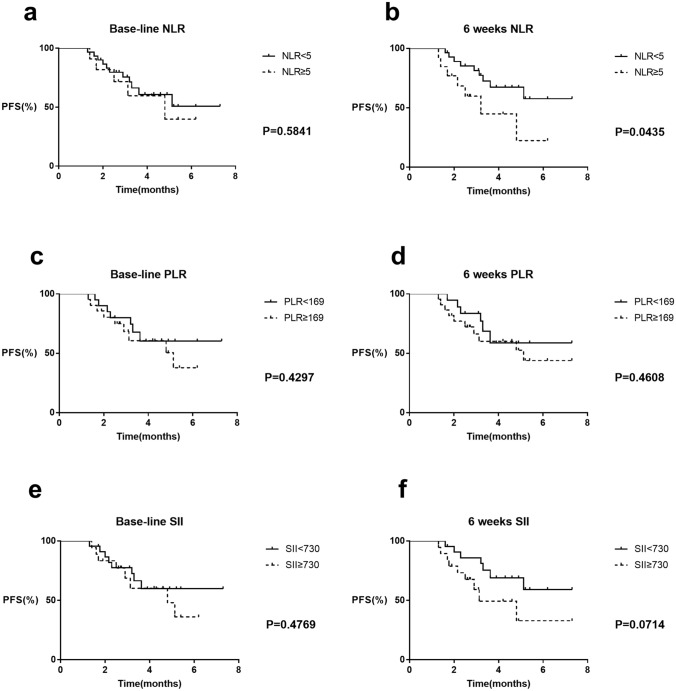
The patients were divided into two groups according to baseline NLR, with the threshold value at 5 as in a previous study [21]. There were 30 patients with NLR < 5 and 11 with NLR ≥ 5. The median PFS of patients with baseline NLR < 5 and ≥ 5 were not reached (NR) and 4.8 months, respectively; the difference was not statistically significant (HR = 0.75, 95% CI 0.24–2.26, P = 0.58; Fig. 1a).
Fig. 1.
PFS curves comparing patients according to NLR, PLR, SII. a PFS curves in patients with baseline NLR < 5 and NLR ≥ 5. b PFS curves in patients with NLR < 5 and NLR ≥ 5 at 6 weeks post treatment. c PFS curves in patients with PLR < 169 and PLR ≥ 169 at baseline. d PFS curves in patients with PLR < 169 and PLR ≥ 169 at 6 weeks post treatment. e PFS curves in patients with SII < 730 and SII ≥ 730 at baseline. f PFS curves in patients with SII < 730 and SII ≥ 730 at 6 weeks post treatment
Patients were then separated into two groups according to NLR at 6 weeks post treatment. There were 13 patients with NLR ≥ 5 and 28 with NLR < 5. The clinical characteristics were comparable between the two groups (Table 2). Median PFS was significantly longer in patients with NLR < 5 than in patients with NLR ≥ 5 (NR vs. 3.2 months, respectively; HR = 0.29, 95% CI 0.09–0.96, P = 0.04; Fig. 1b). In the NLR < 5 group, 9/28 patients had PD at 6 weeks post treatment, and the ORR was 67.9%. In the NLR ≥ 5 group, 7/13 patients had PD at 6 weeks post treatment, and the ORR was 46.2%.
Table 2.
Analysis of clinical characteristics between patients with NLR < 5 and NLR ≥ 5 at 6 weeks post treatment
| Characteristics | 6 weeks NLR < 5 | 6 weeks NLR ≥ 5 | p value |
|---|---|---|---|
| Age | 62 | 60 | 0.652 |
| Sex | 0.548 | ||
| Men | 24 | 12 | |
| Women | 4 | 1 | |
| Smoking | 0.645 | ||
| Yes | 23 | 12 | |
| No | 5 | 1 | |
| ECOG | 0.539 | ||
| 0 | 1 | 1 | |
| 1 | 27 | 12 | |
| Stage | 0.645 | ||
| LD | 5 | 1 | |
| ED | 23 | 12 | |
| Combined chemo | 0.469 | ||
| Yes | 21 | 8 | |
| No | 7 | 5 | |
| Previous line of treatment | 0.103 | ||
| 1 | 14 | 3 | |
| ≥ 2 | 14 | 10 | |
Association between response to anti-PD-1/PD-L1 treatment and PLR at baseline and at 6 weeks post treatment
Patients were divided into two groups with the threshold value of PLR set as 169, as in a previous study [20]. There were 21 patients with PLR < 169 and 20 with PLR ≥ 169 at baseline. Median PFS was comparable in the two groups (NR vs 5.1 months, HR = 0.67, 95% CI 0.25–1.80, P = 0.43; Fig. 1c). At 6 weeks post treatment, there were 22 patients with PLR < 169 and 19 with PLR ≥ 169. No significant difference was found between the patients of the two groups (NR vs 5.1 months, HR 0.69, 95% CI 0.25–1.86, P = 0.46; Fig. 1d).
Association between response to anti-PD-1/PD-L1 treatment and SII at baseline and 6 weeks post-treatment
The 41 patients were dichotomized using an SII threshold value of 730, as in the study by Suh et al. [20]. At baseline, there were 18 patients with SII < 730 and 23 patients with SII ≥ 5. Median PFS was not significantly different between the two groups (NR vs. 4.8 months, respectively; HR = 0.70, 95% CI 0.26–1.89, P = 0.48; Fig. 1e). At 6 weeks post treatment, there were 19 patients with SII < 730 and 22 patients with SII ≥ 730. Median PFS was longer for patients with SII < 730 than for patients with SII ≥ 730, but the difference was not statistically significant (NR vs. 3.13 months, respectively; HR = 0.38, 95% CI 0.013–1.09, P = 0.07; Fig. 1f).
Analysis of trend of NLR during treatment
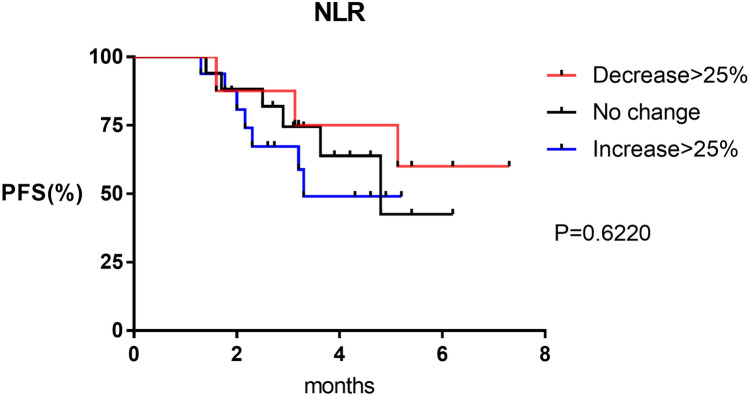
Because NLR at 6 weeks post treatment—but not at baseline—was associated with poorer PFS, we examined whether the change in NLR between the two time points was correlated with response to treatment. Patients were divided into two groups according to clinical response or not. Decrease in NLR was seen in 14/25 (56%) patients in the response group vs. 4/16 (25%) in the non-response group; although the difference was large, it was not statistically significant (P = 0.06; Fig. 2) (Table 3). Further, we divided the patients into three groups according to the percentage change in NLR, with 25% set as the threshold value [22]. PFS was not reached in the > 25% decrease group vs. 4.8 months in the no change group vs. 3.3 months in the > 25% increase group; the difference between the three groups was not statistically significant (P = 0.62; Fig. 3).
Fig. 2.
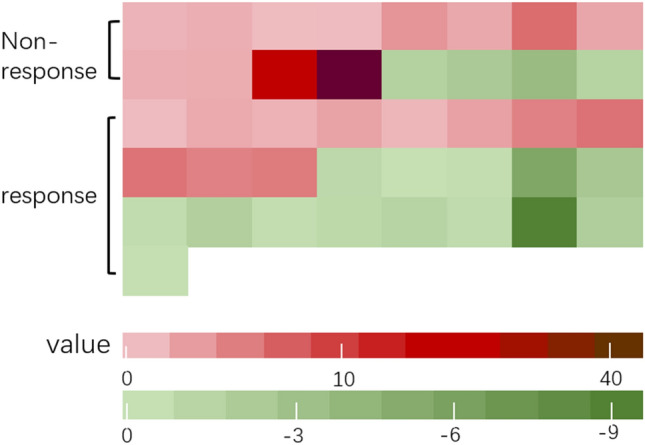
Trend of NLR for patients in non-clinical response group and clinical response group after treatment
Table 3.
Changes of NLR in patients of response and non-response group
| Group | Trend | ||
|---|---|---|---|
| Upward | Downward | Total | |
| Response | 12 | 4 | 16 |
| Non-response | 11 | 14 | 25 |
| Total | 23 | 18 | 41 |
| P value (Fisher) | 0.063 | ||
Fig. 3.
PFS curves comparing patients with post-treatment NLR decrease > 25%, increase > 25% and no change
Discussion
Therapeutic options for SCLC were limited until the IMPower-133 and CASPIAN studies indicated clinical benefit with anti-PD-1/PD-L1 treatment. However, there are no simple methods for identifying SCLC patients likely to respond to the treatment with these agents. NLR and other hematologic parameters have been reported as prognostic biomarkers in NSCLC and other solid tumors treated with immunotherapy [17, 23, 24]. A previous study found that elevated neutrophils stimulated up-regulation of cytokines and chemokines, and possibly contributed to progression of cancer [25]. Meanwhile, decreased lymphocyte production leads to weak immune reaction against tumor cells [26]. These findings suggested that hematologic parameters are markers of inflammation and the adaptive immune response in carcinoma. We therefore hypothesized that these parameters could be predictors of treatment response in SCLC patients receiving anti-PD-1/PD-L1 antibody therapy. In this study, we explored the clinical utility of NLR, PLR, and SII as biomarkers for predicting response to anti-PD-1/PD-L1 antibody in SCLC patients. To the best of our knowledge, this is the first study to examine this relationship.
The prognostic role of pretreatment NLR in cancer patients treated with immunotherapy is still controversial. Ferrucci et al. found that ipilimumab-treated metastatic melanoma patients with baseline NLR < 5 had significantly longer PFS and OS than those with NLR ≥ 5 [27]. In NSCLC patients treating with nivolumab, high pretreatment NLR (≥ 5) was reported to be independently related to poorer OS and PFS, though the authors did not clarify whether it could be used as a predictive marker [17, 28]. Diem et al. found that high baseline NLR was significantly associated with poorer OS and the PFS was also shorter but it was not statistically significant [23]. However, Suh et al. found no different in PFS between those with NLR < 5 and ≥ 5 at baseline among NSCLC patients treated with anti-PD-1 antibody [20]. Consistent with Suh et al., we found no correlation between baseline NLR and response in SCLC patients receiving anti-PD-1/PD-L1 antibody. However, in line with the previous study [20], SCLC patients with NLR < 5 at 6 weeks post treatment had significantly longer PFS than those with NLR ≥ 5. To explore whether the difference between two groups was due to selection bias, propensity score matching (PSM) was intended. However, before the PSM analysis, we found clinical characteristics were comparable between the NLR < 5 group and NLR ≥ 5 group at 6 weeks post-treatment. Thus, these results waived performing PSM in our study. Taken together, NLR at 6 weeks after start of treatment appears to be a useful potential biomarker of response, with potential to augment radiographic assessment in SCLC patients being treated with anti-PD-1/PD-L1 antibody as second- or later-line treatment.
We investigated the value of PLR and SII for predicting response to treatment but found no association between these parameters—at baseline or at 6 weeks post treatment—and PFS of SCLC patients. PLR has been previously reported as a potential inflammatory biomarker reflecting prognosis in SCLC, NSCLC and a number of cancers. However, as for NLR, there is no consensus on the association between PLR and outcomes of immunotherapy. Some studies have shown that in NSCLC patients treated with nivolumab, low baseline PLR is associated with longer PFS and OS [17, 18], but others found no significant difference in survival between NSCLC patients with high and low baseline PLR levels [25, 29]. The reasons for these conflicting results remain to be elucidated, but we speculate that differences between studies in cancer type, treatment methods, sample size, race of patients, and the threshold PLR value used to dichotomize may be responsible.
SII has been considered to be a more promising prognostic marker than the NLR or PLR as it integrates lymphocyte, neutrophil, and platelet counts [26, 30]. A meta-analysis involving 2441 NSCLC patients showed that high SII value predicts poor OS and PFS, and may serve as an adverse prognostic marker in NSCLC patients regardless of the treatment method [19]. SII has also been shown to be a powerful prognostic indicator in a variety of cancers, including hepatocellular carcinoma, colorectal cancer, and esophageal carcinoma [26, 30, 31]. However, in our study, SII at baseline and at 6 weeks post treatment was not associated with PFS. Patients with SII < 730 at 6 weeks post treatment had a longer PFS than those with SII ≥ 730, but the difference was not statistically significant. This lack of statistical significance was probably because our sample was not large enough.
In this study, we also analyzed the changes in NLR during anti-PD-1/PD-L1 treatment. We found decrease of NLR in the majority of patients showing clinical response to treatment, which suggests that decline in NLR might be supplementary evidence of response to anti-PD-1/PD-L1 antibody treatment in SCLC patients. In a previous study on metastatic renal cell carcinoma patients treated with anti-PD-1/PD-L1 antibody, decline of NLR at 6 weeks was associated with longer PFS and higher ORR. The authors also reported that relative NLR change by ≥ 25% from baseline to 6 weeks post treatment was an independent prognostic factor for PFS and OS [22]. However, in the present study, we found no significant difference in PFS between SCLC patients with different degrees of change in NLR. Once again, this may due to the small sample size.
Some limitations exist in this study. First, this was a retrospective analysis of data of a small sample from a single center, and some bias and confounding factors are inevitable. In addition, patients with SII < 730 at 6 weeks post treatment and the trends of NLR change reached marginal association with PFS. These results were likely due to the small sample size. Second, hematologic parameters may have been affected by some concomitant medications, but this was not accounted for in our study. Third, OS was not available for studying association between NLR and survival. Moreover, whether post-treatment NLR has similar relationship with response in first-line immunotherapy treatment needed further investigation. Last but not least, the basic biological and immune mechanism have not been thoroughly elucidated, which preclude deciphering the controversial results of different studies. Nevertheless, our study offers a simple, affordable, and noninvasive method to help physicians predict treatment response at 6 weeks post treatment in SCLC patients receiving anti-PD-1/PD-L1 antibody as second- or later-line treatment in clinical practice.
In conclusion, NLR value at 6 weeks after anti-PD-1/PD-L1 antibody treatment appears to be a promising predictor of response in SCLC patients treated with anti-PD-1/PD-L1 antibodies as second- or later-line treatment. Further prospective studies are needed to investigate the value of NLR as a prognostic biomarker for patients on immunotherapy and the underlying molecular mechanisms in SCLC.
Acknowledgements
This study is supported by the National Major Research Program during the “13th Five-Year Plan” (2018ZX09201013).
Author contributions
Study design: YH. Study conduct: QX, ZH, LX, BQ, XZ. Data collecting: QX, ZH, JZ, BY. Data analysis: QX, ZH, LX, GZ. Data interpretation: QX, ZH and WS. Draft manuscript: QX and ZH. YH takes full responsibility for the integrity of the data analysis.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflict of interest.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Medical Ethics Committee of PLA General Hospital No. S2018-092-01).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Xiong and Ziwei Huang contributed equally to this work.
References
- 1.Owonikoko TK, Behera M, Chen Z, Bhimani C, Curran WJ, Khuri FR, Ramalingam SS. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol. 2012;7(5):866–872. doi: 10.1097/JTO.0b013e31824c7f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 8.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Ozguroglu M, Ji JH, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 10.Leal T, Wang Y, Dowlati A, Lewis DA, Chen Y, Mohindra AR, Razaq M, Ahuja HG, Liu J, King DM (2020) Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. American Society of Clinical Oncology
- 11.Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang JC-H, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind phase III KEYNOTE-604 study. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15(3):426–435. doi: 10.1016/j.jtho.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2019;35(2):329. doi: 10.1016/j.ccell.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 15.Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameratunga M, Chenard-Poirier M, Moreno Candilejo I, Pedregal M, Lui A, Dolling D, Aversa C, Ingles Garces A, Ang JE, Banerji U, et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer. 2018;89:56–63. doi: 10.1016/j.ejca.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Russo A, Russano M, Franchina T, Migliorino MR, Aprile G, Mansueto G, Berruti A, Falcone A, Aieta M, Gelibter A, et al. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv Ther. 2020;37:1145. doi: 10.1007/s12325-020-01229-w. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y, Liao D, Fu X, Gao Q, Zhang Y. Elevated platelet-to-lymphocyte corresponds with poor outcome in patients with advanced cancer receiving anti-PD-1 therapy. Int Immunopharmacol. 2019;74:105707. doi: 10.1016/j.intimp.2019.105707. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Li Y, Chen P, Xu W, Wu Y, Che G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: a meta-analysis. Ann Transl Med. 2019;7(18):433. doi: 10.21037/atm.2019.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim DW, Heo DS, Lee JS. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. doi: 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Lalani AA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, Duquette A, Bosse D, Bellmunt J, Van Allen EM, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Fruh M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandala M, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 25.Fukui T, Okuma Y, Nakahara Y, Otani S, Igawa S, Katagiri M, Mitsufuji H, Kubota M, Hiyoshi Y, Ishihara M. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer. 2019;20(3):208–214. doi: 10.1016/j.cllc.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis F, Marchetti P, Amato G, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112(12):1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Russo A, Franchina T, Ricciardi GRR, Battaglia A, Scimone A, Berenato R, Giordano A, Adamo V. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with nivolumab or docetaxel. J Cell Physiol. 2018;233(10):6337–6343. doi: 10.1002/jcp.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol WJG. 2017;23(34):6261–6272. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(10):2077–2086. doi: 10.1007/s00432-017-2451-1. [DOI] [PubMed] [Google Scholar]