Abstract
Background
We investigated the combined effects of sarcopenia and inflammation on outcomes in patients with HCC treated with nivolumab.
Materials and Methods
We reviewed 102 patients treated with nivolumab between 2017 and 2018. Sarcopenia was diagnosed when the L3 skeletal muscle indices were < 42 cm2/m2 and < 38 cm2/m2 in men and women, respectively. Baseline neutrophil-to-lymphocyte ratio (NLR) and absolute lymphocyte count were used as surrogate markers of inflammation and immune cell reservoir. High NLR (hNLR) was defined as NLR ≥ 3, and severe lymphopenia (sLP) was defined as lymphocyte < 800/μL. The overall survival (OS) and progression-free survival (PFS) were analyzed.
Results
With a median follow-up of 21.9 (interquartile range, 8.3–58.3) months, patients with sarcopenia showed shorter OS than those without sarcopenia (median, 2.9 vs. 7.5 months, respectively). Patients with either hNLR or sLP exhibited inferior survival than those without risk factor (median OS, 2.8 vs. 14.5 months; median PFS, 1.3 vs. 3.7 months, respectively). Among 70 patients treated with RT, benefit of RT was observed in patients with sarcopenia or those without hNLR/sLP (all p < 0.05). After multivariable analysis, RT, hNLR/sLP, albumin–bilirubin (ALBI) grade, and alpha-fetoprotein were significantly associated with OS (all p < 0.05), and hNLR/sLP was also associated with decreased PFS together with ALBI grade, alpha-fetoprotein, and RT (all p < 0.05).
Conclusion
The current study hypothetically demonstrated that the risk group stratified by hNLR/sLP outweighs the significance of sarcopenia in predicting outcomes after nivolumab. Furthermore, patients with sarcopenia might benefit from RT, especially those without risk factors of hNLR/sLP.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02794-3) contains supplementary material, which is available to authorized users.
Keywords: Sarcopenia, Nivolumab, Radiation therapy, Inflammation
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death; however, a limited number of systemic treatment options for advanced/recurrent HCC exists. Sorafenib or lenvatinib are the only standard systemic treatment options currently available for HCC, although their therapeutic effects are limited. The recent prospective phase ½ [1] and 3 trials [2] have demonstrated that nivolumab, an immune checkpoint blockade (ICB) that disrupts programmed cell death protein-1 immune checkpoint signaling, improves the clinical outcomes of patients with HCC. It has been widely implemented in clinical practice since then; however, it has a limited response rate of 10%–15% in HCC [1, 2]. Hence, an increased attention has been focused on clarifying the potential biomarkers of ICB.
Sarcopenia, one of the features of chronic disease, is characterized by the decrease in skeletal muscle mass and muscle deterioration. It has already been well established that sarcopenia has a negative effect in body composition and decreases immunity [3]. With the widespread use of ICB in cancer treatment, both sarcopenia and inflammation are considered important in determining the prognosis and predicting treatment responses of several malignancies [4]. Recently, a newly developed prognostic score based on sarcopenia predicted the treatment response better than traditional prognostic scoring system in patients with advanced stage diseases treated with ICB [5].
We have previously reported that concurrent or history of radiation therapy (RT) with the administration of nivolumab is associated with improved survival outcomes in patients with HCC [6]. Further, the utilization of RT in patients with HCC has increased recently [7–10]. Since RT has an immune-stimulatory effect with releasing tumor antigen, it is anticipated that RT could provoke antitumor immunity even in patients with sarcopenia. Nonetheless, the underlying mechanism between RT and sarcopenia or inflammation in patients with HCC treated with nivolumab is still unknown. Hence, in the present study, we performed an analysis to assess the association between sarcopenia and inflammatory parameters and identify the role of RT in these aspects for patients with HCC treated with nivolumab.
Materials and methods
Study population
Patients with HCC who were treated with nivolumab between March 2017 and December 2018 at Samsung Medical Center were included. Patients were excluded if the follow-up period was < 1 month. We identified and included 102 patients in this study. This study was approved by the institutional review board (No. 2020-03-012), and the protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The need for informed consent was waived owing to the retrospective nature of this study. Patients’ information was collected from medical records, including data on sex, age, Eastern Cooperative Oncology Group performance status score, etiology of HCC, Child–Pugh Class score, and Albumin–Bilirubin (ALBI) score, laboratory test results including complete blood count with differential, intrahepatic, and extrahepatic disease extent, and detailed information on history of previous treatment including RT.
Treatment
Administration of nivolumab was decided after a discussion with the institutional multidisciplinary team that comprised medical oncologists, hepatologists, surgeons, radiation oncologists, and radiologists. In the absence of a reimbursement covered by the Korean National Insurance Program, only patients with limited liver function who could afford the cost were administered with nivolumab.
Intravenous nivolumab (3 mg/kg) [1] was prescribed until the patients experienced unacceptable toxicity and disease progression or refused treatments. The median interval between nivolumab administration and initial diagnosis of HCC was 21.3 (interquartile range [IQR], 6.9–65.1) months.
After the multidisciplinary team discussion, RT was performed to selected patients for mostly palliative purposes. A total of 70 patients (68.8%) received RT before (92.9%) and/or with nivolumab (14.3%) administration. Detailed information on RT is summarized in Supplementary Table S1. The most common sites for RT were the liver (48.6%), followed by the bone (30.0%) and lymph nodes (11.3%). Most patients were treated with photon RT; 10 patients were treated with proton beam RT. With a median total dose of 30.0 (IQR, 20.0–40.0) Gy in median 10 (IQR, 8–10) fractions, the median biologically effective dose using an α/β ratio of 10 for tumor control was 39.0 (IQR, 28.0–56.0) Gy.
Sarcopenia
We used abdominal computed tomography images before nivolumab administration instead of dual-energy X-ray absorptiometry for assessing the body composition of patients. Cross-sectional area (cm2) of the skeletal muscle at the level of the third lumbar spine was evaluated using the in-house software based on MATLAB version R2014a (MathWorks Inc., Natick, MA, USA). The software used in this study is an open-source tool, which is available at the following URL: https://sourceforge.net/projects/muscle-fatarea-measurement/. The skeletal muscle index (SMI) was defined as follows: SMI (cm2/m2) = cross-sectional area (cm2)/height2 (m2). Considering the various criteria currently available for sarcopenia, we used the cutoff values of 42 cm2/m2 for men and 38 cm2/m2 for women based on the guidelines for sarcopenia in liver disease from the Japan Society of Hepatology [11].
Markers of systemic inflammation and immune cell
Absolute counts of lymphocyte (/μL) and neutrophil (/μL) were analyzed prior to first nivolumab administration. As a marker of systemic inflammation, the neutrophil-to-lymphocyte ratio (NLR) was calculated, and a high NLR (hNLR) was defined as NLR ≥ 3.0, which is a widely accepted cutoff value [12, 13]. In addition, a widely accepted cutoff value of 3.0 for NLR resulted in an area under the receiver-operating characteristic curve of 0.699, with a sensitivity of 65.6% and a specificity of 73.9%. We subsequently evaluated the absolute lymphocyte count (ALC) based on the Common Terminology Criteria for Adverse Events version 4.03 guidelines, where ALC from the lower limit of normal range to 800/μL was considered grade 1, 800–500/μL was considered grade 2, and 500–200/μL was considered grade 3. We defined a severe lymphopenia (sLP) as pretreatment ALC < 800/μL (grade 2–4 lymphopenia).
Statistical analyses
All patients were assessed every 3 months with radiologic and laboratory evaluations since the administration of nivolumab. Radiological responses were defined using the Response Evaluation Criteria in Solid Tumors. Disease control rate and overall response rate were determined by the best radiologic response after nivolumab administration: disease control rate included complete response, partial response, and stable disease; overall response rate included complete and partial responses, respectively. The treatment toxicity was graded according to the Common Terminology Criteria for Adverse Events (version 4.03). The baseline characteristics of patients with or without sarcopenia were compared using the Pearson chi-squared or Fisher’s exact test for categorical variables and the Student’s t test or Mann–Whitney U test for continuous variables. Tests were appropriately used after checking distributional assumptions for each variable. The overall survival (OS) and progression-free survival (PFS) were calculated from the date of the first day of nivolumab administration to the date of each event or last follow-up. The Kaplan–Meier method and log-rank test were used to estimate and compare the OS and PFS rates. A Cox proportional hazards model was used when performing a multivariable analysis, which only included factors that showed statistical significance in a univariable analysis. We also evaluated the multicollinearity after calculating a variance inflation factor of < 5 for factors included in the multivariable analysis. A two-sided p value of < 0.05 was considered statistically significant. We used the International Business Machines Corporation (IBM) Statistical Package for the Social Sciences Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses.
Results
Patient characteristics
Patient, tumor, and baseline laboratory test results are summarized in Table 1. With a male predominance (85.3%), the median patient age was 61.3 (IQR, 54.0–69) years. Most patients had chronic hepatitis B virus infection (76.5%), well-compensated liver function (Child–Pugh class A, 81.4%), and extrahepatic metastasis (85.3%) at the time of nivolumab administration. Before nivolumab administration, most patients (97 patients, 95.1%) received tyrosine kinase inhibitor. The median values of body mass index, SMI, and NLR for the entire cohort were 22.9 kg/m2, 48.8, and 3.32, respectively. We ultimately identified 23 patients with sarcopenia (22.5% of the entire cohort). Patients with sarcopenia had worse ALBI score and higher pretreatment alpha fetoprotein levels than those without sarcopenia. Additionally, patients with sarcopenia presented with lower skeletal muscle indices (median, 36.7 vs. 52.4, p < 0.001) and frequent hNLR (78.3% vs. 50.6%, p = 0.019) than those without sarcopenia. After 1 month of nivolumab administration, SMI of patients with sarcopenia decreased more significantly than that of patients without sarcopenia (median ratio, 0.89 vs. 0.96, p = 0.006). There was no difference in the 1-month ALC and NLR values. For further analysis according to the history of RT, baseline characteristics except white blood cell counts and ALC at the time of nivolumab administration were comparable between patients with a history of RT and those without a history of RT (Supplementary Table S2). In addition, sites of RT showed no difference in the presence of sarcopenia, sLP, and hNLR (Supplementary Table S3).
Table 1.
Patient and tumor characteristics stratified by sarcopenia
| Total | No sarcopenia | Sarcopenia | p value | |
|---|---|---|---|---|
| (n = 102) | (n = 79) | (n = 23) | ||
| Age | ||||
| Median (IQR) | 61.3 [54.0; 69.0] | 62.0 [54.1; 69.9] | 59.0 [51.0; 66.0] | 0.222 |
| Sex | ||||
| Male | 87 (85.3) | 70 (88.6) | 17 (73.9) | 0.098 |
| ECOG PS | ||||
| 1–2 | 97 (95.1) | 74 (93.7) | 23 (100.0) | 0.150 |
| Cause of hepatitis | ||||
| HBV | 78 (76.5) | 60 (75.9) | 18 (78.3) | 1.000 |
| HCV | 7 (6.9) | 6 (7.6) | 1 (4.3) | |
| Alcohol | 8 (7.8) | 6 (7.6) | 2 (8.7) | |
| Unknown | 9 (8.8) | 7 (8.9) | 2 (8.7) | |
| CTP class | ||||
| A | 83 (81.4) | 67 (84.8) | 16 (69.6) | 0.128 |
| B or C | 19 (18.6) | 12 (15.2) | 7 (30.4) | |
| ALBI score | ||||
| − 2.50 [− 1.93; − 1.94] | − 2.61 [− 2.85; − 2.00] | − 2.17 [− 2.65; − 1.66] | 0.028 | |
| Grade 1 | 47 (46.1) | 40 (50.6) | 7 (30.4) | 0.141 |
| Grade 2–3 | 55 (53.9) | 39 (49.4) | 16 (69.6) | |
| Intrahepatic tumor burden | ||||
| ≥ 50% of liver | 30 (29.4) | 20 (25.3) | 10 (43.5) | 0.155 |
| Extrahepatic metastasis | ||||
| Yes | 87 (85.3) | 68 (86.1) | 19 (82.6) | 0.740 |
| AFP, ng/mL | ||||
| Median (IQR) | 274.9 [16.6; 17,354.5] | 135.9 [9.1; 9323.0] | 449.8 [235.9; 67,714.2] | 0.019 |
| Previous Treatment | ||||
| Surgery | 43 (42.2) | 36 (45.6) | 7 (30.4) | 0.292 |
| RFA | 31 (30.4) | 25 (31.6) | 6 (26.1) | 0.801 |
| TACE | 64 (62.7) | 52 (65.8) | 12 (52.2) | 0.344 |
| TKI | 97 (95.1) | 76 (96.2) | 21 (91.3) | 0.315 |
| RT | ||||
| Before/concurrent | 70 (68.6) | 54 (68.4) | 16 (69.6) | 1.000 |
| Body weight, kg | 63.8 [59.2; 70.3] | 65.0 [60.2; 71.0] | 59.2 [51.4; 63.2] | 0.001 |
| Height, m | 1.67 [1.63; 1.71] | 1.67 [1.64; 1.71] | 1.69 [1.62; 1.71] | 0.707 |
| BMI, kg/m2 | 22.85 [21.11; 25.13] | 23.89 [21.93; 25.32] | 20.65 [19.42; 22.04] | < 0.001 |
| Baseline | ||||
| SMI, cm2/m2 | 48.80 [43.00; 55.82] | 52.44 [47.41; 56.71] | 36.65 [29.90; 39.60] | < 0.001 |
| WBC count, (× 103/uL) | 5.51 [4.21; 7.08] | 4.97 [3.96; 6.80] | 6.54 [5.55; 8.49] | 0.004 |
| ANC, (× 103/uL) | 3.48 [2.48; 4.96] | 3.16 [2.37; 4.67] | 4.88 [3.02; 6.19] | 0.020 |
| ALC, (× 103/uL) | 1.06 [0.76; 1.58] | 1.06 [0.78; 1.46] | 1.20 [0.77; 1.64] | 0.478 |
| < 800 | 29 (28.4) | 22 (27.8) | 7 (30.4) | 1.000 |
| NLR | 3.32 [2.24; 4.93] | 3.02 [2.23; 4.46] | 4.13 [3.27; 5.64] | 0.109 |
| ≥ 3.0 | 58 (56.9) | 40 (50.6) | 18 (78.3) | 0.019 |
| Post 1 month | ||||
| SMI, cm2/m2 | 45.85 [40.50; 52.15] | 47.00 [41.91; 53.40] | 36.44 [31.00; 42.63] | < 0.001 |
| SMI1month/SMIbaseline | 0.92 [0.83; 1.00] | 0.96 [0.90; 1.17] | 0.89 [0.80; 0.98] | 0.006 |
| ALC, (× 103/uL) | 1.05[0.71; 1.47] | 1.04 [0.70; 1.46] | 1.20 [0.78; 1.42] | 0.904 |
| < 800 | 31 (30.4) | 24 (30.4) | 7 (30.4) | 1.000 |
| ALC1month/ALCbaseline | 1.00 [0.85; 1.21] | 1.04 [0.87; 1.23] | 0.92 [0.77; 1.00] | 0.053 |
| NLR | 3.69 [2.35; 6.01] | 3.48 [2.23; 5.92] | 5.12 [3.13; 6.89] | 0.126 |
| ≥ 3.0 | 64 (62.8) | 46 (58.2) | 18 (78.3) | 0.133 |
| NLR1month/NLRbaseline | 1.10 [0.80; 1.52] | 1.04 [0.78; 1.45] | 1.17 [0.90; 1.72] | 0.269 |
IQR interquartile range, ECOG PS Eastern Cooperative Oncology Group performance status, BMI body mass index, SMI skeletal muscle index, HBV hepatitis B virus, HCV hepatitis C virus, CTP Child-Turcotte-Pugh, ALBI Albumin–Bilirubin score, AFP alpha-fetoprotein, RFA radiofrequency ablation, TACE trans-arterial chemo-embolization, TKI tyrosine kinase inhibitor, RT radiation therapy, WBC white blood cell, ANC absolute neutrophil count, ALC absolute lymphocyte count, NLR neutrophil–lymphocyte ratio
Overall survival
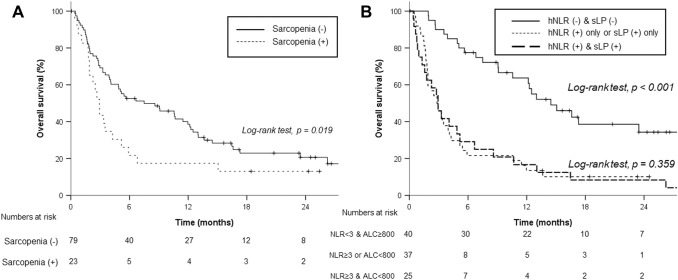
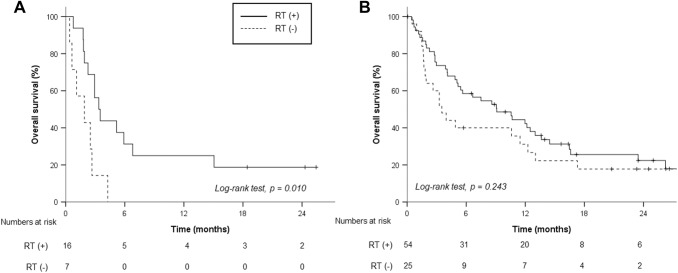
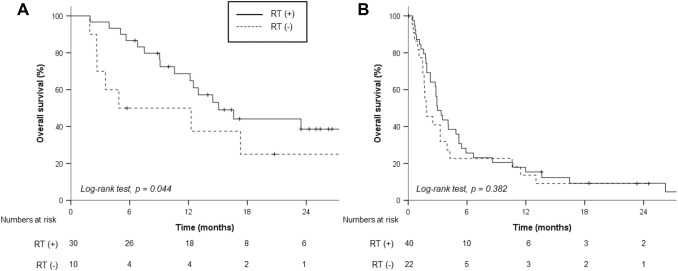
With a median follow-up period of 21.9 (IQR, 8.3–58.3) months, the median OS and 1-year OS rates were 5.1 months and 33.8%, respectively. Patients with sarcopenia showed a lower median OS (2.9 months) than those without sarcopenia (7.5 months, p = 0.036, Fig. 1a). Patients with neither hNLR nor sLP demonstrated significantly better median OS (40 patients, 14.5 months) than those with either hNLR or sLP (37 patients, 2.8 months) and those with both hNLR and sLP (25 patients, 2.9 months, Fig. 1b). In addition, RT differed the OS rates of patients with sarcopenia (RT vs. no RT; 3.4 vs. 1.0 months, p = 0.010, Fig. 2a). Meanwhile, there was no significant difference in accordance with RT for patients without sarcopenia (RT vs. no RT, 9.1 vs. 3.5 months, p = 0.243, Fig. 2b). Although RT sites had no impact on OS outcomes, we observed a nonsignificant but clear trend in favor of concurrent administration of RT and nivolumab for OS (Supplementary Table S3, Supplementary Figure 1A). Subgroup analysis stratified by hNLR and sLP revealed that RT was associated with better OS in patients with both NLR < 3 and ALC ≥ 800/μL (Fig. 3). After a multivariable analysis, both RT (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.25–0.74) and risk factors of either hNLR or sLP at baseline (HR, 3.04; 95% CI, 1.58–5.86) were associated with OS along with the ALBI group and serum alpha fetoprotein level (Table 2). Although relative difference in ALC or NLR between baseline and 1 month after nivolumab administration was not related to OS outcomes, risk factors based on 1-month hNLR/sLP showed borderline significance in OS outcomes (HR, 1.73; 95% CI 0.95–3.14, p = 0.072, Table 2). In addition, hNLR and sLP itself were also associated with poor OS when separately analyzed (Supplementary Table S4).
Fig. 1.
Overall survival rates stratified by sarcopenia status (a) and neutrophil–lymphocyte ratio (NLR) of 3 and absolute lymphocyte count (ALC) of 800/μL
Fig. 2.
Overall survival rates according to radiation therapy (RT) in patients with sarcopenia (a) and without sarcopenia (b)
Fig. 3.
Overall survival rates according to radiation therapy (RT) in patients without risk factors of high neutrophil–lymphocyte ratio and low absolute lymphocyte count (a) and patients with at least one of the risk factors (b)
Table 2.
Prognostic factors for overall survival
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age | (< 60 vs. ≥ 60 years) | 0.67 | 0.43–1.04 | 0.073 | |||
| Sex | (Male vs. female) | 1.32 | 0.71–2.44 | 0.382 | |||
| ECOG PS | (0–1 vs. 2) | 3.21 | 1.29–8.00 | 0.012 | 1.39 | 0.49–3.97 | 0.535 |
| BMI | (≥ 18.5 vs. < 18.5) | 1.10 | 0.40–3.01 | 0.855 | |||
| Sarcopenia | (No vs. yes) | 1.71 | 1.02–2.85 | 0.041 | 1.11 | 0.62–1.97 | 0.727 |
| Viral status | (Others vs. HBV/HCV) | 1.29 | 0.68–244 | 0.434 | |||
| ALBI group | (grade 1 vs. 2–3) | 2.60 | 1.64–4.11 | < 0.001 | 2.65 | 1.55–4.53 | < 0.001 |
| AFP* | 1.24 | 1.07–1.44 | 0.005 | 1.27 | 1.07–1.52 | 0.007 | |
| Intrahepatic tumor burden | (< 50% vs. ≥ 50%) | 2.35 | 1.46–3.77 | < 0.001 | 1.08 | 0.64–1.84 | 0.771 |
| Extrahepatic metastasis | (No vs. yes) | 0.58 | 0.33–1.04 | 0.069 | |||
| Surgery | (No vs. yes) | 0.58 | 0.37–0.92 | 0.020 | 0.80 | 0.48–1.33 | 0.387 |
| RFA | (no vs. yes) | 0.75 | 0.46–1.21 | 0.231 | |||
| TACE | (No vs. yes) | 0.86 | 0.54–1.36 | 0.507 | |||
| TKI | (No vs. yes) | 0.65 | 0.24–1.80 | 0.410 | |||
| RT | (No vs. before/concurrent) | 0.63 | 0.39–0.99 | 0.049 | 0.57 | 0.35–0.94 | 0.028 |
| ALC & NLR risk group+ | (0 vs. 1–2) | 3.38 | 2.06–5.54 | < 0.001 | 2.92 | 1.71–4.96 | < 0.001 |
| SMI1month/SMIbaseline | (Continuous) | 0.94 | 0.37–2.40 | 0.903 | |||
| ALC1month/ALCbaseline | (Continuous) | 1.12 | 0.66–1.89 | 0.680 | |||
| NLR1month/NLRbaseline | (Continuous) | 0.87 | 0.71–1.08 | 0.207 | |||
HR hazards ratio, CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, BMI body mass index, HBV hepatitis B virus, HCV hepatitis C virus, ALBI Albumin–Bilirubin score, AFP alpha-fetoprotein, RFA radiofrequency ablation, TACE trans-arterial chemo-embolization, TKI tyrosine kinase inhibitor, RT radiation therapy, ALC absolute lymphocyte count, NLR neutrophil–lymphocyte ratio, SMI skeletal muscle index
*AFP (log transformed) was treated as a continuous variable
+Risk group refers to patients with ALC < 800 or NLR ≥ 3
Progression-free survival
The median PFS was 1.7 months for the entire group, and there was no difference in median PFS between patients with sarcopenia and those without sarcopenia (1.6 vs. 1.7 months, respectively, p = 0.281, Supplementary Figure 2A). Patients with either hNLR or sLP demonstrated inferior PFS compared to those without risk factors (median PFS, 1.3 vs. 3.7 months, respectively, p < 0.001 Supplementary Figure 2B). Additionally, RT was associated with higher PFS in patients with sarcopenia (RT vs. no RT, 1.8 vs. 0.9 months, respectively, p = 0.045, Supplementary Figure 3A-B). Specifically, concurrent administration of RT and nivolumab showed a better trend in PFS, although RT sites were not relevant (Supplementary Figure 1B, Supplementary Table S3). Clinical benefit of RT was also observed in patients without hNLR or sLP (Supplementary Figure 4A-B). The multivariable analysis demonstrated that the risk factors of hNLR and sLP at baseline attributed to inferior PFS independently (HR, 4.03; 95% CI, 2.06–7.86, p < 0.001, Table 3). Either relative difference of ALC/NLR or risk factors of hNLR/sLP at post-1 month was not associated with PFS outcomes. Further analysis also revealed that both hNLR and sLP independently affected PFS (all p < 0.05, Supplementary Table S4).
Table 3.
Prognostic factors for progression-free survival
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age | (< 60 vs. ≥ 60 years) | 0.78 | 0.51–1.18 | 0.233 | |||
| Sex | (Male vs. female) | 1.19 | 0.67–2.12 | 0.547 | |||
| ECOG PS | (0–1 vs. 2) | 2.87 | 1.15–7.13 | 0.024 | 1.61 | 0.59–4.37 | 0.353 |
| BMI | (≥ 18.5 vs. < 18.5) | 1.09 | 0.44–2.70 | 0.850 | |||
| Sarcopenia | (No vs. yes) | 1.31 | 0.80–2.14 | 0.280 | |||
| Viral status | (Others vs. HBV/HCV) | 1.54 | 0.85–2.77 | 0.153 | |||
| ALBI group | (grade 1 vs. 2–3) | 1.64 | 1.08–2.50 | 0.020 | 1.27 | 1.01–2.24 | 0.049 |
| AFP* | 1.15 | 1.00–1.32 | 0.046 | 0.71 | 0.45–1.11 | 0.066 | |
| Intrahepatic tumor burden | (< 50% vs. ≥ 50%) | 1.56 | 0.99–2.45 | 0.057 | |||
| Extrahepatic metastasis | (No vs. yes) | 0.79 | 0.45–1.41 | 0.430 | |||
| Surgery | (No vs. yes) | 0.89 | 0.58–1.35 | 0.570 | |||
| RFA | (No vs. yes) | 0.82 | 0.52–1.28 | 0.381 | |||
| TACE | (No vs. yes) | 0.76 | 0.49–1.17 | 0.211 | |||
| TKI | (No vs. yes) | 0.72 | 0.29–1.77 | 0.467 | |||
| RT | (No vs. before/concurrent) | 0.66 | 0.42–0.99 | 0.049 | 0.71 | 0.45–1.11 | 0.134 |
| ALC & NLR risk group+ | (0 vs. 1–2) | 2.37 | 1.53–3.67 | < 0.001 | 2.04 | 1.29–3.21 | 0.002 |
| SMI1month/SMIbaseline | (Continuous) | 0.91 | 0.37–2.25 | 0.833 | |||
| ALC1month/ALCbaseline | (Continuous) | 1.06 | 0.62–1.79 | 0.839 | |||
| NLR1month/NLRbaseline | (Continuous) | 0.88 | 0.72–1.07 | 0.191 | |||
HR hazards ratio, CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, BMI body mass index, HBV hepatitis B virus, HCV hepatitis C virus, ALBI Albumin–Bilirubin score, AFP alpha-fetoprotein, RFA radiofrequency ablation, TACE trans-arterial chemoembolization, TKI tyrosine kinase inhibitor, RT radiation therapy, ALC absolute lymphocyte count, NLR neutrophil–lymphocyte ratio, SMI skeletal muscle index
*AFP (log transformed) was treated as a continuous variable
+Risk group refers to patients with ALC < 800 or NLR ≥ 3
Tumor response
Eighty-two patients (80.4%) experienced overall disease progression with a comparable rate of ultimate progressive disease according to sarcopenia (sarcopenia vs. no sarcopenia, 81.0% vs. 78.3%, p = 0.346). The overall rates of progressive disease in extrahepatic and intrahepatic tumor burdens were 56.8% and 50.0%, respectively. Only 8 patients (7.8%) experienced newly developed metastatic lesion after nivolumab administration. Specifically, patterns of failure were similar with regard to sarcopenia (Supplementary Figure 5). For the best response after nivolumab administration, the disease control rate and overall response rate for patients were 34.3% and 10.8%, respectively (Supplementary Table S5). Comparable disease control rate and overall response rate were observed according to the presence of sarcopenia at baseline. However, patients with sLP or hNLR exhibited a lower disease control rate (22.6% vs. 52.5%, p = 0.004) and overall response rate (3.2% vs. 22.5%, p = 0.006) than those without sLP/hNLR. Additionally, RT appeared to result in significantly improved disease control rate (42.9% vs. 15.6%, p = 0.014) and provided an improved but not statistically significant overall response rate (14.3% vs. 3.1%, p = 0.166). In subgroup analysis of RT group, patients with risk factors of sLP or hNLR showed limited response in irradiated area: disease control rate of 12.5% and overall response rate of 2.5% (Supplementary Table S6). On the contrary, patients without sLP/hNLR presented favorable response of RT-treated lesions: disease control rate of 70.0% (p < 0.001) and overall response rate of 36.7% (p = 0.001).
Toxicity
A total of 78 patients (76.5%) experienced grade 1 or more toxic events, and most treatment-related toxic events were confined to grade 1 or 2 (Supplementary Table S7). Comparable incidence of adverse events was observed between patients with and without sarcopenia. Elevated aspartate aminotransferase levels (48.0% of the entire cohort) and anemia (48.0% of the entire cohort) were frequently observed in both groups. There were 11 events of grade 4 toxicities assessed by laboratory studies—increased aspartate aminotransferase levels (n = 3), increased lipase levels (n = 1), increased amylase levels (n = 1), increased bilirubin levels (n = 4), and decreased sodium levels (n = 2). Among the 92 patients who discontinued nivolumab administration, 4 patients discontinued receiving nivolumab because of treatment-related liver failure. Other reasons for nivolumab discontinuation were as follows: presence of progressive disease (n = 80) and lost to follow-up (n = 8).
Discussion
When considering the limited response rate of nivolumab in HCC, potential biomarkers for predicting treatment outcomes have attracted the attention of several physicians. In the current study, we demonstrated that the risk group stratified by both hNLR and sLP might outweigh the significance of sarcopenia in assessing the predictive value of nivolumab in patients with HCC. Furthermore, we showed the potential benefit of RT for patients with sarcopenia, specifically for patients without risk factors of hNLR or sLP.
The detrimental effect of sarcopenia and increased inflammation on outcomes after ICB has been investigated in patients with other solid tumors. Several reports of patients with malignant melanoma or advanced lung cancer have demonstrated that patients with sarcopenia frequently encountered with toxic events [14, 15] or had poor survival outcomes [16–18] after ICB. Several cytokines potentially involved in the development of sarcopenia, such as transforming growth factor-β [19] and interleukin (IL)-6 [20], cause T-cell exhaustion, resulting in reduced efficacy of ICB [4, 21, 22]. In addition, decrease in myokine levels, such as IL-15 and IL-5, as a result of skeletal muscle depletion in sarcopenia, could lead to a poor treatment response to ICB [23, 24].
Besides sarcopenia, chronic inflammatory status related to the development of sarcopenia itself plays a role in ICB resistance. In the current study, patients with sarcopenia had increased levels of inflammatory markers, such as white blood cell and neutrophil count and NLR, which supports the fact that sarcopenia reflects the increased metabolic activity leading to systemic inflammation and muscle depletion [25]. Patients with advanced cancer usually present with an alteration in peripheral blood composition, which comprises both an expansion in the myeloid component and a reduction in the lymphoid component [26]. Since lymphocyte plays a major role in the re-induction of the exhausted T-cell population, sLP has been recognized as a surrogate marker for ICB response [27]. Furthermore, NLR, which is extensively investigated as a marker of systemic inflammatory response and prognosis in cancer [12, 28], could also reflect the relative proportion of circulating lymphocytes. A number of previous studies regarding solid tumors other than HCC have suggested that sLP [28–30] and hNLR [4, 26, 31] could be potential biomarkers of ICB response. A recent study by S. Dharmapuri et al. has demonstrated that hNLR defined as NLR ≥ 5 could predict the poor median survival of 10 months compared to NLR < 5 (5 months, p = 0.0037) in 104 patients with HCC treated with nivolumab [32]. The current study also showed that both risk groups based on hNLR/sLP and hNLR or sLP at baseline could successfully predict survival outcomes and, moreover, response of RT-treated lesions.
In the current cohort, RT is related to improved survival in patients with sarcopenia. Given an immune-stimulatory effect by immunogenic cell death via calreticulin expression at the cell surface [33, 34], RT might overcome the antitumor effect of sarcopenia. Although concurrent administration of RT and nivolumab showed borderline improvement in OS and PFS, half the patients who received nivolumab and RT concurrently had a history of RT. Since the data on the number of patients who only received concurrent nivolumab and RT was not available, further statistical analysis was not performed. Based on the current analysis, we could cautiously suggest local RT to liver or metastatic sites for candidates of nivolumab administration with advanced HCC regardless of sequences, but further studies regarding sequence between RT and nivolumab may help shape a strategy between local RT and nivolumab. In this context, since most patients received RT followed by ICB in the current study, we suggest that a radiation-induced tumor equilibrium, a balance between tumor cell survival and cell death, could provide a window period for immune-modulation [34]. However, RT also could induce immunosuppression by depleting the lymphocyte or lymphoid progenitor cells [35]. We also observed a significant decrease in ALC 1 month after RT, but no remarkable change after nivolumab administration (data not shown). Our results regarding the different impact of RT on the survival in patients stratified by hNLR and sLP also suggest that RT leads to antitumor immune stimulation in accordance to lymphocyte reservoir. This yin and yang of RT combined with ICB on immune response needs further investigation to maximize the synergism between RT and ICB. In addition, recent interests, which highlight the association between RT and immunosuppressive tumor microenvironment including myeloid-derived suppressor cells, need to be identified in future studies [36, 37].
Some limitations of this study should be acknowledged. First, owing to the limited number of patients, the subgroup analysis had a limited statistical power. The statistical significance with limited number of patients need to be interpreted with caution because the observed effect may not result from true biological effect. However, to the best of our knowledge, this is the first study that investigated the effect of sarcopenia and inflammatory markers combined with RT in patients with HCC. Therefore, this study is hypothesis generating for further administration of nivolumab. Although the clinical significance of sarcopenia and hNLR/sLP was observed in the current analysis, the mechanism underlying the combined effect of RT with sarcopenia and hNLR/sLP on the tumor microenvironment needs to be investigated with further preclinical studies with a sarcopenia mouse model. The current ongoing phase II study investigating concurrent nivolumab and RT for advanced HCC (KCT0004483) could help to elucidate the underlying mechanism based on the biomarkers. Relatively poor treatment outcomes in the current analysis could result from advanced disease status and treatment-resistant status even after multiple local and/or systemic treatments. Moreover, we only analyzed the baseline and 1-month SMI, NLR, and ALC prior to first nivolumab administration since subsequent laboratory results are not available for several patients experiencing the early progression of the disease. Although NLR or ALC values of post-nivolumab administration did not significantly affect survival outcomes in the current analysis, further investigation incorporating dynamics of NLR/ALC and nivolumab is needed to clarify this issue. Considering that most patients had progressive disease within 6 months after ICB administration, identifying SMI, NLR, and ALC in advance may be an effective method for stratifying patients at higher risk for poor survival.
Conclusions
In summary, we hypothetically demonstrated that sarcopenia and risk factors of hNLR or sLP directly affect the poor treatment outcomes after nivolumab administration in patients with HCC. Although RT might improve the survival outcomes of patients with sarcopenia, patients with NLR < 3 and ALC ≥ 800/μL mostly benefit from RT in combination with ICB. Further studies should elucidate the underlying mechanisms of crosstalk among sarcopenia, leukocytes, and radiation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ALBI
Albumin–bilirubin
- HCC
Hepatocellular carcinoma
- hNLR
High neutrophil-to-lymphocyte ratio
- HR
Hazard ratio
- ICB
Immune checkpoint blockade
- IQR
Interquartile range
- NLR
Neutrophil-to-lymphocyte ratio
- OS
Overall survival
- PFS
Progression-free survival
- RT
Radiation therapy
- sLP
Severe lymphopenia
- SMI
Skeletal muscle index
Author contributions
Conception, design, data collection, interpretation, and drafting of the manuscript were performed by NK and JIY. Data collection and interpretation were performed by HCP, GSY, CC, JYH, HYL, JL, and MSC. Statistical analysis and editing of the manuscript were performed by JEL and KK. All authors read and approved the final manuscript.
Funding
This study was partly supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03031275).
Data availability
Data availability is limited due to institutional data protection law and confidentiality of patient data.
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This study was approved by the Health Institutional Review Boards of Samsung Medical Center (No. 2020–03-012).
Consent to participate
The requirement for informed consent was waived because of the retrospective nature of this study.
Consent for publication
The requirement for informed consent was waived because of the retrospective nature of this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/s0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–v875. doi: 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 3.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58(11):1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 4.Bilen MA, Martini DJ, Liu Y, Shabto JM, Brown JT, Williams M, et al. Combined effect of sarcopenia and systemic inflammation on survival in patients with advanced stage cancer treated with immunotherapy. Oncologist. 2019 doi: 10.1634/theoncologist.2019-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dercle L, Ammari S, Champiat S, Massard C, Ferté C, Taihi L, et al. Rapid and objective CT scan prognostic scoring identifies metastatic patients with long-term clinical benefit on anti-PD-1/-L1 therapy. Eur J Cancer. 2016;65:33–42. doi: 10.1016/j.ejca.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Yu JI, Lee SJ, Lee J, Lim HY, Paik SW, Yoo GS, Choi C, Park HC. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med. 2019;8(16):6986–6994. doi: 10.1002/cam4.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim N, Cheng J, Jung I, Liang J, Shih YL, Huang WY, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Korean Liver Cancer A, National Cancer C. 2018 Korean liver cancer association-national cancer center korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13(3):227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rim CH, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016;34(3):160–167. doi: 10.3857/roj.2016.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol. 2019;25(20):2416–2429. doi: 10.3748/wjg.v25.i20.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46(10):951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 12.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 13.Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3(12):e172319. doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly LE, Power DG, O'Reilly Á, Donnellan P, Cushen SJ, O'Sullivan K, Twomey M, Woodlock DP, Redmond HP, Ryan AM. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–317. doi: 10.1038/bjc.2016.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Huillard O, Boudou-Rouquette P, et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs. 2017;35(4):436–441. doi: 10.1007/s10637-017-0464-x. [DOI] [PubMed] [Google Scholar]
- 16.Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: a preliminary retrospective study. Sci Rep. 2019;9(1):2447. doi: 10.1038/s41598-019-39120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortellini A, Bozzetti F, Palumbo P, Brocco D, Di Marino P, Tinari N, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep. 2020;10(1):1456. doi: 10.1038/s41598-020-58498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: a "hypothesis-generator" preliminary report. Thorac Cancer. 2019;10(2):347–351. doi: 10.1111/1759-7714.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21(11):1262–1271. doi: 10.1038/nm.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-Induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24(5):672–684. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–5022. doi: 10.1158/0008-5472.Can-18-0118. [DOI] [PubMed] [Google Scholar]
- 23.Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE. Aging, immune senescence, and immunotherapy: a comprehensive review. Semin Oncol. 2018;45(4):187–200. doi: 10.1053/j.seminoncol.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–279. doi: 10.1146/annurev-med-061509-131248. [DOI] [PubMed] [Google Scholar]
- 26.Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ménétrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7(1):85. doi: 10.1186/s40425-019-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6(1):84. doi: 10.1186/s40425-018-0395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8(69):114268–114280. doi: 10.18632/oncotarget.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;105(5):1065–1073. doi: 10.1016/j.ijrobp.2019.08.047. [DOI] [PubMed] [Google Scholar]
- 31.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Dharmapuri S, Özbek U, Lin JY, Schwartz M, Branch A, Ang C. Predictive value of neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) In hepatocellular carcinoma (HCC) patients treated with nivolumab (N) Ann Oncol. 2019;30:v285–v286. doi: 10.1093/annonc/mdz247.071. [DOI] [Google Scholar]
- 33.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Kang C, Jeong S-Y, Song SY, Choi EK. The emerging role of myeloid-derived suppressor cells in radiotherapy. Radiat Oncol J. 2020;38(1):1–10. doi: 10.3857/roj.2019.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton JM, Hanoteau A, Liu H-C, Gaspero A, Parikh F, Gartrell-Corrado RD, et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J Immunother Cancer. 2019;7(1):216–216. doi: 10.1186/s40425-019-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is limited due to institutional data protection law and confidentiality of patient data.