Abstract
High expression of PD-L1 predicts PD-1/PD-L1 inhibitor benefit, meanwhile a few PD-L1-negative patients still benefit from these drugs. In this study, we aimed to explore the underlying cellular and molecular characteristics via single-cell sequencing. Before and after treatment with Pembrolizumab, peripheral blood mononuclear cells (PBMCs) were isolated via Ficoll gradient. Thereafter, single-cell RNA sequencing was performed, and clinical significance was validated with The Cancer Genome Atlas (TCGA) cohort. All 3423 cells of 16 clusters were classified into eight cell types, including NKG7+ T, NKG7+ NK, Naïve T, CDC1C+ dendritic cells, CD8+ T cells, B cells, macrophages and erythrocytes. Cell proportion, the clinical significance of differentially expressed genes and significant pathways of NKG7+ T, NKG7+ NK, Naïve T and CD8+ T cells were analyzed. Ubiquitin-mediated proteolysis/cell cycle/natural killer cell-mediated cytotoxicity were identified as PD-1 blockage-responsive pathways in NKG7+ NK cells. Apoptosis/Th1 and Th2 cell differentiation were proposed as Pembrolizumab-affected pathways in NKT cells. In gene level, ID2, PIK3CD, UQCR10, MATK, MZB1, IL7R and TRGC2 showed a significant correlation with PD-1 expression after TCGA dataset validation, which could possess potential as predictive markers for patients with PD-L1-negative lung squamous cell carcinoma who can benefit from Pembrolizumab.
Supplementary information
The online version of this article (10.1007/s00262-021-02848-0) contains supplementary material, which is available to authorized users.
Keywords: Lung squamous cell carcinoma, Single-cell RNA sequencing, PD-L1-negative, Pembrolizumab, Immune cells
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, in which 85–90% of cases are classified as non-small cell lung cancer (NSCLC) [1]. Lung squamous cell carcinoma is a major histological subtype of NSCLC that accounts for about 25%-30% of cases [2]. The development of PD-1/PD-L1 checkpoint inhibitors has changed the landscape of NSCLC treatment. These antibody-based inhibitors are designed to directly bind to the PD-1 protein or its ligand PD-L1, thereby preventing PD-1 pathway activation [3]. The US Food and Drug Administration (FDA) has approved several PD-1 inhibitors such as Nivolumab and Pembrolizumab for first-line treatment of advanced NSCLC [4, 5]. However, due to the lack of definitive predictive biomarkers, the rational use of these drugs is limited. The expression of PD-L1 is widely detected in different tumor types and has been a marker associated with PD-1 inhibition [6–8]. However, a few PD-L1-negative patients will still benefit from these drugs. The initial establishment of PD-L1 expression as a predictive attempt biomarker could produce variable results [9], influenced by PD-L1 antibodies, assay methods, scoring systems and currently used positive thresholds [10]. Therefore, the identification of new predictive biomarkers will be the key to ensuring the effectiveness and safe of these drugs.
Pembrolizumab has been approved as the first-line treatment option for metastatic NSCLC patients with high PD-L1 expression (tumor proportion score [TPS] ≥ 1%) [11]. A meta-analysis of randomized controlled trials showed that even for PD-L1-negative patients, PD-1 or PD-L1 blocking therapy is a better treatment option [12]. PD-1 inhibitors can enhance antitumor immunity by enhancing the initiation of new anticancer immune responses [13]. However, PD-L1 expression by itself is not sufficient as a choice for routine clinical practice [12]. Therefore, it is of importance to identify other biomarkers to predict the benefit of PD-1 or PD-L1 inhibitors [14].
Compared with bulk sequencing, single-cell RNA sequencing (ScRNA-Seq) provides the information of gene expression in single-cell level and cell heterogeneity in complex tissue, which paves the way for revealing the role of tumor microenvironment in tumor progression and drug resistance [15–17]. By analyzing immune cell marker's expression and dysregulated genes’ function, the cellular and molecular profile and characteristics of lung cancer treatment have been uncovered [18]. In this study, we first reported research of a PD-L1-negative lung cancer patient benefiting from PD-1 antibody treatment. Then, blood peripheral blood mononuclear cells (PBMCs) from the patient before and after PD-1 antibody treatment were applied for ScRNA-Seq. By identifying immune cells marker gene expression, we identified and compared cell type and subtype changes in “after” versus “before” PD-1 antibody treatment. In gene level, dysregulated genes and functional analysis were conducted to obtain molecular alterations in “after” versus “before” PD-1 antibody treatment. This one-patient study uncovered the immune cells and molecular profile of in a PD-L1-negative lung cancer patient benefiting from Pembrolizumab and would shed light on the mechanism and application of PD-L1 inhibitors.
Materials and methods
Clinical characteristics of the lung squamous cell carcinoma (LUSC) patient before and after Pembrolizumab treatment
The ethics committee of Shanghai Chest Hospital approved our study (approval No.KSY2003). The patient was male and 80 years old. Before the study, the patient provided written informed consent. The patient was admitted to the hospital on June 20th, 2018. The patient had a history of pneumothorax and hypertension, and irbesartan hydrochlorothiazide capsules were administered orally to control blood pressure. The patient had a smoking history of 40 years of 20 cigarettes/day. At admission, chest CT showed left lung occupying with mediastinal lymphadenopathy. On June 22nd, bronchoscopy report indicated neoplasm in the upper left lobe ostium completely blocking the lumen with red fluorescence. The pathological examination result shows primary bronchial lung squamous cell carcinoma, central type, left upper lobe, C-T4N2M0, stage IIIB and PD-L1-negative. In view of the fact that the subject is old and Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 2 points, he was not suitable for platinum-doublet chemotherapy (PT-DC), and Pembrolizumab intravenous infusion was administered.
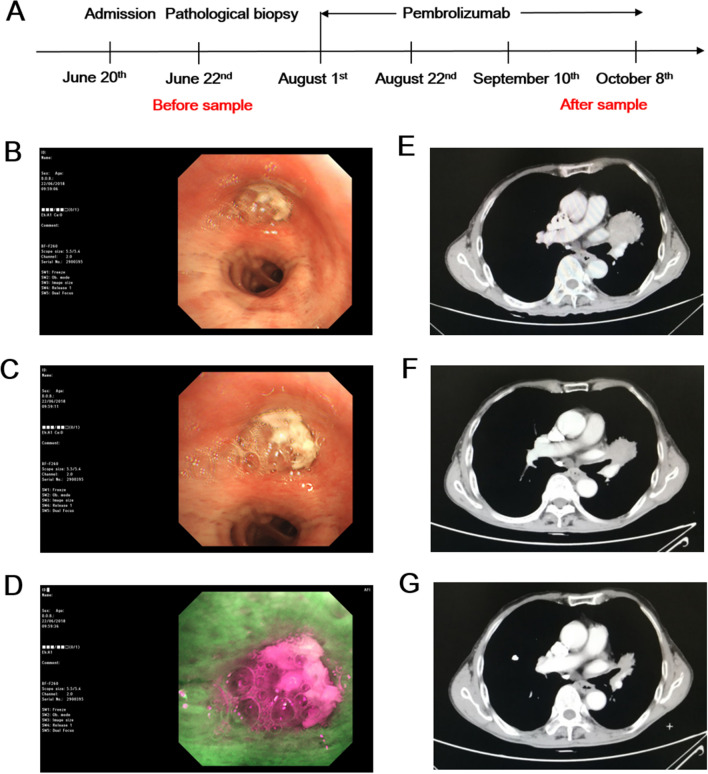
On August 1st and 22nd, 200 mg was administered. On September 10th, chest CT showed a reduction in left lung lesions. On September 17th and October 8th, 200 mg Pembrolizumab intravenous infusion was administered. On October 22nd, chest CT showed that the left lung lesion continued to shrink, and Pembrolizumab is still being administered. There were no adverse events or combined medications reported. Blood samples were collected on June 22nd and October 8th, for Pembrolizumab before and after treatment groups, respectively (Fig. 1a–g). During fluorescent bronchoscopy, after absorbing the fluorescent light, normal bronchial mucosa tissue produces green fluorescence. As tumor tissue contain different fluorescent groups and internal proteins absorb fluorescence with different wavelengths, compared with normal mucosa, the fluorescence intensity in tumor tissue is lower than that of normal tissues, which display red fluorescence. After careful measurement, according to the RECIST evaluation criteria, the longest diameter of the lesion was reduced by 42% after receiving single-drug pembrolizumab treatment, and the best curative effect was judged partial response (PR). The progression-free survival (PFS) was 9 months, and then the nidus increased gradually.
Fig. 1.
Patient with PD-L1-negative lung squamous cell carcinoma benefiting from Pembrolizumab treatment. a Blood samples collection time point. b–d Before treatment, bronchoscopy results of left common branch; left common branch end; left upper lobe (fluorescence). e Before treatment, CT examination results. f After more than one month of treatment, CT examination results. g After two and a half months of treatment, CT examination results
Preparation of PBMCs single-cell suspension
Before and after treatment with Pembrolizumab, blood was drawn and peripheral blood mononuclear cells (PBMCs) were isolated via Ficoll density gradient centrifugation method [19]. Cell viability was assessed by trypan blue staining with cell viability > 85%.
Single-cell RNA sequencing and raw data preprocessing
Single-cell RNA sequencing was achieved by 10× Genomics Single Cell 3′ v3 Reagent Kit, according to the manufacturer's instructions. Single-cell libraries were prepared and sequenced on an Illumina HiSeq X Ten system (Illumina). Using TrimStartingSequence, PolyATrimmer and FilterBam, unqualified raw reads with adapters, duplicates and low sequencing quality were removed. The cleaned data filtered for the low-quality reads and unrelated sequences were imported to CellRanger (version 3.1.0) and aligned to human reference genome (hg19, GRCh37). Cells were sorted according to the barcodes, and the unique molecular identifiers (UMIs) were counted per gene for each cell. The mitochondrial gene at the beginning of MT was marked, and its frequency was counted. Cells with < 500 total expressed genes or with > 7% mitochondria-expressed genes were removed. Principle component analysis (PCA) was performed, and t-distributed stochastic neighbor embedding (t-SNE) was used for dimensionality reduction. Similarly, expressed cells were clustered using Seurat (version 3.1.2) package in R.
Cell type and subclusters identification
We first identified 16 clusters in both datasets of PBMC samples and then used the function FindAllMarkers in Seurat with the statistical analysis method bimod [20]. The highly expressed markers were chosen based on the following criteria: genes with elevated expression in > 50% cells in a cluster, and the expression fold change > 1.5 and adj.p.value < 0.05, compared with other clusters. According to previous study [21] and provided tutorial (https://satijalab.org/seurat/v3.0/immune_alignment.html), FindAllMarkers invokes FindMarkers and could be applied to find markers in two conditions.
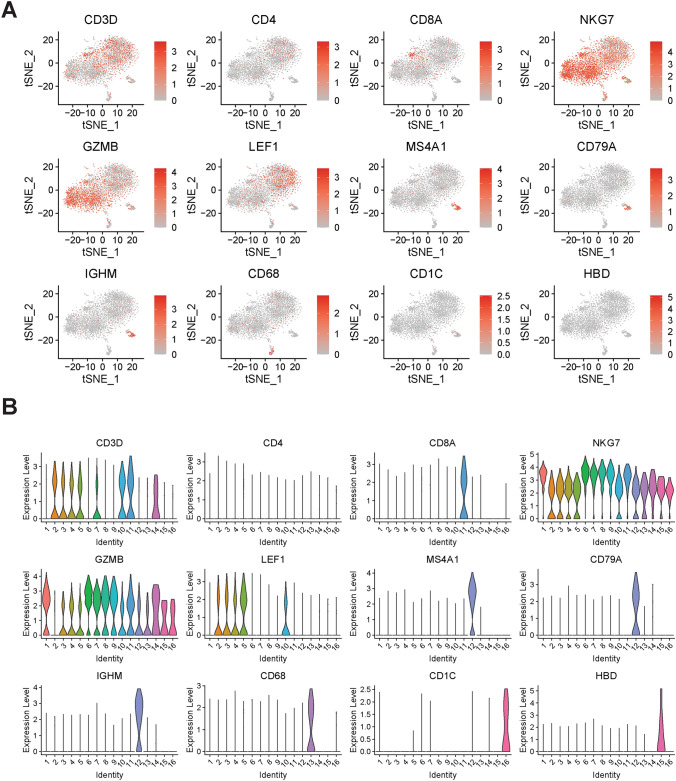
Cell types were identified using canonical markers in t-SNE: T cells-CD3D, CD4 (CD4+ T cells), CD8A (CD8+ T cells), LEF1 and CCR7 (Naïve T cells); NK cells-NKG7 and GZMB; B cells-CD20 (MS4A1), IGHM and CD79; Macrophages-CD68; Dendritic cells-CD1C; Erythrocytes-HBD.
Functional annotation and enrichment analysis
Functional annotation and enrichment analysis, including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, was performed using Clusterprofiler [22], and items with p.adj-value < 0.05 were considered to be significantly enriched.
Prognostic analysis of genes in TCGA lung cancer datasets
Gene expression, expression correlation and prognosis were validated in TCGA lung cancer datasets with GEPIA (http://gepia.cancer-pku.cn/) [23]. Genes with absolute fold change ≥ 2 and p.adj-value < 0.05 were considered significant. Kaplan–Meier survival analysis was applied for evaluating the expression of genes with the prognostic outcome of lung cancer patients. Gene expression correlation with PD-1 was analyzed, and the absolute Pearson correlation coefficient ≥ 0.3 and p-value < 0.05 were considered significant.
Results
Immune cells heterogeneity of PBMCs before and after treatment with Pembrolizumab
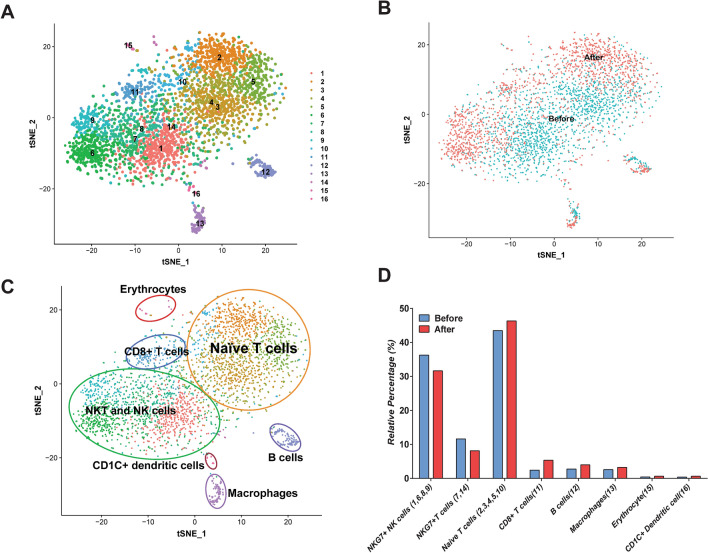
After raw data filtering, a transcriptome of 3423 cells (1850 cells in “before” group and 1573 in “after” group) was acquired. Using the t-SNE clustering, 16 clusters were identified (Fig. 2a). These clusters were classified as “before” and “after” Pembrolizumab treatment (Fig. 2b). Based on the expression of canonical immune cell markers, we classified 16 clusters into 8 cell types: NKG7+ T cells (cluster 7, 14), NKG7+ NK cells (cluster 1, 6, 8, 9), Naïve T cells (cluster 2, 3, 4, 5, 10), CDC1C+ dendritic cells (cluster 16), CD8+ T cells (cluster 11), B cells (cluster 12), macrophages (cluster 13) and erythrocytes (cluster 15) in Fig. 2c. At last, we compared the fraction changes in eight cell types in the “after” group with “before” group and found that the relative percentage of NKG7+ NK cells ad NKG7+ T cells were decreased, and Naïve T cells, CD8+ T cells, B cells and macrophages were increased (Fig. 2d). The t-SNE and violin plot of canonical immune cell markers are shown in Figs. 2b and 3a: T cells-CD3D, CD4 (CD4+ T cells), CD8A (CD8+ T cells), LEF1 (Naïve T cells); NK cells-NKG7 and GZMB; B cells-CD20 (MS4A1), IGHM and CD79; Macrophages-CD68; Dendritic cells-CD1C and Erythrocytes-HBD. In the following analysis, we focused on NKG7+ T cells (cluster 7, 14), NKG7+ NK cells (cluster 1, 6, 8, 9), Naïve T cells (cluster 2, 3, 4, 5, 10) cell types to investigate cellular and molecular characteristics PBMCs before and after treatment with Pembrolizumab.
Fig. 2.
Identification of 16 clusters and immune cell types in “before” and “after” samples. a All 16 clusters were identified and shown with t-SNE plot. b T-SNE plot showing cell distribution in “before” and “after” Pembrolizumab treatment samples. c Identification of immune cell types using t-SNE. Using canonical cell markers: T cells-CD3D, CD4 (CD4+ T cells), CD8A (CD8+ T cells), LEF1 and CCR7 (Naïve T cells); NK cells-NKG7 and GZMB; B cells-CD20 (MS4A1), IGHM and CD79; Macrophages-CD68; Dendritic cells-CD1C; Erythrocytes-HBD, the 16 cell clusters were identified as naïve T cells, NK cells, NKT cells, B cells, Macrophages, Dendritic cells and Erythrocytes. d Relative percentage of immune cell types in blood samples (number of one cell type/number of all cell types) of before and after Pembrolizumab treatment
Fig. 3.
T-SNE and violin plot showing the expression and distribution of 12 canonical immune cell markers in different clusters. We used cell markers to identify cell types, as follows: T cells-CD3D, CD4 (CD4+ T cells), CD8A (CD8+ T cells) and LEF1 (Naïve T cells); NK cells-NKG7 and GZMB; B cells-CD20 (MS4A1), IGHM and CD79; Macrophages-CD68; Dendritic cells-CD1C; Erythrocytes-HBD
NKG7+ NK cells heterogeneity and molecular alterations after Pembrolizumab treatment
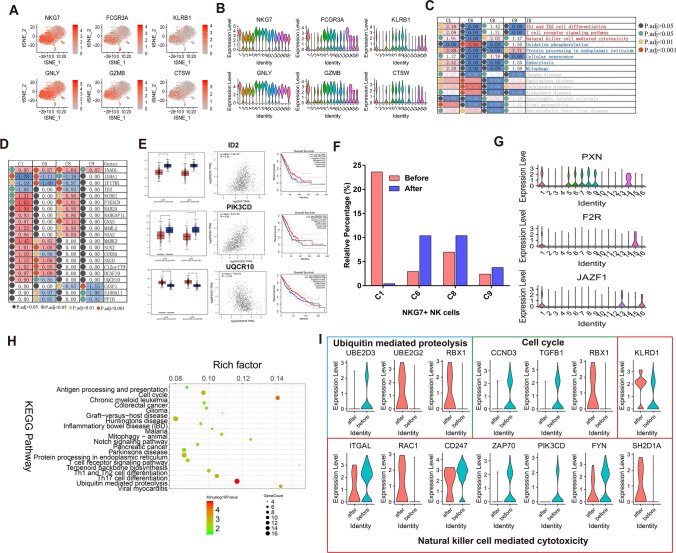
As clusters 1, 6, 7, 8 and 9 showed higher expression of NKG7 while 1, 6, 8, 9 did not express CD3D, we characterized 1, 6, 8, 9 as NKG7+ NK cells. Six highly expressed cell markers in clusters 1, 6, 7, 8 were identified, including natural killer cell granule protein 7 (NKG7), Fc Fragment Of IgG Receptor IIIa (FCGR3A), Killer Cell Lectin Like Receptor B1(KLRB1), Granulysin (GNLY), Granzyme B (GZMB) and Cathepsin W (CTSW) (Fig. 4a, b). To understand the common molecular changes shared by all four NK cell clusters after Pembrolizumab treatment, differentially expressed genes (absolute fold change ≥ 1.5 and p.adj-value < 0.05) and enriched pathways were analyzed. As shown in Fig. 4c, in addition to Th1 and Th2 cell differentiation, T cell receptor signaling pathway and natural killer cell-mediated cytotoxicity, pathways, such as oxidative phosphorylation, protein processing in the endoplasmic reticulum, cellular senescence, endocytosis and mitophagy are significantly enriched in at least two clusters, indicating their potential role in NK cells response of Pembrolizumab treatment. Compared with clusters 1, 8 and 9, although the three pathways were not significantly enriched in cluster 6, oxidative phosphorylation showed the most significant enrichment. In gene level, 21 differentially expressed genes in ≥ 2 clusters were shown in Fig. 4d, such as Inactivation-No-Afterpotential D-Like (INADL), Immunoglobulin Heavy Constant Alpha 1(IGHA1), Interferon Induced Transmembrane Protein 1 (IFITM1), Inhibitor of DNA Binding 2 (ID2) and Nuclear Receptor Corepressor 1 (NCOR1), implying these genes as Pembrolizumab response genes in NK cells and lung cancer. Thereafter, we validated 21 differentially expressed genes expression and correlation with PD-1 in the TCGA lung cancer cohort with GEPIA (http://gepia.cancer-pku.cn/) [23], and only three genes, ID2, PI3KCD and UQCR10 showed significant differential expression(P), correlation with PD-1 and prognostic significance. As shown in Fig. 4e, ID2 showed significantly lower expression in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) tissues, compared to normal tissues (p-value < 0.05). Pearson correlation analysis results suggested that ID2 was positively correlated with PD-1 (p-value = 7.8e−27, R = 0.33). Furthermore, patients with higher ID2 expression exhibited a better prognostic outcome (HR = 0.72, p-value = 0.034). Similarly, significantly lower PIK3CD expression was found both in LUAD and LUSC cancer tissues (p-value < 0.05), and there was a strong positive correlation between PIK3CD and PD-1 (p-value = 9.7e−79, R = 0.55). Overall survival analysis results showed that patients with higher PIK3CD expression had longer survival time than those with lower PIK3CD expression (HR = 0.63, p-value = 0.0027). Moreover, UQCR10 was significantly highly expressed in both LUAD and LUSC tissues than normal tissues (p-value < 0.05) and negatively correlated with PD-1 (p-value = 7.2e−24, R = −0.32). Interestingly, lung cancer patients with higher UQCR10 expression showed significantly longer overall survival time (HR = 0.79, p-value = 0.091).
Fig. 4.
NKG7+ NK cells heterogeneity and molecular alterations after Pembrolizumab treatment. a, b T-SNE and violin plot of six common cell markers in NK cells (cluster 1, 6, 8, 9). c Shared significant enriched Pembrolizumab-responsive pathways in NK cells (cluster 1, 6, 8, 9). d Shared significant enriched Pembrolizumab-responsive genes in NK cells (cluster 1, 6, 8, 9). e Differential expression analysis, Pearson correlation analysis and overall survival analysis of differentially expressed genes (ID2, PIK3CD and UQCR10) using TCGA database. LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma. *p < 0.05. f The relative percentage of cluster 1, 6, 8, 9 before and after treatment with Pembrolizumab. g Violin plot showing the expression distribution of cluster 1 markers PXN, F2R and JAZF1. h Top 20 significant enriched pathways of differentially expressed genes in cluster 1. i Violin plot showing the most significantly altered genes involved in ubiquitin-mediated proteolysis, cell cycle and natural killer cell-mediated cytotoxicity, after Pembrolizumab treatment. Red represents after treatment with Pembrolizumab and blue represents before treatment with Pembrolizumab
Then, we look into the differences in four NKG7+ NK clusters, and the relative percentage changes in cluster 1, 6, 8, 9 in “before” and “after” Pembrolizumab treatment groups are shown in Fig. 4f. We found that the relative percentage of cluster 1 was remarkably decreased after treatment with Pembrolizumab, and the relative percentage of other clusters (6, 8, 9) were all increased after treatment with Pembrolizumab. Hence, we focused on cluster 1 and found three markers specific highly expressed in cluster 1, Paxillin (PXN), Coagulation Factor II Thrombin Receptor (F2R) and Juxtaposed with another zinc finger protein 1 (JAZF1) (Fig. 4g). The differentially expressed genes in cluster 1 were involved ubiquitin-mediated proteolysis, cell cycle, Notch signaling pathway, T cell receptor signaling pathway, Th1 and Th2 cell differentiation and T17 cell differentiation (Fig. 4h). At last, we constructed a violin plot to depict the most significantly altered genes in Ubiquitin-mediated proteolysis, cell cycle and natural killer cell-mediated cytotoxicity before and after treatment with Pembrolizumab (Fig. 4i). In Ubiquitin-mediated proteolysis pathway, UBE2D3 expression was remarkably decreased and UBE2G2 and RBX1 expression was obviously increased after treatment. In cell cycle pathway, loss of CCND3 and TGFB1 expression and high RBX1 expression were found after treatment. As for natural killer cell-mediated cytotoxicity, ZAP70 and PIK3CD expression was distinctly decreased and RAC1 and SH2D1A expression was significantly elevated after treatment. In this part, we identified four NKG7+ NK cell clusters and uncovered their markers, percentage and pathway changes after Pembrolizumab treatment.
NKG7+ T (NKT) cells characteristics after Pembrolizumab treatment
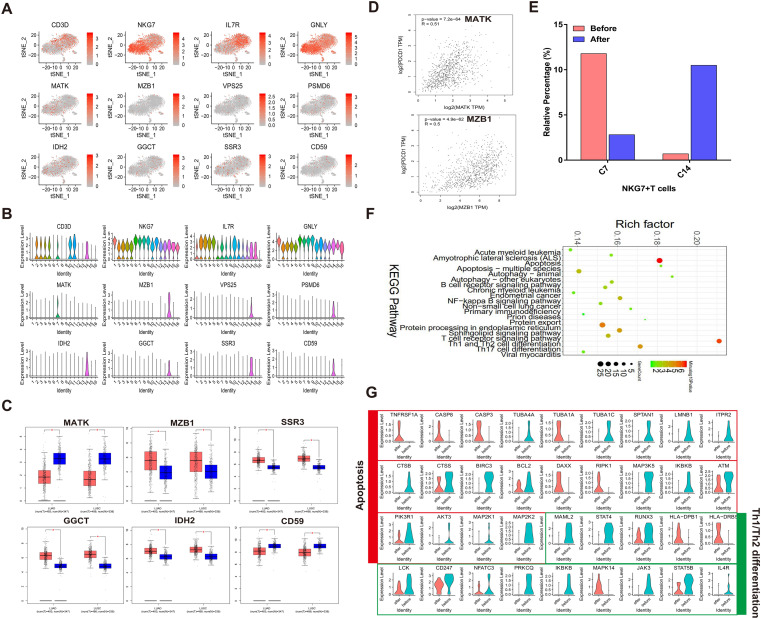
As clusters 7 and clusters 14 both express CD3D and NKG7, we characterized clusters 7 and 14 as NKG7+ T (NKT cells) cells (cluster 7, 14). T-SNE and violin plot of 12 markers like CD3D, NKG7, IL7R, GNLY, MATK, MZB, VSP25, PSMD6, IDH2, GGCT, SSR3 and CD59 were shown in Fig. 5a, b. After validating their expression in lung cancer samples by TCGA database, MATK and CD59 were significantly lowly expressed, while MZB1, SSR3, GGCT and IDH2 were all highly expressed both in LUAD and in LUSC tissues compared to normal tissues (Fig. 5c). Pearson correlation analysis results showed that MATK and MZB1 were positively correlated with PD-1 (MATK: p-value = 7.2e−64, R = 0.51; MZB1: p-value = 4.9e−62, R = 0.5; Fig. 5d). However, no genes showed significant prognostic significance (data not shown). After treatment with Pembrolizumab, the relative percentage of cluster 7 was remarkably decreased, while the relative percentage of cluster 14 was distinctly increased (Fig. 5e). We further analyzed pathways enriched by differentially expressed genes in both clusters. As only 10 genes showed significant differential expression in cluster 7 (data not shown), we focused on the gene expression and pathway alterations in cluster 14. As depicted in Fig. 5f, 988 differentially expressed genes in cluster 14 were significantly associated with apoptosis, autophagy, B cell receptor signaling pathway, NF-κB signaling pathway, T cell receptor signaling pathway, Th1 and Th2 cell differentiation and Th17 cell differentiation. Violin diagrams showed the most significant altered markers in apoptosis and Th1 and Th2 cell differentiation before and after treatment with Pembrolizumab (Fig. 5g). In the apoptosis pathway, TNFRSF1A, CASP8, CASP3, TUBA1A, DAXX and RIPK1 were increased after treatment, while TUBA4A, TUBA1C, SPTAN1, LMNB1, ITPR2, CTSB, BIRC3, MAP3K5, IKBKB, AKT3, MAP2K1 and MAP2K2 was decreased after treatment. For Th1 and Th2 cell differentiation pathway, we found that HLA-DRB5 and MAPK14 expression remarkably increased, while MAML2, STAT4, PRKCQ, IKBKB, JAK3 and IL-4R expression obviously decreased after treatment with Pembrolizumab. In this part, we identified two NKG7+ T cells (NKT) clusters and uncovered their markers, percentage and pathway changes after Pembrolizumab treatment.
Fig. 5.
NKG7+ T (NKT) cells characteristics after Pembrolizumab treatment. a, b The expression of 12 cell markers of NKG7+ T cells (cluster 7, 14). c Validation of cell markers of NKG7+ T cells using TCGA database. LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma. *p < 0.05. d Pearson correlation analysis of cell markers and PD-1 using TCGA database. e The relative percentage of clusters 7, 14 in before and after Pembrolizumab treatment samples. f KEGG pathway enrichment analysis of differentially expressed genes in cluster 14. g The changes in expression of genes in apoptosis and Th1 and Th2 cell differentiation pathways in before and after Pembrolizumab treatment samples
Naïve T cell clusters, markers and pathways changes after Pembrolizumab treatment
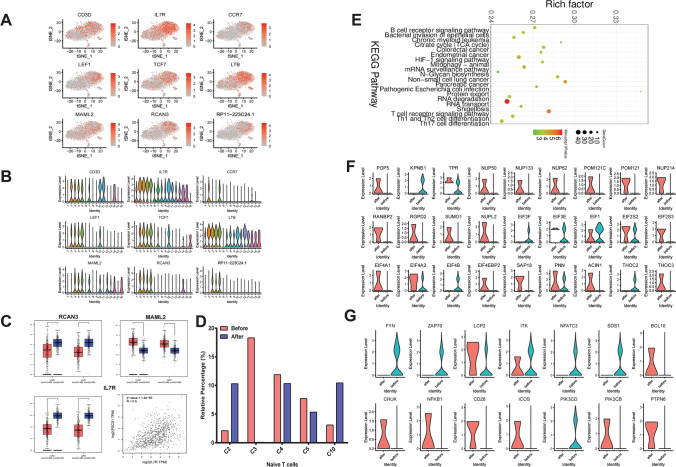
As clusters 2, 3, 4, 5 and 10 all highly express CD3D, IL-7R and naïve T cell maker LEF1, all five clusters were identified as LEF1+ Naïve T cells. Interestingly, only clusters 2 and 5 highly express another naïve T cells marker CCR7. A total of nine makers were shown in t-SNE and violin plots (Fig. 6a, b). Among them, RCAN3 and IL-7R were significantly lowly, and MAML2 was significantly highly expressed in both LUAD and LUSC tissues compared to normal tissues (Fig. 6c) after validation using the TCGA database. Correlation analysis results suggested that IL-7R was positively correlated with PD-1 (p-value = 1.4e−63, R = 0.5; Fig. 6c). However, no genes showed significant prognostic significance (data not shown). After treatment with Pembrolizumab, we found that cluster 3 had the most remarkable changes (Fig. 6d), so we focused on cluster 3 in the following analysis. Pathways of differentially expressed genes in cluster 3 were mainly enriched in B cell receptor signaling pathway, HIF-1 signaling pathway, mRNA surveillance pathway, protein transport, RNA degradation, RNA transport, T cell receptor signaling pathway, Th1 and Th2 cell differentiation and Th17 cell differentiation (Fig. 6e). Figure 6f showed the most significantly altered genes in RNA transport after Pembrolizumab treatment, such as POP5, KPNB1, NUP50 and NUP133. Furthermore, we also depicted the most significantly altered genes in T cell receptor pathway, as shown in Fig. 6g.
Fig. 6.
Naïve T cell clusters, markers and pathways changes after Pembrolizumab treatment. a, b T-SNE and violin plot of 9 cell markers of Naïve T cells (cluster 2, 3, 4, 5, 10). c Differential expression analysis and Pearson correlation analysis of cell markers of Naïve T cells using TCGA database. LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma. *p < 0.05. d The relative percentage of cluster 2, 3, 4, 5, 10 in before and after Pembrolizumab treatment samples. e KEGG enrichment analysis of differentially expressed genes in cluster 3. f, g The changes in expression of genes in RNA transport and T cell receptor pathways, in before and after Pembrolizumab treatment samples
CD8+ T cell characteristics before and after with Pembrolizumab
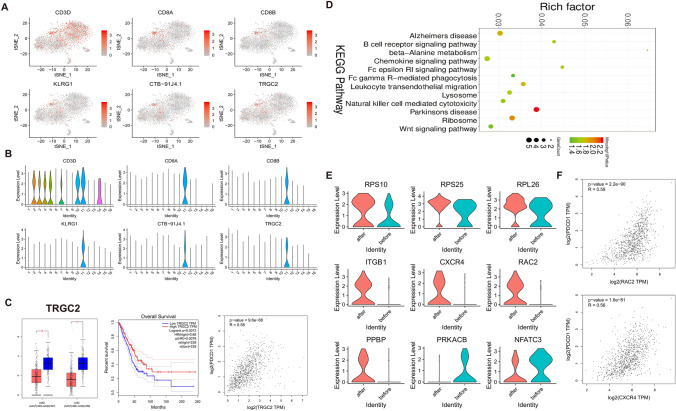
All six cells markers of CD8+ T cells (cluster 11) are shown in Fig. 7a, b. We found that CD8A, CD8B, KLRG1, CTB-91J4.1 and TRGC2 were only expressed in cluster 11. After validation using the TCGA database, TRGC2 was significantly lowly expressed in both LUAD and LUSC tissues compared to normal tissues (Fig. 7c). Furthermore, its low expression indicated a poor prognosis of patients (HR = 0.66, p-value = 0.0072; Fig. 7c). Pearson correlation analysis results suggested that TRGC2 was positively correlated with PD-1 (p-value = 9.6e−88, R = 0.58; Fig. 7c). KEGG enrichment analysis of differentially expressed genes in cluster 11 was performed. We found that cluster 11 was significantly associated with B cell receptor signaling pathway, ribosome, chemokine signaling pathway, lysosome, natural killer cell-mediated cytotoxicity and Wnt signaling pathway (Fig. 7d). Violin diagrams showed the most significantly altered markers in ribosome, chemokine signaling pathway, lysosome and Wnt signaling pathways before and after with Pembrolizumab (Fig. 7e). Among them, we found that RAC2, CXCR4, ITGB1 and PPBP expression was elevated after Pembrolizumab treatment. Pearson correlation analysis results suggested that RAC2 was positively correlated with PD-1 (p-value = 2.2e−90, R = 0.59; Fig. 7f). Moreover, there was positive correlation between CXCR4 and PD-1 (p-value = 1.6e−81, R = 0.56; Fig. 7f).
Fig. 7.
CD8+ T cell characteristics before and after with Pembrolizumab. a, b T-SNE and violin plot of six cell markers of CD8+ T cells (cluster 11). c Differential expression analysis, Pearson correlation analysis and overall survival analysis of cell marker TRGC2 of CD8+ T cells using TCGA database. LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma. *p < 0.05. d KEGG pathway enrichment analysis of differentially expressed genes in cluster 11. e The expression changes in genes involved in ribosome, chemokine signaling pathway, lysosome and Wnt signaling pathways, in before and after Pembrolizumab treatment samples. f Pearson correlation analysis between RAC2 or CXCR4 and PD-1, using TCGA database. LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma. *p < 0.05
Discussion
Pembrolizumab has been recommended as the first line for metastatic NSCLC patients with high PD-L1 expression [tumor proportion score (TPS) ≥ 1%]. However, there are still some PD-L1-negative patients who can benefit from treatment with PD-1 inhibitors including Pembrolizumab, and the underlying mechanism remains unclear. In this study, we performed single-cell RNA sequencing to uncover the immune cells and molecular changes in PBMCs from a PD-L1-negative lung squamous cell carcinoma patient benefiting from Pembrolizumab treatment. Our results, for the first time, revealed the blood immune cells heterogeneity, PD-1 blockage-response genes and pathways for PD-L1-negative patients benefiting from Pembrolizumab treatment.
In this study, a total of 16 clusters of 1850 cells (before treatment) and 1573 cells (after treatment) were identified based on canonical markers expression, which could be obviously distinguished difference between “before” and “after” Pembrolizumab treatment, indicating that Pembrolizumab treatment might significantly change the percentage of these clusters. Thereafter, 16 clusters were classified into NKG7+ NK cells, NKG7+ T (NKT) cells, Naïve T cells, CDC1C+ dendritic cells, CD8+ T cells, B cells, macrophages, erythrocytes, etc. There was a remarkable decrease in NKG7+ NK and NKG7+ T (NKT) cells while an increase in Naïve T cells and CD8+ T cells. A previous study has found that anti-PD-L1 antibody can inhibit PD-L1-negative tumor growth in vivo by activating PD-L1+ NK cells [24]. A recent clinical trial reported that Pembrolizumab plus NK cells can significantly prolong the survival time of advanced PD-L1+ NSCLC patients [25]. Another study found that NSCLC patients benefiting from PD-1 inhibitors showed an increase in the number of CD8+ T cells [26]. The above results indicated that the changes in the percentage of NK cells and T cells could be associated with effective Pembrolizumab treatment.
In this study, we collected whole blood samples and conducted PBMC isolation via the Ficoll density gradient centrifugation method, according to previous studies. The isolated PBMCs were then applied for single cells sequencing. We observed no red blood cells and hemolysis in blood sample collection, and considering the resolution of single-cell sequencing, we identified (according to the expression of marker HBD) only a small proportion of red blood cells (0.43% and 0.29%), as shown in Fig. 2c. In the following analysis, due to the small sample size and examination variance, instead of low proportion of cell types like dendritic cells and red blood cells, we focused on and draw conclusions from cell types with the highest proportion, such as NKT and NK cells, Naïve T cells and CD8+ T cells.
We identified six NK cell markers (NKG7, FCGR3A, KLRB1, GNLY, GZMB and CTSW). As expected, genes in NK cells were significantly enriched lung squamous cell carcinoma-related immune pathways such as Th1 and Th2 cell differentiation [27], T cell receptor signaling pathway [28] and natural killer cell-mediated cytotoxicity [29]. After validation, using TCGA database, ID2 and PIK3CD were significantly lowly expressed both in lung adenocarcinoma and lung squamous cell carcinoma tissues. Both of them were positively correlated with PD-1. Furthermore, their low expression indicated poor overall survival time. It has been reported that ID2 is significantly upregulated in lung squamous cell carcinoma [30, 31]. Its high expression could stimulate the proliferation of lung squamous carcinoma cells through the NF-κB/cyclin D1 pathway in vitro. High UQCR10 expression was found both in lung adenocarcinoma and lung squamous cell carcinoma tissues, which was negatively correlated with PD-1. Patients with low UQCR10 expression had poor overall survival time. Thus, the above three biomarkers could be associated with the benefit of Pembrolizumab treatment. Among cluster 1, 6, 8, 9, cluster 1 had the most significant change in NK cells. Differentially expressed genes in cluster 1 were significantly enriched in lung squamous cell carcinoma-related immune pathways like antigen processing and presentation [32], cell cycle [33], Notch signaling pathway [34], T cell receptor signaling pathway [35], Th1 and Th2 cell differentiation [27] and ubiquitin-mediated proteolysis [36].
Among NKG7+ T cell (cluster 7, 14) markers, MATK and CD59 were significantly lowly expressed and MZB1, SSR3, GGCT and IDH2 were all highly expressed both in lung adenocarcinoma and lung squamous cell carcinoma tissues. We found that there was a positive correlation between MZB1 and PD-1. MZB1 has been reported as a potential prognostic marker for NSCLC [37]. Differentially expressed genes in cluster 14 were significantly associated with apoptosis, autophagy, B cell receptor signaling pathway, NFκB signaling pathway, T cell receptor signaling pathway, Th1 and Th2 cell differentiation and Th17 cell differentiation. In Th1 and Th2 cell differentiation pathway, HLA-DRB5 and MAPK14 expression was remarkably increased and MAML2, STAT4, PRKCQ, IKBKB, JAK3 and IL-4R expression was obviously decreased after treatment with Pembrolizumab. Silencing STAT4 has been found to induce lymphatic metastasis of squamous cell carcinoma by mediating immune suppression [38].
Among Naïve T cell markers, lowly expressed RCAN3 and IL-7R and highly expressed MAML2 were validated both in lung adenocarcinoma and lung squamous cell carcinoma tissues. Furthermore, IL-7R was positively correlated with PD-1. As a previous study, reducing the number of PD-1+ dendritic cells in the tumor enhances the expression of IL-7R on CD8+ T cells [39]. Mechanically, IL-7R activates PI3K/Akt/mTOR pathway in lung cancer via inhibiting Beclin-1 [40]. Differentially expressed genes in cluster 3 were mainly enriched in RNA transport and Th1 and Th2 cell differentiation. It has been confirmed that PD-L1 is differentially mediated by Th1 and Th2 cells [41].
In this study, we found that among CD8+ T cell markers, TRGC2 was significantly lowly expressed both in lung adenocarcinoma and lung squamous cell carcinoma tissues and its low expression indicated a poor prognosis of patients. In addition, TRGC2 was positively correlated with PD-1. However, the function of TRGC2 in lung squamous cell carcinoma remains unclear, which deserves more in-depth research. Our KEGG enrichment analysis results showed that differentially expressed genes in cluster 11 were significantly associated with ribosome, chemokine signaling pathway, lysosome and Wnt signaling pathways before and after with Pembrolizumab. In the above pathways, RAC2, CXCR4, ITGB1 and PPBP expression was elevated after treatment with Pembrolizumab. Among them, RAC2 and CXCR4 were both positively correlated with PD-1. It was found that targeting CXCR4 could enhance the efficacy of anti-PD-1 [42].
Meanwhile, there are some limits in this study. The patient is 80 years old, and ECOG PS = 2, so the platinum-doublet chemotherapy (PT-DC) was not applied. Though PD-L1 shows negative expression, the patient indeed benefited from Pembrolizumab treatment. In two years, we observed only one case and collected blood samples from the case. It is important to note that cell changes relating to the before/after changes are small, and without statistical data from more patients, we could not conclude that the cell changes are of biological significance. Moreover, if samples from more patients could be collected in the future, we will continue to examine and validate the results in this study. Second, we collected blood samples before and after Pembrolizumab treatment and focused on revealing the cellular and molecular changes after Pembrolizumab treatment. In the future, by collecting more samples, a comparative study of investigating the Pembrolizumab response difference in PD-L1-negative patients may provide markers to predict the response for PD-L1-negative patients. At last, the conclusion was based on clinical data, the role of immune cell and genes in PD-L1-negative and Pembrolizumab-responsive lung cancer patients will be studied in vivo and in vitro in the future.
In sum, our studies depicted the heterogeneity of NKG7+ NK cells, NKG7+ T (NKT) cells, Naïve T cells and CD8+ T cells before and after treatment with Pembrolizumab in PD-L1-negative lung squamous cell carcinoma. In gene level, ID2, PIK3CD, UQCR10, MATK, MZB1, IL7R and TRGC2 showed a significant correlation with PD-1 expression after TCGA dataset validation, which could possess potential as predictive markers for patients with PD-L1-negative lung squamous cell carcinoma who can benefit from Pembrolizumab.
Conclusion
Our findings firstly revealed the heterogeneity of immune cells in patients with PD-L1-negative lung squamous cell carcinoma benefiting from Pembrolizumab before and after treatment. Several potential biomarkers of NKG7+ NK cells, NKG7+ T (NKT) cells, Naïve T cells and CD8+ T cells were identified. In this study, ID2, PIK3CD, UQCR10, MATK, MZB1, IL7R and TRGC2 showed a significant correlation with PD-1 expression after TCGA dataset validation. Their differential expression both in TCGA lung cancer samples and PD-L1 antibody treatment “after” group were believed to be used to predict the benefit from Pembrolizumab treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Information1 (XLSX 527 kb)
Abbreviations
- FDA
Food and Drug Administration
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- NK
Natural killer
- NSCLC
Non-small cell lung cancer
- PBMCs
Peripheral blood mononuclear cells
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death 1 ligand 1
- TCGA
The Cancer Genome Atlas
- t-SNE
t-distributed Stochastic Neighbor Embedding
Author contributions
Runbo Zhong (RZ) and Yunbin Zhang (YZ) conducted the single-cell sequencing and bioinformatics analysis. Dongfang Chen (DC) collected the samples and Shuhui Cao (SC) collected the clinical data. Baohui Han (BH) and Hua Zhong (HZ) designed the study and revised the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shanghai (19ZR1449700) and Shanghai Chest Hospital-Collaborative Innovation Project (YJXT20190204).
Availability of data and materials
All relevant data can be acquired by contacting the correspondent author.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The ethics committee of Shanghai Chest Hospital approved our study, and the patient provided written inform consent.
Consent for publication
Informed consent for publication of the manuscript was obtained from all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Runbo Zhong and Yunbin Zhang are equal contribution.
Contributor Information
Baohui Han, Email: 18930858216@163.com.
Hua Zhong, Email: eddiedong8@hotmail.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69(2019):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PA, Jr, Kim ES, Langer CJ, Natale RB, Novello S, Paz-Ares L, Perol M, Reck M, Ramalingam SS, Reynolds CH, Spigel DR, Wakelee H, Thatcher N. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13:165–183. doi: 10.1016/j.jtho.2017.11.111. [DOI] [PubMed] [Google Scholar]
- 3.Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–38. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, Juergens RA, Laurie SA, Nathan FE, Shen Y, Harbison CT, Hellmann MD. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ninomiya K, Hotta K. Pembrolizumab for the first-line treatment of non-small cell lung cancer. Expert Opin Biol Ther. 2018;18:1015–1021. doi: 10.1080/14712598.2018.1522300. [DOI] [PubMed] [Google Scholar]
- 6.Chretien S, Zerdes I, Bergh J, Matikas A, Foukakis T (2019) Beyond PD-1/PD-L1 inhibition: what the future holds for breast cancer immunotherapy. Cancers (Basel) 11 [DOI] [PMC free article] [PubMed]
- 7.Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824–16837. doi: 10.1002/jcp.28358. [DOI] [PubMed] [Google Scholar]
- 8.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2:1217–1222. doi: 10.1001/jamaoncol.2016.0639. [DOI] [PubMed] [Google Scholar]
- 10.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Chmielowski B, Pellissier J, Xu R, Stevinson K, Liu FX. Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naive patients with advanced melanoma in the United States. J Manag Care Spec Pharm. 2017;23:184–194. doi: 10.18553/jmcp.2017.23.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, Choi K, Fromme RM, Dao P, McKenney PT, Wasti RC, Kadaveru K, Mazutis L, Rudensky AY, Pe'er D. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–1308.e1236. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar MP, Du J, Lagoudas G, Jiao Y, Sawyer A, Drummond DC, Lauffenburger DA, Raue A. Analysis of single-cell rna-seq identifies cell-cell communication associated with tumor characteristics. Cell Rep. 2018;25:1458–1468.e1454. doi: 10.1016/j.celrep.2018.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jane-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard A, McCoach CE, Rotow JK, Harris L, Haderk F, Kerr DL, Yu EA, Schenk EL, Tan W, Zee A, Tan M, Gui P, Lea T, Wu W, Urisman A, Jones K, Sit R, Kolli PK, Seeley E, Gesthalter Y, Le DD, Yamauchi KA, Naeger DM, Bandyopadhyay S, Shah K, Cech L, Thomas NJ, Gupta A, Gonzalez M, Do H, Tan L, Bacaltos B, Gomez-Sjoberg R, Gubens M, Jahan T, Kratz JR, Jablons D, Neff N, Doebele RC, Weissman J, Blakely CM, Darmanis S, Bivona TG. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell. 2020;182:1232–12511e222. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuss IJ, Kanof ME, Smith PD, Zola H (2009) Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. Chapter 7 Unit7 1 [DOI] [PubMed]
- 20.McDavid A, Finak G, Chattopadyay PK, Dominguez M, Lamoreaux L, Ma SS, Roederer M, Gottardo R. Data exploration, quality control and testing in single-cell qPCR-based gene expression experiments. Bioinformatics. 2013;29:461–467. doi: 10.1093/bioinformatics/bts714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, 3rd, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive integration of single-cell data. Cell. 2019;177(1888–1902):e1821. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Z, Li C, Kang B, Gao G, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, Zhang J, Benson DM, He K, Caligiuri MA, Yu J. The mechanism of Anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9:1422–1437. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.M. Lin, H. Luo, S. Liang, J. Chen, A. Liu, L. Niu, Y. Jiang, Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest (2020). [DOI] [PMC free article] [PubMed]
- 26.Kim H, Kwon HJ, Han YB, Park SY, Kim ES, Kim SH, Kim YJ, Lee JS, Chung JH. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol. 2019;32:367–375. doi: 10.1038/s41379-018-0142-3. [DOI] [PubMed] [Google Scholar]
- 27.Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS. Characterization of M1/M2 tumour-associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron. 2016;9:1–11. doi: 10.1007/s12307-015-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XC, Wang J, Shao GG, Wang Q, Qu X, Wang B, Moy C, Fan Y, Albertyn Z, Huang X, Zhang J, Qiu Y, Platero S, Lorenzi MV, Zudaire E, Yang J, Cheng Y, Xu L, Wu YL. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun. 2019;10:1772. doi: 10.1038/s41467-019-09762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson BW, Morstyn G. Natural killer (NK)-resistant human lung cancer cells are lysed by recombinant interleukin-2-activated NK cells. Cell Immunol. 1987;106:215–222. doi: 10.1016/0008-8749(87)90165-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Chen Q, Hamajima Y, Sun W, Zheng YQ, Hu XH, Ondrey FG, Lin JZ. Id2 regulates the proliferation of squamous cell carcinoma in vitro via the NF-kappaB/Cyclin D1 pathway. Chin J Cancer. 2012;31:430–439. doi: 10.5732/cjc.011.10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Li M, Ji H, Fang Z. Landscape of transcriptional deregulation in lung cancer. BMC Genom. 2018;19:435. doi: 10.1186/s12864-018-4828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman EJ, Mitchell DC, Garner AL. The RNA-binding protein SART3 promotes miR-34a biogenesis and G1 cell cycle arrest in lung cancer cells. J Biol Chem. 2019;294:17188–17196. doi: 10.1074/jbc.AC119.010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, Felip E, Zeron-Medina J, Garrido P, Brosseau S, Zalcman G, Mazieres J, Caramela C, Lahmar J, Adam J, Chaput N, Soria JC, Besse B. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sellers K, Allen TD, Bousamra M, 2nd, Tan J, Mendez-Lucas A, Lin W, Bah N, Chernyavskaya Y, MacRae JI, Higashi RM, Lane AN, Fan TW, Yuneva MO. Metabolic reprogramming and Notch activity distinguish between non-small cell lung cancer subtypes. Br J Cancer. 2019;121:51–64. doi: 10.1038/s41416-019-0464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, Woo E, Friedmann PS, King EV, Thomas GJ, Sanchez-Elsner T, Vijayanand P, Ottensmeier CH. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osoegawa A, Yoshino I, Tanaka S, Sugio K, Kameyama T, Yamaguchi M, Maehara Y. Regulation of p27 by S-phase kinase-associated protein 2 is associated with aggressiveness in non-small-cell lung cancer. J Clin Oncol. 2004;22:4165–4173. doi: 10.1200/JCO.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 37.Niemira M, Collin F, Szalkowska A, Bielska A, Chwialkowska K, Reszec J, Niklinski J, Kwasniewski M, Kretowski A (2019) Molecular signature of subtypes of non-small-cell lung cancer by large-scale transcriptional profiling: identification of key modules and genes by weighted gene co-expression network analysis (WGCNA). Cancers (Basel) 12 [DOI] [PMC free article] [PubMed]
- 38.Anderson K, Ryan N, Volpedo G, Varikuti S, Satoskar AR, Oghumu S. Immune suppression mediated by STAT4 deficiency promotes lymphatic metastasis in HNSCC. Front Immunol. 2019;10:3095. doi: 10.3389/fimmu.2019.03095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jian M, Yunjia Z, Zhiying D, Yanduo J, Guocheng J. Interleukin 7 receptor activates PI3K/Akt/mTOR signaling pathway via downregulation of Beclin-1 in lung cancer. Mol Carcinog. 2019;58:358–365. doi: 10.1002/mc.22933. [DOI] [PubMed] [Google Scholar]
- 41.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Alterio C, Buoncervello M, Ierano C, Napolitano M, Portella L, Rea G, Barbieri A, Luciano A, Scognamiglio G, Tatangelo F, Anniciello AM, Monaco M, Cavalcanti E, Maiolino P, Romagnoli G, Arra C, Botti G, Gabriele L, Scala S. Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J Exp Clin Cancer Res. 2019;38:432. doi: 10.1186/s13046-019-1420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information1 (XLSX 527 kb)
Data Availability Statement
All relevant data can be acquired by contacting the correspondent author.