Abstract
Introduction
Epstein-Barr virus (EBV) is associated with nasopharyngeal carcinoma (NPC), and provides a target for a dendritic cell (DC) vaccine. CD137 ligand (CD137L) expressed on antigen presenting cells, costimulates CD137-expressing T cells, and reverse CD137L signaling differentiates monocytes to CD137L-DC, a type of DC, which is more potent than classical DC in stimulating T cells.
Methods
In this phase I study, patients with locally recurrent or metastatic NPC were administered CD137L-DC pulsed with EBV antigens (CD137L-DC-EBV-VAX).
Results
Of the 12 patients treated, 9 received full 7 vaccine doses with a mean administered cell count of 23.9 × 106 per dose. Treatment was well tolerated with only 4 cases of grade 1 related adverse events. A partial response was obtained in 1 patient, and 4 patients are still benefitting from a progression free survival (PFS) of currently 2–3 years. The mean pre-treatment neutrophil: lymphocyte ratio was 3.4 and a value of less than 3 was associated with prolonged median PFS. Progressors were characterized by a high frequency of naïve T cells but a low frequency of CD8+ effector T cells while patients with a clinical benefit (CB) had a high frequency of memory T cells. Patients with CB had lower plasma EBV DNA levels, and a reduction after vaccination.
Conclusion
CD137L-DC-EBV-VAX was well tolerated. The use of CD137L-DC-EBV-VAX is demonstrated to be safe. Consistent results were obtained from all 12 patients, indicating that CD137L-DC-EBV-VAX induces an anti-EBV and anti-NPC immune response, and warranting further studies in patients post effective chemotherapy.
Precis.
The first clinical testing of CD137L-DC, a new type of monocyte-derived DC, finds that CD137L-DC are safe, and that they can induce an immune response against Epstein-Barr virus-associated nasopharyngeal carcinoma that leads to tumor regression or prevents tumor progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03075-3.
Keywords: Nasopharyngeal carcinoma, CD137L-DC, Dendritic cell immunotherapy, EBV, Phase 1 clinical trial
Introduction
Nasopharyngeal carcinoma (NPC) is an EBV-driven malignancy with an annual incidence as high as 25–30 per 100,000, especially in ethnic groups from Southern China [1, 2]. If NPC is detected at stage I or II, treatment by chemotherapy and radiation therapy results in > 80% 5-year survival rates. However, many patients are diagnosed with stage III or IV NPC, which have only 50–60% 5-year survival rates. When NPC relapses or when patients develop metastatic disease, chemotherapy and radiation therapy are merely palliative with very low survival rates, resulting in approximately 50,000 deaths per year [3]. The median PFS of metastatic NPC is 5–7 months and overall survival (OS) is 18–24 months [4].
Immunotherapy has hugely improved cancer treatment, and is now the fourth pillar of cancer therapy. Immune checkpoint inhibitors have been approved for treating a variety of cancer types. However, PD-1 blockade therapy benefits only a minority of NPC patients, despite PD-L1 being frequently expressed on NPC cells [5]. Therefore, a new type of immunotherapy for NPC is much-needed to improve patient survival [6].
In NPC, EBV enters latency phase 2 during which the expression of most EBV proteins is silenced as that facilitates escape from immune surveillance. However, EBNA-1, LMP1 and LMP-2 are expressed even in NPC, probably because they are essential for maintaining the infection. These proteins can therefore be targeted with immunotherapy as non-self antigens that have not been tolerized by the host, and several clinical trials are indeed utilizing EBNA-1, LMP1 and LMP-2 [7, 8].
Dendritic cells (DC) are vital antigen presenting cells (APC) that possess strong cross-presentation abilities, and are regarded as central orchestrators in bridging innate and adaptive immunity. Coupled with their importance in immunosurveillance and in establishing immunological memory, their potency in inducing anti-tumor immune responses by activating antigen-specific cytotoxic T cells has led to the generation of DC-based tumor vaccines [9, 10]. As DC account for less than 1% of PBMC, various approaches have been explored for the generation of DC vaccines. The most common approach is the ex vivo expansion of monocyte-derived DC. Peripheral blood monocytes are isolated from an apheresis product, and cultured for several days in the presence of GM-CSF and IL-4 for the differentiation to DC. During this manufacturing process, DC are stimulated with a maturation cocktail, and pulsed with relevant tumor associated antigens or tumor lysates to enable the DC to induce tumor-specific immune responses [11].
To date, DC-based immunotherapies directed against NPC-associated EBV antigens are scarce. While the tolerability and safety profile of DC vaccinations are deemed positive, their correlations with immunological and clinical responses remain uncorroborated. In a phase I study, 16 patients with advanced NPC received autologous DC pulsed with HLA-restricted LMP2a epitope peptides [12]. Epitope specific T cell responses were observed in 9 patients, which were sustained for 3 months post vaccination. Partial clinical responses with signs of tumor regression were found in 2 patients. A similar clinical response rate was observed in a phase 2 study [13]. Sixteen metastatic NPC patients were administered autologous DC that were transduced with an adenovirus encoding a truncated LMP1 and full length LMP2. While no LMP1- and LMP2-specific T cell responses were observed, 3 patients showed clinical benefit (CB), (1 patient with partial response (PR) and 2 patients with stable disease (SD)). Lower EBV-DNA levels were observed in 9 out of 16 NPC patients receiving HLA-A2 restricted LMP2A pulsed DC vaccine in a study by [14]. Patients with lower EBV-DNA levels were correspondingly found to have positive skin delayed type hypersensitivity responses against HLA-A2-restricted LMP2A peptides.
CD137 (TNFRSF9, 4-1BB) is a member of the tumor necrosis factor (TNF) receptor family [15, 16]. High levels of CD137 are expressed on activated T cells, and crosslinking of CD137 delivers potent costimulatory signals to T cells [17]. CD137 agonists enhance T cell activity and immune responses leading to the elimination of even established tumors in mice [18–20], and are currently being tested in clinical trials for tumor immunotherapy [21]. The potency of the CD137 costimulatory signal became further evident by the incorporation of the cytoplasmic CD137 signaling domain in the second and third-generation chimeric antigen receptors (CAR), which has greatly enhanced the persistence of CAR T cells and anti-tumor efficacy [22, 23].
CD137 ligand (CD137L) is expressed on APC, and costimulates CD137-expressing, activated T cells. The CD137 receptor / ligand system is capable of bidirectional signaling. CD137L, just as CD137, is expressed as a transmembrane protein on the cell surface and can transmit an activating signal into APC, a process referred to as reverse signaling [24]. For APC the CD137L signal is activating. Reverse signaling by CD137L enhances proliferation and immunoglobulin secretion by B cells [25]. In hematopoietic progenitor cells CD137L signaling induces proliferation, colony formation, myeloid differentiation, in particular to monocytes and macrophages [26–28]. Peripheral human monocytes are activated by CD137L signaling, evidenced by stronger adhesion, heightened secretion of proinflammatory cytokines [29], increased survival [30], proliferation (Langstein et al., 1999) and enhanced migration [31, 32]. In human monocytes, CD137L mediates activation by combining with CD32a [33] and signaling through protein tyrosine kinases, p38 MAPK, ERK1,2, MEK, PI3-K) and PKA [34].
In addition, CD137L signaling can induce maturation of immature monocyte-derived DC leading to an enhanced expression of costimulatory molecules and of IL-12p70, and an enhanced capacity of the DC to stimulate T cell proliferation, IFN-γ secretion and in vivo migration toward a CCL19 gradient [35].
Furthermore, recombinant CD137 protein which induces CD137L signaling is sufficient as a sole factor to induce differentiation of human monocytes to DC, and these resulting CD137L-DC differ considerably from in vitro generated classical DC (cDC, generated from monocytes cultured with GM-CSF + IL-4) in cell surface marker expression and cytokine secretion. For example, CD137L-DC have a mature phenotype without addition of external maturation factors, and express lower levels of immunoinhibitory molecules PD-L1 and IL-10. Compared to cDC, CD137L-DC exhibit enhanced potency in stimulating antigen-specific cytotoxic T cells against cytomegalovirus, Hepatitis B virus and EBV proteins [36–38].
A gene expression analysis showed that CD137L-DC are highly similar to inflammatory DC isolated from sites of inflammation [39]. This suggests that CD137L signaling is a physiological inducer for the in vivo generation of inflammatory monocyte-derived DC [40]. The higher costimulatory activity of CD137L-DCs is mediated by a higher Akt-mTORC1 activity and an increased Akt-driven glycolysis [41].
Based on these promising findings we aimed to translate the high potency of CD137L-DC to the benefit of NPC patients. We designed our trial based on the positive learning points of the many DC-based immunotherapy trials that have been conducted earlier (10).
As migration of DC to lymph nodes is pivotal for an immunotherapeutic effect [42, 43], we added prostaglandin E2 (PGE2) to the maturation cocktail, despite PGE2 slightly reducing overall DC activation [38]. To further enhance CD137L-DC migration, we preconditioned injection site with tetanus and diphtheria toxoid [44]. In addition, we used intradermal injection as this facilitates migration of CD137L-DC to regional lymph nodes [45].
We find that CD137L-DC are safe. After pulsing with EBV antigens, they can induce an immune response against EBV-associated NPC that leads to tumor regression and prevents tumor progression, and prolongs life in subgroups of patients.
Material and methods
Patient selection
Eligible patients were at least 21 years old with metastatic EBV-encoded RNA (EBER)-positive NPC detected by in-situ hybridisation who were treated with systemic chemotherapy, and had achieved at least SD as the best clinical response. Additional inclusion criteria included Eastern Cooperative Oncology Group performance status of 0 or 1; adequate bone marrow (absolute monocyte count ≥ 0.2 × 109/l; platelet count ≥ 100 × 109/l; hemoglobin ≥ 8 g/dl), renal (creatinine < 1.5 times upper limit of normal), and hepatic functions (aspartate and alanine transaminase ≤ 2.5 times upper limit of normal or ≤ 5 times with liver metastases). Evaluable disease was not required at entry but tumor assessment at follow up was as per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Archival or fresh tissue samples were available. Patients were excluded if they had active autoimmune disease, had received chemotherapy or radiotherapy within 4 weeks of scheduled vaccination, were allergic to diphtheria tetanus toxoid, had acute serious infections, or were receiving corticosteroids of more than 10 mg/day of prednisolone or its equivalent or other immunosuppressive treatment. Written informed consent was obtained before any study-related procedures, and the study protocol was approved by the ethics committee of the institution (National Health Care Group DSRB Ref: 2016/00492), and was conducted in compliance with ethical guidelines in the Declaration of Helsinki, (Clinicaltrials.gov ID: NCT03282617).
Patient treatment
A total of 14 patients were enrolled in this trial. Patients had metastatic NPC and had been treated with systemic chemotherapy. Median lines of prior treatment for metastatic NPC was 1 (range 1–6), the most common being cisplatin and gemcitabine. All patients were of Chinese race with 5 (41.6%) and 7 (58.4%) being of male and female gender, respectively. The mean age was 58 years (Table 1).
Table 1.
Patient condition prior to CD137L-DC-EBV-Vax treatment
| Study ID | Age | Gen-der | Race | Primary cancer | Stage on study entry | No. of meta-static sites | Immediate prior treatment to DC | Prior immune checkpoint inhibitor | Prior treatments in metastasis setting | Best response prior treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| EBV001 | 45 | M | Chinese | NPC | IVB | ? | CT + Bev | Yes | 6 | SD |
| EBV002 | 59 | M | Chinese | NPC | IVB | 2 | CT | No | 1 | PR |
| EBV003 | 65 | F | Chinese | NPC | IVB | 1 | CT | No | 1 | PR |
| EBV004 | 51 | F | Chinese | NPC | IVB | 1 | CT | No | 1 | PR |
| EBV006 | 36 | F | Chinese | NPC | IVB | 1 | CT | No | 1 | CR |
| EBV007 | 73 | F | Chinese | NPC | IVB | 2 | CT | No | 1 | PR |
| EBV008 | 61 | F | Chinese | NPC | IVA | 0 | Anti-EGFR | Yes | 3 | SD |
| EBV009 | 66 | M | Chinese | NPC | IVB | 1 | CRT | No | 4 | PR |
| EBV010 | 63 | F | Chinese | NPC | IVB | 1 | CRT | No | 2 | SD |
| EBV011 | 78 | F | Chinese | NPC | IVB | 1 | CT | No | 1 | SD |
| EBV013 | 43 | M | Chinese | NPC | IVB | 2 | CT | No | 1 | PR |
| EBV014 | 54 | M | Chinese | NPC | IVB | 2 | CT | No | 1 | PR |
CT: Chemotherapy
CRT: Chemo- and Radiotherapy
CT + Bev: Chemotherapy + Bevacizumab
SD: Stable disease
PR: Partial response
CR: Complete response
A dose escalation study was done for the first patient. The first and second injections contained 5 × 106 and 10 × 106 CD137L-DC, respectively, while, the full dose of 16.7 × 106 CD137L-DC was used for all subsequent injections. After CD137L-DC were found to be safe in the dose escalation study, as many CD137L-DC as available were administered per dose for subsequent patients. Two patients did not commence treatment due to disease progression. Three patients did not receive the full 7 vaccine doses, due to trial discontinuation, disease progression and insufficient vaccine generated. Nine patients received the full 7 vaccine doses (range 2–7) with a mean administered cell count of 23.9 × 106.
Eligible patients underwent apheresis at least 3 weeks after the last chemotherapy dose was administered to isolate peripheral blood mononuclear cells (PBMC).
CD137L-DC-EBV-VAX vaccine generation
The production of ex vivo CD137 ligand-generated dendritic cells (CD137L-DC) was performed in the Cell Therapy Facility of Health Science Authorities under GMP conditions (Suppl Fig. 1). To generate CD137L-DC, autologous PBMC were plated onto 145 × 20 mm dishes (639160, Greiner Bio-One, Kremsmünster Austria) pre-coated with 5 µg/ml anti-CD137L antibody (clone 5F4, BioLegend, San Diego, CA) for 7 days. PBMC were isolated from leukapheresis products by Ficoll-Paque Premium (17-5442-02, GE Healthcare) density gradient centrifugation, with a viability of ≥ 80%. Isolated PBMC were re-suspended in RPMI-1640 medium (L0495-500, Biowest, Riverside, MO) supplemented with 10% gamma irradiated fetal bovine serum (FBS, Bovogen, East Keilor Victoria, Australia) and seeded onto anti-CD137L antibody coated dishes at a concentration of 5 × 106 cells/ml. Cells were placed at 37 °C in a humidified incubator with 5% CO2 for 7 days. Non-adherent cells were removed 24 h later, and adhered cells were replenished with media and cultured for another 6 days at 37 °C. To induce maturation, adhered cells were matured with 1 µg/ml R848 (tlrl-r848, InvivoGen, San Diego, California), 50 ng/ml IFN-γ (130-096-482, Miltenyi Biotec, Bergisch Gladbach, Germany) and 1 µg/ml PGE2 (P0409-1MG, Sigma-Aldrich, St. Louis, Missouri) for 18 h. Cells were additionally pulsed with 0.5 µg/ml Peptivator EBV LMP1 (130-095-931), 0.5 µg/ml Peptivator EBV LMP2A (130-093-616) and 0.25 µg/ml Peptivator EBV EBNA1 (130-093-614; all Miltenyi Biotec). 18 h later, EBV peptide-loaded CD137L-DC were harvested using L7hPSC harvesting solution (FP-5013, Lonza, Basel, Switzerland) or TrypLE Select Enzyme (12563, Life Technologies, Carlsbad, California). Harvested cell viability was ≥ 50%, and cells were frozen in 85% RPMI supplemented with 5% human serum albumin (CSL Behring, Australia) and 10% DMSO (Wak Chemie Medical). Cells were frozen in 5–7 aliquots of 5 × 106 – 50 × 106 cells per vial for vaccination and were stored in the vapor phase of liquid nitrogen until release for clinical use. Time requirement from apheresis to freezing of CD137L-DC was 8 days, followed by 3 weeks for quality testing.
Quality control of CD137L-DC vaccine
Generated CD137L-DC-EBV-Vax met with all release specifications, including secretion of TNF ≥ 10 pg/mL and IL-8 ≥ 1 ng/ml. Final CD137L-DC products were assessed for bacterial and fungal sterility, and the presence of mycoplasma and endotoxin. Tests were conducted by the Technischer Überwachungsverein (TÜV) SÜD, Singapore. The viability of PBMC and harvested CD137L-DC was assessed by trypan blue staining, and a minimum of 25 × 106 live cells were required to meet the release criteria.
Vaccination and assessment
Patients received 0.5 ml of tetanus and diphtheria vaccine (Decavac®) intradermally 24 h prior to administration of intradermal CD137L-DC-EBV-VAX at the same injection site. Injections were placed near the inguinal region on alternate sides, and up to 7 vaccines were administered at 2 weekly intervals. For each CD137L-DC-EBV-VAX, approximately 5–50 × 106 cells were administered. Vaccines were thawed and immediately used at the point of administration. Dose delays were allowed for a week to allow recovery from adverse events.
Patients were monitored with history and physical examination, full blood counts, chemistry (including sodium, potassium, urea, creatinine, glucose, calcium, phosphate, albumin, alkaline phosphatase, total bilirubin, lactate dehydrogenase, total protein, serum glutamic pyruvic transaminase/alanine aminotransferase (SGPT/ALT) and thyroid-stimulation hormone (TSH, with Free T4 and Free T3), amylase) on the day of each tetanus and diphtheria vaccine administration and at each follow-up after completion of vaccination. Tumor assessments were scheduled using CT, MRI or PET CT scans at baseline, week 12 (after the 7th vaccination) and every 3–6 months thereafter for follow-up till progression. Measurements of tumor responses was based on RECIST criteria 1.1. PFS was defined as the length of time from first vaccination to the first RECIST defined disease progression.
Immune response monitoring
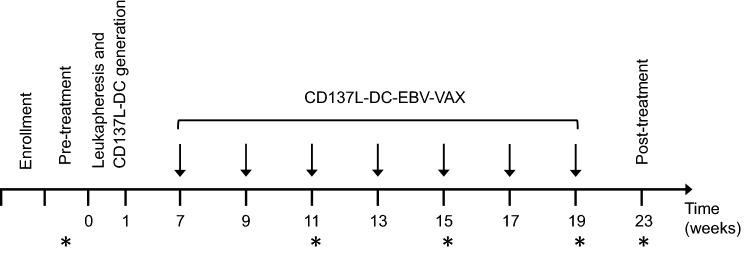
Whole blood was collected from patients at the following time points: Pre-treatment, 3rd, 5th and 7th vaccination and post-treatment (Fig. 1) for the evaluation of immunological response. Plasma and PBMC were isolated for immune monitoring by multi-color flow cytometry and plasma EBV DNA clearance.
Fig. 1.
Study design for CD137L-DC-EBV-VAX treatment and evaluation schedule.* Indicates time point for immune monitoring
Flow cytometry analysis
The average PBMC cell viability of all patient samples used for flow cytometry analysis was ≥ 10%. Patient PBMC were seeded at 105 cells per well in a 96-well V bottom plate and stained with Fixable Viability Stain 440UV (BD 566,332) for the exclusion of dead cells followed by Fc Receptor Binding Inhibitor Polyclonal Antibody, (eBioscience 14–9161-73). PBMC were incubated with fluorescently labeled monoclonal antibodies to determine cell lineage and activation status: T-cells were identified with CD278 (ICOS)-BB515 (Clone DX29, BD 564,549), CD69-BB660 (Clone FN50, BD 624,295), CD197 (CCR7)- PE/Dazzle™ 594 (Clone G043H7, BioLegend 353,236), CD27-BB700 (Clone L128, BD 746,084), CD137-PE/Cyanine7 (Clone 4B4-1, BioLegend 309,818), CD223 (LAG-3)-APC (Clone 7H2C65, BioLegend 369,212), CD25-APC-H7 (Clone M-A251, BD 560,225), CD54-BV421 (Clone HA58, BD 564,077), CD45RA-BV510 (Clone HI100, BioLegend 304,142), CD3-BV570 (Clone UCHT1, BD 624,298), CD38-BV711 (Clone HIT2, BioLegend 303,528), CD127-BV750 (Clone HIL-7R-M21, BD 747,089), HLA-DR-BUV395 (Clone G46-6, BD 565,972), CD8-BUV563 (Clone HIT8a, BD 741,384), CD4-BUV615 (Clone SK3, BD 624,297), CD95-BUV737 (Clone DX2, BD 612,790); NK cells were identified by CD56-BV605 (Clone NCAM16.2, BD 562,780), CD16-BUV805 (Clone 3G8, BD 748,850); B-cell lineage was identified with CD40-PE (Clone 5C3, BioLegend 334,308), IgM-BB630 (Clone G20-127, BD 624,294), CD27-BB700 (Clone L128, BD 746,084), CD80-BB790 (Clone L307.4, BD 624,296), CD24-BV650 (Clone ML5, BD 563,720), CD38-BV711 (Clone HIT2, BioLegend 303,528), CD86-BV785 (Clone IT2.2, BioLegend 305,442), HLA-DR-BUV395 (Clone G46-6, BD 565,972), CD19-BUV563 (Clone HIB19, BD 741,361), IgD-BUV615 (Clone IA6-2, BD 624,297); Monocytes were identified with CD14-AF700 (Clone M5E2, BioLegend 301,822), CD45RA-BV510 (Clone HI100, BioLegend 304,142), CD11c-BUV661 (Clone S-HCL-3, BD 624,285), CD16-BUV805 (Clone 3G8, BD 748,850); Dendritic cells and basophils were identified with CD123-PeCy5 (Clone 9F5, BD 551,065), CD11c-BUV661 (Clone S-HCL-3, BD 624,285), HLA-DR-BUV395 (Clone G46-6, BD 565,972), CD16-BUV805 (Clone 3G8, BD 748,850). Cells were acquired using a BD X-30 FACSymphony (San Jose, CA) with FACS Diva (Version 8.5) (BD Biosciences). Flow cytometry data was analyzed using Flowjo (Treestar) software (BD, version 10.7.1). The 27-color compensation matrix was evaluated in FlowJo by investigating N-by-N view feature as well as the pairwise expression of with all markers. Fluorescence minus one (FMO) experiments ran prior to this study aided with the optimization of the compensation matrix. Samples were concatenated and analyzed using FlowJo plugins (https://flowjo.com/exchange/), namely: Downsample (Version 3.3), UMAP (Version 3.1) and Phenograph (Version 3.0). UMAP was run using the default settings (Elucidean distance function, nearest neighbors: 15 and minimum distance 0.5). PhenoGraph was run using the default number of nearest neighbors (K = 30). Immune cell subsets identified by PhenoGraph were validated through traditional gating strategy. Graphs were made in Prism 9, v9.0.0 (GraphPad Software Inc.).
EBV DNA quantification
Plasma samples were stored at -80 °C, and levels of EBV DNA in plasma were determined based on the Epstein-Barr nuclear antigen (EBNA)-1 gene using the Clarity EBV Quantification Kit (JN Medsys, Singapore).
Results
Toxicity and adverse effects
CD137L-DC-EBV-VAX-based immunotherapy was well tolerated with only 4 cases (33.3%) of grade 1 related adverse events, most commonly observed at the injection site reaction. No G3-4 toxicities were observed. All vaccinations were safely administered with no major toxicity, and none of the patients developed any clinical or biochemical signs of autoimmune disease.
Patient response
To determine clinical outcomes, we measured best response (RECIST), clinical benefit (CB) rate and PFS. CB was defined as the sum of complete response (CR), partial response (PR) or stable disease (SD) for at least 24 weeks from treatment start. CB was seen in 5 cases (42%) with 1 PR and 4 SD of 2–3 years (Table 2). There was no CR, and 58% had progressive disease (Suppl. Figure 2).
Table 2.
Patient condition post CD137L-DC-EBV-Vax treatment
| Study ID | Measurable disease | Evaluable disease on consenting | Pre-treatment albumin | Pre-treatment cortico-steroid | Cells admini-stered × 106 | Best res-ponse | PFS (weeks) from start of treatment | OS (weeks) from start of treatment |
|---|---|---|---|---|---|---|---|---|
| EBV001 | No | Yes | 50 | No | 16.7 | PD | 3 | 10 |
| EBV002 | Yes | Yes | 40 | No | 5.44 | PD | 4 | 75 |
| EBV003 | No | Yes | 46 | Yes – EMLA | 31.13 | SD | 102* | 102* |
| EBV004 | Yes | Yes | 46 | No | 17.23 | PD | 17 | 161* |
| EBV006 | No | No | 41 | No | 50.0 | SD | 136* | 136* |
| EBV007 | Yes | Yes | 38 | Yes – EMLA | 19.19 | PD | 15 | 100 |
| EBV008 | No | Yes | 46 | Yes – EMLA | 12.05 | SD | 107* | 107* |
| EBV009 | Yes | Yes | 48 | No | 22.68 | SD | 112* | 112* |
| EBV010 | Yes | 48 | No | 13.72 | PD | 4 | 64 | |
| EBV011 | Yes | Yes | 43 | Yes – EMLA | 27.89 | PR | 32 | 32 |
| EBV013 | Yes | Yes | 46 | Yes – EMLA | 41.63 | PD | 16 | 81 |
| EBV014 | Yes | Yes | 38 | No | 29.09 | PD | 16 | 71 |
PFS: Progression free survival
EMLA: Lidocaine/prilocaine cream
*: censored
The median PFS was 16.5 weeks (range 3–136 weeks). The lowest PFS (3 weeks) was in a patient with 6 prior lines of treatment including a checkpoint inhibitor. The median OS was 90.5 weeks (range 10–161 weeks) (Table 2, Fig. 2). At present, patients with SD are still alive.
Fig. 2.
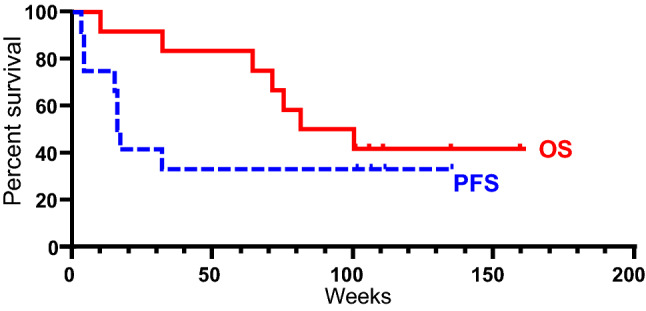
Kaplan Meir curve of patient survival. Red, solid line, represents Overall survival (OS). Blue, dashed line, represents Progression-free survival (PFS)
The mean pretreatment neutrophil: lymphocyte ratio (NLR) was 3.4, and patients with a CB were characterized by a lower pretreatment and lower pre-2nd dose NLR of approximately 2.5, whereas the NLR of patients without a CB was above 4, although this difference was statistically not significant (Table 3). Further, a NLR of less than 3 was associated with prolonged median PFS (42 vs 14 weeks, p = 0.01), (Fig. 3). The hazard ratio was 0.11 (95% CI; 0.02 – 0.65), indicating that a patient with a NLR < 3 had a 89% lower risk of progression than a patient with a N:L ≥ 3 at a given time point.
Table 3.
Mean neutrophil: lymphocyte ratio (NLR) and clinical benefit. Listed are NLS of all 12 treated patients ± standard deviation
| Mean N:L ratio | Clinical Benefit | No clinical benefit | p value |
|---|---|---|---|
| Pretreatment | 2.4 ± 0.7 | 4.1 ± 2.5 | 0.17 |
| Pre-2nd dose | 2.5 ± 1.2 | 4.5 ± 2.1 | 0.09 |
Fig. 3.
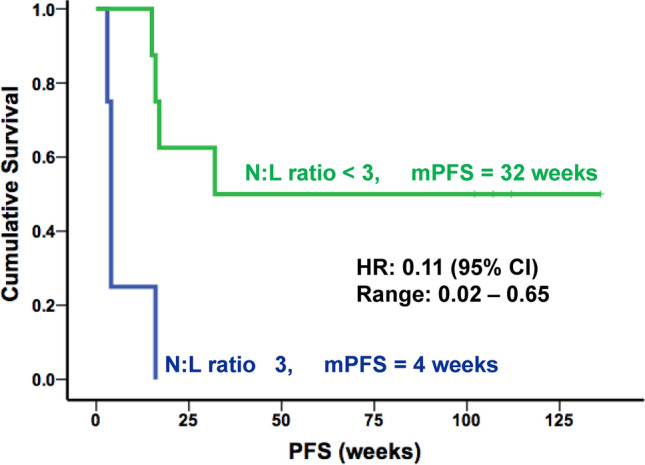
A low neutrophil: lymphocyte (N:L) ratio correlates with survival. The median PFS (mPFS) was significantly longer in patients with a pre-treatment N:L ratio < 3. HR: hazard ratio for risk of progression. CI confidence interval
A 77-year-old Chinese woman with locally advanced NPC (EBV011), involving bilateral cavernous sinuses and diffuse bony metastases at diagnosis, who had been treated with 6 cycles of systemic chemotherapy of cisplatin and gemcitabine with partial response was enrolled on the CD137L-DC-EBV-VAX, and had a successful expansion of CD137L-DC. She received 7 vaccinations with 27.89 × 106 cells per dose. Repeat PET CT scans 2 months after vaccination showed a metabolic response as well as a partial response in the left level 2 cervical lymph node (Suppl. Figure 3), indicating a response to the vaccination therapy. This was associated with a reduction in the plasma EBV DNA copy numbers post-vaccination (350 to 180 copies/ml).
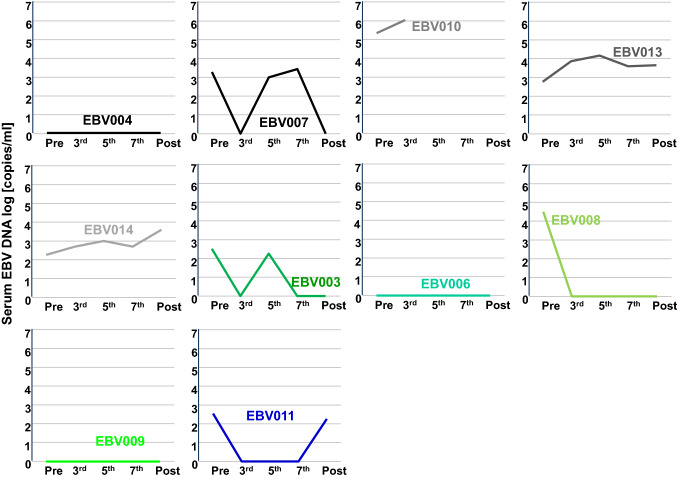
EBV DNA plasma levels
EBV DNA levels in plasma are a surrogate marker for EBV-associated cancers [46]. There were profoundly higher concentrations of EBNA-1 DNA in plasma of patients with progressing NPC than in patients who experienced a CB (Fig. 4). In patients with detectable EBNA-1 DNA levels, a general decrease in plasma levels was observed for patients with CB (responder and SD). In the progressors group, 1 out of 4 patients showed reduced EBNA-1 DNA levels after receiving all 7 vaccinations.
Fig. 4.
EBV DNA levels in patient plasma. Data from patients with progressing disease are in gray and black. Data from patients with stable disease are in green, and the responder is in blue
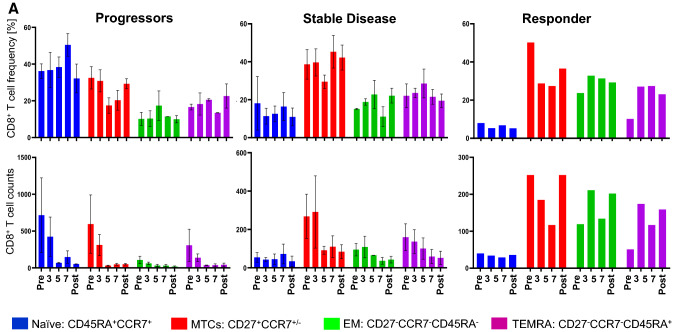
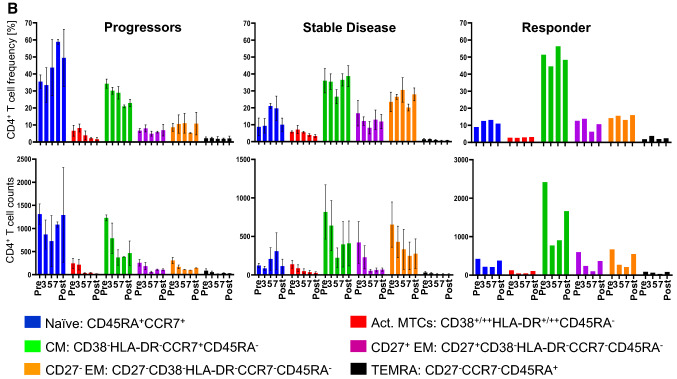
Immunological response
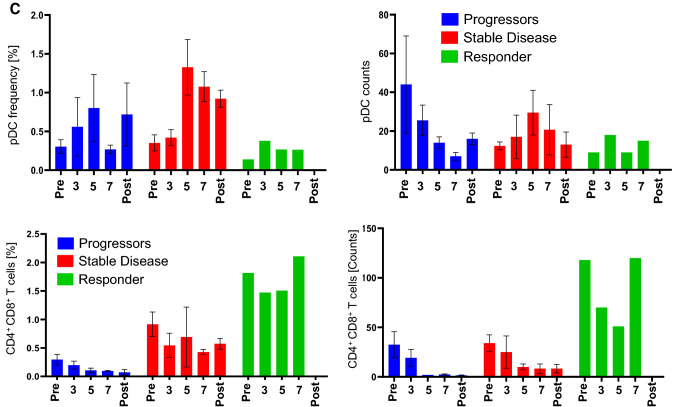
A phenograph analysis, which takes into account all markers for a cell type, revealed that the progressors were characterized by a high frequency of naïve T cells but a low frequency of CD8+ effector T cells at all time points (Fig. 5a). The situation was different for patients with a CB. Those with SD had a low frequency of naïve T cells but a high frequency of CD8+ memory T cells (Fig. 5a) and of CD4+ memory T cells (CD27− EM and Central Memory) (Fig. 5b). The patient with a PR had a high frequency of CD4+ central memory (CM) T cells and of CD4+CD8+ T cells at all time points. Furthermore, the patient showed an increase in effector CD8+ T cell effector memory (EM) and terminally differentiated effector memory (TEMRA) and plasmacytoid DC (pDC) frequencies, especially after the 3rd vaccination (Fig. 5c). There was a stark difference in the starting number (Pre-dose) of CD4/CD8 double positive T cells between progressors, patients with SD and the PR (Fig. 5d). It may be worthwhile to evaluate in future studies whether this could be used as a prognostic marker for disease progression.
Fig. 5.
Flow cytometric analysis of PBMCs from patients treated with CD137L-DC-EBV-VAX. Frequencies (upper panels) and total number (lower panels) of a CD8+ T cell subsets), b CD4+ T cell subsets, c pDCs, and of d CD4/CD8-double positive T cells. Depicted are means ± standard errors
Discussion
DC immunotherapy trials have been conducted in a number of cancers with various DC types. In this first clinical trial with CD137L-DC, the primary objective was to evaluate the safety profile of CD137L-DC-EBV-VAX. Tolerability was demonstrated with only grade 1 immune related events in 4 of the 12 patients. This good tolerability is in line with many other DC-based clinical trials [47, 48].
Although, assessing the safety profile was the primary aim of this trial, further evaluations on the efficacy of CD137L-DC-EBV-VAX were conducted. One of the 12 patients experienced a tumor shrinkage (PR) which puts this objective response rate of 8.5% in the range of other DC immunotherapy trials [49, 50]. More interestingly, in addition to the one PR, 4 patients had a prolonged PFS beyond the expected median of 5–7 months compared to chemotherapy treatment in patients with metastatic NPC. These patients’ PFS have also exceeded the expected median overall survival of about 18–24 months [4]. The 4 patients with SD continue to show no signs of relapse 2–3 years post treatment. It is noteworthy that this therapeutic effect has been achieved by CD137L-DC-EBV-VAX alone, without the concomitant administration of adjuvants that are often required for DC to induce a strong immune response [51]. Therefore, the data from this phase I trial suggest that in selected metastatic patients with low tumor burden (low EBV DNA) and a high risk of relapse, e.g. N3 or T4 disease, a CD137L-DC-EBV-VAX vaccine may induce durable disease control, especially after debulking the tumor with effective chemotherapy.
The only approved DC vaccine, Sipuleucel-T (Provenge), is utilized for treatment of prostate cancer. It did not induce any objective response but it prolonged overall survival by 4.1 months without delaying disease progression [52]. It is well known that the objective response rate for immunotherapies is generally low, but they nevertheless often prolong survival, with an increase in overall median survival for DC vaccines of at least 20%, [49], and a maximum of 344% [53].
Previous DC trials demonstrated that observed immune responses do not necessarily correlate with the clinical response. Only increased circulating TAA-specific CD8+ T cells, eosinophilic blood count, strength of allergic reactions at the DC injection site, and a CD4+ T cell response in sentinel lymph nodes were found to correspond to clinical outcome [48]. Further, the mode of analysis is of importance since the DC-stimulated anti-Her2 CD4+ T cell response correlated with the clinical response significantly only in the sentinel lymph nodes but not in the peripheral blood [54].
CD137L-DCs were found to be more efficacious in vitro than moDCs generated with GM-CSF + IL-4, as they induced stronger cytotoxic immune responses against tumor-associated viruses such as EBV and HBV [37, 38]. In this study, we find that the clinical response initiated by CD137L-DCs is in the range of that of other types of moDCs with a low objective tumor response rate but prolonged patient survival [48, 49]. It is impossible to elucidate why we did not see superior potency of CD137L-DCs in vivo as we had done earlier in vitro. However, comparisons are difficult, and can be misleading due to different cancer types, treatment protocols and patient cohorts.
The fact that patients with a lower pretreatment NLR, i.e. relatively more lymphocytes, had a longer median PFS corroborates the rationale of this study, since it is the lymphocytes that were stimulated by CD137L-DC-EBV-VAX, and that could exert an anti-NPC immune response. Further, neutrophils in cancers are often immunosuppressive, and limit anti-cancer T cell responses [55].
The 4 patients with a PR were characterized by immune parameters that set them apart from the patients who experienced CB, already before the first vaccination. Among those were higher numbers of naive CD4+ and CD8+ T cells. It may well be that some of these cells had suppressive activity.
Pre-treatment concentrations of plasma EBV DNA positively correlate with tumor volume [56]. This may explain that a high EBV DNA load in plasma of our patients was associated with disease progression. Therefore, the observed decrease in plasma EBV DNA concentration during the vaccination period could be an indication of the efficacy of CD137L-DC-EBV-VAX. Alternatively, it could mean that the vaccination works best with a reduced tumor burden, implying that the optimal time for vaccination would be after tumor debulking by an effective chemotherapy.
In most single arm DC studies, the immunological response has not correlated well with the clinical response. However, in this study, we do see a correlation of CB with the T cell (especially memory and effector) response. Therefore, combining CD137L-DC with immune checkpoint inhibitors should be effective. Particularly, since immune checkpoint inhibitors together with cDC already demonstrated synergism in inducing anti-melanoma responses [57, 58]. The combination of CD137L-DC with Wnt3a/GSK3b inhibitors may also be useful as Wnt3b inhibition in T cells is known to boost the memory T cell response [59], or with CD137 agonists since CD137 stimulation promotes effector and memory CD8 T cell responses [60, 61]. Alternatively, adding an anti-VEGF antibody to CD137L-DC treatment could enhance T cell migration into the malignant tissue [62, 63]. Of note, CD137L-DC-EBV-VAX, possibly as a combination therapy, may also be beneficial in other EBV-associated malignancies such as lymphoepithelioma.
The limited efficacy of DC vaccines and its poor correlation to clinical success can be attributed to several factors, ranging from DC generation methods, antigen loading technique and vaccine delivery approaches (Sabado et al., 2016; Garner et al., 2020). In this trial, pulsing CD137L-DC with EBV peptides have proven to be expensive. Thus, alternative strategies that can further enhance the quality and efficacy of DC vaccines should be explored. One approach could be to transfect CD137L-DC with antigen-expressing vectors such as recombinant adenovirus [1].
To date, management of relapsed NPC continues to remain extremely challenging. Palliative therapy remains to be the first-line treatment for relapsed NPC patients, although various experimental treatments, such as targeted therapy and immunotherapy, have been explored [64, 65]. The good safety and tolerability as well as the encouraging patient response and the enhanced immune parameters provide a basis for further developing and testing CD137L-DC vaccines for immunotherapy, and CD137L-DC-EBV-VAX may give relapsed NPC patients a chance for extending health and life.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the patients who participated. We thank the many people who translated an initial PCR fragment to a clinical application, most notably Joachim Langstein, Daniela Drenkard, Shaqireen Kwajah and Zulkarnain Harfuddin. We thank the Life Sciences Institute flow cytometry core facility for excellent assistance. We thank Lingzhi Wang, Amelia Lau, and Shiwen Qiu for help with analysing EBV plasma DNA levels.
Author Contributions
Conception and design: Herbert Schwarz, Boon Cher Goh. Financial support: Herbert Schwarz, Boon Cher Goh. Administrative support: Michelle Poon, Yvonne Ang, Yugarajah Asokumaran, Wan Qin Chong, Yiqing Huang, Kwok Seng Loh, Ross Soo. Provision of study materials: Emily Nickles, Bhushan Dharmadhikari, Li Yating, Lip Kun Tan, Mickey Koh, Liam Pock Ho, Marieta Chan, Madelaine Niam, Melissa Soh, Yen Hoon Luah. Provision of patients: Boon Cher Goh, Chwee Ming Lim, J. Walsh, Liang Piu Koh. Collection and assembly of data: Emily Nickles, Li Yating, Robert J. Walsh, Bhushan Dharmadhikari, John E. Connolly, Nivashini Kaliaperumal, Veonice B. Au. Data analysis and interpretation: Herbert Schwarz, Boon Cher Goh, Emily Nickles, Bhushan Dharmadhikari, Li Yating, John E. Connolly, Nivashini Kaliaperumal, Najwa Binte Said Nasir Talib, Reina Sg. Manuscript writing: Herbert Schwarz. Revision and final approval of manuscript: All authors.
Funding
This research was supported by a grant (NMRC/BnB/018b/2015) from the National Medical Research Council, Singapore.
Declarations
Conflict of interest
There are no conflicts of interest to declare.
Footnotes
Emily Nickles and Bhushan Dharmadhikari authors contributed equally
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Boon Cher Goh, Email: phcgbc@nus.edu.sg.
Herbert Schwarz, Email: phssh@nus.edu.sg.
References
- 1.Smith C, Khanna R. A new approach for cellular immunotherapy of nasopharyngeal carcinoma. Oncoimmunology. 2012;1(8):1440–1442. doi: 10.4161/onci.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao SW, Tsang CM, and Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732). [DOI] [PMC free article] [PubMed]
- 3.Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol. 2015;32(1):54–73. doi: 10.1053/j.semdp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 5.Lv JW, Li JY, Luo LN, Wang ZX, Chen YP. Comparative safety and efficacy of anti-PD-1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: findings from recent advances in landmark trials. J Immunother Cancer. 2019;7(1):159. doi: 10.1186/s40425-019-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow JC, Ngan RK, Cheung KM, Cho WC. Immunotherapeutic approaches in nasopharyngeal carcinoma. Expert Opin Biol Ther. 2019;19(11):1165–1172. doi: 10.1080/14712598.2019.1650910. [DOI] [PubMed] [Google Scholar]
- 7.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(8):798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung CS, Maurer MA, Meixlsperger S, Lippmann A, Cheong C, Zuo J, et al. Robust T-cell stimulation by Epstein-Barr virus-transformed B cells after antigen targeting to DEC-205. Blood. 2013;121(9):1584–1594. doi: 10.1182/blood-2012-08-450775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabado RL, Meseck M, Bhardwaj N. Dendritic cell vaccines. Methods Mol Biol. 2016;1403:763–777. doi: 10.1007/978-1-4939-3387-7_44. [DOI] [PubMed] [Google Scholar]
- 10.Gardner A, de Mingo PA, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. 2020;11:924. doi: 10.3389/fimmu.2020.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines-recent breakthroughs and encouraging clinical results. Front Immunol. 2019;10:766. doi: 10.3389/fimmu.2019.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF, et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Can Res. 2002;62(23):6952–6958. [PubMed] [Google Scholar]
- 13.Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH, et al. A phase II study evaluating the safety and efficacy of an adenovirus-DeltaLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(4):997–1005. doi: 10.1093/annonc/mdr341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Song D, Lu Y, Zhu H, Chen Z, He X. Delayed-type hypersensitivity (DTH) immune response related with EBV-DNA in nasopharyngeal carcinoma treated with autologous dendritic cell vaccination after radiotherapy. J Immunother. 2013;36(3):208–214. doi: 10.1097/CJI.0b013e31828bd87b. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz H, Tuckwell J, Lotz M. A receptor induced by lymphocyte activation (ILA): a new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene. 1993;134(2):295–298. doi: 10.1016/0378-1119(93)90110-O. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz H, Valbracht J, Tuckwell J, von KJ, and Lotz M. ILA, the human 4–1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85(4):1043–52. [PubMed]
- 17.Thum E, Shao Z, Schwarz H. CD137, implications in immunity and potential for therapy. Front Biosci. 2009;14:4173–4188. doi: 10.2741/3521. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7–H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol. 2011;344:245–267. doi: 10.1007/82_2010_81. [DOI] [PubMed] [Google Scholar]
- 19.Vinay DS, Kwon BS. 4–1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014;47(3):122–129. doi: 10.5483/BMBRep.2014.47.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillerey C, Nakamura K, Pichler AC, Barkauskas D, Krumeich S, Stannard K, et al. Chemotherapy followed by anti-CD137 mAb immunotherapy improves disease control in a mouse myeloma model. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed]
- 21.Sanmamed MF, Etxeberria I, Otano I, and Melero I. Twists and turns to translating 4–1BB cancer immunotherapy. Sci Transl Med. 2019;11(496). [DOI] [PubMed]
- 22.Campana D, Schwarz H, Imai C. 4–1BB chimeric antigen receptors. Cancer J. 2014;20(2):134–140. doi: 10.1097/PPO.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 23.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 24.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89(1):21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 25.Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. JLeukocBiol. 2002;72(1):35–42. [PubMed] [Google Scholar]
- 26.Jiang D, Chen Y, Schwarz H. CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages. J Immunol. 2008;181(6):3923–3932. doi: 10.4049/jimmunol.181.6.3923. [DOI] [PubMed] [Google Scholar]
- 27.Jiang D, Yue PS, Drenkard D, Schwarz H. Induction of proliferation and monocytic differentiation of human CD34+ cells by CD137 ligand signaling. Stem Cells. 2008;26(9):2372–2381. doi: 10.1634/stemcells.2008-0158. [DOI] [PubMed] [Google Scholar]
- 28.Jiang D, and Schwarz H. Regulation of granulocyte and macrophage populations of murine bone marrow cells by G-CSF and CD137 protein. PloS one. 2010;5(12):e15565. [DOI] [PMC free article] [PubMed]
- 29.Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol. 1998;160(5):2488–2494. [PubMed] [Google Scholar]
- 30.Langstein J, Schwarz H. Identification of CD137 as a potent monocyte survival factor. JLeukocBiol. 1999;65(6):829–833. doi: 10.1002/jlb.65.6.829. [DOI] [PubMed] [Google Scholar]
- 31.Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, et al. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(2):456–463. doi: 10.1096/fj.05-4739com. [DOI] [PubMed] [Google Scholar]
- 32.Quek BZ, Lim YC, Lin JH, Tan TE, Chan J, Biswas A, et al. CD137 enhances monocyte-ICAM-1 interactions in an E-selectin-dependent manner under flow conditions. Mol Immunol. 2010;47(9):1839–1847. doi: 10.1016/j.molimm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Q, Soe YM, Lim Y, Sobota RM, and Schwarz H. CD137 ligand interacts with CD32a to trigger reverse CD137 ligand signaling. Cellular & molecular immunology. 2020. [DOI] [PMC free article] [PubMed]
- 34.Sollner L, Shaqireen DOKMM, Wu JT, and Schwarz H. Signal transduction mechanisms of CD137 ligand in human monocytes. Cellular signalling. 2007;19(9):1899–908. [DOI] [PubMed]
- 35.Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Latif AH, et al. CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. EurJImmunol. 2008;38(4):1024–1032. doi: 10.1002/eji.200737800. [DOI] [PubMed] [Google Scholar]
- 36.Kwajah MMS, Schwarz H. CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur J Immunol. 2010;40(7):1938–1949. doi: 10.1002/eji.200940105. [DOI] [PubMed] [Google Scholar]
- 37.Harfuddin Z, Kwajah S, Chong Nyi Sim A, Macary PA, and Schwarz H. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. Oncoimmunology. 2013;2(11):e26859. [DOI] [PMC free article] [PubMed]
- 38.Dharmadhikari B, Nickles E, Harfuddin Z, Ishak NDB, Zeng Q, Bertoletti A, et al. CD137L dendritic cells induce potent response against cancer-associated viruses and polarize human CD8(+) T cells to Tc1 phenotype. Cancer immunology, immunotherapy : CII. 2018;67(6):893–905. doi: 10.1007/s00262-018-2144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38(2):336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Harfuddin Z, Dharmadhikari B, Wong SC, Duan K, Poidinger M, Kwajah S, et al. Transcriptional and functional characterization of CD137L-dendritic cells identifies a novel dendritic cell phenotype. Sci Rep. 2016;6:29712. doi: 10.1038/srep29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng Q, Mallilankaraman K, Schwarz H. Increased akt-driven glycolysis is the basis for the higher potency of CD137L-DCs. Front Immunol. 2019;10:868. doi: 10.3389/fimmu.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Can Res. 2003;63(1):12–17. [PubMed] [Google Scholar]
- 43.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Can Res. 1999;59(1):56–58. [PubMed] [Google Scholar]
- 44.Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2007;56(10):1513–1537. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan R, Phua SKA, Soong YL, Oon LLE, Chan KS, Lucky SS, et al. Clinical utility of Epstein-Barr virus DNA and other liquid biopsy markers in nasopharyngeal carcinoma. Cancer Commun (Lond). 2020. [DOI] [PMC free article] [PubMed]
- 47.Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic cell-based immunotherapy: state of the art and beyond. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(8):1897–1906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 48.Huber A, Dammeijer F, Aerts J, Vroman H. Current state of dendritic cell-based immunotherapy: opportunities for in vitro antigen loading of different DC subsets? Front Immunol. 2018;9:2804. doi: 10.3389/fimmu.2018.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15(7):e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 50.Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol. 2010;40(8):2123–2130. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- 51.Mohme M, Neidert MC, Regli L, Weller M, Martin R. Immunological challenges for peptide-based immunotherapy in glioblastoma. Cancer Treat Rev. 2014;40(2):248–258. doi: 10.1016/j.ctrv.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 53.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Can Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 54.Lowenfeld L, Mick R, Datta J, Xu S, Fitzpatrick E, Fisher CS, et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2(pos) DCIS independent of route: results of randomized selection design trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(12):2961–2971. doi: 10.1158/1078-0432.CCR-16-1924. [DOI] [PubMed] [Google Scholar]
- 55.Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40(7):565–583. doi: 10.1016/j.it.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan KC. Plasma Epstein-Barr virus DNA as a biomarker for nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):598–603. doi: 10.5732/cjc.014.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, et al. Phase II study of autologous monocyte-derived mrna electroporated dendritic cells (TriMixDC-MEL) Plus Ipilimumab in patients with pretreated advanced melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(12):1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 58.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115(9):1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, et al. Combination of 4–1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3(2):149–160. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 61.Woroniecka KI, Rhodin KE, Dechant C, Cui X, Chongsathidkiet P, Wilkinson D, et al. 4–1BB Agonism averts TIL exhaustion and licenses PD-1 blockade in Glioblastoma and other intracranial cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(6):1349–1358. doi: 10.1158/1078-0432.CCR-19-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chong WQ, Lim CM, Sinha AK, Tan CS, Chan GHJ, Huang Y, et al. Integration of Antiangiogenic Therapy with Cisplatin and Gemcitabine Chemotherapy in patients with Nasopharyngeal Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(20):5320–5328. doi: 10.1158/1078-0432.CCR-20-1727. [DOI] [PubMed] [Google Scholar]
- 64.Lee V, Kwong D, Leung TW, Lam KO, Tong CC, Lee A. Palliative systemic therapy for recurrent or metastatic nasopharyngeal carcinoma - How far have we achieved? Crit Rev Oncol Hematol. 2017;114:13–23. doi: 10.1016/j.critrevonc.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 65.Perri F, Della Vittoria Scarpati G, Caponigro F, Ionna F, Longo F, Buonopane S, et al. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 2019;12:1583–1591. doi: 10.2147/OTT.S188148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.