Abstract
CD99 is a surface molecule expressed on various cell types including cancer cells. Expression of CD99 on multiple myeloma is associated with CCND1-IGH fusion/t(11;14). This translocation has been reported to be a genetic hallmark of mantle cell lymphoma (MCL). MCL is characterized by overexpression of cyclin D1 and high tumor proliferation. In this study, high expression of CD99 on MCL cell lines was confirmed. Our generated anti-CD99 monoclonal antibody (mAb), termed MT99/3, exerted potent antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) activities against mantle B-cell lymphoma without direct cytotoxic effects. The anti-tumor activities of mAb MT99/3 were more effective in MCL than in other B-cell lymphomas. Moreover, in a mouse xenograft model using Z138 MCL cell line, treatment of mAb MT99/3 reduced tumor development and growth. Our study indicated that mAb MT99/3 is a promising immunotherapeutic candidate for mantle cell lymphoma therapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02789-0) contains supplementary material, which is available to authorized users.
Keywords: Mantle cell lymphoma, CD99, Monoclonal antibody, Cancer immunotherapy, Cytotoxicity
Introduction
CD99, also known as E2, is an extensive O-glycosylated type I single-chain transmembrane protein [1]. CD99 molecules express at different levels on several human cell types, both hematopoietic and non-hematopoietic [2–4]. These molecules display two isoforms; CD99 long form (wild-type) and alternative splicing CD99 short form (truncated) [5]. The expression of CD99 isoforms differs among cell types, and elicits distinct functions [6–8]. Multifunction of CD99 has been demonstrated in both physiological and pathological conditions [9]. It has been implicated in numerous cellular processes including cell apoptosis [10, 11], cell adhesion [3, 5], transendothelial migration of leukocytes [12], cell differentiation [13], T-cell regulation [14, 15] and protein trafficking [16, 17]. Expression of CD99 on particular types of malignancies was also demonstrated [10, 18]. In tumors, CD99 can have either oncogenic or oncosuppressive functions [19]. Among its oncogenic functions, strong expression of CD99 has been proposed as a potential therapeutic target for monoclonal antibody treatment in Ewing’s sarcoma (ES) [20], acute lymphoblastic leukemia (ALL) [21, 22], acute myeloid leukemia (AML) and the myelodysplastic syndromes (MDS) [23]. Monoclonal antibodies (mAbs) targeting CD99 expressed on these cancers are directly cytotoxic, inducing cancer apoptosis in the absence of immune effector cells or complement. In ES and AML, the anti‐tumor effect of anti-CD99 mAb was confirmed in mouse xenografts [20, 23]. These data show that CD99 surface molecules can be targeted by antibodies and may emerge as a promising therapeutic target for other CD99-overexpressing cancers.
B-cell non-Hodgkin lymphomas (NHL) are the most frequent among all hematologic and lymphoid malignancies [24]. Mantle cell lymphoma (MCL) is a subtype of B-cell NHL that probably derives from naive B cells in the mantle zone of lymphatic follicles [25]. MCL represents about 6–8% of NHL cases [26]. MCL was defined as a highly proliferative lymphoma due to a chromosomal translocation t(11;14)(q13;q32). This translocation is a genetic hallmark of MCL that juxtaposes CCND1 gene to the immunoglobulin heavy chain (IGH) gene enhancer region resulting in the overexpression of cyclin D1 and accelerated cell proliferation [27]. Hence, MCL patients have frequent relapses and a median survival of only 3–5 years [28]. The combination of the anti-CD20 antibody (rituximab) with chemotherapeutic drugs such as R-CHOP has improved clinical outcomes of MCL patients [29]. Nevertheless, the disease remains incurable [29]. Currently, only one immunotherapeutic drug, rituximab, is available for MCL treatment; new and effective candidates are needed. Recently, CD99 expression was shown to be retained on CCND1-IGH fusion/t(11;14) myeloma but reduced on t(11;14)-negative myeloma [30]. It is likely that MCL, which is also CCND1-IGH fusion/t(11;14), expresses CD99 at high levels. Targeting CD99 on MCL with a specific antibody might affect tumor growth and lead to novel immunotherapeutic drugs.
In this study, we demonstrated that human mantle cells highly express CD99. Anti-human CD99 mAb (named MT99/3) exerts potent anti-tumor effects via ADCC and CDC on MCL. mAb MT99/3 could reduce tumor development and growth in a Z138 MCL cell xenograft model. This mAb appears to be a candidate antibody drug for treatment of MCL.
Materials and methods
Cell lines
Z138 (obtained from JCRB cell bank, Osaka, Japan), RC-K8, MM1R, L-363 (gift from Dr. Hidekatsu Iha, Oita University Faculty of Medicine), Jurkat (obtained from RIKEN cell bank, Tsukuba, Japan) and Granta-519 cell lines (gift from Dr. Siwanon Jirawatnotai, Department of Pharmacology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand) were maintained in RPMI 1640 medium (Wako, Osaka, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT, USA), 100 U/ml penicillin, and 100 µg/ml streptomycin (10%FBS-RPMI 1640) at 37 °C in a humidified 5% CO2 atmosphere.
For mouse splenocytes, spleens were harvested from female BALB/c nude mice (Japan Clea, Tokyo, Japan) then homogenized in RPMI 1640 and centrifuged. Red blood cells were lysed by NH4Cl hypotonic lysis buffer. After washing, mouse splenocytes were resuspended in 10%FBS-RPMI 1640.
Preparation of monoclonal antibody MT99/3 and Isotype-matched control mAb
Mouse hybridoma clone MT99/3 (anti-human CD99 mAb; IgG2a) was generated in our laboratory [3]. Hybridoma clone 4G2 (anti-dengue viral protein; IgG2a) was obtained from Dr. Prida Malasit (Division of Medical Molecular Biology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand). Hybridoma cells were cultured in Iscove's Modified Dulbecco's Media (IMDM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), 40 μg/ml gentamycin and 2.5 μg/ml amphotericin B (10%FBS-IMDM) at 37 °C in a 5% CO2 incubator. For mAb purification, the hybridoma cells were adapted to grow in hybridoma serum free media (H-SFM; Gibco). Culture supernatants containing mAbs were collected from hybridoma culture and purified by affinity chromatography using HiTrap Protein G HP (GE Healthcare BioSciences AB, Uppsala, Sweden). The concentration of monoclonal antibodies was measured at 280 nm absorbance. The purity of mAbs was monitored by SDS–polyacrylamide gel electrophoresis (SDS-PAGE).
Immunofluorescence staining and flow cytometric analysis
For direct immunofluorescence staining, cell lines (5 × 105 cells) were stained with PE-conjugated anti-human CD99 mAb (Caltag Laboratories Burlingame, CA, USA) or PE-conjugated mouse IgG isotype control mAb (ImmunoTools, Friesoythe, Germany) on ice for 30 min. For indirect immunofluorescence staining, cell lines (5 × 105 cells) were blocked with Fc receptor using 10% human AB serum on ice for 30 min. Cells were then incubated with 10 µg/ml of mAb MT99/3 or isotype mAb or without Abs on ice for 30 min. After washing, bound antibodies were detected using Alexa Flour-488-anti-mouse IgG Abs (H + L chains specific) (Invitrogen Life Technologies, Grand Island, NY, USA). After incubation on ice for 30 min, the excess conjugates were washed out. Stained cells were suspended in propidium iodide (PI) solution (0.5 µg/ml) and analyzed by a FACSCelesta flow cytometer (BD Biosciences, San Jose, CA). Live cells (negative staining for PI) were gated to determine CD99 expression using FlowJo software (Tree Star Inc., Ashland, OR). Gating strategy was shown in Supplementary Fig. 5.
Mouse splenocytes were stained with PE-conjugated anti-mouse CD49b (DX5) mAb (Miltenyi Biotec, Bergisch-Gladbach, Germany) and FITC-conjugated anti-mouse CD19 mAb (Miltenyi Biotec). The percentages of NK cells (DX5+CD19− cells) were determined by flow cytometry (FACSCelesta flow cytometer, BD Biosciences, CA, USA). 4–7% of NK cells were present in the prepared splenocytes.
Protein extraction and Western blot analysis
Cells were lysed using lysis buffer (25 mM HEPES, 10 mM Na4P2O7, 1% Triton X-100, 5 mM EDTA, 100 mM NaF, 2 mM Na3VO4, and protease inhibitor cocktail). Cell lysates were collected and protein concentrations were quantified by a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Thirty micrograms of total protein were loaded in each lane of SDS-PAGE. The proteins were separated by SDS-PAGE and electrically blotted onto a PVDF membrane (Merck Millipore, Tullagreen, Ireland). The primary antibodies used were anti-CD99 mAb (MT99/3) and anti-actin mAbs (C-2) (Santa Cruz Biotechnology, Santa Cruz, CA). HRP-conjugated anti-mouse IgG Abs (Cell Signaling Technology, Inc., Danvers, MA, USA) was used as the conjugate. Immunoreaction bands were developed using Chemi-Lumi One Super reagents (Nacalai Tesque, Kyoto, Japan) and detected by the ImageQuant LAS 4000 system (GE Healthcare).
Direct cytotoxicity and ADCC assay by freshly isolated mouse splenocytes
Cell lines were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR, USA) and used as target cells. CFSE-labeled cell lines (1 × 105 cells) were pre-incubated with mAb MT99/3 or isotype-matched control mAb (20 µg/ml), or kept in culture medium (no mAb) for 15 min at room temperature (RT). After incubation, CFSE-labeled cell lines were co-cultured with mouse splenocytes (effector cells) at E:T ratios of 50:1, 100:1 or without effector cells in 250 µl of 10%FBS-RPMI 1640, and incubated for 4 h at 37 °C in a 5% CO2 incubator. Cells were harvested, washed with PBS and resuspended in PI solution (0.5 µg/ml). The percentage of dead target cells (CFSE+PI+) was determined by a FACSCelesta flow cytometer. The data were analyzed by FlowJo software. Gating strategy was shown in Supplementary Fig. 5.
ADCC assay by IL-2 activated mouse splenocytes
Cell lines were labeled with CFSE and used as target cells. CFSE-labeled cell lines (5 × 104 cells) were pre-incubated with mAb MT99/3 or isotype-matched control mAb (20 µg/ml), or kept in culture medium (no mAb) for 15 min at room temperature (RT). Mouse splenocytes (1 × 107 cells/ml) were treated with mouse IL-2 (mIL-2, PeproTech, Rocky Hill, NJ) at 20 ng/ml and incubated for 3 days at 37 °C in 5% CO2 incubator. After incubation, IL-2 activated mouse splenocytes were harvested and added into CFSE-labeled target cell lines (5 × 104 cells) at E:T ratios of 0:1, 25:1 and 50:1 in 125 µl of 10%FBS-RPMI 1640 at 37 °C in a 5% CO2 incubator for 4 h. Cells were harvested and stained with Ghost Dye Red 780 (Cell Signaling Technology, Danvers, MA). The percentage of dead target cells (CFSE+Ghost Dye+) was determined by a FACSCelesta flow cytometer. The data were analyzed by FlowJo software. Gating strategy was shown in Supplementary Fig. 5.
CDC by MTT assay
Cell lines (1.2 × 105 cells) were seeded into 100 µl of RPMI 1640 medium supplemented with 5% rabbit serum or 5% heat-inactivated rabbit serum in 96-well plates in the presence of mAb MT99/3 or isotype-matched control mAb (20 µg/ml), or kept in culture medium (no mAb). Treated cells were incubated for 2 h at 37 °C in a 5% CO2 incubator. Subsequently, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO, USA) was added into each well at a final concentration of 0.5 mg/ml. Plates were incubated for 4 h at 37 °C in a 5% CO2 incubator. The formazan crystal was dissolved by adding 100 µl of 0.04 N HCl in isopropanol. Absorbance was measured at 570 nm using an iMark microplate reader (Bio-Rad, Hercules, CA, USA).
Z138 mantle cells xenograft
Z138 cells at 2 × 107 cells suspended in 100 µl of 10%FBS-RPMI 1640 were subcutaneously inoculated into whole-body gamma-ray irradiated BALB/c Nude mice (4 Gy). mAbs at 100 µg/100 µl of PBS or PBS only was injected into the peritoneal cavity of each mouse. The antibody was injected into each mouse when palpable tumors were formed (around 3 mm in diameter). The antibodies were injected three times per week, as indicated in Fig. 4. The tumor diameter and tumor volume were determined on the day of Ab injection. The tumor weight was measured after mice were humanely sacrificed.
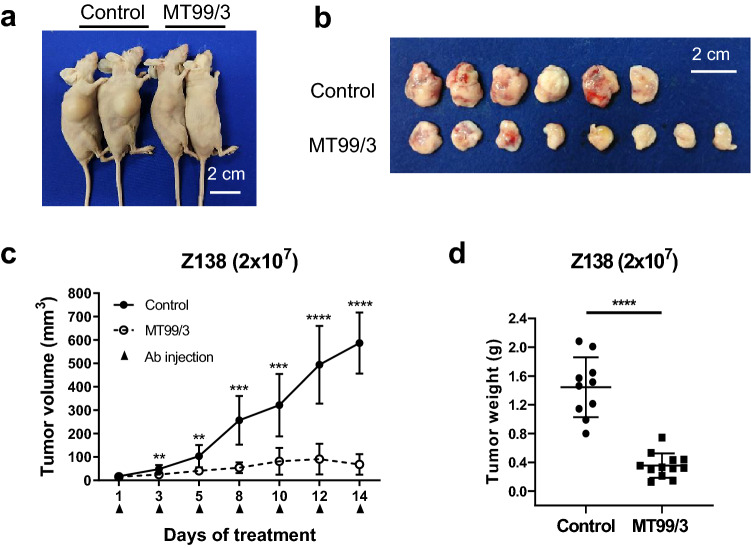
Fig. 4.
Anti-tumor activities of mAb MT99/3 in Z138 xenograft model (2 × 107 cells/100 µl). Z138 mantle cells were subcutaneously inoculated into 4 Gy irradiated BALB/c nude mice. mAb MT99/3 (100 µg/100 µl of PBS) or only PBS was injected into the peritoneal cavity of the mice as specified (control group: n = 10; mAb MT99/3 group: n = 12). a Comparison of tumor size between mAb MT99/3 and PBS control injection in a representative of Z138 xenograft nude mice. b Comparison of tumor size in a representative of Z138 xenograft (day 14 of treatment). c Tumor diameters were measured at the indicated time points and tumor volumes were calculated using the following formula: tumor volume = 3.14 × (W2 × L)/6, where W is short diameter and L is long diameter. d Tumor weight of Z138 xenograft (day 14 of treatment). c, d Values are the mean ± SD. Unpaired t test with Welch's correction was used for comparison, **P < 0.01. ***P < 0.001. ****P < 0.0001
Statistical analysis
Data were expressed as mean ± SEM or mean ± SD as indicated in the figure legends.
All statistical analyses were performed using GraphPad Prism version 8.0.2 (GraphPad Software, CA, USA). The unpaired t-test or one-way or two-way analysis of variance (ANOVA) was used. P < 0.05 was considered significant.
Results
CD99 is highly expressed in t(11;14) translocated B-cell lymphoma
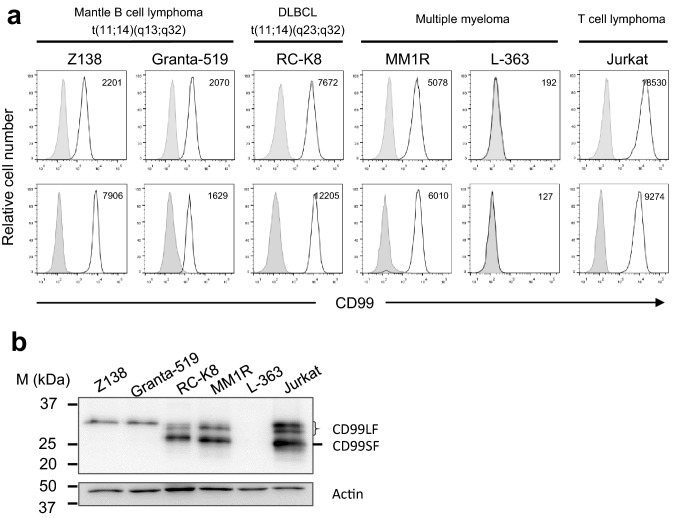
CD99 expression levels on CCND1 overexpressing cells were determined. Human mantle cell lymphoma Z138 and Granta-519 cell lines were used as representatives for the CCND1-IGH/t(11;14) translocated cells. Other human malignant B-cell lines including diffuse large B-cell lymphoma RC-K8 cell line, which has t(11;14) translocation in a different region from mantle cells, as well as two multiple myeloma cell lines, MM1R and L-363, were also evaluated. CD99-expressing Jurkat T-cell line was used as a positive control. Upon immunofluorescence staining, the generated anti-human CD99 mAb clone MT99/3 and commercial anti-human CD99 mAb showed the same immunoreactivity pattern (Fig. 1a). CD99 is strongly expressed in t(11;14) translocated B-cell lymphomas. The two myeloma cell lines showed different CD99 expression patterns; MM1R highly expressed CD99, but L-363 was negative.
Fig. 1.
CD99 expression in malignant B-cell lines. a Direct immunofluorescence staining using commercial anti-CD99 mAb is shown in upper panel. The indicated cells were stained with PE-conjugated anti-CD99 mAb (white peak) or PE-conjugated isotype mAb (gray peak). Indirect immunofluorescence staining using mAb MT99/3 is shown in lower panel. Cells were stained with mAb MT99/3 (white peak) or isotype-matched control mAb (gray peak) followed by Alexa Flour-488-conjugated anti-mouse IgG (H + L) Abs. Mean fluorescence intensity (MFI) of CD99 expression is indicated at right upper corner of histogram graph. b Western blot analysis for CD99 isoform expression of the indicated cells is shown. Actin was used as a loading control. Jurkat T-cell line was used as a positive control of CD99 expression
Two isoforms of CD99, long form (CD99LF; 32 kDa) and short form (CD99SF; 28 kDa), have been reported [19]. As shown in Fig. 1b, human mantle cell lymphoma Z138 and Granta-519 cell lines expressed only CD99LF. RC-K8 and MM1R cell lines expressed both CD99LF and CD99SF. In the flow cytometry results, western blotting analysis confirmed that L-363 was CD99-negative. Jurkat T-cell line, which expressed both CD99 isoforms, was used as a reference in the western blotting experiment.
Anti-CD99 mAb MT99/3 mediates ADCC activities against CD99-positive B lymphoma cells
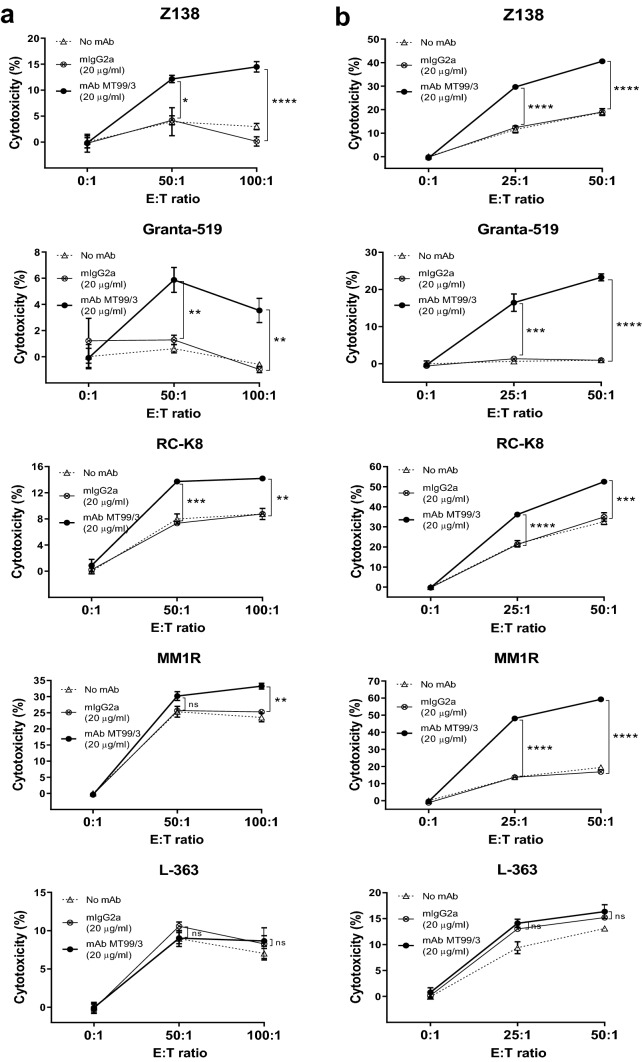
To determine ADCC activity promoted by anti-CD99 mAb MT99/3 on malignant B cells, a panel of B-cell lines was used as target cells. Freshly isolated mouse splenocytes and IL-2 activated mouse splenocytes were employed as effector cells. Upon mAb MT99/3 treatment, significant cell death was observed in two mantle cell lymphoma cell lines, Z138 and Granta-519, as well as diffuse large B-cell lymphoma RC-K8 cell line. This phenomenon was not observed with isotype-matched control treatment (Fig. 2).
Fig. 2.
ADCC activities mediated by anti-CD99 mAb MT99/3. CFSE-labeled Z138, Granta-519, RC-K8, MM1R and L-363 cell lines (target cells) were co-cultured with a freshly isolated mouse splenocytes (effector cells) or b IL-2 activated mouse splenocytes (effector cells) at various E:T ratios in the presence of anti-CD99 mAb (mAb MT99/3) or isotype-match control (mIgG2a) or without Abs (No mAb) for 4 h at 37 °C. The dead cells were analyzed by flow cytometry. Percent cytotoxicity was calculated as [(Dead target cells (%) − spontaneous death (%))/(100 − spontaneous death)] × 100%. The experiments were carried out in triplicate. The values are shown as mean ± SEM. One-way ANOVA followed by Tukey's multiple comparisons test was used for comparison, *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001. ns not statistically significant
To confirm that mAb MT99/3 mediated target cell death was via specific targeting of CD99, CD99-positive MM1R and CD99-negative L-363 cells were used as target cells. mAb MT99/3 boosted the cytotoxic effects of effector cells in killing only the CD99-positive cells (Fig. 2; MM1R), but not the CD99-negative cells (Fig. 2; L-363). In addition, in the absence of effector cells, mAb MT99/3 had no direct cytotoxic effect in any of the cell lines tested (Fig. 2 and Supplementary Fig 1. These results indicated that mAb MT99/3 mediates ADCC activities via specific targeting of CD99 on the target cell surface. Moreover, the ADCC activities mediated by mAb MT99/3 were more potent in IL-2 activated effector cells (Fig. 2b) than in unstimulated effector cells (Fig. 2a). These results suggested that IL-2 could be utilized to enhance the anti-tumor activities of effector cells.
We wondered whether mAb MT99/3-induced target cell death via ADCC activities or whether this mAb could bind to CD99 expressed on effector cells and then activate cytotoxicity of the effector cells. The cross-reactivity of anti-human CD99 mAb (MT99/3) to mouse CD99 (expressed on effector cells) was thus determined. mAb MT99/3 specifically recognized human CD99 expressed on target cells (Fig. 1a), but did not bind to CD99 on mouse splenocytes (Supplementary Fig. 2). The cytotoxic activities of effector cells observed in mAb MT99/3 treatment were, therefore, induced via ADCC. These results demonstrate the ability of mAb MT99/3 to induce target cell death through effector cell functions.
Taken together, our findings indicated that mAb MT99/3 was effective at exerting ADCC activities against CD99-expressing cells, particularly mantle cells.
Anti-CD99 mAb MT99/3 mediates CDC activities against CD99-expressing B lymphoma cells
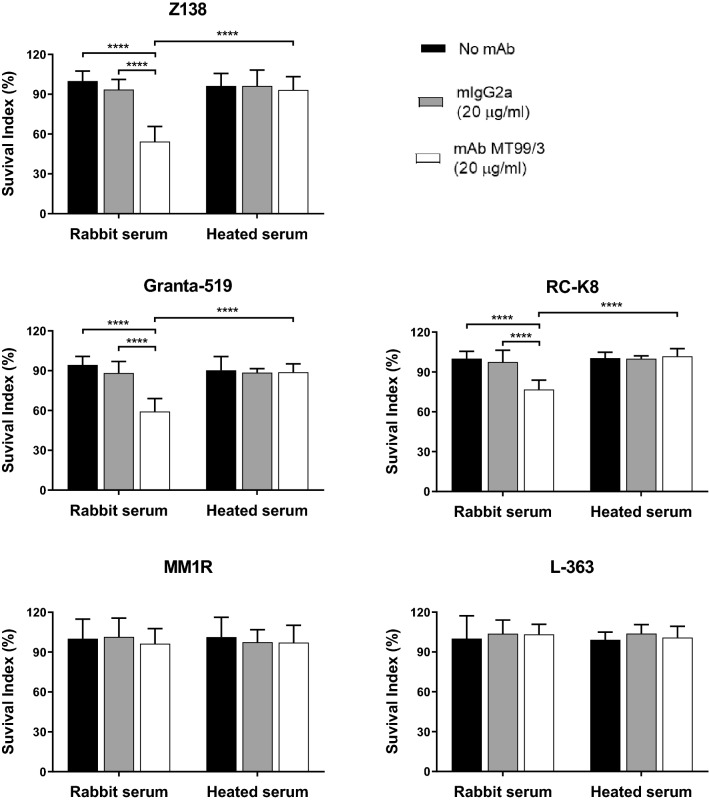
mAb IgG2a isotype-mediated CDC activities have previously been reported [31]. Therefore, we investigated whether mAb MT99/3 (mIgG2a) induces tumor death via CDC. B lymphoma cells were incubated with mAb MT99/3 in the presence of complement, and cell death was determined. mAb MT99/3 could induce cell death in almost all of the CD99-positive cells tested (Fig. 3), except for the MM1R cell line (Fig. 3; MM1R). No target cell death was observed in isotype-matched control mAb treatments. As expected, mAb MT99/3 did not induce CD99-negative L-363 cell death (Fig. 3; L-363). “Complement” refers to the heat-labile serum component. Cell death mediated by mAb MT99/3 was rescued by adding heat-inactivated serum (Fig. 3). To confirm whether CDC activities mediated by mAb MT99/3 induce cell death, cell death determined by PI staining and flow cytometric analysis were carried out in representative CD99-positive and -negative cells. Corresponding to the MTT method, via complement activity, anti-CD99 mAb caused cell death (PI positive cells) in CD99-positive Z138 but not-CD99-negative L-363 cells (Supplementary Fig. 3). Interestingly, among the CD99-positive cell lines, anti-CD99 mAb showed higher CDC activities in both mantle cell lines, Z138 and Granta-519 (Fig. 3).
Fig. 3.
CDC activities mediated by anti-CD99 mAb MT99/3 against B lymphoma cell lines. Z138, Granta-519, RC-K8, MM1R and L-363 cell lines were plated into RPMI 1640 medium supplemented with 5% rabbit serum or heat-inactivated rabbit serum (Heated serum). mAb MT99/3 or isotype-matched control (mIgG2a) were added or kept in culture medium (no mAb) and incubated for 2 h at 37 °C. MTT reagent was added then continuously incubated for 4 h. The formazan crystal was dissolved and the absorbance was measured at 570 nm. Each experiment was performed in triplicated wells. The survival index (%) was calculated by the absorbance of each condition normalized to mean absorbance of no mAb in rabbit serum as 100%. Bar graphs show mean ± SD from six values of two independent experiments. Two-way ANOVA followed by Tukey's multiple comparisons test was used for comparison, ****P < 0.0001
As CD99 molecules are also expressed on the surface of normal blood cells (Supplementary Fig. 2), it is important to examine anti-CD99 mAb mediated CDC activities in normal cells. CDC activities mediated by mAb MT99/3 were further tested in human PBMCs. mAb MT99/3 did not exert CDC activities in PBMCs (Supplementary Fig. 4). These results indicated that mAb MT99/3 mediation of CDC activity would not occur in CD99-positive normal cells.
mAb MT99/3 exerts anti-tumor activities of Z138 xenograft models
To investigate the therapeutic possibility of anti-CD99 mAb, anti-tumor activities mediated by mAb MT99/3 were conducted in vivo. Z138 cells were implanted into the flanks of 4 Gy irradiated nude mice. Antibody was injected into each mouse when palpable tumors were formed (around 3 mm in diameter). Tumor formation was observed in 100% of the control group (Fig. 4), but mAb MT99/3 treatment reduced tumor development. The tumor volume was significantly limited by mAb MT99/3 (Fig. 4b, c). The tumor weights of mice in the mAb MT99/3 treatment groups were significantly lower than in the control group (Fig. 4d). These results indicate that administration of mAb MT99/3 inhibited the tumor growth of Z138 mantle cell lymphomas in xenograft mice.
Discussion
Even if the precise functional mechanism of CD99 is still undefined, CD99 has been shown to have oncogenic functions in several tumor types [19]. During tumor development, CD99 is upregulated and expressed on the cell surface. Hence, CD99 is become a promising therapeutic target for antibody treatment of CD99-overexpressing cancers.
In this study, we demonstrated various expressions of CD99 and its isoforms among B-cell lymphomas. Differences in CD99 expression at each stage of B-cell differentiation have been reported [32]. Immature pre B1 stage has high CD99 expression. During the transition from pre B1 to pre B3 stages, CD99 expression is lost and remains low in naïve B cells. CD99 is upregulated again when naïve B cells are activated and differentiated into plasma cells. However, CD99 downregulation is mainly found on plasma cell neoplasm (multiple myeloma) [30]. Nevertheless, CD99 expression is retained on CCND1-IGH/t(11;14) translocated myeloma samples. In agreement with these reports, we found that mantle cell lines with CCND1-IGH/t(11;14) exhibited high CD99 expression. A DLBCL cell line with a distinct region of t(11;14) also has high CD99 expression. Consistent with a previous report [33], CD99 positivity was investigated in DLBCL patients associated with advanced stage. Two multiple myeloma cell lines showed differential expression levels of CD99; CD99-positive and CD99-negative cells.
Two isoforms of CD99 have been revealed [6, 7]. The expression of CD99 isoforms is dependent on cell type [9]. Hence, CD99 in each cell type provides different functional outcomes due to distinct isoform expression and the specific cellular context [8, 18]. We investigated CD99 isoform expression in B-cell lymphomas. The expression of CD99 isoforms was unique to each cancer type. Co-expression of the two isoforms is required to trigger immature T-cell death by anti-CD99 mAb [6, 34]. Nevertheless, targeting CD99 in Ewing’s sarcoma, which expresses only long form, could induce cell death [10]. We then determined the direct effect of anti-CD99 mAb generated in our laboratory, termed MT99/3. mAb MT99/3 had no direct cytotoxic effect on the tested cells. Monoclonal antibody-induced cell death is influenced by epitope recognition sites [4]. mAb MT99/3 might not have reacted on the bioactive domains for death signals on the CD99 molecules expressed on the tested tumor cells.
Currently, the ADCC and CDC mechanisms of Abs have been highlighted as important for therapeutic efficacy in various cancers [35, 36]. In the present study, mAb MT99/3 was found to exert potent ADCC and CDC activities in mantle cell lymphoma. Anti-human CD99 mAb clone MT99/3 effectively induced killing of mantle cells via the ADCC mechanism. This mAb, however, could not bind to mouse CD99, indicating that it did not directly activate effector cell function. The killing effect might come from the ADCC mechanism. Moreover, mAb MT99/3-induced ADCC activity was not observed in CD99-negative cells. This result confirmed that mAb MT99/3 specifically binds human CD99 molecules expressed on target cells and mediates target cell death, probably by NK-cell-mediated ADCC.
IL-2 has been demonstrated to augment the NK-mediated ADCC response [37]. We therefore compared the mAb MT99/3 mediation of ADCC between mouse splenocytes and IL-2-activated mouse splenocytes. We found that IL-2-activated mouse splenocytes (as effector cells) exerted more potent ADCC activities than unstimulated mouse splenocytes. These results suggested that the combination of IL-2 activation and mAb MT99/3 might be a potential approach to enhance anti-tumor activity in mantle cell lymphoma.
In addition, we demonstrated that mAb MT99/3 could powerfully induce mantle cell death via complement activation, with a greater effect than CD99-positive DLBCL cells. The CDC activities mediated by mAb MT99/3 did not occur in CD99-negative L-363 cells, a CD99-expressing MM1R cell line, as in CD99-positive human PBMC. These findings indicated that the CDC activity of mAb MT99/3 is cell type specific [38]. According to previous reports, multiple myeloma cells increase the levels of complement regulatory proteins CD55 and CD59 at the time of progression during anti-human CD38 antibody (daratumumab) treatment [39]. Higher levels of CD59 were also found in CLL cells that were not cleared from the blood after anti-CD20 antibody (rituximab) therapy [40]. We speculated that mAb MT99/3 might increase complement regulatory proteins levels in some CD99-positive cells after treatment, leading the cells to become resistant to CDC. As no CDC activity occurs in normal blood cells by MT99/3, but an anti-tumor effect is seen specifically in mantle B-cell lymphoma, this mAb offers a potential immunotherapeutic strategy for treatment of mantle cells with low side effects.
ADCC and CDC are important mechanisms of anti-tumor activity in vivo [41]. To confirm the therapeutic effect of mAb MT99/3, mouse xenograft experiments were conducted. We confirmed that mAb MT99/3 also showed anti-tumor activities in a Z138 mouse xenograft model. The administration of mAb MT99/3 reduced tumor development and dramatically inhibited tumor growth. Nevertheless, tumors were not complete eradication. This is probably due to limitation of NK cell number in immunosuppressed nude mice. In this study, in the xenograft model, the ADCC and CDC might contribute in anti-tumor activity. However, in nude mice, ADCC might play a major role and more important than CDC [41–43]. It has been demonstrated that nude mice have less serum C3c levels and complement activation comparing to BALB/cJ and SCID mice [44]. Therefore, CDC in nude mice might be the minor role for anti-tumor effect in vivo. The study of in vivo models is a preclinical validation, bridging in vitro research with human studies for predicting the efficacy of anticancer therapies [45, 46]. The success of antibody treatment in preclinical models is a critical step for using potential antibodies in clinical trials [47]. Furthermore, preclinical models for screening anticancer agents require vigorous clinical correlation [45]; in this case, to reliably predict how mantle cells will respond to mAb MT99/3 treatment in clinic. However, the distribution of CD99 expression among normal blood cells, hematopoietic stem cells and normal tissue including endothelial cells and epithelial cells has been reported [23, 48, 49]. An antigenic sink effect upon anti-CD99 antibody therapy may occur and limits efficiency of therapeutic antibody. Consequently, the appropriated doses for treatment must be concerned.
Taken together, CD99 appears to have potential as a novel therapeutic target for mantle B-cell lymphoma. Targeting CD99 on mantle B cells with anti-CD99 antibody clone MT99/3 exerted potent anti-tumor effects in both in vitro and mouse models. mAb MT99/3 is a promising immunotherapeutic candidate for improving long-term survival in mantle cell lymphoma patients. Humanized mAbs need to be generated before application in patients, to reduce the risk for immunogenicity of the mAbs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CCND1
Cyclin D1
- CD
Cluster of differentiation
- FITC
Fluorescein isothiocyanate
- HRP
Horseradish peroxidase
- PE
Phycoerythrin
Author contributions
NT conceived the study, designed and carried out experiments, analyzed data and drafted the manuscript. GS performed ADCC assay of IL-2 activated splenocytes and participated in in vivo experiments. RK participated in in vivo experiments. WK and SO conceived the study, and were involved in the experimental design, interpretation of results and editing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Distinguished Research Professor Grant (NRCT808/2563) of the National Research Council of Thailand, Chiang Mai University Center of Excellence Project and Japan Student Services Organization (JASSO) scholarship (Grant no. UTB1717401001).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
Mice were bred and cared for in the animal research facility according to institutional guidelines. All experimental procedures in this study were approved by the Institutional Animal Care and Use Committee at Kumamoto University, Japan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Watchara Kasinrerk, Email: Watchara.k@cmu.ac.th.
Seiji Okada, Email: okadas@kumamoto-u.ac.jp.
References
- 1.Aubrit F, Gelin C, Pham D, Raynal B, Bernard A. The biochemical characterization of E2, a T cell surface molecule involved in rosettes. Eur J Immunol. 1989;19(8):1431–1436. doi: 10.1002/eji.1830190813. [DOI] [PubMed] [Google Scholar]
- 2.Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67(7):1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::AID-CNCR2820670712>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Kasinrerk W, Tokrasinwit N, Moonsom S, Stockinger H. CD99 monoclonal antibody induce homotypic adhesion of Jurkat cells through protein tyrosine kinase and protein kinase C-dependent pathway. Immunol Lett. 2000;71(1):33–41. doi: 10.1016/S0165-2478(99)00165-0. [DOI] [PubMed] [Google Scholar]
- 4.Khunkaewla P, Chiampanichayakul S, Yasamut U, Pata S, Kasinrerk W. Production, characterization, and functional analysis of newly established CD99 monoclonal antibodies MT99/1 and MT99/2. Hybridoma (Larchmt) 2007;26(4):241–250. doi: 10.1089/hyb.2007.0504. [DOI] [PubMed] [Google Scholar]
- 5.Hahn JH, Kim MK, Choi EY, Kim SH, Sohn HW, Ham DI, Chung DH, Kim TJ, Lee WJ, Park CK, Ree HJ, Park SH. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. 1997;159(5):2250–2258. [PubMed] [Google Scholar]
- 6.Alberti I, Bernard G, Rouquette-Jazdanian AK, Pelassy C, Pourtein M, Aussel C, Bernard A. CD99 isoforms expression dictates T cell functional outcomes. FASEB J. 2002;16(14):1946–1948. doi: 10.1096/fj.02-0049fje. [DOI] [PubMed] [Google Scholar]
- 7.Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006;281(46):34833–34847. doi: 10.1074/jbc.M605483200. [DOI] [PubMed] [Google Scholar]
- 8.Scotlandi K, Zuntini M, Manara MC, Sciandra M, Rocchi A, Benini S, Nicoletti G, Bernard G, Nanni P, Lollini PL, Bernard A, Picci P. CD99 isoforms dictate opposite functions in tumour malignancy and metastases by activating or repressing c-Src kinase activity. Oncogene. 2007;26(46):6604–6618. doi: 10.1038/sj.onc.1210481. [DOI] [PubMed] [Google Scholar]
- 9.Pasello M, Manara MC, Scotlandi K. CD99 at the crossroads of physiology and pathology. J Cell Commun Signal. 2018;12(1):55–68. doi: 10.1007/s12079-017-0445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerisano V, Aalto Y, Perdichizzi S, Bernard G, Manara MC, Benini S, Cenacchi G, Preda P, Lattanzi G, Nagy B, Knuutila S, Colombo MP, Bernard A, Picci P, Scotlandi K. Molecular mechanisms of CD99-induced caspase-independent cell death and cell-cell adhesion in Ewing's sarcoma cells: actin and zyxin as key intracellular mediators. Oncogene. 2004;23(33):5664–5674. doi: 10.1038/sj.onc.1207741. [DOI] [PubMed] [Google Scholar]
- 11.Jung KC, Kim NH, Park WS, Park SH, Bae Y. The CD99 signal enhances Fas-mediated apoptosis in the human leukemic cell line. Jurkat FEBS Lett. 2003;554(3):478–484. doi: 10.1016/S0014-5793(03)01224-9. [DOI] [PubMed] [Google Scholar]
- 12.Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, Muller WA. Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. J Exp Med. 2015;212(7):1021–1041. doi: 10.1084/jem.20150354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Zhou X, Wang Z, Li F, Liu F, Zhong L, Li X, Han X, Wu Z, Chen S, Zhao T. CD99 triggers upregulation of miR-9-modulated PRDM1/BLIMP1 in Hodgkin/Reed-Sternberg cells and induces redifferentiation. Int J Cancer. 2012;131(4):E382–394. doi: 10.1002/ijc.26503. [DOI] [PubMed] [Google Scholar]
- 14.Laopajon W, Pata S, Takheaw N, Surinkaew S, Khummuang S, Kasinrerk W. Triggering of CD99 on monocytes by a specific monoclonal antibody regulates T cell activation. Cell Immunol. 2019;335:51–58. doi: 10.1016/j.cellimm.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Waclavicek M, Majdic O, Stulnig T, Berger M, Sunder-Plassmann R, Zlabinger GJ, Baumruker T, Stockl J, Ebner C, Knapp W, Pickl WF. CD99 engagement on human peripheral blood T cells results in TCR/CD3-dependent cellular activation and allows for Th1-restricted cytokine production. J Immunol. 1998;161(9):4671–4678. [PubMed] [Google Scholar]
- 16.Bremond A, Meynet O, Mahiddine K, Coito S, Tichet M, Scotlandi K, Breittmayer JP, Gounon P, Gleeson PA, Bernard A, Bernard G. Regulation of HLA class I surface expression requires CD99 and p230/golgin-245 interaction. Blood. 2009;113(2):347–357. doi: 10.1182/blood-2008-02-137745. [DOI] [PubMed] [Google Scholar]
- 17.Sohn HW, Shin YK, Lee IS, Bae YM, Suh YH, Kim MK, Kim TJ, Jung KC, Park WS, Park CS, Chung DH, Ahn K, Kim IS, Ko YH, Bang YJ, Kim CW, Park SH. CD99 regulates the transport of MHC class I molecules from the Golgi complex to the cell surface. J Immunol. 2001;166(2):787–794. doi: 10.4049/jimmunol.166.2.787. [DOI] [PubMed] [Google Scholar]
- 18.Manara MC, Bernard G, Lollini PL, Nanni P, Zuntini M, Landuzzi L, Benini S, Lattanzi G, Sciandra M, Serra M, Colombo MP, Bernard A, Picci P, Scotlandi K. CD99 acts as an oncosuppressor in osteosarcoma. Mol Biol Cell. 2006;17(4):1910–1921. doi: 10.1091/mbc.e05-10-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manara MC, Pasello M, Scotlandi K. CD99: a cell surface protein with an oncojanus role in tumors. Genes (Basel) 2018 doi: 10.3390/genes9030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scotlandi K, Perdichizzi S, Bernard G, Nicoletti G, Nanni P, Lollini PL, Curti A, Manara MC, Benini S, Bernard A, Picci P. Targeting CD99 in association with doxorubicin: an effective combined treatment for Ewing's sarcoma. Eur J Cancer. 2006;42(1):91–96. doi: 10.1016/j.ejca.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. 2001;166(8):4931–4942. doi: 10.4049/jimmunol.166.8.4931. [DOI] [PubMed] [Google Scholar]
- 22.Husak Z, Printz D, Schumich A, Potschger U, Dworzak MN. Death induction by CD99 ligation in TEL/AML1-positive acute lymphoblastic leukemia and normal B cell precursors. J Leukoc Biol. 2010;88(2):405–412. doi: 10.1189/jlb.0210097. [DOI] [PubMed] [Google Scholar]
- 23.Chung SS, Eng WS, Hu W, Khalaj M, Garrett-Bakelman FE, Tavakkoli M, Levine RL, Carroll M, Klimek VM, Melnick AM, Park CY. CD99 is a therapeutic target on disease stem cells in myeloid malignancies. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aaj2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Park HJ, Park EH, Ju HY, Oh CM, Kong HJ, Jung KW, Park BK, Lee E, Eom HS, Won YJ. Nationwide statistical analysis of lymphoid malignancies in Korea. Cancer Res Treat. 2018;50(1):222–238. doi: 10.4143/crt.2017.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94(11):1488–1492. doi: 10.3324/haematol.2009.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora M, Gowda S, Tuscano J. A comprehensive review of lenalidomide in B-cell non-Hodgkin lymphoma. Ther Adv Hematol. 2016;7(4):209–221. doi: 10.1177/2040620716652861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7(10):750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 28.Dreyling M, Amador V, Callanan M, Jerkeman M, Le Gouill S, Pott C, Rule S, Zaja F, European Mantle Cell Lymphoma N Update on the molecular pathogenesis and targeted approaches of mantle cell lymphoma: summary of the 12th annual conference of the European Mantle Cell Lymphoma Network. Leuk Lymphoma. 2015;56(4):866–876. doi: 10.3109/10428194.2014.940584. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Wang M, Romaguera J. Current regimens and novel agents for mantle cell lymphoma. Br J Haematol. 2014;167(1):3–18. doi: 10.1111/bjh.13000. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Yellapantula V, Fenelus M, Pichardo J, Wang L, Landgren O, Dogan A, Roshal M. Tumor suppressor CD99 is downregulated in plasma cell neoplasms lacking CCND1 translocation and distinguishes neoplastic from normal plasma cells and B-cell lymphomas with plasmacytic differentiation from primary plasma cell neoplasms. Mod Pathol. 2018;31(6):881–889. doi: 10.1038/s41379-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokrass MJ, Liu MF, Lindorfer MA, Taylor RP. Activation of complement by monoclonal antibodies that target cell-associated beta(2)-microglobulin: implications for cancer immunotherapy. Mol Immunol. 2013;56(4):549–560. doi: 10.1016/j.molimm.2013.05.242. [DOI] [PubMed] [Google Scholar]
- 32.Dworzak MN, Fritsch G, Buchinger P, Fleischer C, Printz D, Zellner A, Schollhammer A, Steiner G, Ambros PF, Gadner H. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. 1994;83(2):415–425. doi: 10.1182/blood.V83.2.415.415. [DOI] [PubMed] [Google Scholar]
- 33.Lee SP, Park S, Park J, Hong J, Ko YH. Clinicopathologic characteristics of CD99-positive diffuse large B-cell lymphoma. Acta Haematol. 2011;125(3):167–174. doi: 10.1159/000322551. [DOI] [PubMed] [Google Scholar]
- 34.Bernard G, Breittmayer JP, de Matteis M, Trampont P, Hofman P, Senik A, Bernard A. Apoptosis of immature thymocytes mediated by E2/CD99. J Immunol. 1997;158(6):2543–2550. [PubMed] [Google Scholar]
- 35.Bakema JE, van Egmond M. Fc receptor-dependent mechanisms of monoclonal antibody therapy of cancer. Curr Top Microbiol Immunol. 2014;382:373–392. doi: 10.1007/978-3-319-07911-0_17. [DOI] [PubMed] [Google Scholar]
- 36.Redman JM, Hill EM, AlDeghaither D, Weiner LM. Mechanisms of action of therapeutic antibodies for cancer. Mol Immunol. 2015;67(2 Pt A):28–45. doi: 10.1016/j.molimm.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7(5):105. doi: 10.21037/atm.2019.01.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, Sotto JJ, Leroux D, Bensa JC, Plumas J. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101(3):949–954. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 39.Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, Groen RW, van Duin M, Sonneveld P, Minnema MC, Zweegman S, Chiu C, Bloem AC, Mutis T, Lokhorst HM, Sasser AK, van de Donk NW. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–970. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]
- 40.Bannerji R, Kitada S, Flinn IW, Pearson M, Young D, Reed JC, Byrd JC. Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistance. J Clin Oncol. 2003;21(8):1466–1471. doi: 10.1200/JCO.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Kato Y, Kunita A, Fukayama M, Abe S, Nishioka Y, Uchida H, Tahara H, Yamada S, Yanaka M, Nakamura T, Saidoh N, Yoshida K, Fujii Y, Honma R, Takagi M, Ogasawara S, Murata T, Kaneko MK. Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts antitumor activity against human podoplanin through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Monoclon Antib Immunodiagn Immunother. 2017;36(1):20–24. doi: 10.1089/mab.2016.0045. [DOI] [PubMed] [Google Scholar]
- 42.Takei J, Ohishi T, Kaneko MK, Harada H, Kawada M, Kato Y. A defucosylated anti-PD-L1 monoclonal antibody 13-mG2a-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Biochem Biophys Rep. 2020;24:100801. doi: 10.1016/j.bbrep.2020.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe M, Ohishi T, Kuzuoka M, Nudelman ED, Stroud MR, Kubota T, Kodairo S, Abe O, Hirohashi S, Shimosato Y, et al. In vitro and in vivo antitumor effects of murine monoclonal antibody NCC-ST-421 reacting with dimeric Le(a) (Le(a)/Le(a)) epitope. Cancer Res. 1991;51(8):2199–2204. [PubMed] [Google Scholar]
- 44.Guhad FA, Jensen HE, Hau J. Complement activation in SCID and nude mice is related to severity of tissue inflammation in the Candida mastitis model. FEMS Microbiol Lett. 2000;192(1):27–31. doi: 10.1111/j.1574-6968.2000.tb09354.x. [DOI] [PubMed] [Google Scholar]
- 45.Dhandapani M, Goldman A. Preclinical cancer models and biomarkers for drug development: new technologies and emerging tools. J Mol Biomark Diagn. 2017 doi: 10.4172/2155-9929.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gengenbacher N, Singhal M, Augustin HG. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat Rev Cancer. 2017;17(12):751–765. doi: 10.1038/nrc.2017.92. [DOI] [PubMed] [Google Scholar]
- 47.Feng L, Wang W, Yao HP, Zhou J, Zhang R, Wang MH. Human tumor xenografts in mouse as a model for evaluating therapeutic efficacy of monoclonal antibodies or antibody-drug conjugate targeting receptor tyrosine kinases. Methods Mol Biol. 2015;1233:151–159. doi: 10.1007/978-1-4939-1789-1_14. [DOI] [PubMed] [Google Scholar]
- 48.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3(2):143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 49.Choi G, Roh J, Park CS. CD99 is strongly expressed in basal cells of the normal adult epidermis and some subpopulations of appendages: comparison with developing fetal skin. J Pathol Transl Med. 2016;50(5):361–368. doi: 10.4132/jptm.2016.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.