Abstract
Background
To understand how to improve the effect of immune checkpoint inhibitors in uveal melanoma (UM), we need a better understanding of the expression of PD-1 and PD-L1, their relation with the presence of tumor-infiltrating lymphocytes (TILs), and their prognostic relevance in UM patients.
Materials and methods
Expression of PD-1 and PD-L1 was assessed in 71 UM tissue samples by immunohistochemistry and quantitative real-time PCR (qRT-PCR), and further validated by western blotting. The effect of interferon gamma (IFN-γ) on PD-1/PD-L1 expression was determined on four UM cell lines.
Results
Immunoreactivity of PD-1 was found in 30/71 cases and of PD-L1 in 44/71 UM samples. Tumor-infiltrating lymphocytes were found in 46% of UM tissues. PD-1 was expressed on TILs while tumor cells expressed PD-L1. UM with and without TILs showed expression of PD-1 in 69% and 18% cases, respectively (p = 0.001). Similarly, PD-L1 was found in 75% of UM with TILs and in 50% of cases without TILs, respectively (p = 0.03). DFS rate were lower in patients with TILs with expression of PD-1 and PD-L1, but the rate of DFS was higher with expression of PD-L1 in patients without TILs. After treatment of UM cell lines with IFN-γ, PD-1 expression was induced in all UM cell lines whereas PD-L1 expression was found at a lower level in untreated cells, while expression also increased following treatment with IFN-γ.
Conclusion
Our study suggests that increased infiltration with TILs promotes the aggressive behavior and suppresses the immune response of UM cells, thereby inhibiting immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02773-8) contains supplementary material, which is available to authorized users.
Keywords: Uveal melanoma, Immunotherapy, Tumor-infiltrating lymphocytes, Interferon gamma
Introduction
Melanomas of the uveal tract constitute 5% of all melanomas [1]. Uveal melanoma (UM) is the most common intraocular primary malignancy in adults [1]. It develops from neural crest-derived uveal melanocytes [2]. Genetic alterations such as monosomy 3 (M3), polysomy of 8q and loss of BAP1 in primary UM are associated with poor prognosis.[3].
Treatment modalities such as brachytherapy, external beam radiotherapy, and photon-based radiation, are often able to preserve vision in the affected eye [4–6]. Enucleation of the affected eye is done when the tumor is too large for one of the other treatments [7]. Local treatments is often sufficient to control the primary UM but do not prevent metastases to the liver [8]. Immunotherapy is rapidly becoming a mainstream treatment of various cancers; nonetheless, less than 30% of patients benefit from this approach. There are presently no standard proven treatments for metastatic UM patients [9]. Therefore, there is an urgent need to develop novel immunotherapeutic agents for patients [10].
The eye is one of a few areas of the body with ‘immune privilege’, where both innate and adaptive immunity are suppressed by many known mechanisms [11]. The emerging field of checkpoint receptors has discovered a hidden, and powerful, ability of the adaptive immune system that can be used to reduce tumor growth. The tumor microenvironment has malignant tumor cells, non-malignant stromal cells and immune cells that form the structure of the tumor and provide access to blood vessels that contribute to tumor development [12]. Part of the immune cells is referred to as the tumor-infiltrating lymphocytes (TILs). The primary host defense mechanisms against cancer are probably immunologic. Multiple tumor antigens can be recognized by TILs, and this represents an important component of the anti-tumor immune response [13].
Several studies have evaluated the role of TILs in the clinical outcome and prognosis prediction to the response of existing checkpoint inhibitors in cancer [14–19]. The existence of TILs is associated with better outcome in many solid tumors; however, TILs correlate with poor prognosis in UM; how TILs promote this tumor progression has not yet been determined properly [20].
Current treatments for metastatic tumors rely on modification of the immune system to kill tumor cells. Immunotherapies aimed at inhibition of cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) have been successfully exploited in the treatment of different malignancies, providing long-lasting clinical responses [21]. PD-1 molecules are not only expressed on the surface of activated T cells but also on B cells and bind to the programmed death ligand 1 (PD-L1). This then functionally impairs the activated T cell, preventing it from mounting an effective immune response against tumor antigens. The poor outcome of anti-PD-1 and anti-CTLA-4 agents in metastatic UM may be due to several features such as a low mutational burden or an immune-suppressive microenvironment of the liver, both considered immune escape mechanisms [22].
Studies have demonstrated that inhibitors of PD-1 and PD-L1 as well as other immune checkpoint blockade therapies result in an increase in interferon-gamma (IFN-γ) production, which in turn inhibits the growth of tumor cells [23, 24]. Expression of PD-L1 is low on the surface of T cells, B cells, macrophages, and dendritic cells. In some tumors, PD-L1 is not constitutively expressed, but rather depends on proinflammatory signals that are released by T cells such as IFN-γ. In tumors, TILs are often the main source of IFN-γ, enhancing the expression of PD-1 and PD-L1 [25]. Recently, Mauguso et al. established that defects in IFN-γ signaling induce the resistance to immunotherapy in B16 melanoma cells [26]. UM cell lines expressed PD-L1 by suppressing IL-2 production after the induction of IFN-γ [27]. However, the role of TILs with regard to PD-1 and PD-L1 has been suggested but not yet demonstrated.
The aim of our study is to elucidate the role of TILs in the expression of PD-1 and PD-L1 in UM, for which we defined a group with TILs (group I, n = 33) and a group of UM without TILs (group II, n = 38)) and correlated expression with clinicopathological parameters and patient outcome. In addition to this, we performed in vitro studies on UM cell lines by treating them with interferon-gamma and assessed the level of PD-1/PD-L1 along with cytokines known to be associated with infiltrating T lymphocytes.
Materials and methods
Sample size
Patient samples were collected in the Department of Ocular Pathology, Dr. Rajender Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India. The in vitro studies were performed at the Department of Ophthalmology, University of California, Irvine (UCI), USA. This study was performed on human tissues after the approval by Institutional Ethics Committee (IEC), All India Institute of Medical Sciences (AIIMS), New Delhi (Ref. No. IEC-424/RP-6/2016). Written consent for participation in this study and providing tissue were obtained from all the patients. All procedures conformed to the tenets of the Declaration of Helsinki. The follow-up period was 6–65 months (mean = 41 months). Formalin-fixed tissue samples were used for immunohistochemistry. Fresh tumor samples were immediately used for RNA and protein extraction and stored at − 80 °C for further experiments.
Clinicopathological details
Demographic, clinical, and radiological data were obtained from the patient’s medical records. Data regarding clinical features such as gender and age at presentation, largest tumor basal diameter, tumor thickness, scleral and ciliary body invasion, extraocular tumor extension and AJCC clinical staging (AJCCc) were collected. Gross examination of enucleated eyes included tumor size, location, the presence of retinal detachment, iris neovascularization, or vitreous hemorrhage, and orbital involvement. Hematoxylin and eosin slides were reviewed for histopathological characteristics such as the cell type, scleral invasion, ciliary body involvement, number of mitoses per 0.25 mm2 at × 40 magnification, counting 40 high-power fields, number of infiltrating macrophages, microvascular density (MVD), largest basal diameter (LBD) of > 15 mm [28], and tumor thickness > 8 mm [29] and necrosis. Infiltrating macrophages were identified using ≤ 50% CD68-positive macrophages whereas MVD was determined by counting ≤ 50 vessels/0.25 mm2 using immunostaining for CD34 epitope [30, 31]. Loss of BAP-1 staining was also identified by immunohistochemistry. Routine clinical and laboratory examinations were performed during follow up of the patients.
Detail of UM cell lines
We obtained UM cell lines from Prof. Martine Jager (co-author), Leiden University Medical Center, Leiden, The Netherlands. The 92.1 cell line was derived from a primary UM that had grown into the right orbit of a 76-year-old woman. The right globe had been displaced superotemporally by the large UM tumor that involved the optic nerve, inferior rectus, lateral rectus, and superior rectus muscles. The 92.1 cells exhibit disomy 3, tetrasomy of chromosome 6p and tetrasomy of chromosome 8. It carries an EIF1AX mutation, and expresses the BAP1 protein [32].
The MEL270 cell line was derived from the primary choroidal melanoma of a 79-year-old patient. Cell lines OMM2.3 and OMM2.5 were derived from liver metastases of the same case. The patient had also developed metastases in the ribs and the iliac wing [33, 34]. Sanger sequencing showed a GNAQ Q209 mutation as well as heterogeneity with only one signal of chromosome 3, but two copies of 8q and 8p. These cell lines show no mutations in BAP1, EIFIAX, or SF3B1.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue Sects. (4 µm) were taken for immunohistochemistry using ImmPACT AEC (Vector Laboratories; Burlingame, CA). Sections were deparaffinized and rehydrated and subjected to heat-induced epitope retrieval using citrate buffer (pH 6.0) for 30 min. Blocking was done by 3% hydrogen peroxide for 30 min. After washing with tris-buffered saline (TBS; pH 7.4), sections were incubated with corresponding primary antibody against PD-1 [Clone: D4W2J (Cell Signaling)] or PD-L1 [Clone: E1L3N (Cell Signaling)] overnight at 4 °C. Sections were again washed three times with TBS. Enzyme-conjugated secondary incubation was carried out in the dark for 30 min at room temperature. Immunoreactivity was developed using AEC (3-amino-9-ethylcarbazole) Red chromogen and the reaction was stopped in distilled water. Sections were counterstained with hematoxylin and mounted with aqueous mount media (BDH, Poole, UK). Finally, sections were examined by light microscopy. A corresponding antibody-positive control (placenta) and retinal pigment epithelium were taken as controls and a negative control (without primary antibody) was run with every experiment.
Immunoreactivity scoring
Semi-quantitative scoring for expression of PD-1 and PD-L1 proteins was independently done using a light microscope by two authors (MKS and LS), and consensus was reached under the supervision of the experienced pathologist (SK) regarding the immunohistochemistry score. The score for staining intensity was considered as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The staining percentage for tumor cells was scored as 0 as < 5%, 1 as 5–25%, 2 as 26–50%, 3 as 51–75% and 4 as > 75%. Immunoreactivity scores (IRS) were obtained in each case by multiplying the score for staining intensity and percentage positivity of tumor cells. The tumor was considered as negative when the final score was ≤ 4 and positive when > 4 [35].
The density of tumor-infiltrating lymphocytes (TILs) was determined using primary antibodies directed against CD3, CD4, and CD8. Immunoreactivity was developed using AEC Red chromogen. Their presence was scored as 1 (few), 2 (moderate) or 3 (many) [20]. Cases with a low staining for CD3, CD4, as well as CD8 were classified as “negative” (1), whereas moderate (2) and numerous (3) TILs were together grouped as “positive”. We grouped our cohort on the basis of cases having positive expression of CD3, CD4 and CD8 as cases of UM with TILs (group I) and negative expression of CD3, CD4 and CD8 as cases of UM without TILs (group II).
RNA extraction, cDNA synthesis, and qRT-PCR
RNA extraction was performed on 71 fresh tumor samples and 20 controls (normal uveal tissue) from staphylomatous eyes as non-tumor samples using RNA isolation kit (Purelink RNA Kit, Ambion, USA) as per the manufacturer’s protocol. Quantity and purity of RNA was measured on Nanodrop spectrophotometer. An OD260/280 of 1.8–2.0 indicates the purity of RNA devoid of any contamination of protein. Total RNA was judged to be intact if discreet 28S and 18S ribosomal RNA bands were observed on 1% agarose gel electrophoresis.
Concentration of 100 ng/µL of RNA was reverse transcribed into cDNA using Verso cDNA synthesis kit (Thermo Scientific, California, USA). The mRNA level of PD-1 and PD-L1 gene was determined by qRT-PCR using SYBR Green Master Mix (Thermo, Invitrogen, USA) in tumor and control samples in triplicates. Hypoxanthine phosphoribosyl transferase (HPRT) was used as housekeeping gene for the experiment. Reactions were carried out in a final volume of 10 μl. KiCqStart® SYBR® green primers (Sigma, St. Louis, MO) were used to examine the expression of PD-1 (PDCD1; Ref# NM_005018), PD-L1 (CD274; Ref# NM_014143) and cytokines such as IL-2 (Ref# NM_000586), IL-4 (Ref# NM_172348), L-21 (Ref# NM_001207006), IL-6 (Ref# NM_000600), IL-1β (Ref# NM_000576) and TNF-α (Ref# NM_000594). QuantiTect SYBR® green primer assay (QIAGEN®, USA) was used for TGF-β (Ref# QT00080934) and IFN-γ (Ref# QT00066675). The PCR conditions were as follows: 95 °C for 10 min, followed by 35 cycles of 95 °C for 30 s, 60 °C (PD-1)/57 °C (PD-L1) for 30 s and 72 °C for 30 s. Each PCR reaction was followed by continuous melt curve analysis. No template control (NTC) was also included in each PCR run to assess contamination.
Data analysis was performed after normalisation to the reference gene to calculate a fold-change value using the ∆∆Ct method which was calculated by subtracting ∆Ct of the uveal melanoma samples from ∆Ct of the control samples. ∆Ct was the difference between the threshold cycles (Cts) of the target gene (PD-1 and PD-L1 gene) and the housekeeper gene (HPRT gene). Fold change was calculated using the following formula: Fold change = 2ΔΔCt. Fold change value ≥ 1 was considered as ‘upregulation’, whereas fold change value < 1 was considered as ‘downregulation’ of the gene.
Western blotting
A nuclear and cytoplasmic extraction kit (Thermo Scientific, USA) was used for performing protein extraction in 16 representative fresh uveal melanoma tissue samples (8 each from group I and group II) to validate the immunohistochemistry. Protein concentration was determined by Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific, USA). From the total protein, 25 µg was separated and resolved by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (MDI Membrane Technologies, Pasadena, CA, USA) at 90 V for 2 hr. Membranes were subjected to Ponceau S staining for the detection of transferred protein and then washed with water for blocking. Immunoblot was blocked using 5% bovine serum albumin (BSA) in Tris-buffered saline-Tween20 (TBST) and subsequently incubated with primary antibody against PD-1 (1:2000), PD-L1 (1:2000) and β-actin (1:5000; Abcam) overnight at 4 °C. Blots were then washed three times with 1 × TBST for 5 min each and incubated with the corresponding secondary antibodies conjugated with HRP (HRP labeled, 1:5000; Cell Signaling Technology) for 1 h at room temperature. Dilutions of primary and secondary antibodies were prepared in TBST. Finally, blots were washed again with TBST, and protein bands were visualized by enhanced chemiluminescence method using Western BLoT Ultra-Sensitive HRP Substrate kit (Takara Bio USA, Inc.).
Cell line and growth condition
A normal retinal pigmented epithelial cell line, ARPE-19, was grown at UCI, USA and cultured in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% of fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific) and 2% of a penicillin/streptomycin mixture (Sigma-Aldrich, St. Louis, MO, USA). One vial (1 ml) of established uveal melanoma cell lines (92.1, MEL270, OMM2.3, and OMM2.5) was used for the experiments [32–34]. All UM cells were grown in RPMI 1640, Dutch Modified (Life Technologies, Cat. # 22,409–015) supplemented with 10% FBS, 3 mM l-glutamine (1%, Life Technologies, Cat. # 35,050–038), 2% penicillin/streptomycin (10 ml, Life Technologies, Cat. # 15,140–122).
Trypan blue exclusion assay for cell viability after treatment with IFN-γ
Cells were grown in culture media and counted with a cell counter. After counting, cells were seeded in 6-well plates (Corning Life Science) at a density of 5 × 105 cells/well for 24 h. Media were replaced and treated with culture media containing 100 international units (IU)/ml of IFN-γ (ImmunoTools, Germany) and incubated for 48 hr. Cell viability was assessed by trypan blue exclusion assay on untreated and treated cells using the automated Vi-Cell Viability Analyzer (Vi-Cell; Beckman Coulter, Fullerton, CA). Untreated and treated cells were subsequently prepared for RNA extraction and qRT-PCR (protocol as described above) to determine the mRNA expression level of PD-1, PD-L1 and cytokines gene associated with T lymphocytes such as IL-2, IL-4, IL-21, IL-1, IL-1β, TNF-α, TGF-β, IFN-γ.
Statistical analysis
All tests were two sided to evaluate the statistical association of immunohistochemical and mRNA expression of PD-1 and PD-L1 with clinicopathological parameters and patient outcome. A statistical significance for all tests were considered as p < 0.05. All statistical analyses were performed using Stata 9 software (Stata Corp LP, College Station, TX, USA). Log-rank test for equality of survivor functions was performed for the estimation of disease-free survival (DFS) of the patient with a positive or negative expression of protein markers. Hazard ratios and their 95% confidence intervals (CI) were noted for each parameter and independent prognostic factors were identified by univariate and multivariate analysis through Cox proportional-hazards models. Survival curves were drawn by Kaplan–Meier method. Graphs were drawn by GraphPad Prism software. The p value was adjusted for multiple comparisons using the Benjamini–Hochberg (BH) correction.
Results
Patient characteristics
Demographical, clinica,l and pathological features were noted for all 71 patients. Enucleation was performed on 62 UM cases, while 9 cases underwent an exenteration. No patient underwent brachytherapy or radiotherapy. UM cases were divided on the basis of TILs: UM with TILs (group I; n = 33; CD3 + or CD4 or CD8 +) and UM without TILs (group II; n = 38; all CD3-/CD4-/CD8-). Group I (with TIL) showed a significantly higher age, more often LBD of > 15 mm, and tumor thickness > 8 mm, more frequently an epithelioid cell type, mitotic count (median = 2; mean ± SD = 3.5 ± 3.4), infiltrating macrophages (p < 0001) and microvascular densities (p = 0.007), and an absence of BAP-1 staining (p < 0.001) using Mann–Whitney U test (Table 1).
Table 1.
Clinicopathological correlation between group I and group II uveal melanoma patients using Mann–Whitney Wilcoxon Test
| Clinicopathological parameters (N = 71) | N = 71 (%) | UM with Tumour Infiltrating Lymphocytes (Group I) N = 33 (%) | UM without Tumour Infiltrating Lymphocytes (Group II) N = 38 (%) | *p value | **p value |
|---|---|---|---|---|---|
| Largest tumor basal diameter | |||||
| ≤ 15 mm | 22 (30%) | 6 (18%) | 16 (42%) | 0.031 | 0.071 |
| > 15 mm | 49 (69%) | 27 (81%) | 22 (57%) | ||
| Tumour thickness | |||||
| ≤ 8 mm (36) | 36 (50%) | 12 (36%) | 24 (63%) | 0.025 | 0.067 |
| > 8 mm (35) | 35 (49%) | 21 (63%) | 14 (36%) | ||
| Epithelioid cell type | |||||
| Absent | 31 (43%) | 7 (21%) | 24 (63%) | < 0.001 | 0.016 |
| Present | 40 (56%) | 26 (78%) | 14 (36%) | ||
| Mitotic count | |||||
| < 4/40HPF | 43 (60%) | 12 (36%) | 31 (81%) | < 0.001 | 0.008 |
| ≥ 4/40HPF | 28 (39%) | 21 (63%) | 7 (18%) | ||
| Infiltrating macrophages | |||||
| ≤ 50% CD68 positivity | 40 (56%) | 9 (27%) | 31 (81%) | < 0.001 | 0.005 |
| > 50% CD68 positivity | 31 (43%) | 24 (72%) | 7 (18%) | ||
| Microvascular density | |||||
| ≤ 50 vessels/0.25mm2 | 38 (53%) | 12 (36%) | 26 (68%) | 0.007 | 0.022 |
| > 50 vessels/0.25mm2 | 33 (46%) | 21 (63%) | 12 (31%) | ||
| BAP1 staining | |||||
| Present | 29 (40%) | 6 (18%) | 23 (60%) | < 0.001 | 0.004 |
| Absent | 42 (59%) | 27 (81%) | 15 (39%) | ||
*p value before Benjamini–Hochberg correction
**p value adjusted after Benjamini–Hochberg correction
Bold signifies statistically significant value
Immunoexpression of PD-1 and PD-L1 and their correlation with clinicopathological parameters
Expression of PD-1 and PD-L1 protein was evaluated in all 71 FFPE tissue samples (Fig. 1). Immunoreactivity of PD-1 was found in 30/71 cases, whereas PD-L1 was seen in 44/71 samples (Fig. 2a, f). Expression of PD-1 was present in TILs while tumour cells expressed PD-L1. PD-1 expression was mainly found in UM with TILs (group I; 23/33 cases) whereas few cases showed positivity in UM without TILs (group II; 7/38 cases). PD-L1 was found in the majority of UM with TILs (group I; 25/33 cases) and in 50% of UM without TILs (group II; 19/38 cases).
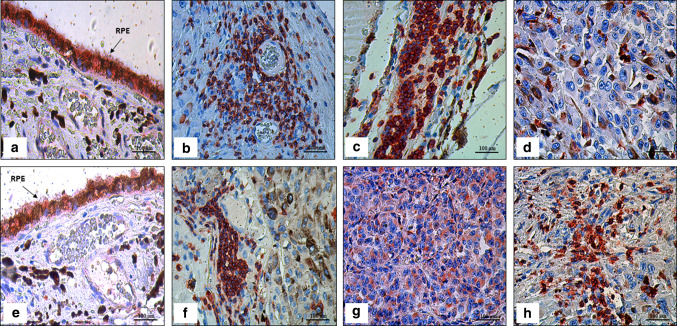
Fig. 1.
Immunoexpression in uveal melanoma (UM) tissue samples with and without tumor infiltrating lymphocytes (TILs) using AEC (3-amino-9-ethylcarbazole) red chromogen, and then counterstained with hematoxylin. a Immunoexpression of PD-1 in retinal pigment epithelium (RPE; arrow) as an internal control (× 400); (b) Immunoexpression of PD-1 in TILs near the intratumoral blood vessels (× 400); (c) Immunoexpression of PD-1 in TILs (× 400); (d) Weak immunoexpression of PD-1 in mixed cell type (× 400); (e) Immunoexpression of PD-L1 in retinal pigment epithelium as an internal control (× 400); (f) Immunoexpression of PD-L1 in TILs (× 400); (g) Cytoplasmic immunoexpression of PD-L1 in epithelioid UM (× 400); (h) Strong immunoexpression of PD-L1 in mixed cell type (× 400)
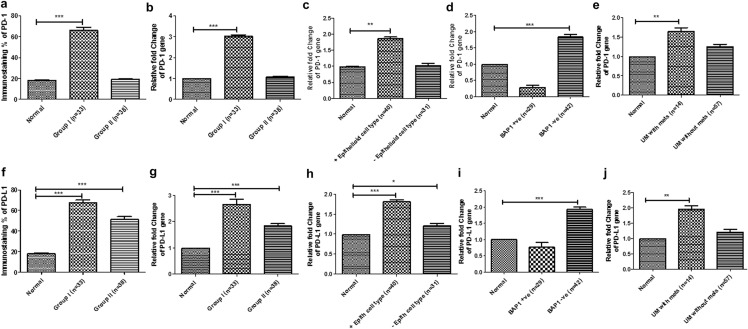
Fig. 2.
Bar graph representing immunoexpression and relative mRNA fold change using quantitative real-time PCR (qRT-PCR). a immunoexpression of PD-1 in group I and group II of uveal melanoma cases; relative mRNA fold change of PD-1 in (b) group I and group II, (c) cases with epithelioid cell type, (d) expression of BAP-1, and (e) cases with UM with metastasis using qRT-PCR (*P < 0.05, **P < 0.01, ***P < 0.001); (f) immunoexpression of PD-L1 in group I and group II of uveal melanoma cases. The relative mRNA fold change of PD-L1 (g) in group I and group II, (h) cases with epithelioid cell type, (i) expression of BAP-1, and (j) cases with UM with metastasis using real-time PCR (*P < 0.05, **P < 0.01, ***P < 0.001)
The association of PD-1 and PD-L1 expression with clinicopathological parameters was analyzed using Fisher’s exact test. In group I, PD-1 expression was correlated significantly with ciliary body invasion (5/11 cases; p = 0.032), epithelioid cell type (21/26 cases; p = 0.008), infiltrating macrophages (21/24 cases; p < 0.001), absence of BAP-1 staining (17/27 cases; p = 0.047) and liver metastasis (4/9 cases; p = 0.043). PD-L1 expression also correlated significantly with higher AJCC tumor staging (13/14 cases, p = 0.049), infiltrating macrophages (16/24 cases; p = 0.047) and liver metastasis (9/9; p = 0.047)). The other parameters were not significantly correlated to PD-1 and PD-L1 expression in group I. Similarly, in group II, PD-1 expression was correlated significantly with ciliary body invasion (3/6 cases; p = 0.030), epithelioid cell type (5/14 cases; p = 0.036), and liver metastasis (3/5 cases; p = 0.010), whereas PD-L1 was related with increased tumor pigmentation (11/28 cases; p = 0.027) and infiltrating macrophages (6/7 cases; p = 0.036). The other parameters were not significantly correlated with PD-1 and PD-L1 expression in group II (Table 2).
Table 2.
Correlation of PD-1 and PD-L1 immunoexpression with the clinicopathological parameters in group I and group II uveal melanoma patients
| Clinicopathological parameters | N = 33 | Uveal melanoma with tumor infiltrating lymphocytes (TILs) (Group I) | N = 38 | Uveal melanoma without tumor infiltrating lymphocytes (TILs) (Group II) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-1 Immunoexpression | PD-L1 Immunoexpression | PD-1 Immunoexpression | PD-L1 Immunoexpression | |||||||||||||||
| Absent (10) | Present (23) | *p value | **p value | Absent (8) | Present (25) | *p value | **p value | Absent (31) | Present (7) | *p value | p-value | Absent (19) | Present (19) | *p value | **p value | |||
| JCC clinical staging | ||||||||||||||||||
| T1–T2 | 19 | 6 | 13 | 0.85 | 1.943 | 7 | 12 | 0.049 | 0.261 | 25 | 20 | 5 | 0.72 | 0.886 | 12 | 13 | 0.73 | 0.973 |
| T3–T4 | 14 | 4 | 10 | 1 | 13 | 13 | 11 | 2 | 7 | 6 | ||||||||
| Ciliary body invasion | ||||||||||||||||||
| Absent | 22 | 4 | 18 | 0.032 | 0.171 | 5 | 17 | 0.77 | 0.821 | 32 | 28 | 4 | 0.030 | 0.240 | 16 | 16 | 1.00 | 1.067 |
| Present | 11 | 6 | 5 | 3 | 8 | 6 | 3 | 3 | 3 | 3 | ||||||||
| Tumor pigmentation | ||||||||||||||||||
| Decrease | 10 | 2 | 8 | 0.39 | 1.040 | 1 | 9 | 0.20 | 0.533 | 10 | 8 | 2 | 0.88 | 0.939 | 2 | 8 | 0.027 | 0.432 |
| Increase | 23 | 8 | 15 | 7 | 16 | 28 | 23 | 5 | 17 | 11 | ||||||||
| Epithelioid cell type | ||||||||||||||||||
| Absent | 7 | 5 | 2 | 0.008 | 0.064 | 1 | 6 | 0.48 | 0.768 | 24 | 22 | 2 | 0.036 | 0.192 | 13 | 11 | 0.50 | 1.000 |
| Present | 26 | 5 | 21 | 7 | 19 | 14 | 9 | 5 | 6 | 8 | ||||||||
| Infiltrating macrophages | ||||||||||||||||||
| ≤ 50% CD68 positivity | 9 | 7 | 2 | 0 | 9 | 31 | 27 | 4 | 0.06 | 18 | 13 | |||||||
| > 50% CD68 positivity | 24 | 3 | 21 | < 0.001 | 0.016 | 8 | 160 | 0.047 | 0.752 | 7 | 4 | 3 | 0.240 | 1 | 6 | 0.036 | 0.288 | |
| BAP1 staining | ||||||||||||||||||
| Present | 6 | 0 | 6 | 2 | 4 | 0.747 | 23 | 20 | 3 | 0.29 | 0.580 | 12 | 11 | 0.74 | 0.911 | |||
| Absent | 27 | 10 | 17 | 0.047 | 0.150 | 6 | 21 | 0.56 | 15 | 11 | 4 | 7 | 8 | |||||
| Liver Metastasis | ||||||||||||||||||
| Absent | 24 | 5 | 19 | 0.043 | 0.172 | 8 | 16 | 0.047 | 0.37 | 33 | 29 | 4 | 0.010 | 0.160 | 18 | 15 | 0.15 | 0.600 |
| Present | 9 | 5 | 4 | 0 | 9 | 5 | 2 | 3 | 1 | 4 | ||||||||
*p value before Benjamini–Hochberg correction
**p value adjusted after Benjamini–Hochberg correction
Bold signifies statistically significant value
mRNA expression of PD-1 and PD-L1 and their correlation with clinicopathological parameters
mRNA expression was evaluated in all 71 fresh tumor tissues, which contained both tumor and tumor-infiltrating lymphocytes. PD-1 and PD-L1 mRNA expression was found in 26/71 cases and 39/71 cases, respectively. We found a significantly higher expression of PD-1 mRNA expression with tumor thickness, epithelioid cell type, high number of infiltrating macrophages, high microvascular densities, and absence of BAP1 staining. When looking at PD-L1 mRNA expression, we observed significant correlations with high tumor thickness, high mitotic count, and high numbers of infiltrating macrophages. The correlation of PD-L1 mRNA expression with AJCC clinical staging (p = 0.051), microvascular densities (p = 0.09) and liver metastasis (p = 0.07) did not reach statistical significance. (Supplementary Table 1) (Table 3).
Table 3.
Clinical significance of clinicopathological and molecular parameters of uveal melanoma by univariate and multivariate analysis for disease-free survival (DFS) (Cox proportional-hazards model)
| Clinicopathological parameters | Disease-free survival (DFS) | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| Hazard ratio (95% confidence interval) | *p value | Hazard ratio (95% confidence interval) | *p value | |
| Largest tumor basal diameter (LBD > 15 mm) | 8.4 (1.03–12.93) | 0.047 | 1.66 (0.34–7.95) | 0.52 |
| Tumor thickness greater than 8 mm | 3.67 (1.03–12.93) | 0.043 | 1.20 (0.23–6.09) | 0.82 |
| AJCC clinical staging | 2.92 (0.94–9.08) | 0.06 | – | – |
| Scleral invasion | 3.02 (0.96–10.63) | 0.05 | – | – |
| Ciliary body invasion | 2.72 (0.80–9.28) | 0.10 | – | – |
| Epithelioid cell type | 1.73 (0.52–5.72) | 0.36 | – | – |
| Infiltrating macrophage (> 50% CD68 positivity) | 3.33 (1.00–11.09) | 0.05 | – | – |
| Microvascular density (> 50vessels/0.25 mm2) | 2.86 (0.86–9.51) | 0.08 | – | – |
| CD3+/CD4+/CD8+ (TILs) | 4.25 (1.20–15.03) | 0.025 | 2.82 (0.92–8.68) | 0.06 |
| PD-1 immunoexpression | 3.44 (1.05–11.19) | 0.040 | 3.78 (0.53–53.05) | 0.046 |
| PD-L1 immunoexpression | 12.13 (1.49–98.65) | 0.020 | 9.70 (1.04–90.48) | 0.039 |
| PD-1 mRNA expression | 2.5 (0.77–8.02) | 0.12 | – | – |
| PD-L1 mRNA expression | 2.75 (0.78–9.68) | 0.11 | – | – |
Bold signifies statistically significant value
Validation of immunohistochemical expression of PD-1 and PD-L1 by western blotting
Western blotting was performed to validate the immunoreactivity of PD-1 and PD-L1 protein in the lysate of uveal melanoma tissue samples along with normal controls. Immunoblot analysis of eight representative samples from group 1 and group 2 each were analyzed along with the positive control (β-actin). Prominent bands were found at 60 kDa (PD-1), 50 kDa (PD-L1) and 42 kDa (β-actin). In group I, expression of PD-1 was found in 5/8 samples, whereas PD-L1 was found in 6/8 samples. In group II, expression of PD-1 and PD-L1 was found in 2/8 and 5/8 samples, respectively. These results were consistent in both group of samples with our immunohistochemical results (Supplementary Fig. 1).
Association of clinicopathological parameters and PD-1/PD-L1 expression with patient outcome
We evaluated disease-free survival with clinicopathological and molecular variables for the prognostic outcome in patients with uveal melanoma by Cox proportional-hazards model. The 5-year disease-free survival (DFS) rate in patients expressing PD-1 in the immunohistochemical staining were 70%, and those not expressing PD-1 were 85%, while for PD-L1 these numbers were 68% and those not expressing PD-L1 were 96%. In univariate analysis for DFS, the hazard ratio was higher in cases with loss of BAP-1 (HR 2.47; p = 0.014), tumor thickness > 8 mm (HR 3.67; p = 0.043), largest basal diameter > 15 mm (HR 8.4; p = 0.047), and the presence of TILs expressing CD3 + /CD4 + /CD8 + (HR 4.25; p = 0.025). Immunoreactivity of PD-1 (HR 3.44; p = 0.040) and PD-L1 (HR 12.13; p = 0.020) was associated with poor outcome in univariate analysis for these patients. While performing multivariate analysis for all these parameters together, only PD-1 (HR 3.78; p = 0.046) and PD-L1 (HR 9.70; p = 0.039) immunoreactivity was statistically significant for reduced disease-free survival (Supplementary Table 2).
Our study analyzed the DFS rate separately in both groups of the patients. The DFS rate was lower in patients with the presence of PD-1 (DFS 65%, p = 0.013) and PD-L1 (DFS 71%, p = 0.20) mRNA expression. DFS rate in group I having UM with TILs were lower in patients with positive immunoreactivity and mRNA expression of PD-1 and PD-L1 but the rate of DFS was seen significantly better in patients with expression of PD-L1 in group II (Supplementary Table 3).
Expression of PD-1 and PD-L1 after the treatment with IFN-γ
To assess the ideal working concentration of IFN-γ, we performed an initial concentration titration experiment wherein cell lines were treated with different doses of IFN-γ i.e., 0, 100, and 500 IU/ml for 48 h followed by measurement of viable cell numbers using trypan blue assay. The optimal concentration for the IFN-γ used was 100 IU/ml for treatment in ARPE cell line and UM cell lines (Fig. 3a). After 48 h incubation with IFN-γ, RNA was extracted from untreated and treated cells and followed by qRT-PCR to assess the expression of PD-1, PD-L1 and cytokines. Before treatment, no expression of PD-1 was noticed in any of the cell lines or control cells. IFN-γ treatment induced the expression of PD-1 and PD-L1 in treated cells as compared to untreated cells (Fig. 3b–d). The PD-L1 levels were increased in 92.1 (1.7-fold, p = 0.003), MEL270 (1.6-fold, p = 0.001), OMM2.3 (2.5-fold, p = 0.001) and OMM2.5 (2.6-fold, p = 0.001) compared to ARPE-19. Expression levels of the PD-1 gene were higher in 92.1 (1.3-fold, 0.022), MEL270 cells (1.2-fold, p = 0.016) compared to ARPE-19 cells, but higher in metastatic-derived OMM2.3 (1.7-fold, p = 0.002) and OMM2.5 (1.6-fold, p = 0.001). There was an increased expression of IL-2, IL-4, IL-21, IL-6, and IFN-γ as compared to IL-1β, TGFβ, and TNFα in untreated cells after the treatment (Supplementary Fig. 2).
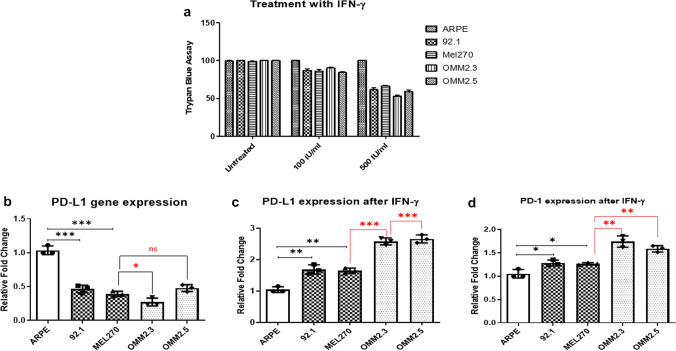
Fig. 3.
Treatment effect of interferon-gamma (IFN-γ) on uveal melanoma in-vitro using Trypan blue dye exclusion assay; (a) Trypan blue dye exclusion assay at concentration of 100 IU/ml and 500 IU/ml; (b) relative mRNA fold change of PD-L1 gene expression; (c) relative mRNA fold change of PD-L1 gene expression after treatment of IFN-γ; (d) relative mRNA fold change of PD-1 gene expression after treatment of IFN-γ. There was no expression of PD-1 on uveal melanoma cell lines without the treatment of IFN-γ. (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
Metastatic UM is still a challenge for medical oncologists as there is no treatment to increase the overall survival in patients. UM proofs itself to be an immunotherapy-resistant tumor [36]. There is a renewed interest in studying tumor-infiltrating lymphocytes as the predictor and potential prognostic response to immunotherapy in patient with metastasis. TILs are a key immune component in the tumor microenvironment of UM, as they express several immune checkpoints. There are various ongoing clinical trials targeting PD-1/PD-L1 pathway that might be effective for the treatment modalities in cancer, with durable responses and reduced toxicity but no definite results have been achieved in UM. To understand the role of TILs in UM, we evaluated the immunohistochemical and mRNA expression of immune checkpoint markers (PD-1 and PD-L1) in UM with TILs and without TILs and their correlation with clinicopathological parameters and patient outcome. In-vitro studies validated this data in UM cell lines by qRT-PCR.
The clinical profile of UM patients with tumor-infiltrating lymphocytes were different from the profile of patients without tumor-infiltrating lymphocytes. In our study, we found PD-L1 expression not only in tumor cells, but also in on TILs. Overall patients with positive expression of PD-L1 immunoreactivity (68%) and mRNA expression of PD-1 (65%) had a significantly shortened disease-free survival. This expression was more pronounced even in low TIL group I. The survival of the patients was better and favorable with PD-L1 expression (both by IHC and qRT-PCR) in cases without TILs having significantly longer disease-free survival. The prognostic value of PD-L1 in cases without TILs is in concordance with recent findings in UM, in which a high expression of PD-L1 with less TILs were linked to favorable outcome [37]. These findings are contrary to other studies where PD-L1 expression was correlated with poor outcome in solid tumors including cutaneous melanoma. This suggests that PD-1/PD-L1 interaction inhibit the T-cell activation and cytokine production leading to T-cell apoptosis to promote an immune-escape state due to factors which include local ocular immune privilege and the lack of a lymphatic system. However, owing to the small case numbers in group I and group II, these results need to be validated in a larger study cohort before drawing conclusions.
Several studies suggest that TIL infiltration in UM appears to be associated with a poor prognosis [38–42]. Poor prognostic factors are always important and help in management adjuvant therapy for metastatic diseases. Correlation of immune markers with histopathological and clinical parameters might be useful in improving the immunotherapeutic approaches for treating UM patients [43, 44]. In our study, the high expressions of PD-1 and PD-L1 in cases with TILs were associated with loss of BAP-1, epithelioid cell type, ciliary body invasion, CD68 positivity and liver metastasis, all parameters associated with a bad prognosis in UM patients. These results suggest an influence the immunosuppressive tumor microenvironment in the development of UM metastases.
Pardoll suggests the term “adaptive immune resistance” for dampening inflammation after the induction of PD-L1 in cancer to protect themselves from T-cell mediated cytotoxicity or inhibition of cytokine production [45]. Inflammatory infiltrates are characterized by an increased expression of cytokines produced by T lymphocytes. It is known that T lymphocytes are capable of producing cytokines, therefore, the levels of cytokines could be representative of T-lymphocyte infiltration [46]. IFN-γ, an immunostimulatory cytokine, constitutes a primary causative factor of triggering or promoting the inflammatory response. The obligate role of IFN-γ in both tumor immune surveillance and immune evasion suggest its importance as a new immunotherapeutic strategy in UM [25]. We validated our result using in-vitro study on UM cell lines. We found a different pattern of cytokine levels produced by UM Cell lines after the treatment of IFN-γ. There was an induction of PD-1, PD-L1 expression and different level of cytokines produced by T-lymphocytes in all UM cell lines before and after treatment with IFN-γ. These results are in line with Yang et al. where they found a constitutive expression of PD-L1 in approximately half of the primary UM cell lines and in only 20% of the metastatic UM cell lines, but showed expression of PD-L1 after exposure to IFN-γ in primary and metastatic UM cell lines [47]. Therefore, cytokines activating the immune system such as IFN-γ might be used to reverse the immune escape and thus to potentiate the efficacy of immunotherapy in UM.
This supports the hypothesis that UM cells have the capacity to sense the presence of an inflammatory response in the form of IFN-γ and respond by upregulating molecules such as PD-L1 that launch a counter attack that extinguishes immune-mediated inflammation directed against the UM [48]. These findings suggest that unravelling the mysteries of immune privilege may have important implications for designing therapeutic modalities for managing malignancies such as UM that have adopted immune privilege as a strategy for escaping immune surveillance.
To conclude, TILs play an important immune cell for achieving successful immunotherapeutic strategies in the treatment of UM patients. Our finding suggests that PD-1 and PD-L1 expression in TILs showed poor outcome that helps in identifying a subgroup of UM patients who may benefit from immunotherapy. However, a limitation of the study is the requirement of longer patient follow-up. This should be further validated and confirmed in a large cohort of the patients for future immunotherapeutic clinical trials in UM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work was supported by Department for Science and Technology (DST), Govt. of India for providing National Post-Doctoral fellowship (N-PDF) to Dr. Lata Singh and conducting this research (NPDF/2016/000903).
Abbreviations
- AJCC
American Joint Committee on Cancer
- IHC
Immunohistochemistry
- IFN-γ
Interferon gamma
- MVD
Microvascular density
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SDS-PAGE
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TILs
Tumor-infiltrating lymphocytes
- UM
Uveal melanoma
Author contributions
Conception and study design: LS and MKS; clinicopathological data analysis: LS, MKS and SK; in vitro data analysis: LS and MCK; enucleated sample: RM and NL; follow-up of the patients: NL and SB; pathology slide review: SK and SS; cell lines provider: MJJ; manuscript editing: MCK, MJJ, LS, MKS and SK; all the authors finally approved the manuscript version to be published.
Funding
The authors have no proprietary or commercial interest in any materials discussed in this article.
Compliance with ethical standards
Conflict of interest
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Ethics approval
Human subjects were included in this study. Informed consent was obtained from all patients before participation in this study. All procedures were approved by the institutional ethics committee, All India Institute of Medical Sciences (AIIMS), New Delhi, India (Ref. No. IEC-424/RP-6/2016) and were conducted in accordance with the tenets of the Declaration of Helsinki for experiments involving humans.
Human and animal rights
This research involved the human participants.
Informed consent
Consent for publication was obtained from all patients for collection of tissue samples prior to the surgery.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005;18(1):75–84. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Kashyap S, Meel R, Singh L, Singh M. Uveal melanoma. Semin Diagn Pathol. 2016;33(3):141–147. doi: 10.1053/j.semdp.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seibel I, Cordini D, Rehak M, Hager A, Riechardt AI, Boker A, Heufelder J, Weber A, Gollrad J, Besserer A, Joussen AM. Local recurrence after primary proton beam therapy in uveal melanoma: risk factors, retreatment approaches, and outcome. Am J Ophthalmol. 2015;160(4):628–636. doi: 10.1016/j.ajo.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Weis E, Salopek TG, McKinnon JG, Larocque MP, Temple-Oberle C, Cheng T, McWhae J, Sloboda R, Shea-Budgell M. Management of uveal melanoma: a consensus-based provincial clinical practice guideline. Curr Oncol. 2016;23(1):e57–64. doi: 10.3747/co.23.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan P, Cohen V, Coupland S, Curtis K, Damato B, Evans J, Fenwick S, Kirkpatrick L, Li O, Marshall E, McGuirk K, Ottensmeier C, Pearce N, Salvi S, Stedman B, Szlosarek P, Turnbull N. Uveal melanoma UK national guidelines. Eur J Cancer. 2015;51(16):2404–2412. doi: 10.1016/j.ejca.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Rospond-Kubiak I, Damato B. The surgical approach to the management of anterior uveal melanomas. Eye (Lond) 2014;28(6):741–747. doi: 10.1038/eye.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira PR, Odashiro AN, Lim LA, Miyamoto C, Blanco PL, Odashiro M, Maloney S, De Souza DF, Burnier MN., Jr Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. 2013;7:1669–1682. doi: 10.2147/opth.S28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato T. Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol. 2010;37(2):127–138. doi: 10.1053/j.seminoncol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Komatsubara KM, Carvajal RD. Immunotherapy for the treatment of Uveal melanoma: current status and emerging therapies. Curr Oncol Rep. 2017;19(7):45. doi: 10.1007/s11912-017-0606-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhou R, Caspi RR. Ocular immune privilege. Biol Rep. 2010;1:2. doi: 10.3410/b2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27(3):184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkert C, Loibl S, Noske A, Roller M, Muller B, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 15.Dieci M, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu M, Delaloge S, Curigliano G. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tougeron D, Fauquembergue E, Rouquette A, Le Pessot F, Sesboue R, Laurent M, Berthet P, Mauillon J, Di Fiore F, Sabourin JC, Michel P, Tosi M, Frebourg T, Latouche JB. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22(9):1186–1195. doi: 10.1038/modpathol.2009.80. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Won HS, Sun S, Hong JH, Ko YH. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97(32):e11769. doi: 10.1097/md.0000000000011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Avi R, Farhi R, Ben-Nun A, Gorodner M, Greenberg E, Markel G, Schachter J, Itzhaki O, Besser MJ. Establishment of adoptive cell therapy with tumor infiltrating lymphocytes for non-small cell lung cancer patients. Cancer Immunol Immunother. 2018;67(8):1221–1230. doi: 10.1007/s00262-018-2174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang W-T, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishna Y, McCarthy C, Kalirai H, Coupland SE. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum Pathol. 2017;66:159–166. doi: 10.1016/j.humpath.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 22.Rossi E, Schinzari G, Zizzari IG, Maiorano BA, Pagliara MM, Sammarco MG, Fiorentino V, Petrone G, Cassano A, Rindi G, Bria E, Blasi MA, Nuti M, Tortora G. Immunological backbone of uveal melanoma: is there a rationale for immunotherapy? Cancers (Basel) 2019;11:8. doi: 10.3390/cancers11081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pötzl J, Roser D, Bankel L, Hömberg N, Geishauser A, Brenner CD, Weigand M, Röcken M, Mocikat R. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-γ and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer. 2017;140(9):2125–2133. doi: 10.1002/ijc.30646. [DOI] [PubMed] [Google Scholar]
- 24.Hallermalm K, Seki K, De Geer A, Motyka B, Bleackley RC, Jager MJ, Froelich CJ, Kiessling R, Levitsky V, Levitskaya J. Modulation of the tumor cell phenotype by IFN-gamma results in resistance of uveal melanoma cells to granule-mediated lysis by cytotoxic lymphocytes. J Immunol. 2008;180(6):3766–3774. doi: 10.4049/jimmunol.180.6.3766. [DOI] [PubMed] [Google Scholar]
- 25.Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7(9):4509–4516. doi: 10.1002/cam4.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547(7664):413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wierenga APA, Cao J, Luyten GPM, Jager MJ. Immune checkpoint inhibitors in uveal and conjunctival melanoma. Int Ophthalmol Clin. 2019;59(2):53–63. doi: 10.1097/iio.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 28.Daniels AB, Veverka KK, Patel SN, Sculley L, Munn G, Pulido JS. Computing uveal melanoma basal diameters: a comparative analysis of several novel techniques with improved accuracy. Int J Retina Vitreous. 2019;5:2. doi: 10.1186/s40942-018-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade OA, Mehta S, Forte L, Long A, Dellacava EF, Kaplan B, Shields JA. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127(8):989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 30.Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42(7):1414–1421. [PubMed] [Google Scholar]
- 31.Makitie T, Summanen P, Tarkkanen A, Kivela T. Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 1999;40(11):2471–2480. [PubMed] [Google Scholar]
- 32.Jager MJ, Magner JA, Ksander BR, Dubovy SR. Uveal melanoma cell lines: where do they come from? (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2016;114:T5. [PMC free article] [PubMed] [Google Scholar]
- 33.De Waard-Siebinga I, Blom DJ, Griffioen M, Schrier PI, Hoogendoorn E, Beverstock G, Danen EH, Jager MJ. Establishment and characterization of an uveal-melanoma cell line. Int J Cancer. 1995;62(2):155–161. doi: 10.1002/ijc.2910620208. [DOI] [PubMed] [Google Scholar]
- 34.Chen PW, Murray TG, Uno T, Salgaller ML, Reddy R, Ksander BR. Expression of MAGE genes in ocular melanoma during progression from primary to metastatic disease. Clin Exp Metastasis. 1997;15(5):509–518. doi: 10.1023/a:1018479011340. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. doi: 10.1186/s12943-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for immune-therapy. Ann Transl Med. 2016;4(9):172. doi: 10.21037/atm.2016.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoroquiain P, Esposito E, Logan P, Aldrees S, Dias AB, Mansure JJ, Santapau D, Garcia C, Saornil MA, Belfort Neto R, Burnier MN. Programmed cell death ligand-1 expression in tumor and immune cells is associated with better patient outcome and decreased tumor-infiltrating lymphocytes in uveal melanoma. Mod Pathol. 2018;31(8):1201–1210. doi: 10.1038/s41379-018-0043-5. [DOI] [PubMed] [Google Scholar]
- 38.de la Cruz PO. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990;65(1):112–115. doi: 10.1002/1097-0142(19900101)65:1<112::aid-cncr2820650123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 39.Whelchel JC, Farah SE, McLean IW, Burnier MN. Immunohistochemistry of infiltrating lymphocytes in uveal malignant melanoma. Invest Ophthalmol Vis Sci. 1993;34(8):2603–2606. [PubMed] [Google Scholar]
- 40.Bronkhorst IH, Vu TH, Jordanova ES, Luyten GP, Burg SH, Jager MJ. Different subsets of tumor-infiltrating lymphocytes correlate with macrophage influx and monosomy 3 in uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53(9):5370–5378. doi: 10.1167/iovs.11-9280. [DOI] [PubMed] [Google Scholar]
- 41.Bronkhorst IH, Jager MJ. Inflammation in uveal melanoma. Eye (Lond) 2013;27(2):217–223. doi: 10.1038/eye.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gezgin G, Dogrusoz M, van Essen TH, Kroes WGM, Luyten GPM, van der Velden PA, Walter V, Verdijk RM, van Hall T, van der Burg SH, Jager MJ. Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment. Cancer Immunol Immunother. 2017;66(7):903–912. doi: 10.1007/s00262-017-1991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue M, Shang J, Chen B, Yang Z, Song Q, Sun X, Chen J, Yang J. Identification of prognostic signatures for predicting the overall survival of uveal melanoma patients. J Cancer. 2019;10(20):4921–4931. doi: 10.7150/jca.30618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaliki S, Shields CL, Shields JA. Uveal melanoma: estimating prognosis. Indian J Ophthalmol. 2015;63(2):93–102. doi: 10.4103/0301-4738.154367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dearman R, Moussavi A, Kemeny D, Kimber I. Contribution of CD4+ and CD8+ T lymphocyte subsets to the cytokine secretion patterns induced in mice during sensitization to contact and respiratory chemical allergens. Immunology. 1996;89(4):502–510. doi: 10.1046/j.1365-2567.1996.d01-778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY. PD-L1: PD-1 interaction contributes to the functional suppression of T-cell responses to human uveal melanoma cells in vitro. Invest Ophthalmol Vis Sci. 2008;49(6):2518–2525. doi: 10.1167/iovs.07-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog Retin Eye Res. 2009;28(5):329–347. doi: 10.1016/j.preteyeres.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.