Abstract
Introduction
TCR and BCR repertoire diversity plays a critical role in tumor immunity. Thus, analysis of TCR and BCR repertoires might help predict the clinical efficacy of anti-PD-1 treatment.
Methods
Blood samples from 30 patients with non-small cell lung cancer (NSCLC) treated with anti-PD-1 antibody were collected before and six weeks after treatment initiation. The clinical significance of TCR and BCR repertoire diversity in peripheral blood was evaluated in all the enrolled patients (n = 30) or in the subset with (n = 10) or without (n = 20) EGFR/ALK mutation.
Results
TCR and BCR diversity was significantly correlated at baseline (R = 0.65; P = 1.6 × 10–4) and on treatment (R = 0.72; P = 1.2 × 10–5). Compared to non-responders (SD or PD), responders (PR) showed significantly decreased TCR and BCR diversity after treatment in the EGFR/ALK wild-type subset (P = 0.0014 and P = 0.034, respectively), but not in all the enrolled patients. The post-treatment reduction in TCR and BCR repertoire diversity was also significantly associated with the occurrence of adverse events in the EGFR/ALK wild-type subset (P = 0.022 and P = 0.014, respectively). Patients with more reduced TCR diversity showed better progression-free survival (PFS) in the EGFR/ALK wild-type subset (P = 0.011) but not in the mutant subset.
Conclusions
These findings suggest the clinical significance of changes in peripheral TCR and BCR repertoire diversity after anti-PD-1 treatment in patients with NSCLC without EGFR/ALK mutation. Monitoring of the peripheral TCR and BCR repertoires may serve as a surrogate marker for the early detection of EGFR/ALK wild-type NSCLC patients who would benefit from anti-PD-1 treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02900-z.
Keywords: T cell receptor (TCR) repertoire, B cell receptor (BCR) repertoire, Anti-PD-1 antibody, Non-small cell lung cancer (NSCLC), EGFR/ALK
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide [1]. Most patients with advanced lung cancer relapse after they develop resistance to cytotoxic agents. Recently, immune checkpoint inhibitors (ICIs), which target suppressive pathways in T cells to enhance antitumor responses, have demonstrated high efficacy in the treatment of several cancers [2–5]. After randomized phase 3 trials were performed in patients with non-small cell lung cancer (NSCLC), nivolumab and pembrolizumab, which are monoclonal antagonist antibodies to programmed cell death protein 1 (PD-1), have become a standard treatment for patients with previously treated advanced NSCLC [6–8]. However, the response rate to these PD-1 inhibitors is only approximately 20–30%. Therefore, considering the limited clinical efficacy, immune-related adverse events (AEs) and high cost, patient selection may be recommended. Anti-PD-1 antibodies have been suggested to inhibit the interaction between PD-1 and its ligands, programmed cell death ligand 1 and 2 (PD-L1 and PD-L2), and then restore the antitumor activity of tumor-infiltrating lymphocytes [4, 5]. Thus, PD-L1 expression in tumors has been established as a predictive biomarker for ICI [5, 9, 10]. However, as several studies have shown the clinical benefits of PD-1/PD-L1 antibodies even in patients lacking PD-L1 expression in tumors, developing other predictive biomarkers is an urgent priority [5, 11].
T and B cells play important roles in the adaptive immune response against cancer; the clonal diversity of T cell receptors (TCRs) and B cell receptors (BCRs) may be closely associated with the antitumor immune response. Although changes in TCR repertoires during ICI treatment have been already studied in various cancers [12–17], no reports regarding the clinical significance of BCR repertoires have been published yet. In addition, the clinical significance of TCR and BCR repertoires has not been compared between NSCLC patients with and without EGFR/anaplastic lymphoma kinase (ALK) mutation. Here, we evaluated the clinical roles of TCR and BCR repertoires in patients with advanced NSCLC who were treated with anti-PD-1 antibody.
Materials and methods
Patients
The study included patients with NSCLC who were treated with anti-PD-1 antibody (nivolumab or pembrolizumab) at Kurume University (Kurume, Japan) between February 2016 and August 2017. The Institutional Review Board of Kurume University approved the study protocol (Approval number: Kurume University 15210). Written informed consent was received from all participants prior to inclusion in the study. The patients underwent assessment at baseline and received nivolumab (3 mg/kg of body weight, every 2 weeks) or pembrolizumab (200 mg, every 3 weeks) intravenously without combined chemotherapy. They received treatment until progressive disease (PD) or intolerable toxicity developed. Lesions were evaluated using chest and abdominal computed tomography (CT) and cranial CT or magnetic resonance imaging (MRI). Antitumor response [partial response (PR), stable disease (SD) and PD] was assessed based on the best overall response according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. AE severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, and their causal association with anti-PD-1 treatment was determined by the investigators.
TCR and BCR repertoire analyses
After enrollment, blood samples were collected from the patients before and six weeks after the initiation of anti-PD-1 antibody treatment. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and frozen until analysis. Total RNA was isolated from the PBMCs and purified using RNeasy Plus Universal Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA amounts and purity were measured using Agilent 2200 TapeStation (Agilent Technologies, Palo Alto, CA).
Next-generation sequencing analysis of TCR beta chain and BCR IgG heavy chain was performed using an unbiased TCR/BCR repertoire analysis technology developed by Repertoire Genesis Inc. (Osaka, Japan). In brief, unbiased adaptor-ligation PCR was performed according to previous reports [18, 19]. Total RNA was converted to complementary DNA (cDNA) using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The BSL-18E primer containing polyT18 and a NotI site was used for cDNA synthesis. After cDNA synthesis, double-stranded (ds)-cDNA was synthesized using E. coli DNA polymerase I (Invitrogen), E. coli DNA Ligase (Invitrogen) and RNase H (Invitrogen). ds-cDNAs were blunted with T4 DNA polymerase (Invitrogen). P10EA/P20EA adapter was ligated to the 5ʹ end of the ds-cDNA and then cut with the NotI restriction enzyme. After removal of the adapter and primer using the MinElute Reaction Cleanup kit (Qiagen), the first PCR was performed with KAPA HiFi DNA Polymerase (Kapa Biosystems, Woburn, MA) using constant region-specific 1st PCR and P20EA primers. PCR conditions were as follows: 98 °C for 20 s, 65 °C (TCR beta) or 60 °C (BCR IgG) for 30 s and 72 °C for 1 min (20 cycles). The second PCR was performed with 2nd PCR and P20EA primers under the same PCR conditions. Amplicons were prepared by amplifying the products of the second PCR using Tag PCR and P22EA-ST1-R primers. After PCR amplification, index (barcode) sequences were added by amplification with Nextera XT index kit v2 setA or setD (Illumina, San Diego, CA). The indexed amplicon products were mixed in an equal molar concentration and quantified using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA). Sequencing was performed using the Illumina MiSeq paired-end platform (2 × 300 bp). The information of each primer is shown in Supplementary Table 1.
Table 1.
Patient characteristics
| All (n = 30) | EGFR/ALK wild type (n = 20) | ||
|---|---|---|---|
| Gender | Male | 21 | 17 |
| Female | 9 | 3 | |
| Age (years) | Median | 70.5 | 68.5 |
| Range | 49–89 | 49–89 | |
| Smoking status | Never | 9 | 3 |
| Smoker | 21 | 17 | |
| Histology | Non-squamous | 26 | 16 |
| Squamous | 4 | 4 | |
| PD-L1 status | 0% | 8 | 4 |
| 1–49% | 10 | 8 | |
| > 50% | 8 | 4 | |
| Unknown | 4 | 4 | |
| Treatment line | 1 | 3 | 3 |
| 2 | 12 | 11 | |
| > 3 | 15 | 6 | |
| Tumor response | PR | 13 | 8 |
| SD | 6 | 4 | |
| PD | 11 | 8 |
ALK anaplastic lymphoma kinase, PD-L1 programmed cell death ligand 1, PR partial response, SD stable disease, PD progressive disease
Data analyses
All paired-end reads were classified according to index sequences. Sequence assignment was performed by determining sequences with the highest identity in a dataset of reference sequences from the international ImMunoGeneTics information system® (IMGT) database (http://www.imgt.org). Data processing, assignment and aggregation were automatically performed using Repertoire Genesis (RG) software originally developed by Repertoire Genesis Inc. (Osaka, Japan). RG implemented a program for sequence homology searches using BLASTN, an automatic aggregation program, a graphics program for gene usage and CDR3 length distribution. Nucleotide-level sequence identities between the query and entry sequences were automatically calculated. Parameters that increased sensitivity and accuracy [E-value threshold, minimum kernel and high-scoring segment pair (HSP) score] were carefully optimized for the respective repertoire analysis. Nucleotide sequences of the CDR3 regions that ranged from conserved cysteine at position 104 (Cys104) of IMGT nomenclature to conserved phenylalanine or tryptophan at position 118 (Phe118 or Trp118) were translated to deduced amino acid sequences. A unique sequence read (USR) was defined as a sequence read having no identity in the assignment of gene segments and deduced amino acid sequences of CDR3 with the other sequence reads. The copy number of identical USRs in each sample was automatically counted using RG software and then ranked in the order of the copy number. Percentage occurrence frequencies of sequence reads with V, D, J and C genes in total sequence reads were also calculated.
Statistics
Progression-free survival (PFS) was calculated as the number of days from the start of anti-PD-1 antibody treatment to the date of documentation of treatment failure (death or disease progression) or the date of censoring at the final follow-up examination. PFS was estimated using the Kaplan–Meier method, and intergroup comparisons were assessed using the Cox proportional hazard model with SAS9.4 software (SAS Institute, Cary, NC).
TCR and BCR repertoire diversity before and after treatment was evaluated using two different methods, the Shannon–Weaver (S–W) index [20] and inverse Simpson's (invSimp) index [21], to consider the influence of the number of unique receptors (richness) and their relative abundance (evenness). Two-sided P values evaluated using the Wilcoxon rank sum test were used to compare TCR and BCR repertoire diversity or clonality. Spearman’s rank correlation coefficient was used to evaluate the correlation between the TCR and BCR repertoire diversity. P < 0.05 was considered statistically significant. The box plot in the figures represents the interquartile range (box) with the median, 25th and 75th percentile and a vertical line showing the lowest and highest data points, excluding any outliers. All scatter, bar and box plots were depicted using the package “ggplot2” [22] with R version 4.0.2 [23].
Results
Patient characteristics
A total of 30 patients with NSCLC who were treated with anti-PD-1 antibody (nivolumab or pembrolizumab) monotherapy between February 2016 and August 2017 were included (Table 1). The median patient age was 70.5 (range, 49–89) years. Of the 30 patients, 21 (70%) and 9 (30%) were male and female, respectively; 21 (70%) were current or former smokers; 26 (87%) and 4 (13%) had non-squamous and squamous cell carcinomas, respectively; and 9 (30%) and 1 (3%) had EGFR mutation and ALK rearrangement, respectively. Of the 26 patients whose tissue samples were available, baseline PD-L1 expression was weakly (1%–49% of tumor cells) and strongly (> 50% of tumor cells) positive in 10 (38%) and 8 (31%) patients, respectively. Anti-PD-1 antibody was administered as the first-, second-, and third- or further-line treatment in 3 (10%), 12 (40%) and 15 (50%) patients, respectively. Of the 30 patients, the best overall responses of PR, SD and PD were observed in 13 (43%), 6 (20%) and 11 (37%) patients, respectively.
Significant correlation between TCR and BCR repertoire diversity
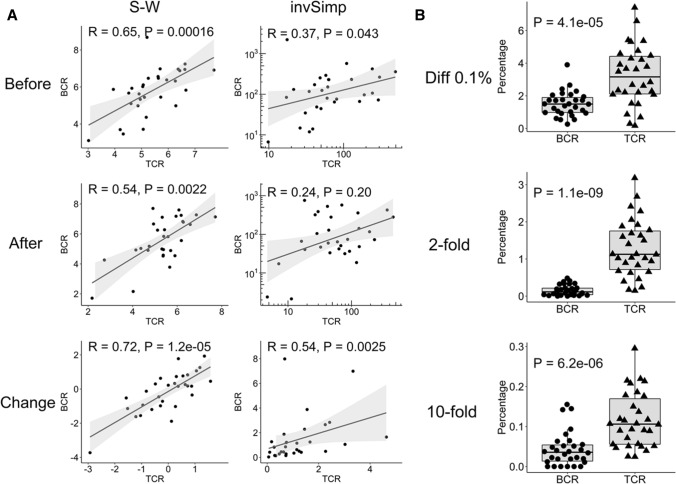
Before and after anti-PD-1 treatment in each patient, the TCR and BCR repertoire diversity was evaluated using two different methods, the S–W index and invSimp index. As shown in Fig. 1a, TCR and BCR repertoire diversity was significantly correlated before and after anti-PD-1 treatment when evaluated using the S–W index (before, R = 0.65, P = 1.6 × 10–4; after, R = 0.54, P = 0.0022 ) but not substantially when evaluated using the invSimp index (before, R = 0.37, P = 0.043; after, R = 0.24, P = 0.20). In addition, more interestingly, a stronger correlation between the TCR and BCR repertoire diversity was observed in post-treatment changes with the S–W index (R = 0.72, P = 1.2 × 10–5) and invSimp index (R = 0.54, P = 0.0025). Nevertheless, it should be noted that the frequencies of the TCR clones that increased by more than 0.1% or by more than two or ten times after treatment were significantly higher than those of the BCR clones (P = 4.1 × 10–5, P = 1.1 × 10–9, or P = 6.2 × 10–6, respectively), suggesting that clonal expansion in T cells was greater than that in B cells (Fig. 1b).
Fig. 1.
Correlation between TCR and BCR repertoire diversity. a The diversity of TCR or BCR repertoire was evaluated using two different methods, S–W index and invSimp index, before and after anti-PD-1 treatment. The correlation between the TCR and BCR diversity before and after treatment as well as that between post-treatment changes in TCR and BCR diversity was statistically analyzed by Spearman’s rank correlation coefficient. Each dot indicates an individual patient. A solid line with gray band shows linear correlation with 95% confidence intervals. For invSimp index, double logarithmic scaling plot was used before and after treatment, and log10 fold change values were used for the post-treatment changes. b The frequencies of TCR and BCR clones that increased by more than 0.1% (Diff 0.1%) or by more than twofold or tenfold after treatment were assessed and compared. Statistical significances were tested by paired Wilcoxon rank sum test
No clinical relevance of TCR or BCR repertoire diversity in all the enrolled NSCLC patients
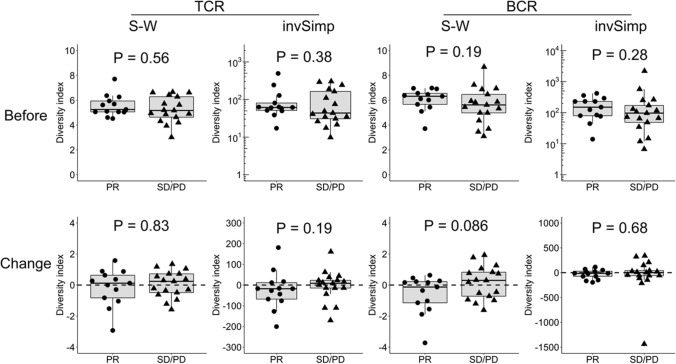
We examined the relationship between the TCR and BCR repertoire diversity and antitumor response in all the enrolled patients (n = 30) (Fig. 2). TCR diversity was not significantly different between responders (PR; n = 13) and non-responders (SD or PD; n = 17) at baseline (S–W, P = 0.56; invSimp, P = 0.38) and on treatment (S–W, P = 0.83; invSimp, P = 0.19). Similarly, BCR diversity was also not significantly different between the responders and non-responders at baseline (S–W, P = 0.19; invSimp, P = 0.28) and on treatment (S–W, P = 0.086; invSimp, P = 0.68).
Fig. 2.
No significant association between the TCR or BCR repertoire diversity and antitumor response in all the enrolled patients with NSCLC. TCR or BCR repertoire diversity before treatment and their post-treatment changes was compared between the responders (PR; n = 13) and non-responders (SD or PD; n = 17) in all the enrolled patients with NSCLC (n = 30). Statistical significances were tested using the unpaired Wilcoxon rank sum test
Clinical significance of TCR or BCR repertoire diversity in the subset of patients without EGFR/ALK mutation
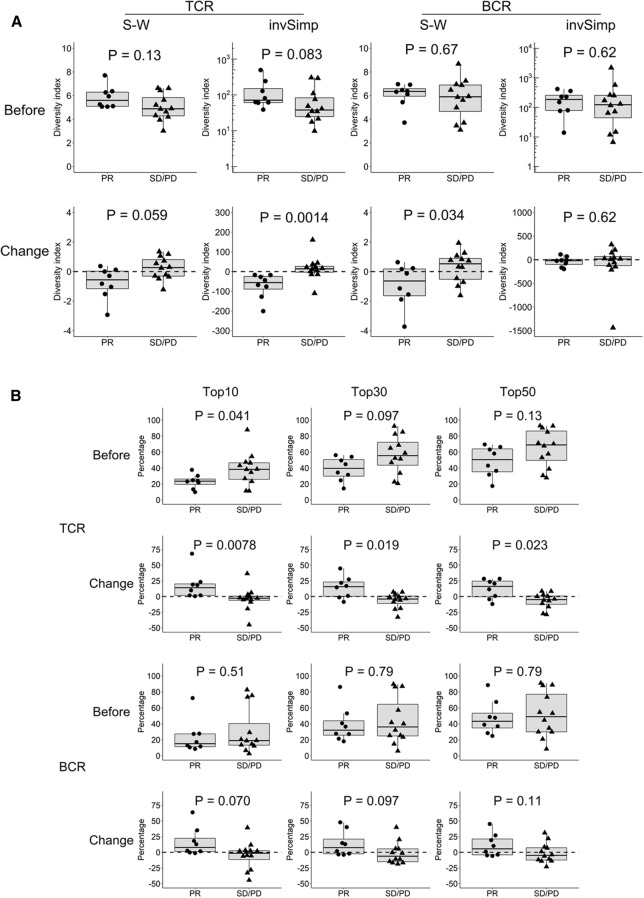
Clinical responses to anti-PD-1 antibody have been reported to be substantially different between ICI-treated NSCLC patients with and without driver gene mutations, such as EGFR mutations or ALK rearrangements [24, 25]. Therefore, we evaluated the clinical significance of the TCR or BCR repertoire diversity in the subset of patients without EGFR/ALK mutation (n = 20). The relevant patient characteristics are shown in Table 1. Pre-treatment TCR diversity tended to be higher in the responders (PR; n = 8) than in the non-responders (SD or PD; n = 12), although not statistically significant (invSimp, P = 0.083) (Fig. 3a). However, after anti-PD-1 treatment, TCR diversity was significantly decreased in the responders compared to that in the non-responders (invSimp, P = 0.0014) (Fig. 3a). In addition, evaluation of TCR clonal distribution supported these results (Fig. 3b). The responders showed greater TCR diversity before anti-PD-1 treatment; the frequencies occupied by the top 10 TCR clones in the responders were significantly lower than those in the non-responders (P = 0.041). However, on treatment, the frequencies of the top 10, 30 and 50 TCR clones in the responders were significantly increased, compared to those in the non-responders (P = 0.0078, P = 0.019 and P = 0.023, respectively). These results suggest that the responders had more diverse T cells before treatment but exhibited a stronger skewing in TCR clones after treatment.
Fig. 3.
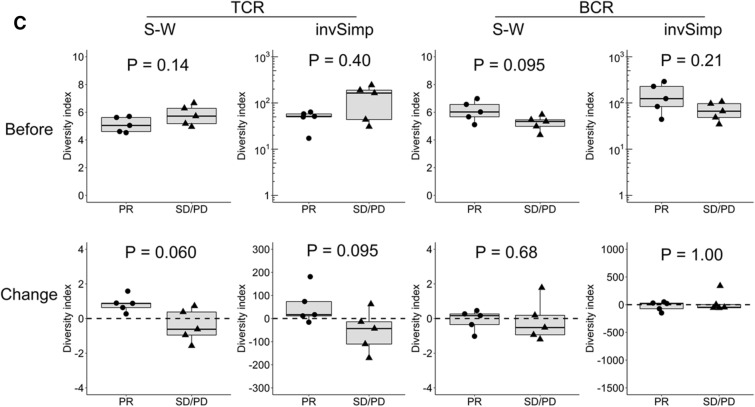
Significant association between the TCR or BCR repertoire diversity and antitumor response in the patient subset without EGFR/ALK mutation a TCR or BCR repertoire diversity before treatment and their post-treatment changes were compared between the responders (PR; n = 8) and non-responders (SD or PD; n = 12) in the subset without EGFR/ALK mutation (n = 20). b The frequencies occupied by top 10, 30 and 50 TCR or BCR clones before treatment and their post-treatment changes were compared between the responders (PR; n = 8) and non-responders (SD or PD; n = 12) in the subset without EGFR/ALK mutation (n = 20). c TCR or BCR repertoire diversity before treatment and their post-treatment changes were compared between the responders (PR; n = 5) and non-responders (SD or PD; n = 5) in the subset with EGFR/ALK mutation (n = 10). Statistical significances were tested using the unpaired Wilcoxon rank sum test
Similarly, BCR diversity showed significant changes in the subset without EGFR/ALK mutation. Before anti-PD-1treatment, BCR diversity was not significantly different between the responders and non-responders, whereas after treatment, it was significantly decreased in the responders compared to the non-responders (S–W index, P = 0.034) (Fig. 3a). In addition, the frequencies of the top 10 BCR clones tended to be increased after treatment in the responders, although not statistically significant (P = 0.070) (Fig. 3b).
We also assessed TCR and BCR repertoire diversity in patients harboring EGFR/ALK mutation (n = 10). As shown in Fig. 3c, there was no significant difference in the pre-treatment TCR diversity between the responders (PR; n = 5) and non-responders (SD or PD; n = 5). However, interestingly, TCR diversity tended to be increased in responders compared to non-responders after treatment (S–W, P = 0.060), suggesting that the clinical significance of TCR diversity alteration might be substantially different between NSCLC patients with and without EGFR/ALK mutation. In contrast, BCR diversity showed no significant roles at baseline or on treatment in the EGFR/ALK mutant subset (Fig. 3c).
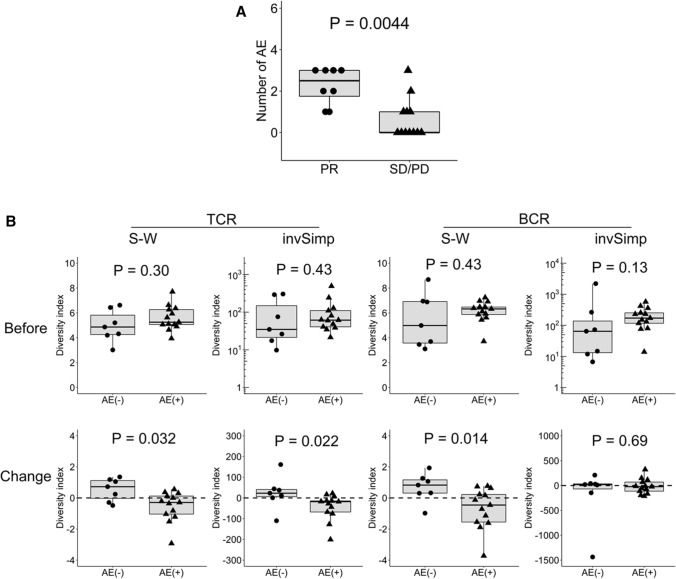
Association between AE occurrence and TCR or BCR repertoire diversity
As shown in Fig. 4a, the antitumor response was significantly associated with the number of AEs in the subset without EGFR/ALK mutation (P = 0.0044). We examined the association between the occurrence of AEs and TCR or BCR repertoire diversity in this subset (Fig. 4b). Before treatment, TCR and BCR diversity was not significantly different between the patients with (n = 13) and without (n = 7) AEs. However, after treatment, the patients with AEs showed significant reduction in TCR (S–W index, P = 0.032; invSimp, P = 0.022) and BCR (S–W index, P = 0.014) diversity.
Fig. 4.
Significant association between the TCR or BCR repertoire diversity and AE occurrence in the patient subset without EGFR/ALK mutation. a The number of AEs was compared between the responders (PR; n = 8) and non-responders (SD or PD; n = 12) in the subset without EGFR/ALK mutation (n = 20). b TCR or BCR repertoire diversity before treatment and their post-treatment changes in the subset without EGFR/ALK mutation were compared between the patients with (n = 13) and without (n = 7) AEs. Statistical significances were tested using the unpaired Wilcoxon rank sum test
Difference between patients with and without EGFR/ALK mutation
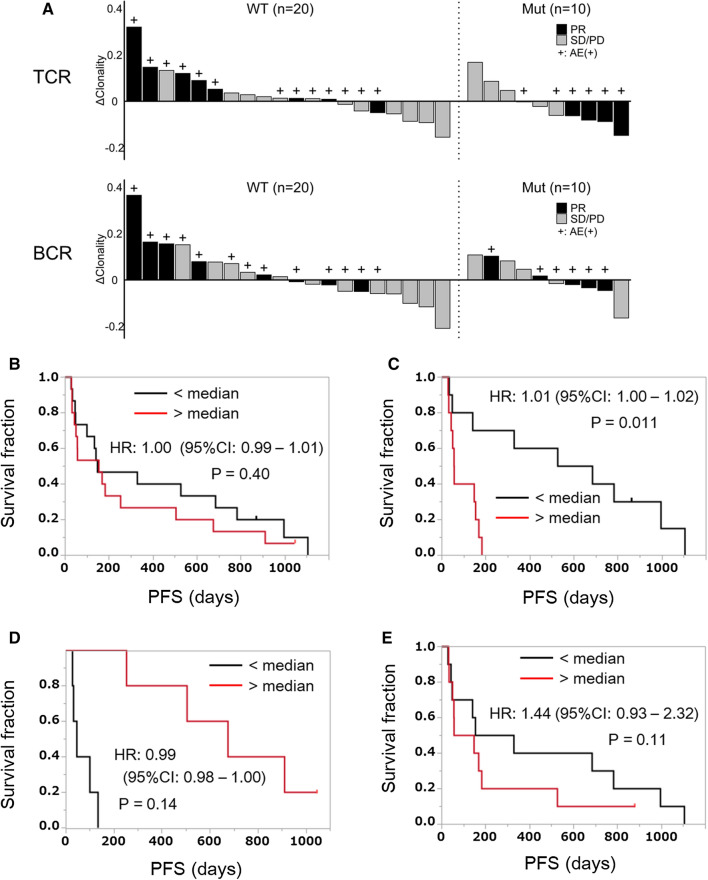
Figure 5a illustrates the changes in TCR (upper) or BCR (lower) clonality after treatment as well as antitumor responses or AE occurrence in each patient in the subset with or without EGFR/ALK mutation. In the subset without mutation, patients with increased TCR or BCR clonality after treatment tended to have better clinical effects and/or AE occurrence. Conversely, in the subset with mutation, post-treatment decrease in TCR clonality tended to be related to better clinical effects and AE occurrence.
Fig. 5.
Difference in the clinical significance of the TCR or BCR repertoire diversity between patients with and without EGFR/ALK mutation. a Post-treatment changes in TCR (upper) and BCR (lower) clonality as well as antitumor responses or AE occurrence were shown in each patient in the subset with (Mut, n = 10) and without (WT, n = 20) EGFR/ALK mutation. The clonality index was calculated as [1—the normalized S–W index]. Each vertical bar indicates an individual patient arranged in the order of post-treatment change of the clonality index (ΔClonality). b All the enrolled patients (n = 30) were divided into two groups based on the median value of post-treatment changes in the TCR repertoire diversity (evaluated by invSimp index). Kaplan–Meier plots of PFS for low and high groups were shown. The difference was evaluated statistically using the Cox proportional hazard model, and hazard ratio (HR), 95% confidence interval (95%CI) and P value were shown. c Patients without EGFR/ALK mutation (n = 20) were divided into two groups based on the median value of post-treatment changes in the TCR repertoire diversity (evaluated by invSimp index). Kaplan–Meier plots of PFS for low and high groups were shown. The difference was evaluated statistically using the Cox proportional hazard model, and HR, 95% CI and P value were shown. d Patients with EGFR/ALK mutation (n = 10) were divided into two groups based on the median value of post-treatment changes in the TCR repertoire diversity (evaluated by invSimp index). Kaplan–Meier plots of PFS for low and high groups were shown. The difference was evaluated statistically using the Cox proportional hazard model, and HR, 95% CI and P value were shown. e Patients without EGFR/ALK mutation (n = 20) were divided into two groups based on the median value of post-treatment changes in the BCR repertoire diversity (evaluated by S–W index). Kaplan–Meier plots of PFS for low and high groups were shown. The difference was evaluated statistically by the Cox proportional hazard model, and HR, 95% CI and P value were shown
The patients were divided into two groups depending on the post-treatment changes in the TCR repertoire diversity, and PFS was compared between these two groups. As shown in Fig. 5b, there was no significant difference in PFS between the high and low groups in all the enrolled patients [hazard ratio (HR), 1.00; 95% confidence interval (95%CI), 0.99–1.01; P = 0.40]. However, in the subset without EGFR/ALK mutation, the PFS was significantly longer in the low group than in the high group (HR, 1.01; 95%CI, 1.00–1.02; P = 0.011) (Fig. 5c). In contrast, in the subset with mutation, the low group tended to show worse PFS than the high group, although not statistically significant due to the small number of patients (HR, 0.99; 95%CI, 0.98–1.00; P = 0.14) (Fig. 5d). These results suggest that the clinical effects of TCR diversity changes after anti-PD-1 treatment might vary depending on the presence/absence of driver mutation in NSCLC. The changes in the BCR repertoire diversity after treatment exhibited no statistically significant effects on PFS in the subset without mutation (HR, 1.44; 95% CI, 0.93–2.32; P = 0.11) (Fig. 5e), suggesting that TCR diversity might have a stronger clinical effect than BCR diversity.
Discussion
We demonstrated the following novel findings in the present study: (1) The clinical roles of TCR and BCR diversity in peripheral blood were substantially different between ICI-treated NSCLC patients with and without EGFR/ALK mutation. (2) The changes of not only TCR but also BCR repertoire diversity in peripheral blood were significantly associated with tumor responses and/or AE occurrence after ICI treatment in EGFR/ALK wild-type patients; and (3) TCR and BCR diversity in peripheral blood was significantly correlated at baseline and on ICI treatment. To our knowledge, this is the first study to examine both TCR and BCR repertoire diversity in peripheral blood and compare their clinical significance between ICI-treated NSCLC patients with and without EGFR/ALK mutation.
Previous studies have reported the features of TCR repertoires in patients with NSCLC treated with anti-PD-1 antibody [12, 13]. Han et al. demonstrated that patients with high TCR diversity in peripheral PD-1+CD8+ T cells before ICI treatment showed better response to ICI and PFS compared with those with low diversity and that patients with increased PD-1+CD8+ TCR clonality after ICI treatment had a longer PFS [12]. These findings may be consistent with ours, although we analyzed the TCR repertoire in total T cells but not in selected T cell subsets. Similarly, increased TCR clonality in peripheral blood after anti-PD-1 treatment was reported to be significantly associated with better clinical responses in various cancers, including melanoma [14–16] and urothelial cancer [17]. Interestingly, Zhang et al. demonstrated that in neoadjuvant PD-1 blockade in patients with resectable NSCLC, tumors with major pathological responses were enriched with T cell clones that had peripherally expanded after treatment [13]. It has recently been suggested that T cell clones expanded within tumors after ICI treatment are not derived from pre-existing tumor-infiltrating T cells but instead consist of a distinct repertoire of novel T cell clones that may have just recently entered the tumor from outside, most probably from the peripheral compartment [26, 27]. Based on these results, including those reported in our study, the analysis and monitoring of the TCR repertoire in peripheral blood may be useful as a surrogate marker for the early detection of patients who would benefit from ICI treatment.
Interestingly, the current study demonstrated that patients with better antitumor responses showed a significant reduction in the TCR repertoire diversity after anti-PD-1 treatment only in the subset without EGFR/ALK mutation. In addition, patients with decreased TCR diversity after treatment had a longer PFS in the EGFR/ALK wild-type subset but not in the EGFR/ALK-mutant subset. This is the first report to demonstrate the difference in the clinical significance of peripheral TCR diversity between NSCLC patients with and without EGFR/ALK mutation. Based on our findings, the types of antigens recognized by the T cell clones expanded after ICI treatment might be different between patients with and without EGFR/ALK mutation. It is possible that in EGFR/ALK wild-type tumors with larger numbers of tumor-specific non-synonymous passenger mutations, T cells that can recognize neoantigens derived from them expand and contribute to tumor control after ICI treatment, whereas in EGFR/ALK-mutant tumors with fewer non-synonymous mutations, “bystander” T cells that recognize tumor-unrelated antigens may expand selectively [28, 29]. To precisely understand the difference of TCR repertoire features between patients with and without EGFR/ALK mutation, further studies characterizing the antigen specificity of clonally expanded T cells would be recommended.
There have been no reports regarding the relationship between the BCR repertoire diversity and clinical responses after ICI treatment. Interestingly, this study demonstrated that reduction in the BCR repertoire diversity was significantly associated with antitumor responses after treatment. In addition, the BCR repertoire diversity in peripheral blood was shown to be significantly correlated with the TCR repertoire diversity at baseline and on treatment. Notably, the clinical significance of the BCR repertoire diversity was demonstrated only when evaluated using the S–W index, which is more strongly influenced by rare clones and clone richness, while that of the TCR repertoire diversity was detected more clearly when evaluated using the invSimp index, which gives more importance to common clones and clone evenness. These findings suggest that the TCR and BCR repertoire shows different clonal distributions and responses to ICI treatment. Indeed, we demonstrated that the increase in T cell clonality after treatment was higher than that in B cell clonality.
As a subset of B cells have been reported to express PD-1 on their cell surface [30, 31], anti-PD-1 antibody may directly react with PD-1-expressing B cells and drive their activation/proliferation, giving rise to a skewed BCR repertoire. Alternatively, B cells might be stimulated indirectly by helper T cells that are activated by anti-PD-1 antibody. Recently, several studies have reported the clinical roles of B cells in cancer patients treated with ICI [30–35]. Das et al. reported that early changes in B cells following ICI treatment, which were characterized by a decline in circulating B cells and an increase in CD21lo B cells and plasmablasts, may help identify melanoma patients at increased risk of immune-related AEs [30]. In addition, Xiao et al. demonstrated that PD-1/PD-L1 blockade inhibits PD-1+ regulatory B cells that have suppressive function by producing IL-10 and inducing effector T cell dysfunction in hepatoma [31]. Furthermore, recent studies have demonstrated that the presence of B cells in highly specialized compartments called tertiary lymphoid structures (TLS) within the tumor microenvironment before ICI treatment was associated with an improved clinical response [32–34] and that during treatment TLS are more prevalent in good responders to ICI than in poor responders [33]. Although these findings suggest the critical roles of B cell activation in ICI treatment, the precise mechanism remains unelucidated. As B cells are known to function in tumor immunity through several different mechanisms, such as antigen presentation to effector T cells, production of tumor-specific antibodies and secretion of immune-regulatory factors [35], further studies are warranted to clarify the exact roles of B cell clones that have expanded after treatment.
This study also demonstrated a significant association between AE occurrence and the TCR or BCR repertoire diversity after anti-PD-1 treatment in patients with NSCLC without EGFR/ALK mutation. Although no reports have been published regarding such a relationship after anti-PD-1 therapy, our findings might be expected because clinical efficacy is reported to be significantly associated with AE occurrence after treatment in various cancers, including NSCLC [36, 37]. As immune-related AEs are suggested to be associated with T cell activation or B cell-mediated autoantibody production [38, 39], selectively expanded T or B cell clones after treatment might react to autoantigens expressed in normal tissues and cause AEs.
This study has some limitations. First, our findings are exploratory due to the limited number of available samples, because we enrolled only the patients treated with anti-PD-1 antibody monotherapy, but not those treated with combination therapy with anti-PD-1 antibody and chemotherapy, to exclude the effects of chemotherapeutic agents. Second, the antigen specificity of clonally expanded T and B cells was not assessed. Further investigations via large-scale prospective studies are thus warranted to confirm the present results and clarify the clinical roles of changes in the TCR and BCR repertoire after ICI treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Junya Otake (Kanagawa Cancer Center Research Institute) for sample handling and data acquisition.
Abbreviations
- AE
Adverse event
- ALK
Anaplastic lymphoma kinase
- BCR
B cell receptor
- CT
Computed tomography
- ICI
Immune checkpoint inhibitor
- InvSimp index
Inverse Simpson's index
- NSCLC
Non-small cell lung cancer
- PBMCs
Peripheral blood mononuclear cells
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PFS
Progression-free survival
- PR
Partial response
- RG
Repertoire Genesis
- SD
Stable disease
- S–W index
Shannon–Weaver index
- TLS
Tertiary lymphoid structures
- TCR
T cell receptor
Authors’ contributions
KA and TS designed the study. HS, KY, KA and TS obtained financial support for this study. YN, YI and HH contributed to data acquisition. NM, TH and KA collected patient samples and completed the follow-up. YN, TM, YI and KM conducted statistical analyses. YN, TM, YI, NM, HS, KY, KM, KA and TS analyzed and interpreted the data. YN, TM and TS wrote the manuscript, and NM and KA provided critical revisions of the manuscript. All authors approved the final version of the manuscript. YN, TM, YI and NM contributed equally as first authors.
Funding
This study was supported by AMED under Grant Number JP20ae0101076 (TS, KA, HS, KY) and JSPS KAKENHI Grant Number JP18K19490 (TS).
Code availability
Not applicable.
Compliance with ethical standards
Conflicts of interest
TM is an employee of Repertoire Genesis, Inc. YN has received personal fees from MSD, Ono, Chugai, Eli Lilly, Bristol-Myers Squibb and Nippon Boehringer Ingelheim, and grants from Takeda, Bristol-Myers Squibb and Eli Lilly. HS has received personal fees from Ono, Nippon Boehringer Ingelheim and Novartis, and grants from Chugai, AstraZeneca and MSD. KY has received personal fees from Ono, Chugai and Bristol-Myers Squibb. KA has received grants and personal fees from AstraZeneca, MSD, Bristol Myers Squibb, Ono and Chugai. TS has received grants from BrightPath Biotherapeutics. The other authors have declared that no conflict of interest exists.
Ethics approval and consent to participate
The Institutional Review Board of Kurume University approved the study protocol (Approval number: Kurume University 15210). Written informed consent was received from all participants prior to inclusion in the study.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and analyzed during the current study are available on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yoshiro Nakahara, Takaji Matsutani, Yuka Igarashi and Norikazu Matsuo have contributed equally to this work.
References
- 1.Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. New Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 11.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42(4):587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J, Duan J, Bai H, et al. TCR repertoire diversity of peripheral PD-1 + CD8 + T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res. 2020;8(1):146–154. doi: 10.1158/2326-6066.CIR-19-0398. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Ji Z, Caushi JX, et al. Compartmental analysis of T-cell clonal dynamics as a function of pathologic response to neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Clin Cancer Res. 2020;26(6):1327–1337. doi: 10.1158/1078-0432.CCR-19-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan SA, Courtier A, Cheng PF, et al. Peripheral blood TCR repertoire profiling may facilitate patient stratification for immunotherapy against melanoma. Cancer Immunol Res. 2019;7(1):77–85. doi: 10.1158/2326-6066.CIR-18-0136. [DOI] [PubMed] [Google Scholar]
- 15.Fairfax BP, Taylor CA, Watson RA, et al. Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26(2):193–199. doi: 10.1038/s41591-019-0734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valpione S, Galvani E, Tweedy J, et al. Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer. 2020;1(2):210–221. doi: 10.1038/s43018-019-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder A, Nathanson T, Funt SA, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med. 2017;14(5):e1002309. doi: 10.1371/journal.pmed.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitaura K, Shini T, Matsutani T, Suzuki R. A new high-throughput sequencing method for determining diversity and similarity of T cell receptor (TCR) α and β repertoires and identifying potential new invariant TCR α chains. BMC Immunol. 2016;17(1):38. doi: 10.1186/s12865-016-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitaura K, Yamashita H, Ayabe H, Shini T, Matsutani T, Suzuki R. Different somatic hypermutation levels among antibody subclasses disclosed by a new next-generation sequencing-based antibody repertoire analysis. Front Immunol. 2017;8:389. doi: 10.3389/fimmu.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon CE, Weaver W. The mathematical theory of communication. Champaign: The University of Illinois Press; 1949. [Google Scholar]
- 21.Simpson EH. Measurement of diversity. Nature. 1949;163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 22.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 23.R Development Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna. Available at: http://www.R-project.org/.
- 24.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calles A, Riess JW, Brahmer JR. Checkpoint blockade in lung cancer with driver mutation: choose the road wisely. Am Soc Clin Oncol Educ Book. 2020;40:372–384. doi: 10.1200/EDBK_280795. [DOI] [PubMed] [Google Scholar]
- 26.Yost KE, Satpathy AT, Wells DK, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25(8):1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu TD, Madireddi S, de Almeida PE, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579(7798):274–278. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 28.Toki MI, Mani N, Smithy JW, et al. Immune marker profiling and programmed death ligand 1 expression across NSCLC mutations. J Thorac Oncol. 2018;13(12):1884–1896. doi: 10.1016/j.jtho.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoni Y, Becht E, Fehlings M, et al. Bystander CD8 + T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 30.Das R, Bar N, Ferreira M, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest. 2018;128(2):715–720. doi: 10.1172/JCI96798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao X, Lao XM, Chen MM, et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 2016;6(5):546–559. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 32.Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 33.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petitprez F, de Reyniès A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 35.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 36.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237–247.e231. doi: 10.1016/j.cllc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 2019;58(Suppl 7):59–67. doi: 10.1093/rheumatology/kez308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.