Abstract
Background
Natural killer (NK) cell-based immunotherapy is a promising treatment approach for multiple myeloma (MM), but obtaining a sufficient number of activated NK cells remains challenging. Here, we report an improved method to generate ex vivo expanded NK (eNK) cells from MM patients based on genetic engineering of K562 cells to express OX40 ligand and membrane-bound (mb) IL-18 and IL-21.
Methods
K562-OX40L-mbIL-18/-21 cells were generated by transducing K562-OX40L cells with a lentiviral vector encoding mbIL-18 and mbIL-21, and these were used as feeder cells to expand NK cells from peripheral blood mononuclear cells of healthy donors (HDs) and MM patients in the presence of IL-2/IL-15. Purity, expansion rate, receptor expression, and functions of eNK cells were determined over four weeks of culture.
Results
NK cell expansion was enhanced by short exposure of soluble IL-18 and IL-21 with K562-OX40L cells. Co-culture of NK cells with K562-OX40L-mbIL-18/-21 cells resulted in remarkable expansion of NK cells from HDs (9,860-fold) and MM patients (4,929-fold) over the 28-day culture period. Moreover, eNK cells showed increased expression of major activation markers and enhanced cytotoxicity towards target K562, U266, and RPMI8226 cells.
Conclusions
Our data suggest that genetically engineered K562 cells expressing OX40L, mbIL-18, and mbIL-21 improve the expansion of NK cells, increase activation signals, and enhance their cytolytic activity towards MM cells.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02982-9.
Keywords: Multiple Myeloma, NK cells, OX40L, IL-18, IL-21
Introduction
Trials of immunotherapies using natural killer (NK) cells have increased due to the cytotoxic activity of NK cells against abnormal cells including cancer cells and the possibility of allogenic treatment with low risk of side effects such as graft-versus-host disease (GvHD) [1–3]. NK cell-based immunotherapy in combination with immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies is an emerging therapeutic approach to treat multiple myeloma (MM) [4–7]. Although allogenic NK cells show promise in MM patients, autologous NK cells appear to be dysfunctional, and the level of dysfunction appears to be correlated with more advanced disease [8, 9]. Some pre-clinical studies have suggested that NK cell dysfunction can be reversed by ex vivo expansion/activation of NK cells [10, 11]. Therefore, NK cell expansion to obtain a sufficient number of cells with high cytotoxicity is essential for effective treatment. However, expansion of NK cells is expensive because of the continuous requirement for cytokines during cultivation. Thus, development of a cost-effective expansion method for highly cytotoxic NK cells from both MM patients and healthy donors is highly desirable.
To increase NK cell expansion and cytotoxic activity, diverse cytokine combinations including common γ‐chain cytokines (i.e., IL-2, IL-15, and IL-21) have been investigated [12–16], and genetically engineered (GE) K562 cells expressing co-stimulatory factors (i.e., 41BB ligand, OX40 ligand) have been developed [17, 18]. Recently, K562 cells expressing membrane-bound (mb) forms of cytokines (i.e., K562-mbIL-21, K562-mbIL-15) [19, 20] were designed to stimulate NK cells during expansion to reduce costs associated with providing cytokines during culture. Our group previously reported an effective NK cell expansion protocol using K562-OX40L cells cultured in the presence of soluble IL-21 for the first day of culture, which we described as “short exposure” to IL-21 [18]. Stimulation of NK cells with cells expressing membrane-bound forms of cytokines should have similar effects to a short exposure to cytokines because irradiated-K562 feeder cells are not detected within a few days of NK expansion [21]. Thus, K562 cells expressing mbIL-21 and other membrane-bound forms of cytokines having short exposure effect could benefit NK cell expansion. Recently, the effects of IL-18 on the expansion and phenotype of human NK cells were reported [22]; the results of this previous study suggest that IL-18 is a candidate membrane-bound cytokine that can benefit NK cell expansion.
In this study, we confirmed the beneficial effects of short exposure of NK cells to IL-18 and IL-21. Based on these results, a novel K562 cell line expressing membrane-bound forms of IL-18/IL-21 and OX40L (K562-OX40L-mbIL-18/-21) was developed. Next, we examined the effects of K562-OX40L-mbIL-18/-21 feeder cells on expansion and activation of NK cells from MM patients compared to healthy donors (HDs).
Materials and methods
Healthy donors (HDs) and MM patients
Informed consent was obtained from MM patients and HDs in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2017–187 and 2019–073).
Cell culture and cytokines
K562 and MM cell lines (U266 and RPMI8226) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (Lonza, Walkersville, MD, USA) at 37 °C in a humidified 5% CO2 incubator. Recombinant human interleukin (IL)-2, IL-15, IL-21 (all from PeproTech, Rocky Hill, NJ, USA), and IL-18 (MBL International, Woburn, MA, USA) were used to expand NK cells.
Reagents
Fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 monoclonal antibody (mAb) and allophycocyanin (APC)-conjugated anti-human CD56 mAb were used to evaluate NK cell purity. Phycoerythrin (PE)-conjugated anti-human CD16, CD69, NKG2D, NKp30, NKp44, NKp46, CD94, CD158a, and CD158b mAbs were used to examine the surface expression of NK cell receptors. PE-conjugated anti-human IFN-γ and granzyme B mAb were used for intracellular staining. PE-conjugated anti-human CD107a mAb was used as a surrogate marker of degranulation. All fluorescent mAbs were from BD Biosciences (San Jose, CA, USA), except for PE-conjugated antihuman IFN-γ (eBioscience, San Diego, CA, USA).
Plasmid construct
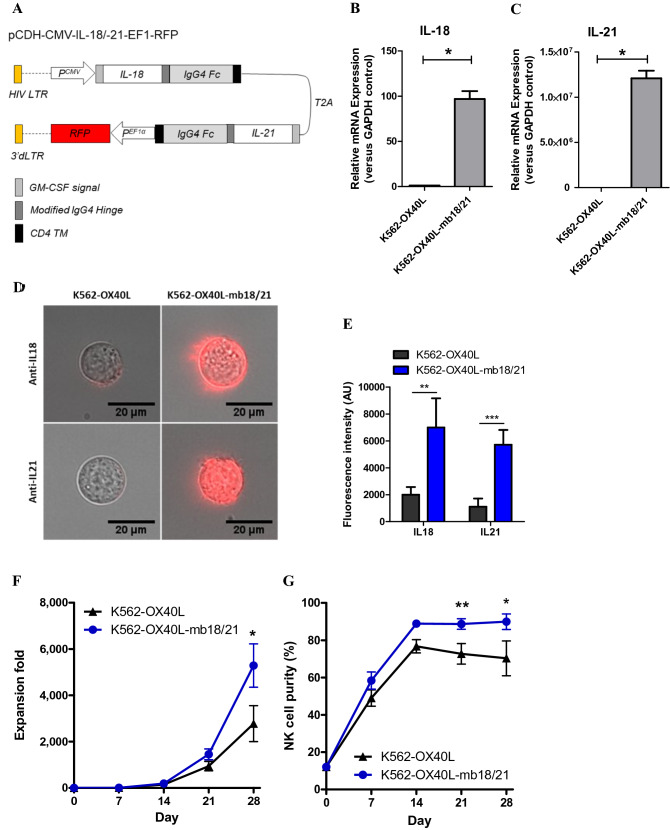
mbIL-18 and mbIL-21 were synthesized as reported previously [23]. To generate mbIL-18, mbIL-21, and granulocyte–macrophage colony-stimulating factor (GM-CSF) signal peptide sequences, coding sequences of mature human IL-18, IL-21, a modified 12-aa IgG4 hinge region, the 5’ end of the human immunoglobulin γ-4 chain CH2 and CH3 regions, and human CD4 transmembrane domain were sequentially linked. mbIL-18/-21 full sequence was synthesized and verified by DNA sequencing after each fused sequence was linked through the T2A self-cleavage site, as shown in Fig. 2a. Then, the synthetic mbIL-18/-21 sequence was ligated between NheI and NotI sites of the pCDH-CMV-EF1-RFP vector (System Biosciences, Palo Alto, CA, USA) to generate pCDH-CMV-mbIL-18/-21-EF1-RFP.
Fig. 2.
Development of K562-OX40L-mbIL-18/-21 cells. (a) Illustration of the lentiviral gene used to obtain an GE-K562 cell line producing mbIL-18 and mbIL-21. IL-18 (b) and IL-21 (c) mRNA expression by K562-OX40L and K562-OX40L-mbIL-18/-21 cells was determined by RT-PCR with GAPDH serving as a control (n = 4). (D) Representative merged images showing cells (DIC) and mbIL-18 and mbIL-21 (red). Quantified fluorescence intensity indicating amounts of mbIL-18 and mbIL-21 (E) (n = 13–19). Expansion fold (f) and NK cell purity (g) of NK cells when K562-OX40L or K562-OX40L-mbIL-18/-21 cells were used as feeder cells (n = 7). In all cases, means and SEM were plotted. *: p < 0.05, **: p < 0.01, ***: p < 0.001
Lentiviral transduction and production of transformed K562 cells co-expressing OX40L, mbIL-18, and mbIL-21
293FT cells were co-transfected with pCDH-CMV-mbIL-18/-21-EF1-RFP and a lentiviral packaging mixture (pLP1, pLP2, and pLP/VSVG; Thermo Scientific) to produce lentiviral vectors expressing mbIL-18/-21. Transfections were performed using D-fectin (Lugen Sci Co. Ltd., Bucheon, South Korea) according to the manufacturer’s instructions. At 48 h post-transfection, viral supernatant was collected, and the viral titer was determined using the Lenti-X qRT-PCR titration kit (Takara Bio Inc., Otsu, Shiga, Japan). K562-OX40L cells, which were made in a previous study (18), were seeded into 6-well plates at 3 × 105 cells/well, and then transduced with lentiviral supernatant for 24 h in the presence of polybrene (8 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) at a multiplicity of infection of 50. Twenty-four hours post-incubation, the virus-containing medium was removed and replaced with 2 ml of fresh culture medium. When cell confluency was greater than 90%, the cell culture was expanded to a T-25 flask. Transduction efficiency was evaluated daily under an inverted fluorescence microscope. Two weeks after transduction, GFP-positive cells were sorted using a BD FACSAria TM III flow cytometer and maintained in RPMI 1640 with 10% FBS.
Rq-PCR detection of mbIL-18 and mbIL-21
Total RNA for mRNA Rq-PCR analysis was isolated using an RNeasy Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions and quantified using an IMPLEN Nanophotometer P330 (IMPLEN, Munich, Germany). Isolated RNA was converted to cDNA using a QuantiTect Reverse Transcription Kit (Qiagen). All PCR reactions were performed using a QuantiTect SYBR Green PCR Kit (Qiagen) and a Rotor-Gene Q (Qiagen) in standard 20-µl reaction volumes. Amplification primers and their target mRNAs are shown in Supplementary Table 1. All samples were processed in triplicate to increase the reliability of the results obtained. Each assay was performed using positive and negative controls. The threshold cycle (Ct) value of each gene was measured for each sample. The Ct value of GAPDH was used as an endogenous reference for normalization purposes. The values thus obtained were normalized to the negative control and expressed as fold changes.
Short exposure to IL-18 and/or IL-21
NK cells from HDs were expanded from peripheral blood mononuclear cells (PBMCs) by co-culture with 100 Gy gamma-irradiated GE-K562 cells (K562-OX40L) cells, as described previously [18]. To investigate the effects of short exposure of cytokines to NK cells, soluble IL-21 and/or IL-18 was added to complete media once on day 0 of the culture of K562-OX40L cells, and PBMCs were co-cultured with irradiated K562-OX40L cells in the presence of IL-2 (10U – 100 U/mL)/IL-15 (5 ng/mL) with short exposure to IL-18 (100 ng/mL) alone, IL-21 (5 ng/mL) alone or the combination of IL-18 (100 ng/mL) and IL-21 (5 ng/mL).
Ex vivo NK cell expansion using K562-OX40L-mbIL-18/-21 feeder cells
NK cells were expanded from PBMCs of HDs and MM patients by co-culture with 100 Gy gamma-irradiated GE-K562 (K562-OX40L, K562-OX40L-mbIL-18/-21) cells as described previously, with slight modifications [17, 24]. Briefly, PBMCs were isolated from heparinized peripheral blood from HDs and MM patients using density-gradient centrifugation with Ficoll-Hypaque (d = 1.077, Lymphoprep™; Axis-Shield, Oslo, Norway). PBMCs were co-cultured with irradiated K562-OX40L and K562-OX40L-mbIL-18/-21 cells in a 24-well plate with RPMI 1640 medium (10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 4 mmol/L L-glutamine) containing 10 U/mL recombinant human IL-2. After day 7, the concentration of IL-2 was increased from 10 U/mL to 100 U/mL, and 5 ng/mL soluble IL-15 was also added to the medium. Irradiated GE-K562 feeder cells were used for re-stimulation on days 7 and 14. The medium was replaced every 2–3 days. eNK cells were continuously cultured until day 28. The expansion rate of NK cells is presented as “expansion fold”, which was determined by dividing the absolute number of NK cells at time points of interest by the respective number on day 0.
Surface and intracellular staining and flow cytometry analysis
Expression of NK cell receptors by eNK cells on days 0 and 14 was examined by flow cytometry using FITC-conjugated anti-human CD3 mAb, APC-conjugated anti-human CD56 mAb, and PE-conjugated anti-human CD16, CD69, NKG2D, NKp30, NKp44, NKp46, CD94, CD158a, and CD158b mAbs. Briefly, eNK cells (2 × 105) were washed with FACS buffer (PBS containing 1% FBS) and stained with mAbs for 15 min, washed again with FACS buffer, and acquired using a FACS Calibur and analyzed by FlowJo. Intracellular staining with the use of the BD Cytofix/Cytoperm kit (BD Biosciences) was performed to measure IFN-γ production and granzyme B expression. Briefly, eNK cells (1 × 105) were incubated in a 96-well U-bottom plate in the presence of brefeldin A (BD Biosciences) at 37 °C and 5% CO2 for 5 h. Cells were then harvested, washed with FACS, and stained with anti-human CD3 and CD56 mAbs for 20 min on ice. After washing, fixation, and permeabilization, NK cells were further stained with PE-conjugated anti-human IFN-γ and granzyme B mAbs on ice for 30 min, washed, and analyzed using a FACS Calibur flow cytometer.
CD107a degranulation
eNK cells (5 × 105) were incubated with or without 5 × 105 target cells (K562, U266, RPMI8226) in a 96-well U-bottom plate in the presence of 5 μL PE-conjugated anti-human CD107a. Monensin and brefeldin A (BD Biosciences) were added after 1 h, and the plate was incubated for an additional 4 h. NK cells were then stained with anti-human CD3 and CD56 Abs before acquisition.
Cytotoxicity assay
Cytotoxicity of eNK cells on day 14 against target cells (K562, U266, RPMI8226) was measured by CFSE-based assay for 4 h as described previously [18]. Briefly, target cells were stained with 0.5 μM CFSE in FACS buffer for 10 min at 37 °C, washed twice with complete media, and then 5 × 104 target cells were placed in a 96-well U-bottom plate in triplicate and mixed with eNK cells at 0.5:1, 1:1, and 2:1 effector-to-target (E:T) ratios. Plates were centrifuged at 1500 rpm for 3 min and then incubated at 37 °C in a 5% CO2 incubator for 4 h. Mixed cells were transferred to FACS tubes after incubation. One microliter of 1 mg/mL propidium iodide (PI) (Sigma-Aldrich, St. Louis, MO, USA) was added to each tube before acquisition. Cells were acquired on a FACS Calibur and analyzed using Kaluza software. Percentages of dead target cells (CFSE-positive and PI-positive) were calculated after subtracting the percentage of target cells that had died spontaneously.
Statistics
Statistical analyses of the differences between groups with regard to purity, fold expansion, receptor expression, degranulation, and cytotoxicity of eNK cells were carried out using the Mann–Whitney U-test. P values < 0.05 were considered significant.
Results
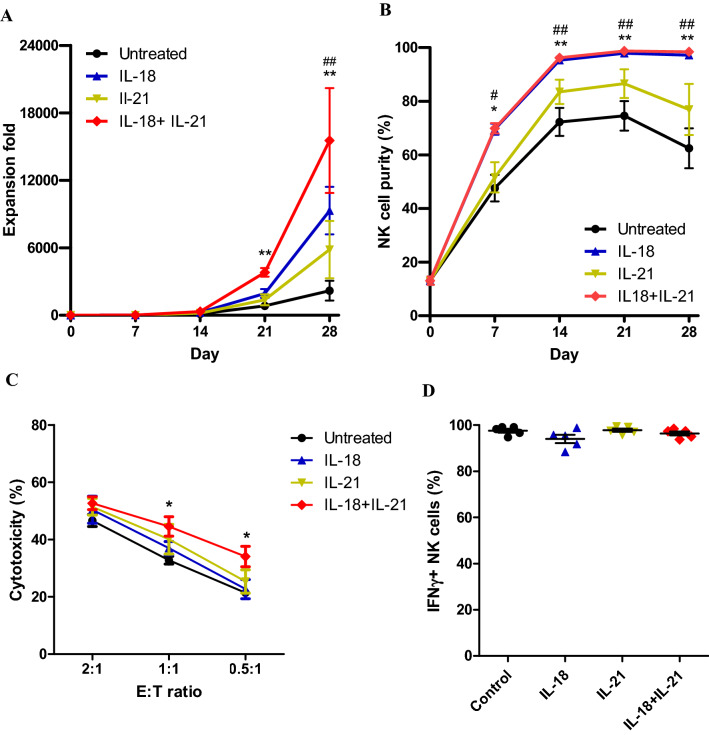
Effects of short exposure of NK cells to IL-18 and IL-21
In our previous study, we showed that co-culture of NK cells with K562 cells expressing OX40L and short exposure to IL-21 had beneficial effects on NK cell expansion. To further increase NK cell expansion in a cost-effective manner, we evaluated if short exposure of NK cells to IL-18, which has been reported to promote the expansion of NK cells [22], improved NK cell expansion. Because short exposure of NK cells to IL-18, like IL-21, benefited their expansion (data is not shown), we exposed NK cells to both IL-18 and IL-21 in the presence of K562-OX40L feeder cells. NK cells were exposed to IL-18 alone, IL-21 alone and combination of IL-18 and IL-21 once on the first day of NK cell expansion in the presence of K562-OX40L cells. Remarkable increase in NK cell expansion was observed after 21 days the only in combination of IL-18 and IL-21 treatment (Fig. 1a). IL-18 treatment and combination of IL-18 and IL-21 increased NK cell purity after day 7 and maintained high (> 97%) after day 14 (Fig. 1b). For the NK cell activity evaluation, direct cytotoxicity to K562 and intracellular amount of IFN-γ of NK cells were measured. The only combination of IL-18 and IL-21 treatment showed increased activity in the cytotoxicity (Fig. 1c) and there was no difference in IFN-γ amount (Fig. 1d). Altogether, short exposure of NK cells to combination of IL-18 and IL-21 was the synergy between recombinant IL-21 and IL-18 in promoting NK cell expansion.
Fig. 1.
Effects of short exposure to IL-18 and IL-21 on NK cell expansion and activities. PBMCs were co-cultured with irradiated K562-OX40L and IL-18, IL-21 alone or in combination of IL-18 and IL-21 added on day 0. NK cell expansion fold was calculated every week for 4 weeks of culture. Expansion fold (a) and NK cell purity (b) of expanded NK cells during culture. Direct cytotoxicity against to K562 (c) and intracellular IFN-γ (d) of expanded NK cells on day 7. Mean and SEM were plotted. (n = 5 in the all conditions; * represents statistical difference between IL-18 + IL-21 and untreated, # representing IL-18 and untreated; *, #: p < 0.05; **, ##: p < 0.01)
Development of a genetically engineered K562 cell line expressing OX40L and membrane-bound IL-18 and IL-21
Because irradiated K562 feeder cells disappear within 3 days of NK expansion [21], NK cells should only be exposed to cytokines expressed by irradiated feeder cells for a short period of time. Based on our finding that short exposure of NK cells to IL-18 and IL-21 improved their expansion, we decided to add membrane-bound forms of IL-18 and IL-21 to the K562-OX40L cell line that we had previously developed. To do this, lentiviral vector containing IL-18 and IL-21 genes tagged with IgG4 Fc (membrane targeting) and the RFP reporter gene were designed (Fig. 2a). After transduction of the K562-OX40L cell line with viral vector, cells expressing high levels of RFP were sorted and named K562-OX40L-mbIL-18/-21. To evaluate the expression of mbIL-18 and mbIL-21, mRNA and surface protein expression levels of the transduced genes were measured. Increased mRNA expression levels of mbIL-18 and mbIL-21 were detected for K562-OX40L-mbIL-18/-21 cells, but not K562-OX40L cells (Fig. 2b, c). Higher amounts of IL-18 and IL-21 were consistently found to be expressed on the surface of K562-OX40L-mbIL-18/-21 cells than K562-OX40L cells as measured by fluorescence imaging (Fig. 2d, e). Next, we evaluated the effects of K562-OX40L-mbIL-18/-21 cells on NK cell expansion when used as feeder cells. NK cell expansion was increased to a greater extent after 21 days of co-culture with K562-OX40L-mbIL-18/-21 cells than K562-OX40L cells (Fig. 2f), and NK cell purity was also greater in the K562-OXL-mbIL-18/-21 (n = 7 donors) co-culture (Fig. 2g). K562-OX40L-mbIL-18/-21 cells had similar effects on NK cell expansion and NK cell purity as short exposure of NK cells to soluble IL-18 and IL-21.
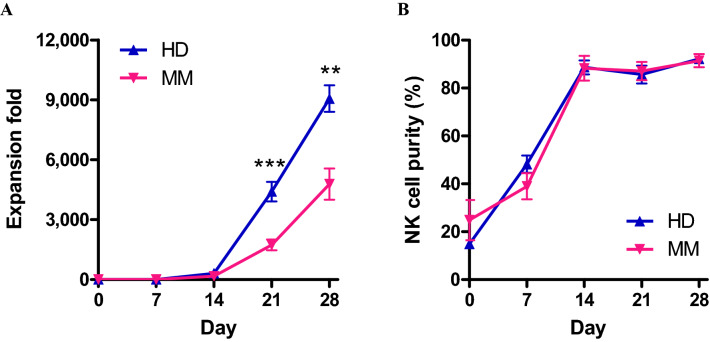
Expansion of NK cells from PBMCs of HDs and MM patients by co-culture with K562-OX40L-mbIL-18/-21 cells
We used K562-OX40L-mbIL-18/-21 cells to expand NK cells from PBMCs of HDs and MM patients. We evaluated the fold expansion and purity of eNK cells from HDs and MM patients after co-culture with K562-OX40L-mbIL-18/-21 cells for 28 days. Fold-expansion of NK cells derived from MM patients was lower than that of HDs up to day 21. Nevertheless, on day 28, 5,000-fold expansion of NK cells from MM patients was observed (Fig. 3a). Moreover, NK cells expanded from both HDs and MM patients showed similarly elevated NK cell purity during the culture period (Fig. 3b). Although the expansion rate of NK cells derived from MM patients was lower than that of NK cells derived from HDs, our results suggest that K562-OX40L-mbIL-18/-21 feeder cells are an effective tool for NK cell expansion from not only HDs, but also MM patients.
Fig. 3.
Comparison of NK cell expansion from PBMCs of HD and MM patients using K562-OX40L-mbIL-18/-21 cells. Comparison of expansion rate (a) and purity (b) of NK cells from HDs and MM patients (n = 9) expanded by co-culture with 100 Gy-irradiated K562-OX40L-mbIL-18/-21 cells over 4 weeks of culture. ** p < 0.01; *** p < 0.001
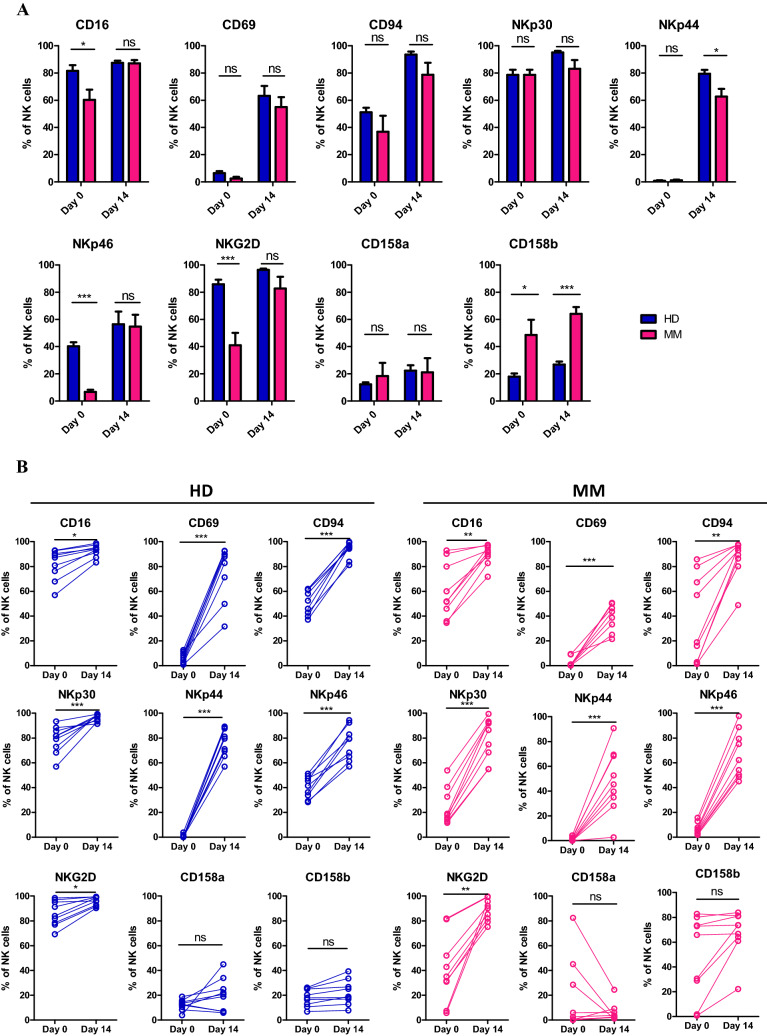
Phenotypic characteristics of NK cells expanded in the presence of K562-OX40L-mbIL-18/-21 cells
NK cells expanded by the K562-OX40L-mbIL-18/-21 feeder cells showed high NK purity (> 90%) and fully functional characteristics confirmed by receptor expression levels (CD16, CD69, CD94, NKp30, NKp44, NKp46, and NKG2D as activating receptors and CD158a and CD158b as inhibitory receptors) from day 14. Therefore we compared the characteristics of expanded NK cells on day 0 and day 14 as before and after expansion time point. The characteristics of NK cells expanded from HDs and MM patients were measured at indicated time points to determine the activation status of NK cells. Before expansion by co-culture with K562-OX40L-mbIL-18/-21 cells, NK cells from MM patients showed decreased expression of some activating receptors (CD69, NKp46 and NKG2D) (Fig. 4a). Although there was no statistical difference in inhibitory receptor expression, considerably increased expression of CD158b was observed in MM patient-derived NK cells. Notably, expression of all NK activation markers was significantly increased in NK cells from HDs and MM patients on day 14 compared to baseline (Fig. 4b). During this process, expression level differences in NK cell activating receptors between MM patient- or HD-derived NK cells disappeared (Fig. 4a). No differences in the expression of inhibitory receptors were observed (Fig. 4b). The active characteristics of expanded NK cells were retained throughout the expansion period similar to day 14 (data not shown).
Fig. 4.
Receptor expression levels in eNK cells obtained from HDs and MM patients. Comparison of receptor expression levels of NK cells between HDs and MM patients (a), and before and after expansion (b). HDs, n = 9; MM patients, n = 9, *p < 0.05; **p < 0.01; ***p < 0.001
Increased activities of NK cells from both HDs and MM patients after expansion
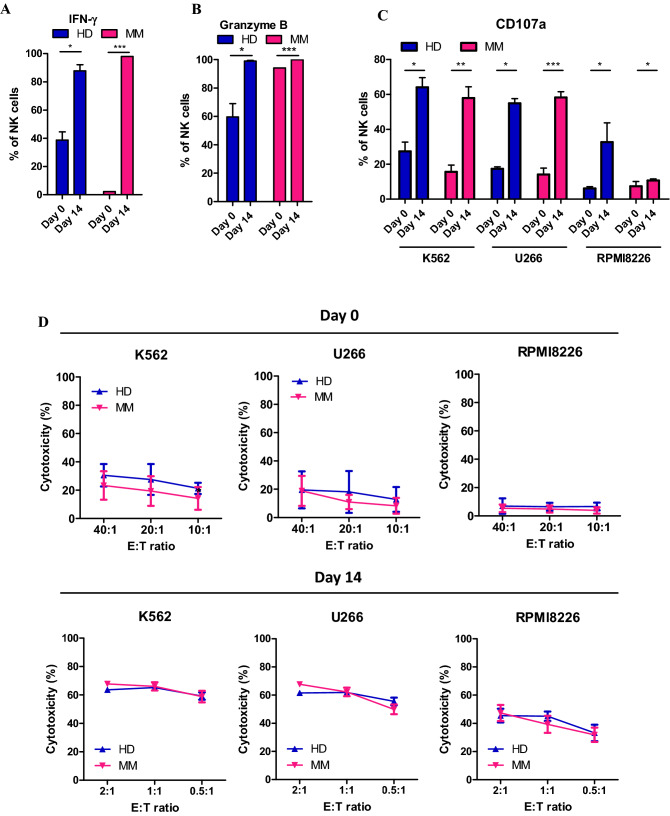
Because the activities of eNK cells should also increase with increased expression of activating receptors, we evaluated two major activities of NK cells: immune regulation by cytokine release and cytotoxicity. We measured intracellular IFN-γ to evaluate immune regulation activity and intracellular granzyme B, a protein involved in the cytotoxic function of NK cells, to evaluate cytotoxicity. During NK cell expansion, levels of intracellular IFN-γ and granzyme B were increased in cell cultures from both HDs and MM patients (Fig. 5a, b). Interestingly, NK cells from MM patients expressed little IFN-γ (~ 2%) before expansion; however, nearly all NK cells expressed IFN-γ (~ 98%) after expansion. CD107a degranulation assay and flow cytometry-based cytotoxicity assay were performed to evaluate the direct killing ability of NK cells (Fig. 5c, d). Before and after expansion, NK cells from HDs and MM patients were incubated with target cells, such as K562 and myeloma cells (U266, RPMI8226). In all cases, a higher CD107a + positive population and higher cytotoxicity were observed for eNK cells. These results indicate that NK cells with increased activities were successfully generated from PBMCs of HDs and MM patients.
Fig. 5.
Cytokine release and cytotoxic activity of eNK cells obtained from HDs and MM patients. Comparison of intracellular expression of IFN-γ (a) and granzyme B (b) of NK cells before and after expansion in HDs and MM patients. CD107a assay (c) and flow cytometry-based cytotoxicity assay (d) using K562, U266, and RPMI8226 cells before and after NK cell expansion. Means and SEM were plotted (n = 4–9). *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
In the present study, we showed that NK cell expansion was remarkably enhanced by using K562-OX40L as feeder cells and short exposure to soluble IL-18/IL-21. Based on this finding, we developed GE-K562-OX40L-mb-IL-18/-21 cells as feeder cells to enhance NK cell expansion and activation without the need to provide soluble IL-18/IL-21. By co-culture with K562-OX40L-mb-IL-18/-21 cells, NK cells from the PBMCs of HDs and MM patients expanded vigorously. Compared with NK cells from HDs, NK cells from MM patients initially showed low expression of activating receptors (NKp46, NKG2D) and high expression of an inhibitory receptor (CD158b). However, after NK cell expansion, the expression levels of activating receptors were similar in HDs and MM patients while expression of inhibitory receptors remained unchanged, indicating successful upregulation of activating receptors in MM patients. Increased activity of eNK cells from MM patients was also confirmed by cytotoxicity assays, CD107a degranulation, IFN-γ production, and granzyme B expression measurements.
Several GE-K562 feeder cell lines expressing NK cell activation signals (4-1BBL, OX40L) as well as cytokines (mbIL-15, mbIL-21) have been developed to expand highly activated NK cells ex vivo [17–19, 25]. GE-K562 cells expressing cytokines can reduce the cost of NK cell expansion. However, gamma-irradiated K562 feeder cells disappear within 3 days due to the cytotoxicity of NK cells [21]. For NK cell expansion, cytokines expressed by GE feeder cells should only be expressed for a short time (IL-12) rather than continuously (i.e., IL-2 or IL-15). Lee’s group used gamma ray-irradiated GE-K562 feeder cells expressing mbIL-21 to induce NK cell expansion; a mean 47,946-fold expansion was achieved after 21 days, which is a 58-fold increase compared to co-culture with GE-K562 cells expressing mbIL-15 [19, 25]. Therefore, co-culture of NK cells with IL-21-expressing GE-K562 cells is equivalent to short-term exposure to IL-21.
Recently Senju et al. reported that IL-18 not only promoted the expansion of NK cells, but also changed the phenotype of NK cells to active [22]. Thus, IL-18 is a good cytokine candidate for the expansion of active NK cells. In this study, we treated cells with soluble IL-18 once at the beginning of culture (day 0) to confirm that short exposure to IL-18 benefits NK cell expansion in our culture system using K562-OX40L cells and short exposure to IL-21. We found that short exposure to IL-18, along with IL-21, enhanced NK cell expansion and improved NK cell expansion relative to IL-21 alone.
Based on this finding, we next developed a GE-K562-OX40L cell line expressing IL-18 and IL-21 (K562-OX40L-mbIL-18/-21), and then used K562-OX40L-mbIL-18/-21 as feeder cells for NK cell expansion from MM- and HD-derived PBMCs. Twenty-eight days after the start of expansion, NK cell expansion was greater for PBMCs co-cultured with K562-OX40L-mbIL-18/-21 cells than K562-OX40L cells, regardless of whether the PBMCs were from healthy donors or MM patients. However, compared to HD-derived PBMCs, less expansion was noticed in MM patient-derived PBMCs. This might be due to differences in initial NK cell numbers between HDs and MM patients who had more than 60% CD3−CD56+ lymphocytes (data not shown). The relatively high percentage of NK cells in two MM patients might have been caused by prior therapy with an immunomodulating dose of cyclophosphamide. It has been reported that compared with NK cells from the blood of HDs, there are an increased number of circulating NK cells in patients with active MM and monoclonal gammopathy of undetermined significance [26–28]. The eNK cells from HDs and MM patients co-cultured with K562-OX40L- mbIL-18/-21 cells had a comparable expansion rate to that reported in other studies using K562-41BBL-mbIL-15 feeder cells [11, 29], and higher NK cell purity than that reported for the OKT3 plus IL-2 (500 U/mL) culture system [10] (Supplementary Table 2). In MM patients, impaired NK cell effector function due to the immune-suppressive tumor microenvironment has been reported [30]. Plasma cells and T regulatory cells in MM patients secrete high levels of TGF-β, resulting in downregulation of multiple NK-activating receptors and impairment of NK cytotoxicity [31–33]. Frauriat et al. showed normal NCR and NKG2D expression, but decreased 2B4 and CD16 expression in the peripheral blood of MM patients (8), while Costello et al. demonstrated drastic downregulation of NKp30, NKG2D, and CD244/2B4/p38 in bone marrow [34]. Reduced DNAM-1 expression was also noted in MM patients [35]. Consistent with these reports, we found significantly decreased expression of activating receptors NKp46, NKG2D, and CD69 in the PBMCs of MM patients compared to HD patients before culture. However, eNK cells generated from MM patients showed increased expression of activation markers (CD69, NKp30, NKp44, NKp46, and NKG2D) and cytotoxicity towards K562 and MM cells similar to the cytotoxicity observed for HD-derived NK cells. Our results, in agreement with data from Alici et al. [10], indicate that the initially impaired effector function of eNK cells from MM patients can be overcome and that NK cell function can be rescued. Recently Dean Lee group found that NK cells expanded using K562 expressing membrane bounded IL-15 reached senescence stage very early than NK cells expanded using K562 expressing membrane bound IL-21 which is caused by elevation of telomere length in expanded NK cells [19]. In our study, we also found effects of short exposure to IL-18 and IL-21 on NK cell expansion using either soluble and membrane bounded form of the cytokines. The addition of IL-18 to the cell culture markedly enhanced the NK cell purity and resulted in improved expansion rate. Moreover, IL-21 was reported to induce elevation of telomere length of expanded NK cells [15, 18, 19]. It can be speculated that K562-OX40L cells expressing both IL-18 and IL-21 may have more potential to stimulate NK cells expansion and activation than K562-OX40L cells expressing IL-18 or IL-21 alone. However, further studies are required to confirm detail mechanisms about the synergic effect of OX40L, IL-18, and IL-21 on NK cell expansion.
In conclusion, co-culture of NK cells from HD and MM patients with K562-OX40L-mbIL-18/-21 cells expressing membrane bound IL-18/IL-21 resulted in outstanding NK cell expansion. Furthermore, eNK cells from MM patients, who have dysfunctional NK cells, demonstrated strong cytolytic activity, thus we expect that eNK cells generated by K562-OX40L-mbIL-18/-21 cells may be a potential therapy for MM.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JL Thangaraj, SH Kweon, M-T Phan, MC Vo, and TH Chu performed the research and analyzed data; J Kim, I Hwang, JH Park, J Doh and SH Lee analyzed data; JH Kim, SH Kim, and JM Lee generated genetically engineered K562-OX40L-mb18/-21 cells; JS Doh, D Cho, and JJ Lee designed the research study and JL Thangaraj, SH Kweon, M-T Phan JH Park, JS Doh, GY Song, SY Ahn, SH Jung, HJ Kim, D Cho, and JJ Lee co-wrote the paper.
Funding
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2018R1A2B6006200, 2020R1A2C2010098).
Declarations
Conflicts of interest
The authors have no conflicts of interest to report. The authors alone are responsible for the content and writing of the paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
JayaLakshmi Thangaraj, Minh-TrangThi Phan, SoonHo Kweon authors contributed equally to this work
Contributor Information
Duck Cho, Email: duck.cho@skku.edu.
Je-Jung Lee, Email: drjejung@chonnam.ac.kr.
References
- 1.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002 doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:955–959. doi: 10.1200/jco.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 5.Benson DM, Jr, Bakan CE, Zhang S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–6391. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J, Tricot GJ, Garg TK, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahaweni NM, Bos GMJ, Mitsiades CS, Tilanus MGJ, Wieten L. Daratumumab augments alloreactive natural killer cell cytotoxicity towards CD38+ multiple myeloma cell lines in a biochemical context mimicking tumour microenvironment conditions. Cancer Immunol Immunother. 2018;67:861–872. doi: 10.1007/s00262-018-2140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauriat C, Mallet F, Olive D, Costello RT. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia. 2006;20:732–733. doi: 10.1038/sj.leu.2404096. [DOI] [PubMed] [Google Scholar]
- 9.Jurisic V, Srdic T, Konjevic G, Markovic O, Colovic M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol (Northwood, London, England) 2007;24:312–317. doi: 10.1007/s12032-007-0007-y. [DOI] [PubMed] [Google Scholar]
- 10.Alici E, Sutlu T, Björkstrand B, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–3162. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 11.Garg TK, Szmania SM, Khan JA, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica. 2012;97:1348–1356. doi: 10.3324/haematol.2011.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weigent DA, Stanton GJ, Johnson HM. Interleukin 2 enhances natural killer cell activity through induction of gamma interferon. Infect Immun. 1983;41:992–997. doi: 10.1128/IAI.41.3.992-997.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, Croce CM, Baumann H, Caligiuri MA. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 15.Lim D-P, Jang Y-Y, Kim S, et al. Effect of exposure to interleukin-21 at various time points on human natural killer cell culture. Cytotherapy. 2014;16:1419–1430. doi: 10.1016/j.jcyt.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Wagner J, Pfannenstiel V, Waldmann A, et al. A two-phase expansion protocol combining interleukin (IL)-15 and IL-21 improves natural killer cell proliferation and cytotoxicity against rhabdomyosarcoma. Front Immunol. 2017 doi: 10.3389/fimmu.2017.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kweon S, Phan M-TT, Chun S, et al. Expansion of human NK cells using K562 cells expressing OX40 ligand and short exposure to IL-21. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012 doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Can Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek HJ, Kim JS, Yoon M, Lee JJ, Shin MG, Ryang DW, Kook H, Kim SK, Cho D. Ex vivo expansion of natural killer cells using cryopreserved irradiated feeder cells. Anticancer Res. 2013;33:2011–2019. [PubMed] [Google Scholar]
- 22.Senju H, Kumagai A, Nakamura Y, et al. Effect of IL-18 on the expansion and phenotype of human natural killer cells: application to cancer immunotherapy. Int J Biol Sci. 2018;14:331–340. doi: 10.7150/ijbs.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh H, Figliola MJ, Dawson MJ, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Can Res. 2011;71:3516–3527. doi: 10.1158/0008-5472.can-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan MT, Lee SH, Kim SK, Cho D. Expansion of NK Cells using genetically engineered K562 feeder cells. Methods Mol Biol. 2016 doi: 10.1007/978-1-4939-3684-7_14. [DOI] [PubMed] [Google Scholar]
- 25.Somanchi SS, Lee DA. Ex vivo expansion of human nk cells using K562 engineered to express membrane bound IL21. Methods Mol Biol. 2016 doi: 10.1007/978-1-4939-3684-7_15. [DOI] [PubMed] [Google Scholar]
- 26.Famularo G, D'Ambrosio A, Quintieri F, Di Giovanni S, Parzanese I, Pizzuto F, Giacomelli R, Pugliese O, Tonietti G. Natural killer cell frequency and function in patients with monoclonal gammopathies. J Clin Lab Immunol. 1992;37:99–109. [PubMed] [Google Scholar]
- 27.Pessoa de Magalhães RJ, Vidriales MB, Paiva B, et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2013;98:79–86. doi: 10.3324/haematol.2012.067272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viel S, Charrier E, Marçais A, Rouzaire P, Bienvenu J, Karlin L, Salles G, Walzer T. Monitoring NK cell activity in patients with hematological malignancies. Oncoimmunology. 2013 doi: 10.4161/onci.26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szmania S, Lapteva N, Garg T, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother. 2015 doi: 10.1097/cji.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125:3049–3058. doi: 10.1182/blood-2014-11-568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittari G, Vago L, Festuccia M, Bonini C, Mudawi D, Giaccone L, Bruno B. Restoring natural killer cell immunity against multiple myeloma in the era of new drugs. Front Immunol. 2017;8:1444. doi: 10.3389/fimmu.2017.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA, Schlossman RL, Anderson KC. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood. 1996;87:1928–1938. doi: 10.1182/blood.V87.5.1928.1928. [DOI] [PubMed] [Google Scholar]
- 33.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 34.Costello RT, Boehrer A, Sanchez C, Mercier D, Baier C, Le Treut T, Sebahoun G. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology. 2013;139:338–341. doi: 10.1111/imm.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Sherbiny YM, Meade JL, Holmes TD, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Can Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.can-06-4230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.