Abstract
AML is the most common blood cancer in adults with a high relapse and an overall poor survival rate. NK cells have been demonstrated to have the capacity to eradicate AML blast, and an impaired NK cell function is involved in AML development and progression. Immune checkpoints are involved in immune escape in various cancers. Immune checkpoints blockade therapy mainly aimed to unleash CD8+T cells function, but NK cells have emerged as new target. However, immune checkpoints profile on NK cells has not been observed in AML patients. Here, we studied the immune checkpoints expression of NK cells from AML patients at initial diagnosis and found increased PD-1, TIGIT and TIM-3 expression compared to NK cells from healthy donors. Further analysis showed that TIGIT expressing NK cells from AML patients had a dysfunctional phenotype, as TIGIT+NK cells exhibit lower antileukemia effect, cytokine production and degranulation compared to TIGIT−NK cells. TIGIT blockade could significantly enhance the function of NK cells. Moreover, AML patients with high frequency of TIGIT+NK cells had higher frequency of poor prognosis risk. Further analysis found that IL-10 upregulated TIGIT expression on NK cells. Thus, TIGIT blockade alone or in combination with other therapy might be potential strategy to treat AML.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02978-5.
Keywords: AML, NK, TIGIT, CD226, Dysfunction
Introduction
Acute myeloid leukemia (AML) is the most common adult leukemia characterized by the uncontrolled proliferation of low differentiated myeloid cells in the bone marrow [1]. And it is a heterogeneous disease with a high rate of relapse and poor overall survival [2, 3]. The conventional therapeutic options for AML patients are induction chemotherapy followed by post-remission therapy [4]. New treatment including NK (Natural Killer) cell-based immunotherapy to treat AML patients is under intensive evaluation [5].
NK cells are innate immune cells playing a key role in host defense against infection and cancer [6]. Without prior activation, NK cells exert rapid and potent killing to virus-infected cells and cancer cells, such as AML blast cells. Significant impaired function of NK cells in AML patients can facilitate escape of AML blast cells from immune surveillance[7, 8, 9]. The mechanisms of NK cell defects and AML evasion to escape from NK mediating killing have been studied. For instance, downregulation of activating receptors such as natural cytotoxicity receptors (NCRs), NKG2D and CD226, and upregulation of inhibitory receptors such as NKG2A contributed to the impaired NK function and correlated with poor prognosis of AML[10, 11]. Several immune checkpoints involving NK cell dysfunction other than above molecules have been identified, including PD-1, CTLA-4, TIGIT, CD96, Lymphocyte Activation Gene-3 (LAG-3) and T-cell immunoglobulin and mucin domain-3 (TIM-3) et al. [12]. However, these immune checkpoints expression profile on NK cells in AML patients has not been investigated.
Blockade of immune checkpoints which are involved in immune escape in various cancers is one type of immunotherapy. It mainly aimed to unleash CD8+T cells function, but NK cells have immerged as new target during immune checkpoints blockade therapy [12, 13]. In AML, the effects of the revolutionized PD-1/PD-L1 blockade combined with other therapy are under the evaluation [7]. NK cells are equipped with different receptors including stimulatory or inhibitory receptors, and their function is tightly regulated by these receptors [14, 15]. In addition to the classical NK cell receptors such as killer immunoglobulin-like receptors (KIRs), and NKG2A, the immune checkpoints such as PD-1, TIGIT, TIM-3, and LAG-3 have been shown to cause NK cell dysfunction in various cancers or chronic infections [12]. Recently, it has been shown that TIGIT expressing NK cells are dysfunctional and blockade of TIGIT prevents NK cell exhaustion, as well as promotes NK cell-mediated antitumor effects in numerous mouse models of cancer (colon, breast, melanoma and fibrosarcoma) [16]. Which lead us to ask what kind of immune checkpoints are expressed on NK cells from AML patients, and which receptors are related with the dysfunction of NK cells?
Here, we found the abnormal expression of immune checkpoints that included increased frequency of PD-1+, TIGIT+ and TIM-3+ on NK cells from AML patients at the time of diagnosis. We previously found that increased TIGIT expressing CD8+T cells with low expression of CD226 were dysfunctional in AML patients [17]. Therefore, we further focused on TIGIT expressing NK cells and found those NK cells had the dysfunctional phenotype compared with healthy controls. When we added the anti-TIGIT blockade, the killing leukemia capacity of NK cells was enhanced. Most importantly, AML patients with high frequency of TIGIT+NK cells had higher frequency of poor prognosis risk, and with high TIGIT and CD155 expression had poor overall survival (OS) using TCGA database. Further analysis found that IL-10 upregulated TIGIT expression on NK cells.
Materials and methods
Subjects and Ethics statement
Whole blood from 23 patients with AML (clinical information showed in Table 1) at initial diagnosis before induction and 30 aged-matched healthy donors (HDs) (16 males and 14 females) were collected from Guangdong General Hospital, and diagnosis was established according to cytological criteria based on the French–American–British (FAB) classification and bone marrow cell morphology. Healthy donors were critically selected on the basis of clinical records and laboratory examinations and had no acute or chronic infectious diseases, autoimmune diseases or tumors.
Table 1.
Comparison clinical data from AML patients based on high and low TIGIT expressing NK frequency
| Total (n = 23) |
Lower TIGIT (%) (n = 10) |
Higher TIGIT (%) (n = 13) |
P-Value |
|---|---|---|---|
| Age, (years) | |||
| M + SD | 42 ± 6.72 | 47.7 ± 4.44 | 0.465 |
| Range | 16–77 | 13–70 | |
| Gender | |||
| Male | 5 | 5 | 0.685 |
| Female | 5 | 8 | |
| WBC, × 109/L | |||
| M + SD | 35.9 ± 14.73 | 90.15 ± 23.84 | 0.087 |
| Range | 0.67–109.9 | 1.22–259.4 | |
| HGB, g/L | |||
| M + SD | 62.16 ± 9.664 | 77.38 ± 6.763 | 0.198 |
| Range | 0.569–111 | 36–128 | |
| PLT, × 109/L | |||
| M + SD | 80.4 ± 17.65 | 55.31 ± 15.64 | 0.300 |
| Range | 16–157 | 8–213 | |
| PB Lymphocyte, % | |||
| M + SD | 32.6 ± 9.178 | 20.62 ± 6.206 | 0.275 |
| Range | 6–88 | 1–66 | |
| PB Blast, % | |||
| M + SD | 36.22 ± 9.063 | 58.56 ± 7.497 | 0.073 |
| Range | 4–76 | 15–97 | |
| BM Lymphocyte, % | |||
| M + SD | 11.55 ± 4.309 | 8.462 ± 3.547 | 0.583 |
| Range | 2.5–48 | 2–50 | |
| BM Blast, % | |||
| M + SD | 55 ± 7.885 | 71.65 ± 6.683 | 0.120 |
| Range | 20–92.5 | 21–93 | |
| FLT3-ITD gene mutation | |||
| Negative | 8 | 11 | > 0.999 |
| Positive | 2 | 2 | |
| Prognosis risk grading | |||
| Good/medium | 8 | 4 | 0.036 |
| Bad | 2 | 9 | |
M + SD mean + standard deviation; WBC white blood cell; HGB Hemoglobin; PLT Platelet; PB peripheral blood; BM bone marrow; ITD internal tandem duplication. 23 patients (13 of high-TIGIT, 10 of low-TIGIT) are shown. Quantitative data were compared two sample means by unpaired t-test, and qualitative data were analyzed by chi-square test. P < 0.05 was considered significant
Cell preparation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of healthy donors (HDs) and AML patients by using Ficoll–Hypaque density gradient centrifuge. Purified NK cells were obtained by negative selection from PBMCs using EasySep™ Human NK Cell Isolation Kit (Stemcell) according to the manufacturer’s protocol. For cell stimulation studies, PBMCs or purified NK cells were stimulated with IL-2 (100 U/mL, Peprotech), IL-12 (100 U/mL, Peprotech) for 24 h. In some experiments, functional antihuman TIGIT antibody (5 μg/mL, eBioscience), antihuman CD226 antibody (5 μg/mL, eBioscience), or IgG isotype control (5 μg/mL, R&D Systems) were added to the culture medium for blocking TIGIT pathway. K562 cell line was obtained from Dr. Jinbao Liu in Guangzhou Medical University (Guangzhou, China). K562, PBMCs and NK cells were cultured in RPMI-1640 medium(GIBICO, Grand, Island, NY, USA) containing 10% FBS (Sijiqing, China), 100U/ml penicillin, 100U/ml streptomycin, 1 mM sodium pyruvate, 55 mM 2-mercaptoethanol (2-ME) (all from GIBICO).
Phenotype analysis of NK cells and CD155/CD112 expression on AML blast by flow cytometry
50 ul whole blood were stained with fluorescence dye labeled Abs specific for human CD45, CD3, CD56, TIGIT, NKG2D, CD33, CD96, TIGIT, CD226, LAG-3, CD107a, PD-1, TIM-3, CD69, CD200R, CD112, CD155 (purchased form BD Biosciences, eBioscience, or Biolegend) 30 min at room temperature. Red blood cells were lysed with ammonium chloride (NH4Cl) for 10 min. Cells were washed and re-suspended in 200μL cold FACS buffer, and followed by analysis with Cytoflex (Beckman coulter, all the cells were recorded). For CTLA4 staining, fixed and permeabilized, and stained with fluorescence labeled anti-CTLA4 antibody. For CD155 and CD112 expression, CD45+CD33+ ML blast cells were analyzed. Data analysis was performed using CytExpert version 2.0 software (Beckman coulter) [16].
Detection of CD107a and intracellular staining for IFN-γ
FITC labeled anti-CD107a antibody was added into the cells and cultured for 6 h in the presence of brefeldin A (10ug/ml; Sigma-Aldrich). The cells were isolated and stained with cell surface markers. Then, the cells were fixed and permeabilized, and stained with APC conjugate anti-IFN-γ antibody (eBioscience). After washing, the pellets were re-suspended in 300μL cold FACS buffer. The cells were analyzed with Cytoflex (Beckman coulter). Data analysis was performed using CytExpert version 2.0 software (Beckman coulter).
Cytotoxicity assay
Purified NK cells were stimulated with or without IL-2 (100 U/mL) and IL-12 (100 U/mL) for 72 h. After culture, the NK cells were collected as effector cells. K562 cells were labeled with CFSE (eBioscience) and used as target cells. Effector cells and target cells were co-incubated at effector to target (E:T) ratio 10:1 in 96 wells plate for 4 h at 37 °C with 5% CO2. Control wells including only target cells were assayed to determine spontaneous cell death. After washing, the cells were suspended in 200μL, 1 × binding buffer (BD), and analyzed immediately by flow cytometry and the dead target cells were indicated as CFSE high 7-AAD+ cells [18].
TCGA Transcriptomic Analysis
The TCGA-LAML cohort data sets are available through the Cancer Genome Atlas (TCGA) data portal (https://portal.gdc.cancer.gov/). We downloaded the fragments per kilobase of transcript per million mapped reads-based gene expression for analysis with gene annotations using GENCODE version 35. Fragments per kilobase of transcript per million mapped reads-based gene expression of RNA-Seq data were transformed to log2 + 1. Correlation between IL10 expression and TIGIT in AML patients was assessed using a Spearman correlation test. For survival analysis of IL10 gene expression, AML patients were divided into 2 groups of IL10 high (> = cutoff point) and IL10 low (< cutoff point) patients based on the comparison with cutoff point of IL10 expression value, which determined by survminer package, and Kaplan–Meier estimates for OS and log-rank tests then were calculated. Univariate cox regression was used to calculate p value and Hazard ratio (HR) of IL10 gene expression.
For survival analysis of combined TIGIT, CD112 and CD155 gene expression, cox regression was used to calculate the combined risk score for those three genes. AML patients were then divided into 2 groups of risk score high (> = cutoff point) and risk score low (< cutoff point) patients based on the comparison with cutoff point of risk score value, which determined by survminer package, and Kaplan–Meier estimates for OS and log-rank tests then were calculated. Univariate cox regression was used to calculate p value and Hazard ratio (HR) of risk score group [19; 20].
Statistical analysis
Statistical differences were calculated with an unpaired Student’s t-test (two unpaired groups) or a paired Student’s t-test (two paired groups). Statistical differences among three or more groups were analyzed by one-way ANOVA with Tukey's multiple comparison tests. Results are expressed as mean ± SEM. Simple correlations were summarized using the Pearson correlation coefficient (r). A value of P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software version 5.
Results
Abnormal immune checkpoints expression on NK cells from AML patients at newly diagnosis
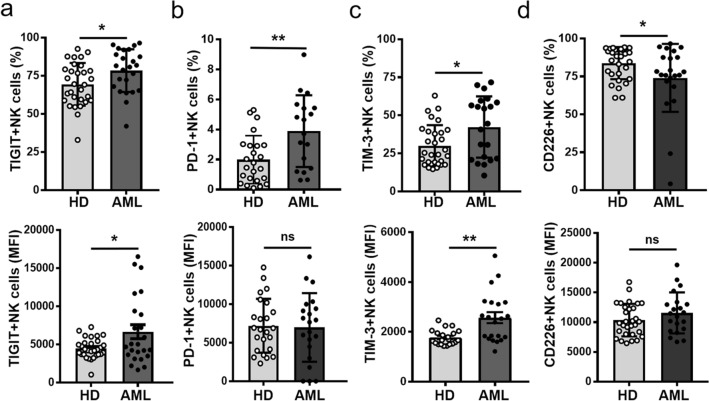
We firstly evaluated the frequency and subsets of NK cells in blood and found that the percentage of NK cells from AML patients was significantly lower compared to healthy donors (HD), which was mainly due to the decrease of CD56dim NK cells (Fig. S1). We further determined the immune checkpoints expression including TIGIT, PD-1, CTLA-4, TIM-3, CD226, CD200R and LAG-3 on NK cells from AML patients at newly diagnosis and both age-matched healthy donors (HD) controls. Flow cytometry analysis revealed that the frequency of NK cells expressing the inhibitory receptors TIGIT, PD-1, or TIM-3 and the expression levels (MFI) of TIGIT or TIM-3 on these cells from AML patients were significantly higher than those from healthy donors (HD) (Fig. 1a–c, S2). By contrast, the frequency of stimulatory receptor CD226 expression on NK cells was markedly decreased (Fig. 1d, S2). In addition, no significant differences of CD200R, CTLA-4 or LAG-3 expression on NK cells were observed (Fig. S3). These results demonstrated that there is abnormal immune checkpoints expression on NK cells from AML patients at newly diagnosis, including increased expression of TIGIT, PD-1 and TIM-3, but reduced CD226 expression.
Fig. 1.
Abnormal immune checkpoints expression on NK cells in AML patients. Quantification of the frequency (top) and expression level (MFI) (bottom) of TIGIT a, PD-1 b, TIM-3 c, CD226 d on CD45+CD3−CD56+NK cells from PBMCs of AML patients or HD by flow cytometry. Each symbol represents one subject, and the data are shown as the mean (± SEM). HD, healthy donors. P values, unpaired two-tailed t-test (a, b, c, d), *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, no significant difference
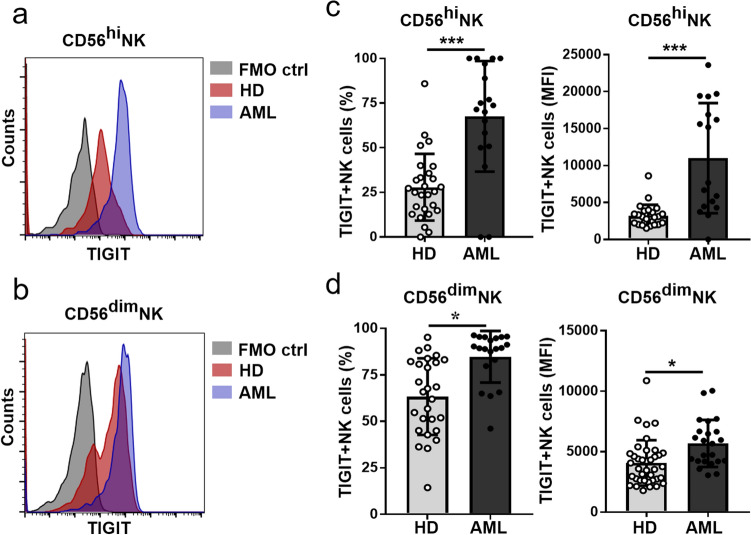
Upregulation TIGIT expression on NK cells with dysfunctional phenotype from AML patients
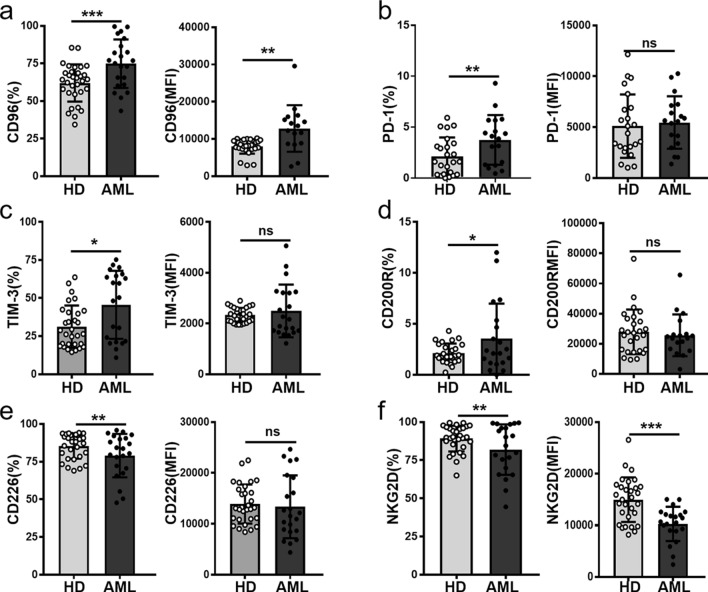
Since recent publication found that the NK cells expressing TIGIT are dysfunctional and blockade of TIGIT promotes NK cell-mediated antitumor effects in several mouse models of cancers [16]. We further determined TIGIT expression on NK subsets and found that TIGIT frequency and expression levels (MFI) on both CD56hi and CD56dim NK subsets from AML patients were significantly increased (Fig. 2). We further determined the phenotype of those TIGIT expressing NK cells including inhibitory receptors CD96, PD-1, TIM-3, CD200R and activating receptors CD226, NKG2D. Compared with HD controls, there were higher frequencies of TIGIT+ NK cells expressing the inhibitory receptors CD96, PD-1, TIM-3, or CD200R as well as the expression levels of CD96 on those cells from AML patients (Figs. 3a–d). By contrast, the frequencies of activating receptors CD226, NKG2D expressed on TIGIT+NK cells were remarkably decreased (Figs. 3e, f). The above results suggested that TIGIT+NK in AML patients might be dysfunctional and involved in the immune escape of AML blast cells.
Fig. 2.
Upregulation TIGIT expression on both CD3−CD56hi and CD3−CD56dim NK cells in AML patients. Representative histogram of TIGIT expression on CD3−CD56hi a and CD3−CD56dim b NK cells. Quantification of frequency (top) and MFI (bottom) of TIGIT expression on NK subsets including CD45+CD3−CD56hi c and CD45+CD3−CD56dim NK cells d from HD and AML patients by flow cytometry. Each symbol represents one subject, and the data are shown as the mean (± SEM). HD, healthy donors. P values, unpaired two-tailed t-test (a, b, c, d), *P < 0.05, ***P < 0.001
Fig. 3.
TIGIT expressing NK cells with dysfunctional phenotype from AML patients. TIGIT + NK cells were first gated and then the frequency and MFI of CD96 a, PD-1 b, TIM-3 c, CD200R d, CD226 e, NKG2D f expression on these cells from AML patients and HDs were quantified. Each symbol represents one subject, and the data are shown as the mean (± SEM). HD, healthy donors. P values, unpaired two-tailed t-test (a, b, c, d), *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, no significant difference
Lower IFN-γ production and degranulation of TIGIT+ NK cells compared with TIGIT−NK cells
To determine whether TIGIT+NK cells have lower function, we compared the cytokine production and degranulation capacity of TIGIT+ NK and TIGIT− NK cells. We found that TIGIT+ NK cells displayed lower IFN-γ production and reduced degranulation than those by TIGIT− NK cells under IL-12 stimulation (Fig. 4a). And there was a negative correlation between IFN-γ production or degranulation with TIGIT expression on NK cells (Fig. 4b). Moreover, the activation of TIGIT+NK cells decreased compared to TIGIT−NK (Fig. 4c), and there was a negative correlation between the activation and TIGIT expression on NK cells (Fig. 4d). Monocytes with high expression CD155 and CD112 might ligate to TIGIT inhibitory signaling to reduce IFN-γ production (Fig. S4a). We further purified NK cells and cultured with K562 cells which highly expressed TIGIT ligands CD155 and CD112 (Fig. S4a). Similar phenomena were observed: TIGIT+NK cells produced lower IFN-γ and CD107a, and there was a negative correlation between IFN-γ production or degranulation with TIGIT expression on NK cells (Figs. S4b, c). Taken together, our results demonstrated that TIGIT+NK cells have lower function.
Fig. 4.
Lower expression of IFN-γ, CD107a by TIGIT+NK cells a Comparison of IFN-γ and CD107a production by TIGIT− and TIGIT+NK cells under stimulation with rIL-12. b The negative correlation between the frequency of TIGIT expressing NK cells with IFN-γ or CD107a expression by NK cells. c Comparison of CD69 expression on TIGIT− and TIGIT+NK cells after stimulation with rIL-12. d The correlation between the frequency of TIGIT expressing NK cells with CD69 expression on NK cells. HD, healthy donors. Each symbol represents one subject and the data are shown as the mean (± SEM). P values, unpaired two-tailed t-test a, c; the Spearman’s rank test was employed for correlation analyses (b, d), *, P < 0.05; **, P < 0.01
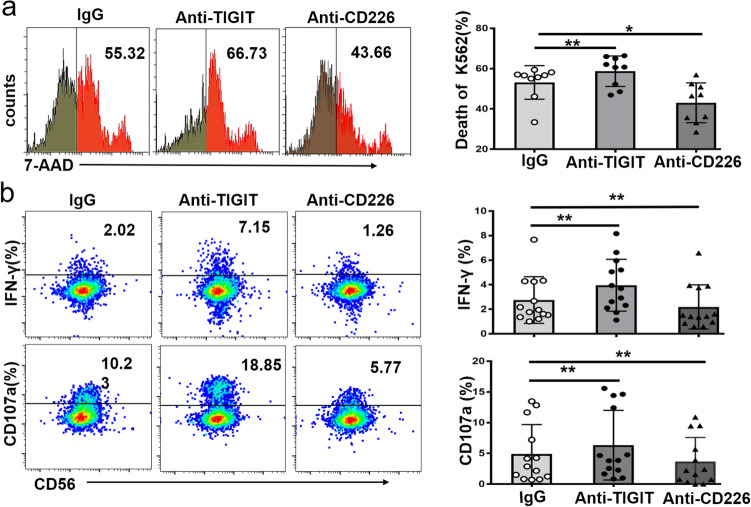
Enhanced cytotoxicity of NK cells by blocking TIGIT
We further evaluated whether blockade of TIGIT pathway could enhance the cytotoxicity of NK cells to leukemia cells. TIGIT blockade significantly enhanced NK cytotoxicity to leukemia cell line K562 (Fig. 5a). Conversely, CD226 blockade remarkably reduced NK cytotoxicity (Fig. 5a). Previous study demonstrated that TIGIT signal pathway mainly inhibits IFN-γ production to suppress NK function [21]. Accordingly, we found that blocking TIGIT enhanced cytotoxicity of NK cells due to increased IFN-γ production and degranulation of NK cells (Fig. 5b). Furthermore, TIGIT+NK cells from AML patients expressed higher activating receptors CD226 and NKG2D compared to TIGIT−NK (Fig. S5), which suggested that there might be enhanced antileukemia effects of NK by TIGIT blockade in vivo in AML patients.
Fig. 5.
TIGIT blockade enhance cytotoxicity of NK cells. Purified NK cells were cultured with IL-12 for 72 h, and were co-cultured with CFSE-labelled K562 at effector to target (E:T) ratio 10:1 for 4 h under the isotype control Ab (IgG), anti-TIGIT neutralizing Ab, and anti-CD226 neutralizing Ab. The cytotoxicity of effector NK cells was determined by 7-AAD + CFSE labeled K562 cells. a Quantification of the frequency of 7AAD+ CFSE labeled K562 cells in the presence of anti-TIGIT Ab or anti-CD226 Ab blockade. b IFN-γ and CD107a expression by effector NK cells in the presence of anti-TIGIT Ab or anti-CD226 Ab blockade. HD, healthy donors. Each symbol represents one subject and the data are shown as the mean (± SEM). P values, one-way ANOVA followed by Dunnett’s multiple-comparisons test (a, b), *, P < 0.05; **, P < 0.01
High frequency of TIGIT+NK cells predicts poor prognosis of AML patients
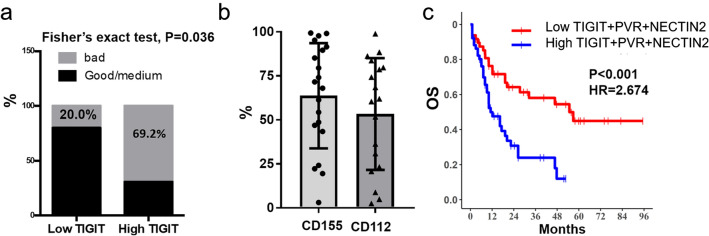
Next, we asked whether the increased frequency of TIGIT+NK with dysfunctional phenotype was correlated with the prognosis of AML. Clinical data from AML patients were compared based on the low and high frequency of TIGIT+NK cells [15, 16]. We found that compared with low TIGIT+NK frequency, the AML patients with the high TIGIT+ NK frequency had higher frequency of poor prognosis risk (Table 1, Fig. 6a). Since the ligands of TIGIT were CD112 and CD155, we further determined those ligands expression on AML blast. Majority of patients expressed more than half of CD112 or CD155 [Fig. 6b]. Using LAML cohorts from TCGA database, survival analysis of combined TIGIT, CD112 and CD155 gene expression based on the risk score demonstrated that patients with high TIGIT and CD155 expression were associated with poor overall survival (OS)[Fig. 6c]. The above results indicated that the increased TIGIT expression on NK cells from AML patients are involved in the AML blast escape and high TIGIT+NK cell frequency can predict poor prognosis.
Fig. 6.
Patients with high TIGIT+ NK frequency had higher percentage of poor prognosis risk. a AML patients were divided into two groups based on the low and high frequency of TIGIT + NK cells as shown in Table 1, frequency of bad prognosis in each group was calculated, and Fisher’s exact test was done. b CD155 or CD112 expression on CD45+CD33+ AML blast by flow cytometry. c Cox regression was used to calculate the combined risk score for those three genes. AML patients were then divided into 2 groups of risk score high (> = cutoff point) and risk score low (< cutoff point) patients based on the comparison with cutoff point of risk score value, which determined by survminer package, and Kaplan–Meier estimates for OS and log-rank tests then were calculated. Univariate cox regression was used to calculate p value and Hazard ratio (HR) of risk score group
Given the significant increase of TIGIT expression on NK cells we observed in AML patients, we further explored the factors leading to elevated TIGIT expression on these cells. Previous studies have demonstrated that the levels of IL-10 and IL-6 significantly increased in AML patients [22]. In LAML cohorts from TCGA database, a high IL10 gene expression but not IL6 (data not shown) was associated with poor overall survival (OS) [Fig. 7a], and further analysis found that IL10 was correlated with TIGIT expression [Fig. 7b]. The above results led us to ask that whether IL-10 could upregulate TIGIT expression on NK cells. To test this, we cultured PBMCs with IL-10, and found that stimulation with IL-10 but not IL-6 led to significant increase in TIGIT expression on NK cells [Fig. 7c, d]. These findings suggest that the TIGIT expression on NK cells from AML patients may be due to (at least in part) to increased cytokine milieu such as IL-10.
Fig. 7.
IL-10 upregulates TIGIT expression on NK cells. a Kaplan–Meier survival curves showing OS in patients from LAML cohorts stratified by high and low IL10 expression (Statistics: log-rank tests). b correlation of IL-10 and TIGIT from LAML cohorts (Statistics: univariate cox regression). c and d PBMCs were cultured in medium, IL-10 or IL-6 for 3 days, then TIGIT frequency and MFI by NK cells were determined by FACS. **, P < 0.01
Discussion
Immune checkpoints blockade alone or combined with other therapy unleash the immune cells function to kill cancer cells [23, 24]. NK cells as one of the major cytotoxic effectors have been pushed into the spotlight for cancer treatment. Here, we mainly evaluated expression of the immune checkpoints including TIGIT, PD-1, TIM-3, CD200R, CTLA-4 and LAG-3 on NK cells from AML at newly diagnosis. Significantly increased frequency of TIGIT expressing NK cells lead us mainly focus on those NK subsets and found that they had the dysfunctional phenotype in AML at the newly diagnosis. Moreover, AML patients with high frequency of TIGIT+ NK cells had higher frequency of poor prognosis risk. Using TIGIT blockade in this study could significantly enhance NK cytotoxicity and cytokine production in vitro, which indicate that TIGIT blockade might enhance NK cells against AML blasts in vivo [16, 17, 25].
We and others previously found that increased TIGIT expressing CD8+T with dysfunction in AML patients was correlated with poorer prognosis [14, 26]. TIGIT is expressed on both T and NK cells, and compete with CD226 to bind with higher affinity to the same ligands CD155 and CD112 [27, 28, 29]. Here, we further observed increased percentage of TIGIT+NK cells among both CD56hi and CD56dim NK subsets and their dysfunctional phenotype in AML compared with healthy donors. Emerging studies found that high expression of CD155 and CD112 in AML blasts confers the negative prognosis to AML patients [30, 31]. This was in consistent with our finding that AML patients with high TIGIT+NK percentage at newly diagnosis had poor prognosis. Preclinical study demonstrated that tumor infiltrating TIGIT+NK were exhausted in several transplant mouse tumor models, and blockade of TIGIT could directly unleash the NK antitumor function and indirectly promote CD8+T antitumor response [16]. Therapeutic blockade of TIGIT alone or in combination with anti-PD-1 or anti-PD-L1 blockade is currently tested in several clinical trials. Furthermore, blockade of TIGIT-CD112/CD155 axis could enhance T cells cytotoxicity against leukemia cells [25]. Our results concur these findings, as we found that blockade of TIGIT could enhance NK cells antileukemia effect, and that AML patients with high TIGIT expression have poor prognosis [32]. However, it still needs to be studied whether TIGIT blockade alone or in combination with other immunotherapy can enhance both NK and CD8+T cytotoxicity against AML blast in vivo.
We observed that NK cells in AML patients at newly diagnosis had an upregulated PD-1 expression. It has been reported that PD-L1/PD-1 axis involved in inhibition of NK function, and blockade of this axis elicited a strong NK response in vivo in certain types of mouse tumor models [33]. Blockade of immune checkpoints (such as PD-1) could enhance cytokine expanded NK cytotoxicity against AML blast in vitro [34]. We found increased PD-1 expression on TIGIT+ NK cells in AML patients, which indicated that PD-1 might be involved in the dysfunction of TIGIT expressing NK cells in AML patients [15]. AML blast upregulated PD-L1 expression during or after therapy, which suggested PD-1 blockade with other therapy in several clinical trials in AML patients could enhance NK cytotoxicity against AML blast [35].
TIM-3 expressing NK cells are increased in several solid tumors such as melanoma, esophageal cancer and lung adenocarcinoma, and might be a marker for dysfunctional NK cells, since TIM-3 blockade could reverse NK cell dysfunction from those patients [36, 37, 38]. Here, we found that NK cells upregulated TIM-3 expression in AML patients at newly diagnosis. It has been reported that TIM-3-Galectin-9 are involved in AML blast escape and associated with chemotherapy resistant [39, 40]. TIM-3 is expressed on leukemia stem cells and blockade of TIM-3 might reverse NK cytotoxicity directly and Fc mediated killing LSCs [41]. Further observation of TIM-3 blockade in AML in vivo should be evaluated. It has been reported that upregulation of CD200 on AML blast cells could suppress NK cells function, we did not observe increased CD200R expression on AML NK cells [42].
In conclusion, we evaluated immune checkpoints expression on NK cells and found that increased TIGIT expressing NK cells with dysfunction phenotype from AML patients, and high frequency of TIGIT+NK predict poor prognosis in AML patients. Thus, TIGIT might be a good candidate to unleash both NK and CD8+T cytotoxicity against AML blast.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81971482, to ES), the Science and Technology Program of Guangzhou (No.007095050049 to ES), and the Science and Technology Planned Project of Bureau of Education of Guangzhou (No. 1201610221, to ES).
Author contributions
ES, MZ and GL conceived the experiments. GL, ZQ and JY performed the study. ES, GL and ZQ analyzed the data. XL, JC, and QL assisted to perform the experiments. YL assisted to do the statistical analysis. LX, WL, TL, QL and XX collected the samples. ES and GL wrote the paper. All authors contributed to the manuscript review. All authors contributed to the article and approved the submitted version.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Declaration
Conflict of interest
YL was employed by Shenzhen Withsum Technology Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This study was conducted in compliance with the Declaration of Helsinki and was approved by the ethics committee of Guangdong General Hospital (Guangzhou, China).
Footnotes
Erxia Shen is the first corresponding author.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guanfang Liu, Qi Zhang and Jingying Yang contributed equally to this work.
Change history
7/6/2021
Erxia Shen is the first corresponding author
Change history
5/8/2023
A Correction to this paper has been published: 10.1007/s00262-023-03455-x
Contributor Information
Maohua Zhou, Email: zmhuagz@126.com.
Erxia Shen, Email: erxia_shen@gzhmu.edu.cn.
References
- 1.Prada-Arismendy J, Arroyave JC, Rothlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31:63–76. doi: 10.1016/j.blre.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD, Diagnosis and management of AML in adults, ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, Garcia-Manero G, Konopleva M, Ravandi F. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11:41. doi: 10.1038/s41408-021-00425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F, Wang R, Feng W, Chen C, Yang X, Wang L, Hu Y, Ren Q, Zheng G. Characteristics of NK cells from leukemic microenvironment in MLL-AF9 induced acute myeloid leukemia. Mol Immunol. 2018;93:68–78. doi: 10.1016/j.molimm.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Miller JS, Lanier LL. Natural killer cells in cancer immunotherapy. Annual Rev Cancer Biol. 2019;3:77–103. doi: 10.1146/annurev-cancerbio-030518-055653. [DOI] [Google Scholar]
- 7.Baragano Raneros A, Lopez-Larrea C, Suarez-Alvarez B. Acute myeloid leukemia and NK cells: two warriors confront each other. Oncoimmunology. 2019;8:e1539617. doi: 10.1080/2162402X.2018.1539617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26:2019–2026. doi: 10.1038/leu.2012.87. [DOI] [PubMed] [Google Scholar]
- 9.Dulphy N, Chretien AS, Khaznadar Z, Fauriat C, Nanbakhsh A, Caignard A, Chouaib S, Olive D, Toubert A. Underground adaptation to a hostile environment: acute myeloid leukemia vs. Nat Killer Cells Front Immunol. 2016;7:94. doi: 10.3389/fimmu.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90:109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 11.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 12.Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. 2020;11:167. doi: 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 14.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 15.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R, Tian Z. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Bu J, Zhou M, Sido J, Lin Y, Liu G, Lin Q, Xu X, Leavenworth JW, Shen E. CD8(+)T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin Immunol. 2018;190:64–73. doi: 10.1016/j.clim.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Fu X, Liu Y, Li L, Li Q, Qiao D, Wang H, Lao S, Fan Y, Wu C. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO− natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41–49. doi: 10.1111/j.1365-2567.2011.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Jiang T, Zhou W, Li J, Li X, Wang Q, Jin X, Yin J, Chen L, Zhang Y, Xu J, Li X. Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat Commun. 2020;11:1000. doi: 10.1038/s41467-020-14802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzi V, Lakkala J, Devarajan R, Savolainen ER, Koistinen P, Heljasvaara R, Pihlajaniemi T. Vanin 1 (VNN1) levels predict poor outcome in acute myeloid leukemia. Am J Hematol. 2018;93:E4–E7. doi: 10.1002/ajh.24925. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015;45:2886–2897. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Correa B, Bergua JM, Campos C, Gayoso I, Arcos MJ, Banas H, Morgado S, Casado JG, Solana R, Tarazona R. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–891. doi: 10.1016/j.cyto.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamm H, Klingler F, Grossjohann EM, Muschhammer J, Vettorazzi E, Heuser M, Mock U, Thol F, Vohwinkel G, Latuske E, Bokemeyer C, Kischel R, Dos Santos C, Stienen S, Friedrich M, Lutteropp M, Nagorsen D, Wellbrock J, Fiedler W. Immune checkpoints PVR and PVRL2 are prognostic markers in AML and their blockade represents a new therapeutic option. Oncogene. 2018;37:5269–5280. doi: 10.1038/s41388-018-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H. T-Cell immunoglobulin and ITIM Domain (TIGIT) associates with CD8+ T-Cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res : An Offic J Am Assoc Cancer Res. 2016;22:3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 28.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Sánchez MV, Fuster JL, Campillo JA, Galera AM, Bermúdez-Cortés M, Llinares ME, Ramos-Elbal E, Pascual-Gázquez JF, Fita AM, Martínez-Banaclocha H, Galián JA, Gimeno L, Muro M, Minguela A. Expression of NK cell receptor ligands on leukemic cells is associated with the outcome of childhood acute leukemia. Cancers. 2021;13:2294. doi: 10.3390/cancers13102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastaglio S, Wong E, Perera T, Ripley J, Blombery P, Smyth MJ, Koldej R, Ritchie D. Natural killer receptor ligand expression on acute myeloid leukemia impacts survival and relapse after chemotherapy. Blood Adv. 2018;2:335–346. doi: 10.1182/bloodadvances.2017015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattori N, Kawaguchi Y, Sasaki Y, Shimada S, Murai S, Abe M, Baba Y, Watanuki M, Fujiwara S, Arai N, Kabasawa N, Tsukamoto H, Uto Y, Yanagisawa K, Saito B, Harada H, Nakamaki T. Monitoring TIGIT/DNAM-1 and PVR/PVRL2 Immune checkpoint expression levels in allogeneic stem cell transplantation for acute myeloid leukemia. Biol Blood Marrow Transplant. 2019;25:861–867. doi: 10.1016/j.bbmt.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, Iannello A, Mathur N, Jardine KE, Kirn GA, Bell JC, McBurney MW, Raulet DH, Ardolino M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B, Zhao W, Li H, Chen Y, Tian H, Li L, Zhang L, Gao C, Zheng J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol, Immunotherapy : CII. 2016;65:305–314. doi: 10.1007/s00262-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannopoulos K. Targeting immune signaling checkpoints in acute myeloid leukemia. J Clin Med. 2019;8:236. doi: 10.3390/jcm8020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, Zhang Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Li Y, Lian J, Yang H, Li F, Zhao S, Qi Y, Zhang Y, Huang L. TNF-alpha-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Transl Med. 2019;17:165. doi: 10.1186/s12967-019-1917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goncalves Silva I, Yasinska IM, Sakhnevych SS, Fiedler W, Wellbrock J, Bardelli M, Varani L, Hussain R, Siligardi G, Ceccone G, Berger SM, Ushkaryov YA, Gibbs BF, Fasler-Kan E, Sumbayev VV. The tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dama P, Tang M, Fulton N, Kline J, Liu H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer. 2019;7:175. doi: 10.1186/s40425-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, Akashi K. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, Darley RL. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.